Supplemental Digital Content is available in the text
Abstract
MYC and BCL2 translocations as well as TP53 deletion/mutation are known risk factors in diffuse large B-cell lymphoma (DLBCL) but their interplay is not well understood.
In this retrospective cohort study, we evaluated the combined prognostic impact of TP53 deletion and mutation status, MYC and BCL2 genomic breaks in tumor samples of 101 DLBCL patients. The cohort included 53 cases with MYC rearrangements (MYC+).
TP53 deletions/mutations (TP53+) were found in 32 of 101 lymphomas and were equally distributed between MYC+ and MYC− cases (35.8% vs. 27.1%). TP53+ lymphomas had lower responses to treatment than TP53− (complete remission 34.4% vs. 60.9%; P = 0.01). TP53 alteration was the dominant independent prognostic factor in multivariate analysis (P = 0.01). Overall survival (OS) varied considerably between subgroups with different genomic alterations: Patients with sole MYC translocation, and interestingly, with triple MYC+/BCL2+/TP53+ aberration had favorable outcomes (median OS not reached) similar to patients without genomic alterations (median OS 65 months). In contrast, patients with MYC+/BCL2+/TP53− double-hit lymphomas (DHL) (28 months), MYC+/BCL2−/TP53+ lymphomas (10 months) or sole TP53 mutation/deletion (12 months) had a poor median OS. Our findings demonstrate differences in OS of DLBCL patients depending on absence or presence of single or combined genetic alterations of MYC, BCL2, and TP53. Cooccurrence of TP53 and BCL2 aberrations ameliorated the poor prognostic impact of single TP53+ or BCL2+ in MYC positive patients.
This pilot study generates evidence for the complex interplay between the alterations of genetic pathways in DLBCL, which goes beyond the concept of DHL. The variable survival of DLBCL patients dependent on single or combined alterations in the TP53, MYC, and BCL2 genes indicates the need for comprehensive genomic diagnosis.
INTRODUCTION
Clinical risk stratification and treatment decisions in diffuse large B-cell lymphoma (DLBCL) are still based on the International Prognostic Index (IPI).1 However, increasing evidence suggests that the prognosis is strongly dependent on concomitant genetic alterations.2–6 Many studies verified the importance of translocations of the MYC and BCL2 genes as well as mutations or deletions of the TP53 gene.7–14 However, the clinical impact of simultaneous occurrence of these genetic changes is not well understood, even in the era of whole genome sequencing.15–20
TP53 plays an important role in regulation of cell cycle and cell proliferation. Based on its capability to induce apoptosis upon desoxyribonucleic acid (DNA) damage it acts as a tumor suppressor. Mutations in the TP53 gene abrogate genetic stability and lead to uncontrolled proliferation of oncogene driven tumor cells. TP53 mutations in aggressive B-cell lymphomas are found at frequencies of 33% in Burkitt lymphoma (BL), 21% to 23% in DLBCL, and 29% to 80% in transformed follicular lymphoma.8,9,21 The association of TP53 mutation with inferior overall survival (OS), transformation into aggressive lymphoma and resistance to chemotherapy has been reported21–24; however, recent research often focuses on genetic changes of MYC, BCL2, and BCL6.3,10,13,25,26
The MYC translocation is the hallmark of BL. The balanced translocation between the MYC locus (8q24) and an immunoglobulin gene, most commonly IGH (14q32) results in overexpression of the MYC protein. Albeit being the hallmark of BL, MYC translocations are also found with a frequency of 5% to 15% in DLBCL and in 50% of B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma (BCLU).27 These aggressive B-cell lymphomas have a poor outcome in many studies.3,4,28,29 Lymphomas with concurrent MYC and BCL2 translocations (double-hit lymphoma, DHL) as well as triple hit lymphomas with additional breaks including the BCL6 gene have been investigated in many studies.3,7,10,11,17,25,30–32 DHL are associated with aggressive, often widespread extranodal disease, dismal prognosis, are often refractory to standard chemotherapy13,28,29,33,34 and represent a distinct entity. An overexpression of MYC, BCL2 protein regardless of the underlying genetic hit has also been identified as poor prognostic factor30; however, reported results are not uniform.30,35,36
Despite of the increasing focus on DHL so far, only few studies included the TP53 status in this setting.11,12,37 We have previously studied the interaction of MYC, BCL2, and TP53 in a mouse model and in a small number of patients with BL and DLBCL.38
Here we investigated the prognostic value of TP53 deletions and mutations in patients with and without MYC and/or BCL2 structural aberrations in a large retrospective series of patients treated with immunochemotherapy. The study provides novel insights into the complex interplay of TP53, MYC, and BCL2 alterations in aggressive lymphomas.
METHODS
For this retrospective study we analyzed 2 similar-sized DLBCL cohorts with and without MYC break (N = 53 vs. 48). Inclusion criteria were: 18 years of age or older, known medical history, diagnosed and treated at one of the participating institutions, rituximab containing treatment was administered. Transplant and human immunodeficiency virus (HIV) associated lymphomas were excluded. Clinical and demographical data were collected. Clinical data included previous medical history, date of diagnosis, histological subtypes, art and duration (including number of cycles) treatments, date and quality of response, date of relapse and death, blood tests and observation time.
In a first step patients diagnosed at the Medical University of Vienna with known MYC status at diagnosis were selected (N = 34). Additional 9 cases with MYC translocation were contributed from the Portuguese Institute of Oncology, Lisbon and 7 from other Austrian hospitals. HIV associated lymphomas were not included.
In a second step, 51 cases of the Medical University of Vienna with available tissue were screened for MYC translocation retrospectively, 3 were positive. The 48 MYC negative patients comprised the second group.
In a third step, BCL2 and TP53 fluorescence in situ hybridization (FISH) as well as TP53 sequencing has been performed.
For final analysis, only patients with information about all three (MYC, BCL2, and TP53) genes were included. Lymphomas were designated as “positive” on the basis of genetic results (MYC+: translocations; BCL2+: translocations; TP53+: deletions and/or mutations) regardless of protein expression.
All cases were reviewed by 2 independent pathologists and diagnoses were adjusted according to the WHO Classification 2008,39 cell of origin (COO) has been defined according to the Hans algorithm.
The final cohort consisted of 101 DLBCL (including 16 BCLU) treated with rituximab containing regimens. Patient's clinical and demographical characteristics are presented in Table 1. Patients were followed until January 2015 or until death, whatever occurred first. Analysis was performed after availability of molecular information. Mean observation period (date of diagnosis to last follow up) was 27.45 months (range 1–128 months; standard deviation 28.22 months).
TABLE 1.
Clinical and Genetic Patient Characteristics
Ethical approval was granted by the Ethics Committee of the Medical University of Vienna (#1051/2013).
Immunohistochemistry (IHC)
IHC was done on whole tissue sections (N = 64) and on sections from tissue microarrays (TMA; N = 37) consisting of 3 representative 0.8 mm cores of tumor tissue. IHC was performed on formalin fixed, paraffin-embedded sections on the automated Leica Bond III Immunostainer (Leica Biosystems, Nussloch, Germany) using routine protocols. According to Kaserer et al,40 TP53 staining (P53 antibody Clone DO-7, DAKO, Glostrup, Denmark) was interpreted as positive if at least 30% of nuclei of tumor cells showed a strong or moderate staining and when there was a sheet like, diffuse staining pattern within the whole tumor tissue or at least within parts of the tumor tissue.
For BCL2 (N = 98) and MYC (N = 83) staining, the following antibodies were used: BCL2 (Clone 124, DAKO), MYC (Clone Y69, Epitomics, Burlingame, CA). Cut-off values for BCL2 and MYC were 50% and 40%, respectively.10 Samples with weakly stained tumor cells were interpreted as negative, regardless of the percentage of positively stained cells.
Interphase FISH Analysis
FISH was done on formalin-fixed, paraffin-embedded tissue except in 18 cases, where fresh tissue was available. Following probes were used: LSI MYC Dual Color, Break Apart Rearrangement Probe (Vysis, Downer's Grove, LSI IGH/BCL-2 Dual Color, Dual Fusion Translocation Probe (Vysis) LSI TP53 (17q13.1) Single Color probe and centromere-17-specific probe (Vysis).41 In each case a total of 200 interphase nuclei was counted. The cut-off for positivity was determined at 10% of nuclei showing aberrant hybridization signals).
Sequencing of TP53
TP53 mutation is associated with increased TP53 protein expression42 and TP53 mutational status was assessed in all TP53 IHC positive cases. TP53 sequencing was performed using ABI Big dye terminator version 1.1 cycle sequencing kit as described previously.38
Sequencing analyses covered exons 4 to 11 and flanking intron regions. Purified DNA fragments were run on an ABI 330 Genetic Analyzer. Sequences were analyzed using the SeqScape analysis software program Versions 2.5 and 2.7 (Applied Biosystems/Life Technologies; Carlsbad, California, USA).
Statistical Methods
Statistical analysis was performed using IBM SPSS Statistics 21.0 (IBM Corporation, Armonk, NY) software. Statistical methods comprised the Chi-square analysis or Fisher exact test to compare baseline characteristics, Kaplan–Meier estimates for survival functions, log-rank test for a comparison of survival distributions and multivariate Cox proportional hazards regression models for the identification of significant and independent prognostic factors for OS. P values (P) of <0.05 (2-sided) were considered statistically significant.
The endpoints were to investigate the incidence of sole/combined genetic aberrations, to characterize clinical and demographic features of the sub-sets as well as to investigate the prognostic influence of sole/combined genetic aberrations in the whole cohort and in the sub-sets.
First, patient's clinical and demographical characteristics were collected. Then all patients with or without MYC-translocation and in a second step the 2 sub-sets with or without TP53 aberrations were compared using the Chi-square analysis or Fisher exact test.
Second, the influence of clinical and molecular parameters on OS was determined using Kaplan–Meier OS curves and the log-rank test. Univariate and multivariate analysis were performed using Cox regression models.
Covariates available for analysis were presence or absence of MYC+, BCL+, and TP53+ (regardless of combined aberrations), age, serum lactate dehydrogenase levels (LDH) (U/ml), bone marrow (BM) infiltration (numeric) and factor variables such as sex, age-group (>60 years or younger), IPI high (3–5) versus low (1–2), LDH high versus low, presence or absence of BM infiltration, localization (nodal/extranodal), transformation, COO, and first-line therapy. BM infiltration and LDH were considered as numeric as well as factor variables. Transformation and combined genetic aberrations were excluded from further considerations in the Cox models due to a not sufficient number of cases.
RESULTS
TP53 Deletions/Mutations
TP53 FISH and TP53 IHC were performed in all samples. FISH analysis revealed a TP53 heterozygote deletion in 12 cases (11.9%). Patient samples with positive IHC were selected for further sequencing (N = 49). Missense mutations were found in 29 tumor samples (28.7%). Altogether, 32 (31.7%) patients had TP53 deletions and/or mutations. The majority of these patients had TP53 mutation only (N = 20, 19.8%), 9 (8.9%) patients had both a deletion and a mutation on the remaining allele, and 3 patients (3%) had a deletion but no material available for sequencing (Supplementary Table 1). TP53 mutations are displayed in Supplementary Table 1.
There were no major differences in the characteristics of patients with or without TP53 alteration at diagnosis regarding sex, age, clinical stage (CS), IPI, BM infiltration, LDH, COO (Table 1). Standard rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) therapy and high-dose methotrexate (HD-MTX) containing treatments were equally used in TP53+ and TP53− patients.
Patients with TP53+ showed significantly more primary resistance to treatment (complete remission (CR) rate 34.4% vs. 60.9%, progressive disease (PD) 34.4% vs. 15.9%) (P = 0.01). Patients with a TP53+ lymphoma showed a significantly shorter OS (median 12 months vs. not reached; hazard ratio (HR) 2.206, 95% confidence interval (95% CI) 1.183–4.117; P = 0.01) (Table 2; Figure 1A).
TABLE 2.
Uni- and Multivariate Analyses Determining the Influence of Clinical and Molecular Parameters at Diagnosis on Overall Survival of 101 Patients
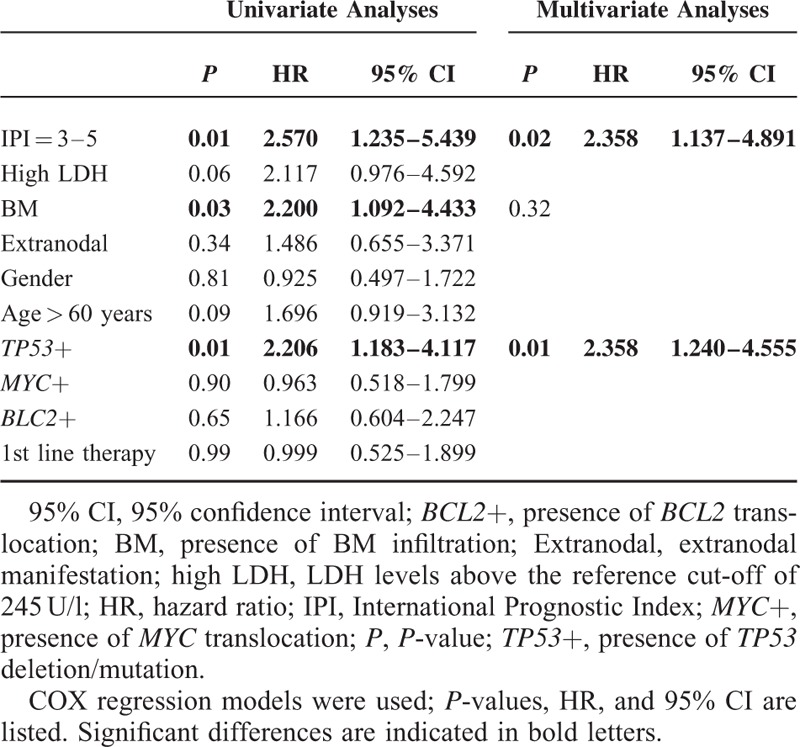
FIGURE 1.
TP53 alteration is associated with significant shorter overall survival in the whole cohort while its prognostic value is attenuated in patients with MYC translocation. Kaplan–Meier plots showing (A) overall survival of all patients (N = 101) according toTP53 deletion/mutation; (B) overall survival of MYC negative patients (N = 48) according to TP53 deletion/mutation; and (C) overall survival of MYC positive patients (N = 53) according to TP53 deletion/mutation. The negative prognostic impact of TP53 alteration is reduced and loses significance in patients with a concurrent MYC translocation. P: P-value.
Relationship Between MYC Translocations and TP53 Deletions/Mutations
MYC+ cases were similarly distributed between the TP53+ and TP53− cohorts (59.4% vs. 49.3%). Vice versa, there were 35.8% TP53+ cases in the MYC+ cohort versus 27.1% in MYC− patients (Table 1). These data indicate that TP53+ occur at similar frequency in lymphomas irrespective of the absence or presence of MYC+.
We next compared the impact of TP53 status on survival of MYC+ or − patients. TP53+ patients without MYC+ had a significantly shorter OS (median OS 12 months vs. 65 months; HR 3.510, 95% CI 1.374–8.967; P = 0.01) (Figure 1B). Interestingly, there was no significant impact of TP53+ in the MYC+ group on OS (median 10 months vs. not reached; HR 1.840, 95% CI 0.759–4.461; P = 0.18) (Figure 1C). We noted that a considerable number of combined TP53+/MYC+ lymphomas were also BCL2+ (triple+) (N = 7, 13.2%) (Table 1). This observation together with the loss of impact of TP53+ in MYC+ patients prompted us to perform a further subgroup analysis including BCL2+.
Relationship Between BCL2 Translocations and TP53 Mutation/Deletions
BCL2+ were detected in 28 patients (27.7%). They rarely occurred alone (4/28) but mainly in conjunction with additional aberrations. BCL2+ were frequently associated with MYC+ lymphomas (DHL: 15/28), followed by TP53+ and MYC+ (7/28) and TP53+ (2/28). They were evenly distributed between TP53+ (9/32, 28.1%) and TP53− (19/69, 27.5%) patients (Table 1). BCL2/TP53 directed analysis revealed a differential clinical outcome in the TP53+ and TP53− subgroups. In TP53− patients, BCL2+ had a poor prognostic impact (median OS 28 months vs. not reached; HR 2.439, 95% CI 1.065–5.582; P = 0.04) (Figure 2A). Interestingly, in TP53+ patients an additional BCL2+ even reversed prognosis indicating an equalizing effect of the two genetic defects (median OS not reached vs. 10 months; HR 0.308, 95% CI 0.086–1.102; P = 0.07) (Figure 2B).
FIGURE 2.
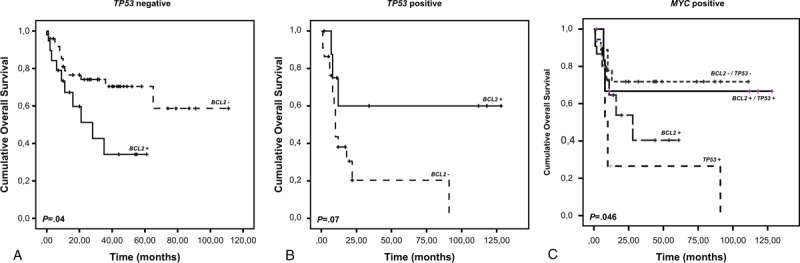
Additional BCL2 translocation abrogates the negative prognostic impact of TP53 aberration. Overall survival of (A) TP53 negative patients (N = 69) according to BCL2 translocations; (B) TP53 positive patients (N = 32) according to BCL2 translocations; The presence of BCL2 translocation has opposite impact on overall survival in TP53 negative (inferior) and positive (superior) patients; (C) MYC positive patients (N = 53) according to combined genetic aberrations. Patients with cooccurrence of TP53, BCL2 and MYC aberrations (triple-hit) have a good prognosis. P: P-value.
Outcome of Patients According to Combined TP53, MYC, and BCL2 Genetic Analysis
A similarly unexpected result was observed when MYC+ patients were analyzed for OS in relation to TP53 and BCL2. Patients with sole MYC+ TP53−/BCL2− (N = 18) had the best OS (median not reached) (Figure 2C). However, the OS of the 7 patients with a triple MYC+/TP53+/BCL2+ aberration was almost equally favorable (median OS not reached). In contrast, patients with MYC+/BCL2+ or MYC+/TP53+ had a shorter median survival of 28 or 10 months, respectively. Patients with sole TP53+ (N = 11) had an OS of 12 months while patients without any of the 3 alterations (N = 31) had an OS of 65 months (data not shown).
Univariate and Multivariate Analysis of Clinical and Genetic Factors
Results of univariate analysis for OS are shown in Table 2. In multivariate analysis high IPI (HR 2.358, 95% CI 1.137–4.891; P = 0.02) and TP53+ (HR 2.358, 95% CI 1.240–4.555; P = 0.01) remained independent poor prognostic factors. Because of the small sample size and low statistic power a Cox regression model describing the interaction between BCL2+ and TP53+ was performed in which the reversal of relative risk was also seen in the combination (Table 3). This effect remained after adjustment for IPI.
TABLE 3.
Unadjusted and IPI Adjusted COX Regression Models of Overall Survival Comparing Single and Combined BCL2 and TP53 Aberrations in the MYC Positive Patient Group (N = 53)
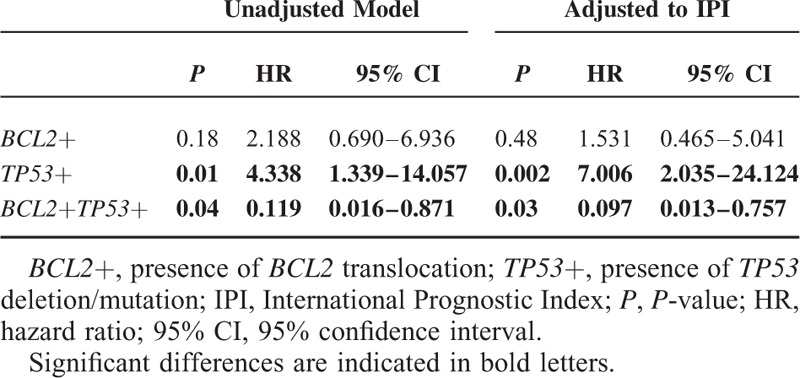
Double-Hit Protein Score (DHS) in Molecular Double-Hit and Triple-Hit Lymphomas
Protein staining for all MYC, BCL2, and TP53 was available in 80 samples. Nine (60%) of the DHL had also a protein expression for both MYC and BCL2 (DHS 2), 3 patients (20%) had a DHS of 1 (2 patients due to BCL2 expression, 1 due to MYC expression), the other 3 patients were positively stained for BCL2 protein, but MYC protein staining was not available. Among the triple-hit lymphomas 4 patients (57.14%) were with DHS of 2, 2 (14.29%) with DHS2 and 1 patient had BCL2 protein, but MYC staining was not done (Supplementary Table 2). Some representative examples are shown in Figures 3 and 4.
FIGURE 3.
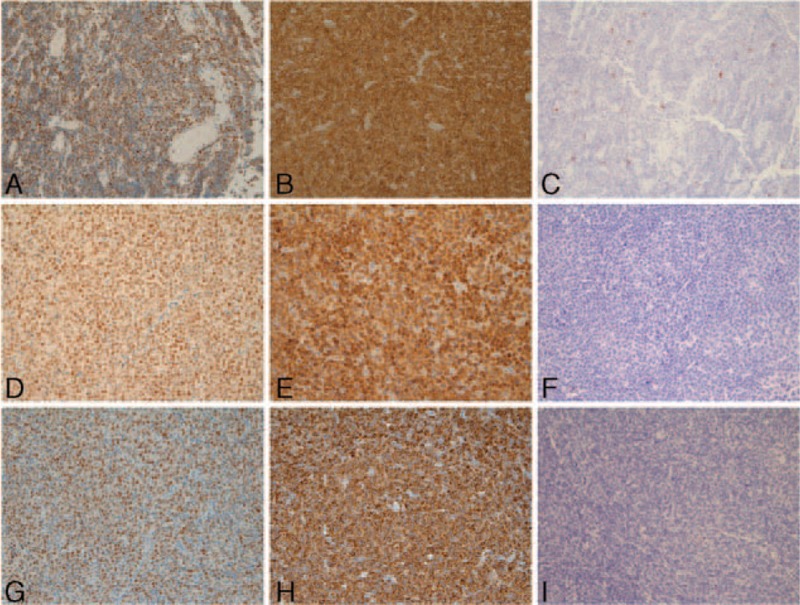
Immunohistochemistry staining profiles of exemplary double-hit patients. Three cases of MYC+/BCL2+ lymphomas. (A–C) Case #31; (D–F) case #37; (G–I) case #86. Panels A/D/G: positive staining for MYC protein; panels B/E/H: positive staining for BCL2; panels C/F/I: negative staining for TP53.
FIGURE 4.
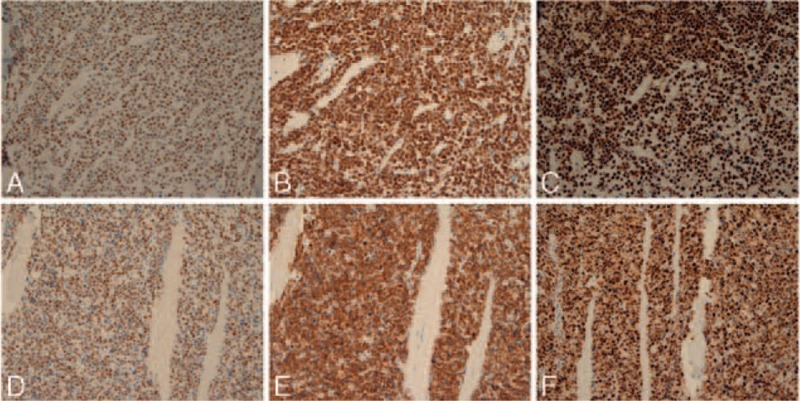
Immunohistochemistry staining profiles of exemplary triple-hit patients, Two cases of MYC+/BCL2+/TP53+ lymphomas. (A–C) Case #83; (D–F): case #26. Panels A and D: positive staining for MYC protein; panels B and E: positive staining for BCL2; panels C and F: positive staining for TP53.
DISCUSSION
The poor prognostic impact of combined MYC and BCL2 translocations (“DH”) in DLBCL has been described in several studies.3,7,33,34,43–45 Likewise, TP53 mutation or deletion has been identified as poor prognostic factor in aggressive lymphomas.8,9,42 However, there are only few reports which integrate all 3 markers11,12,26,46 and data on the prognostic impact on cooccurrence of these aberrations are scarce. Here we have compiled a sufficiently large series of patients allowing for clinical outcome analysis. Our data show a complex interdependence of TP53+, MYC+, and BCL2+ with unexpected results.
Within our cohort TP53+ was the major outcome determinant, with a significant impact on OS as a sole parameter, and remaining significant in multivariate analysis. TP53+ were found in almost one-third of patients with equal distribution between the MYC+ and MYC− subgroups, as well as in the IHC assessed germinal center like (GCB) versus nongerminal center like (nGCB) groups. Clinical characteristics did not differ between TP53+ and TP53− patients at diagnosis. Importantly, more TP53+ patients were identified by subsequent sequencing analysis than by FISH only (19.8% vs. 11.9%). The frequency in our cohort was slightly higher than the reported 21% to 23% of TP53+ in unselected DLBCL.8,9,47 Xu-Monette et al42 reported a TP53 mutation prevalence of 21.9% in de novo DLBCL and described TP53 mutation as independent poor prognostic factor in the GCB subtype and, to a lesser extent, in the nGCB group. Gebauer et al26 investigated the prevalence of TP53 mutations in DHL and found an elevated frequency within MYC+/BCL2+ DHL with an incidence of 35.5% (6/17), compared to 6.25% (1/16) in MYC+/BCL6+ DHL. Information on clinical outcome was not provided. Hu et al found a similar incidence of TP53 mutations in MYC/BCL2 protein positive and negative DLBCL. They observed a slight cumulative adverse impact on OS when MYC/BCL2 protein coexpression was combined with TP53 mutation; however, these data were not significant and MYC and BCL2 status was assessed by IHC and not on a genetic level.11 Another single institutional study based on IHC37 reported a significant correlation of TP53 and MYC protein expression (P = 0.001) in a series of 85 DLBCLs. An enhanced negative impact on OS was observed when both proteins were overexpressed. Recently, the DHS based on IHC of the MYC and BCL2 proteins has shown significant discriminative power.30 We also evaluated the DHS on our samples and found considerable overlap (data not shown). When patients with simultaneous expression of MYC and BCL2 proteins were analyzed together with TP53+ a similar survival pattern as with genetic triple-hits was observed.
Sole MYC+ were associated with a particularly good outcome in our study. Patients with DHL had an inferior survival. A negative prognostic impact of MYC breaks in DLBCL, and particularly of the MYC+/BCL2+ DHL treated with both CHOP48,49 and R-CHOP regimens11,50–52 has been established in the literature. Despite this evidence the matter is not completely resolved. Some other reports30,31,36 could not demonstrate a poor prognostic impact of a sole MYC translocation or MYC protein expression in DLBCL, but only when BCL2 (regardless of translocation or protein expression) was also altered (double-hit “DH”).30,31 In another study based on IHC37 the authors have not detected significant differences in OS between MYC and BCL2 protein positive cases. Recently, Horn et al found a different biological risk profile in a young high-risk DLBCL cohort.35 Breaks in the BCL2 gene served here as the strongest negative prognostic marker in uni- and multivariate analysis for OS and PFS, whereas MYC break only showed a tendency toward inferior OS in these young patients.35 The discrepancies in published results are probably due to the complexity of DLBCL biology14,53 and the method of detection of MYC, BCL2, or TP53 alterations at the protein or genetic level.
Most strikingly, TP53+ lost its negative influence in the presence of concurrent BCL2+ in our cohort. The combined aberration (BCL2+ in combination with TP53+) even improved survival. This interesting equalizing effect of BCL2+ in combination with a TP53+ was first observed by our group in an Eμ-myc-induced lymphoma mouse model.38 In this genetic murine model disease latency was significantly influenced by the nature of the “second-hit,” which was either defined as high expression of BCL2 or disruption of the TP53 pathway.38 The latter led to disease progression through escape from immunosurveillance as shown in in vitro and in vivo experiments. Schuster et al38 were able to ameliorate the negative effect of TP53-deficiency by enforced overexpression of BCL2, which resulted in reinduction of immunologic control mediated by functional NK and T cells. Although, this effect has potentially contributed to our results, there might be other explanations: In a healthy cell proapoptotic TP53 and antiapoptotic BCL2 are well-balanced counterparts. P53 may induce apoptosis by transcriptional activity through upregulation of proapoptotic BH3-only family members PUMA and NOXA and by nontranscriptional mechanisms through direct binding to BCL2 and its family proteins leading to BAX activation.54 If this balance is destroyed by loss of TP53 function through deletion/mutations or by overexpression of BCL2 protein, uncontrolled cell proliferation may occur as a consequence. Concurrent TP53 and BCL2 aberrations may thus render cells vulnerable and more sensitive for immunochemotherapy. However, we could not observe a higher Ki-67 proliferation index in this group of DLBCL patients (data not shown).
Generally accepted treatment recommendations for DLBCLs with MYC and BCL2 translocations are still lacking. Since there is a consensus that DHL have an aggressive clinical course and poor response to conventional immunochemotherapy,7,11,18,20,29,33,45,51 many patients with DHL have received high-dose methotrexate containing (HD-MTX) treatment. In our series, HD-MTX containing regimens were not superior to R-CHOP, particularly in patients over 60 years (data not shown). This is in line with previous results from the MRC/NCRI LY1055 and data published by Snuderl et al.28 Dose-adjusted EPOCH-R (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and rituximab) which has already been shown to be efficient in the treatment of adults with sporadic and immunodeficiency associated BL56 and DLBCL with low and intermediate IPI,57,58 produced also impressive remission in a small series of DHL.59 Recently reported data indicate that BCL2 expression measured by IHC allows better prognostic discrimination than FISH.59 It will be interesting to evaluate patients failing this therapy for additional genetic events, including TP53+. The findings from our study may also support the development of novel drugs, such as BH3 mimetics/BCL2 inhibitors.60 A very recent study has evaluated the clinical effect of ibrutinib on DLBCL outcome. Interestingly, activated B-cell type lymphomas responded much better than GCB lymphomas.61 It will be interesting to analyze the TP53 status in this study.
Every retrospective study has some caveats. Patients were not uniformly treated but all patients received rituximab containing therapy. Our cohort was mainly collected in a referral center, possibly selecting for more advanced or aggressive cases. This might explain the somewhat higher number of TP53+ lymphomas. We did not exclude transformed follicular lymphoma (N = 8), thus possibly accounting for the accumulation of DHL. With the goal to focus on the most frequent genetic hits (TP53+, MYC+, and BCL2+) BCL6 aberrations and MYC translocation partners were not assessed, since the inclusion of further factors would have caused the loss of statistic power in such a small sample size. Our patient group with concurrent TP53+ and BCL2+ is still small (7 MYC+/BCL2+/TP53+ and 2 MYC-/BCL2+/TP53+ patients). Nevertheless, this unique series of DLBCL patients with genetic work-up allowed important insights into the interdependence of genetic lesions as prognostic markers. The results force the necessity for comprehensive genetic and molecular diagnostics beyond the recognition of DH.
In conclusion, our data show that TP53+ occur in approximately one-third of all DLBCL and are associated with poor prognosis. However, subgroups with very different prognosis depending on the occurrence or co-occurrence of genomic aberrations of TP53, MYC, and/or BCL2 can be defined. These findings provide further evidence for more extensive molecular or genetic diagnostics in aggressive lymphomas.
Supplementary Material
Acknowledgments
The authors would like to thank Bettina Pichlhöfer, Tina-Maria Holper, Barbara Neudert, Andrea Alvarez-Hernandez, Yuliya Grin for excellent technical assistance as well as Dr. D.I. Michael Melcher for statistical support. All persons listed above gave permission to the first and/or the last author.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, BCL2 = B-cell CLL/lymphoma 2, BCL6 = B-cell CLL/lymphoma 6, BCLU = B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma, BL = Burkitt lymphoma, BM = bone marrow, COO = cell of origin, CR = complete remission, CS = clinical stage, DH = double-hit, DHL = double-hit lymphoma, DHS = double-hit score, DLBCL = diffuse large B-cell lymphoma, DNA = desoxyribonucleic acid, EPOCH-R = etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone and rituximab, FISH = fluorescence in situ hybridization, GCB = germinal center like, HD-MTX = high-dose methotrexate, HIV = human immunodeficiency virus, HR = hazard ratio, IGH = immunoglobulin heavy locus, IHC = immunohistochemistry, IPI = International Prognostic Index, LDH = lactate dehydrogenase, MYC = v-myc avian myelocytomatosis viral oncogene homolog gene, nGCB = non-germinal center like, OS = overall survival, P = P-value, PD = progressive disease, PR = partial remission, R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone, SD = stable disease, TMA = tissue microarray, TP53 = tumor protein p53.
This study was supported by a grant of the Theodor Koerner Fonds awarded to Edit Porpaczy.
The authors have no conflicts of interests to disclose.
REFERENCES
- 1.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329:987–994. [DOI] [PubMed] [Google Scholar]
- 2.Roschewski M, Staudt LM, Wilson WH. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol 2014; 11:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood 2011; 117:2319–2331. [DOI] [PubMed] [Google Scholar]
- 4.Slack GW, Gascoyne RD. MYC and aggressive B-cell lymphomas. Adv Anat Pathol 2011; 18:219–228. [DOI] [PubMed] [Google Scholar]
- 5.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015; 125:22–32. [DOI] [PubMed] [Google Scholar]
- 6.Sehn LH. Introduction to a clinical review series on aggressive B-cell lymphoma. Blood 2015; 125:1–2. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 2009; 114:2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung KJ, Horsman DE, Gascoyne RD. The significance of TP53 in lymphoid malignancies: mutation prevalence, regulation, prognostic impact and potential as a therapeutic target. Br J Haematol 2009; 146:257–269. [DOI] [PubMed] [Google Scholar]
- 9.Young KH, Leroy K, Moller MB, et al. Structural profiles of TP53 gene mutations predict clinical outcome in diffuse large B-cell lymphoma: an international collaborative study. Blood 2008; 112:3088–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013; 121:2253–2263. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013; 121:4021–4031.quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiskvik I, Beiske K, Delabie J, et al. Combining MYC, BCL2 and TP53 gene and protein expression alterations improves risk stratification in diffuse large B-cell lymphoma. Leuk Lymphoma 2014; 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Pillai RK, Sathanoori M, Van Oss SB, et al. Double-hit B-cell lymphomas with BCL6 and MYC translocations are aggressive, frequently extranodal lymphomas distinct from BCL2 double-hit B-cell lymphomas. Am J Surg Pathol 2013; 37:323–332. [DOI] [PubMed] [Google Scholar]
- 14.Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood 2013; 122:3884–3891. [DOI] [PubMed] [Google Scholar]
- 15.Morin RD, Gascoyne RD. Newly identified mechanisms in B-cell non-Hodgkin lymphomas uncovered by next-generation sequencing. Semin Hematol 2013; 50:303–313. [DOI] [PubMed] [Google Scholar]
- 16.Morin RD, Mungall K, Pleasance E, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood 2013; 122:1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedberg JW. Double-hit diffuse large B-cell lymphoma. J Clin Oncol 2012; 30:3439–3443. [DOI] [PubMed] [Google Scholar]
- 18.Dunleavy K, Grant C, Wilson WH. Using biologic predictive factors to direct therapy of diffuse large B-cell lymphoma. Ther Adv Hematol 2013; 4:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffe ES, Pittaluga S. Aggressive B-cell lymphomas: a review of new and old entities in the WHO classification. Hematology Am Soc Hematol Educ Program 2011; 2011:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheah CY, Oki Y, Westin JR, et al. A clinician's guide to double hit lymphomas. Br J Haematol 2015; 168:784–795. [DOI] [PubMed] [Google Scholar]
- 21.Zainuddin N, Berglund M, Wanders A, et al. TP53 mutations predict for poor survival in de novo diffuse large B-cell lymphoma of germinal center subtype. Leuk Res 2009; 33:60–66. [DOI] [PubMed] [Google Scholar]
- 22.Preudhomme C, Fenaux P. The clinical significance of mutations of the P53 tumour suppressor gene in haematological malignancies. Br J Haematol 1997; 98:502–511. [DOI] [PubMed] [Google Scholar]
- 23.Simonitsch-Klupp I, Hauser I, Ott G, et al. Diffuse large B-cell lymphomas with plasmablastic/plasmacytoid features are associated with TP53 deletions and poor clinical outcome. Leukemia 2004; 18:146–155. [DOI] [PubMed] [Google Scholar]
- 24.Krug U, Ganser A, Koeffler HP. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene 2002; 21:3475–3495. [DOI] [PubMed] [Google Scholar]
- 25.Akyurek N, Uner A, Benekli M, et al. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 2012; 118:4173–4183. [DOI] [PubMed] [Google Scholar]
- 26.Gebauer N, Bernard V, Gebauer W, et al. TP53 mutations are frequent events in double-hit B-cell lymphomas with MYC and BCL2 but not MYC and BCL6 translocations. Leuk Lymphoma 2015; 56:179–185. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Anastasi J, Cohen KS, et al. The impact of MYC expression in lymphoma biology: beyond Burkitt lymphoma. Blood Cells Mol Dis 2010; 45:317–323. [DOI] [PubMed] [Google Scholar]
- 28.Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol 2010; 34:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carbone A, Gloghini A, Aiello A, et al. B-cell lymphomas with features intermediate between distinct pathologic entities. From pathogenesis to pathology. Hum Pathol 2010; 41:621–631. [DOI] [PubMed] [Google Scholar]
- 30.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012; 30:3460–3467. [DOI] [PubMed] [Google Scholar]
- 31.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012; 30:3452–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thieblemont C, Briere J. MYC, BCL2, BCL6 in DLBCL: impact for clinics in the future? Blood 2013; 121:2165–2166. [DOI] [PubMed] [Google Scholar]
- 33.Le Gouill S, Talmant P, Touzeau C, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 2007; 92:1335–1342. [DOI] [PubMed] [Google Scholar]
- 34.Dunleavy K. Double-hit lymphomas: current paradigms and novel treatment approaches. Hematology Am Soc Hematol Educ Program 2014; 2014:107–112. [DOI] [PubMed] [Google Scholar]
- 35.Horn H, Ziepert M, Wartenberg M, et al. Different biological risk factors in young poor-prognosis and elderly patients with diffuse large B-cell lymphoma. Leukemia 2015; 29:1564–1570. [DOI] [PubMed] [Google Scholar]
- 36.Visco C, Tzankov A, Xu-Monette ZY, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica 2013; 98:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Y, Bulbul MA, Ji L, et al. p53 expression is a strong marker of inferior survival in de novo diffuse large B-cell lymphoma and may have enhanced negative effect with MYC coexpression: a single institutional clinicopathologic study. Am J Clin Pathol 2014; 141:593–604. [DOI] [PubMed] [Google Scholar]
- 38.Schuster C, Berger A, Hoelzl MA, et al. The cooperating mutation or “second hit” determines the immunologic visibility toward MYC-induced murine lymphomas. Blood 2011; 118:4635–4645. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow SH, International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 40.Kaserer K, Schmaus J, Bethge U, et al. Staining patterns of p53 immunohistochemistry and their biological significance in colorectal cancer. J Pathol 2000; 190:450–456. [DOI] [PubMed] [Google Scholar]
- 41.Wiesner T, Streubel B, Huber D, et al. Genetic aberrations in primary cutaneous large B-cell lymphoma: a fluorescence in situ hybridization study of 25 cases. Am J Surg Pathol 2005; 29:666–673. [DOI] [PubMed] [Google Scholar]
- 42.Xu-Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2012; 120:3986–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Lin P, Young KH, et al. MYC/BCL2 double-hit high-grade B-cell lymphoma. Adv Anat Pathol 2013; 20:315–326. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol 2012; 25:145–156. [DOI] [PubMed] [Google Scholar]
- 45.Niitsu N, Okamoto M, Miura I, et al. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 2009; 23:777–783. [DOI] [PubMed] [Google Scholar]
- 46.Akay OM, Aras BD, Isiksoy S, et al. BCL2, BCL6, IGH, TP53, and MYC protein expression and gene rearrangements as prognostic markers in diffuse large B-cell lymphoma: a study of 44 Turkish patients. Cancer Genet 2014; 207:87–93. [DOI] [PubMed] [Google Scholar]
- 47.Leroy K, Haioun C, Lepage E, et al. p53 gene mutations are associated with poor survival in low and low-intermediate risk diffuse large B-cell lymphomas. Ann Oncol 2002; 13:1108–1115. [DOI] [PubMed] [Google Scholar]
- 48.Yoon SO, Jeon YK, Paik JH, et al. MYC translocation and an increased copy number predict poor prognosis in adult diffuse large B-cell lymphoma (DLBCL), especially in germinal centre-like B cell (GCB) type. Histopathology 2008; 53:205–217. [DOI] [PubMed] [Google Scholar]
- 49.Klapper W, Stoecklein H, Zeynalova S, et al. Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL). Leukemia 2008; 22:2226–2229. [DOI] [PubMed] [Google Scholar]
- 50.Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 2009; 114:3533–3537. [DOI] [PubMed] [Google Scholar]
- 51.Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010; 28:3360–3365. [DOI] [PubMed] [Google Scholar]
- 52.Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Ann Oncol 2014; 25:2124–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lossos IS. Molecular pathogenesis of diffuse large B-cell lymphoma. J Clin Oncol 2005; 23:6351–6357. [DOI] [PubMed] [Google Scholar]
- 54.Ploner C, Kofler R, Villunger A. Noxa: at the tip of the balance between life and death. Oncogene 2008; 27 Suppl. 1:S84–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood 2008; 112:2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med 2013; 369:1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol 2008; 26:2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purroy N, Bergua J, Gallur L, et al. Long-term follow-up of dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) in untreated patients with poor prognosis large B-cell lymphoma. A phase II study conducted by the Spanish PETHEMA Group. Br J Haematol 2014. [DOI] [PubMed] [Google Scholar]
- 59.Dunleavy K, Fanale M, LaCasce A, et al. Preliminary report of a multicenter prospective phase II study of DA-EPOCH-R in MYC-rearranged aggressive B-cell lymphoma, 1242014 presented at the 56th ASH Annual Meeting and Exposition; Abstract No. 395 https://ash.confex.com/ash/2014/webprogram/Paper74656.html. [Google Scholar]
- 60.Kunkalla K, Liu Y, Qu C, et al. Functional inhibition of BCL2 is needed to increase the susceptibility to apoptosis to SMO inhibitors in diffuse large B-cell lymphoma of germinal center subtype. Ann Hematol 2013; 92:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015; 21:922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


