Abstract
The combined hyperhomocysteinemia condition is a feature of the Chinese hypertensive population. This study used the case-control method to investigate the association between plasma homocysteine and the C677T gene polymorphism of its key metabolic enzyme, 5, 10-methylenetetrahydrofolate reductase (MTHFR), and early renal damage in a hypertensive Chinese Han population.
A total of 379 adult essential hypertensive patients were selected as the study subjects. The personal information, clinical indicators, and the C677T gene polymorphism of MTHFR were texted. This study used the urine microalbumin/urine creatinine ratio (UACR) as a grouping basis: the hypertension without renal damage group (NRD group) and the hypertension combined with early renal damage group (ERD group).
Early renal damage in the Chinese hypertensive population was associated with body weight, systolic pressure, diastolic pressure, urea nitrogen, serum creatinine, cystatin C, uric acid, aldosterone, and glomerular filtration rate. The homocysteine level and the UACR in the TT genotype group were higher than those in the CC genotype group. The binary logistic regression analysis results showed that after sex and age were adjusted, the MTHFR C677T gene polymorphism was correlated with early renal damage in hypertension in both the recessive model and in the additive model.
Plasma homocysteine and the C677T gene polymorphism of its key metabolic enzyme MTHFR might be independent risk factors of early renal damage in the hypertensive Chinese Han population.
INTRODUCTION
Data have shown1 that the incidence of hypertension in the world has reached 26% worldwide, and this number may continue to grow in the next few years. Hypertension usually results in clinically targeted organ damage in the heart, brain, kidney, and retina, thus severely endangering human health. It is worth noting that in the Chinese hypertensive population, the incidence of renal damage is approximately 20.87%, whereas it is only 7.43% in the nonhypertensive population.2 Thus, the increase in blood pressure may increase the incidence of renal damage. Renal damage in hypertension is an important cause of end stage renal disease.3 However, because it has a slow disease onset, the disease is occult, and the clinical symptoms of patients are not obvious; consequently, the disease is already late in its course when patients seek treatment. Identifying the risk factors of renal damage in hypertension can assist in screening the high-risk population in early stages to perform effective interventions and increase the quality of life of patients. Currently, based on existing studies, urine microalbumin is usually used as an indicator for early renal damage in clinical situations.4
The combined hyperhomocysteinemia condition is a feature of the Chinese hypertensive population. Because of the influences of genetic and environmental factors, the average plasma homocysteine level of adult hypertensive patients in China is 15 μmol/L, and approximately 75% of patients (91% of men and 63% of women) have the complication of an increase in plasma homocysteine levels.5 The increase of plasma homocysteine is associated with the C677T gene polymorphism in its key metabolic enzyme 5, 10-methylenetetrahydrofolate reductase (MTHFR).6 The frequency of the mutant T allele in the MTHFR C677T gene in the Chinese population is 41%,7 which is higher than in other populations. There have been more studies on the function of homocysteine in cardiovascular and cerebrovascular diseases in recent years. Homocysteine produces toxic effects on vascular endothelial cells to induce vascular endothelial dysfunction. In addition, homocysteine is easily oxidized in the blood to produce a large amount of free radicals to damage vascular endothelial cells. This may be the pathogenic basis of a variety of diseases caused by homocysteine, and this mechanism may influence the functions of the kidney endothelia and glomerular basement membrane cells; therefore, the glomerular filtration membrane charge selectivity and pore size change can cause the presence of urine proteins.
Hyperhomocysteinemia is an independent risk factor of myocardial infarction and stroke in cardiovascular and cerebrovascular diseases.8,9 Therefore, identifying the association between homocysteine and the C677T gene polymorphism in its key metabolic enzyme MTHFR and early renal damage in hypertension may explain the high incidence of renal damage in the Chinese hypertensive population from a new angle. The rate of the increase of plasma homocysteine levels in hypertensive patients showed a positive correlation with target organ damage; this was even more prominent in patients with stroke after hypertension.9 However, current studies on homocysteine and early renal damage in hypertension generally have small sample sizes and are single-indicator analyses. The individual or synergistic effects of other hypertension-associated risk factors such as body weight, smoking, blood glucose, and blood lipid clinical factors with homocysteine on early renal damage in hypertension remain unknown. Furthermore, studies on the combined detection of the C677T gene polymorphism in MTHFR, the key metabolic enzyme of homocysteine, are rare.
This study used the case-control method to detect plasma homocysteine, the C677T gene polymorphism in its key metabolic enzyme MTHFR, urine microalbumin, and a variety of clinical indicators that might be associated with hypertension, such as liver function, kidney function, blood glucose, blood lipids, hypersensitive C-reactive protein, and renin-angiotensin system hormones, in hypertensive patients with or without early renal damage. In addition, combined with possible influencing factors such as sex, age, body weight, smoking and drinking history, family history of hypertension, and time of hypertension history, the risk factors of early renal damage in the Chinese hypertensive population were analyzed to provide a theoretical basis for the early prevention of clinical renal damage in hypertension.
METHODS
Population
A total of 379 adult essential hypertensive patients who were diagnosed in the Cardiology clinic or during hospitalization between January 2012 and December 2014 were selected as the study subjects. This study included 231 men with an average age of 55.67 ± 11.77 years and 148 women with an average age of 60.78 ± 11.33 years. The personal information of the patients including sex, age, body weight, smoking and drinking history, family history of hypertension, and time of hypertension history was recorded using a questionnaire survey. A history of smoking and drinking was defined as a patient who smoked and drank and did not quit smoking and drinking. A family history of hypertension referred to a patient whose immediate family members had essential hypertension. The time of hypertension history was calculated from the first diagnosis of hypertension.
Inclusion and Exclusion Criteria
Hypertension was defined according to the 2010 edition of the Chinese hypertension guidelines.10 An adult systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg was diagnosed as hypertension. Urine microalbumin can be used as a sensitive indicator of early renal damage in hypertension. This study used the urine microAlbumin/urine creatinine ratio (UACR) that is commonly used in clinical studies and is easy to detect as a grouping basis. A UACR between 30 and 300 mg/g was considered the presence of early renal damage in hypertension.11
Grouping: the hypertension without renal damage group (NRD group): systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg and a UACR <30 mg/g (208 cases with 131 men and 77 women); the hypertension combined with early renal damage group (ERD group): systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg and a UACR between 30 and 300 mg/g (171 cases with 100 men and 71 women).
Exclusion criteria: secondary hypertension, UACR >300 mg/g, renal parenchymal or vascular lesions, severe heart failure or renal failure, cardiovascular and cerebrovascular diseases occurring within 6 months (such as myocardial infarction, arrhythmia, and stroke), tumors, and a variety of recent severe infections.
Methods
Urine microalbumin was measured using a BN ProSpec specific protein analyzer (Siemens, Germany). Plasma homocysteine was determined using an OP-162 microfluorescence detector (Opulen Medical, China). Liver functions (alanine aminotransferase and aspartate aminotransferase), kidney function indicators (uric acid, creatinine, urea nitrogen, and cystatin C), blood glucose (fasting blood glucose and glycated hemoglobin), blood lipids (triglycerides, total cholesterol, high-density lipoprotein, and low-density lipoprotein), and hypersensitive C-reactive protein were detected using a MODULAR PP automatic biochemistry analyzer (Hitachi, Japan). Renin-angiotensin system hormones (renin, angiotensin I, angiotensin II, and aldosterone) were detected using radioimmunoassays.
Detection of MTHFR C677T genotypes: human total blood DNA was extracted using the phenol–chloroform method. The genotypes of MTHFR C677T were detected using the Taqman probe technology. The physical location of the MTHFR gene in the HapMap database was chr1: 11768374 to 11788702. The C677T location was rs1801133. The Taqman probes were synthesized by ABI (USA), and the genotypes were automatically analyzed using the ABI7900 fluorescent quantitative PCR analyzer.
Statistical Analysis
A statistical software package (SPSS, version 17.0, Chicago, IL) was used for the analyses. The measurement data were expressed as the mean ± standard deviation
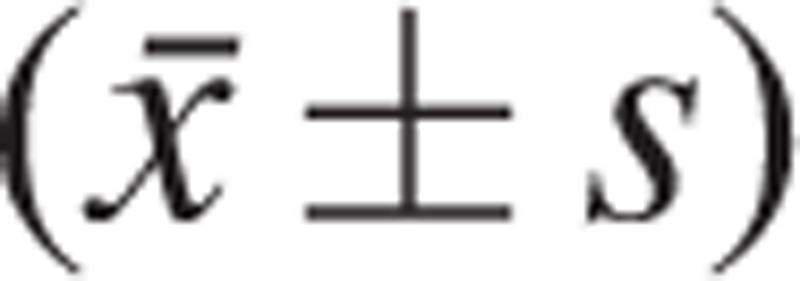 |
. The significance of differences between the groups was determined by χ2 tests for categorical variables. Comparison between the 2 groups was performed using the 2 independent samples t test. Data from 3 groups were compared using one-way analysis of variance (ANOVA). Correlations were determined by Pearson's rank correlation test. The analysis of risk factors was performed using the binary logistic regression. Statistical significance was set at P < 0.05.
RESULTS
Comparison of Basic Information Between These 2 Groups
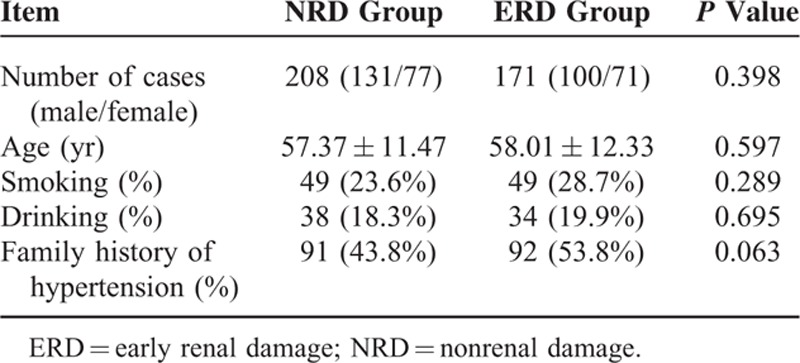
There were 208 cases in the NRD group; the average age was 57.37 ± 11.47 years, and there were 131 men and 77 women included in this study. There were 171 cases in the ERD group; the average age was 58.01 ± 12.33 years, and there were 100 men and 71 women. A comparison between the NRD group and the ERD group showed that sex, age, smoking history, drinking history, and family history of hypertension were all similar and that any differences were not statistically significant (P > 0.05) (Table 1).
TABLE 1.
Comparison of Basic Information Between These 2 Groups
Comparison of Clinical Biochemistry Indicators Between These 2 Groups
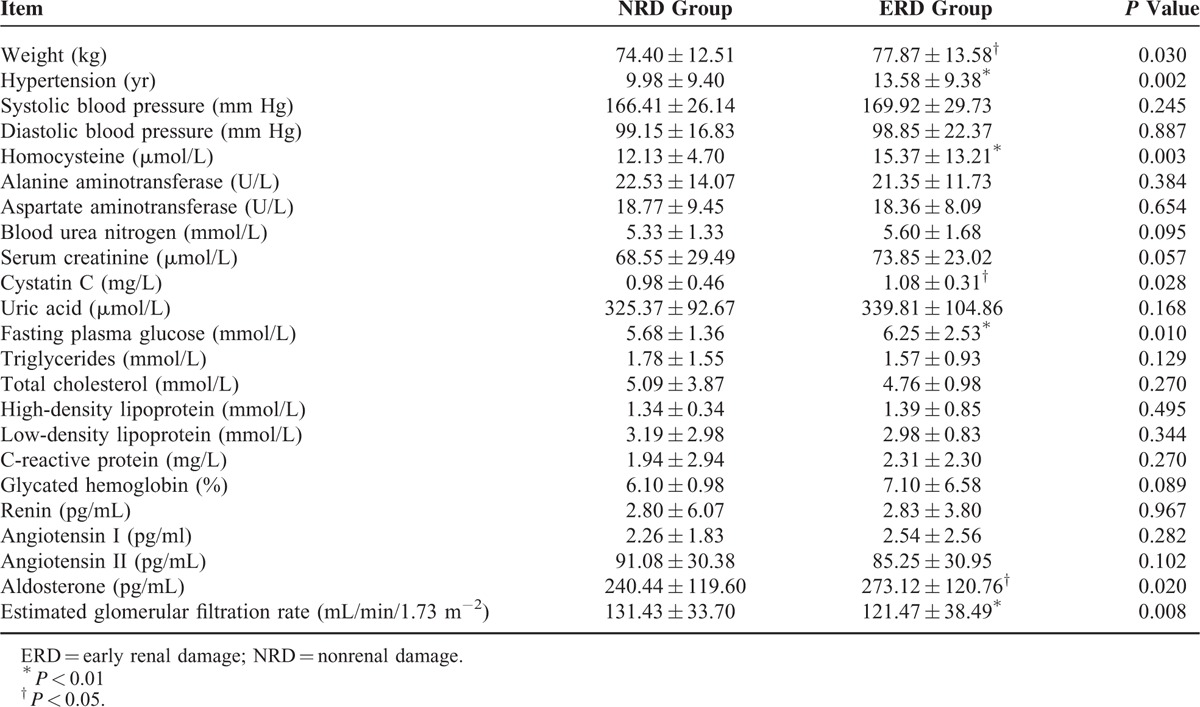
The biochemistry indicators between these 2 groups were compared using the t test. Compared with the NRD group, the ERD group had a higher body weight (74.40 ± 12.51 kg, 77.87 ± 13.58 kg, P < 0.05), a longer hypertension history (9.98 ± 9.40 years, 13.58 ± 9.38 years, P < 0.01), and increased plasma homocysteine (12.13 ± 4.70 μmol/L, 15.37 ± 13.21 μmol/L, P < 0.01), cystatin C (0.98 ± 0.46 mg/L, 1.08 ± 0.31 mg/L, P < 0.05), fasting blood glucose (5.68 ± 1.36 mmol/L, 6.25 ± 2.53 mmol/L, P < 0.01), and aldosterone (240.44 ± 119.60 pg/mL, 273.12 ± 120.76 pg/mL, P < 0.05) levels, whereas the glomerular filtration rate decreased (131.43 ± 33.70 mL/min/1.73 m−2, 121.47 ± 38.49 mL/min/1.73 m−2, P < 0.01). The differences of the other clinical biochemistry indicators did not reach statistical significance (P > 0.05) (Table 2).
TABLE 2.
Comparison of Clinical Biochemistry Indicators Between These 2 Groups
Pearson's Correlation Analysis
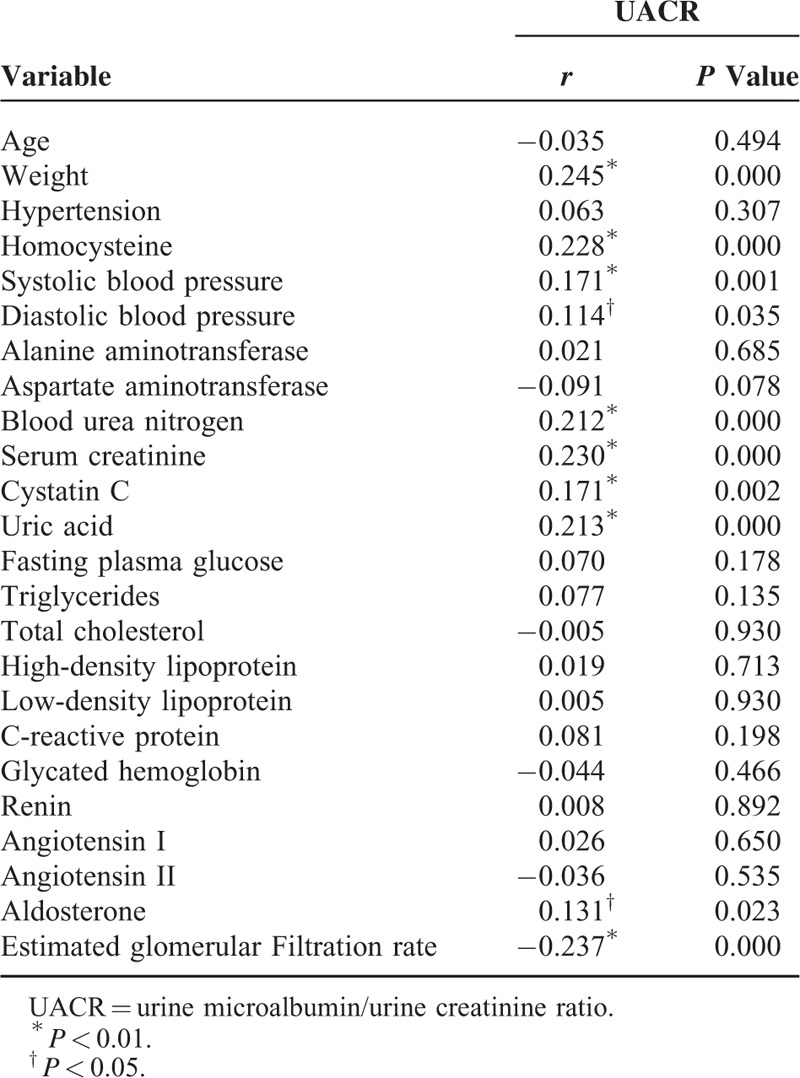
All of the biochemistry indicators and the UACR were used for Pearson's correlation analysis. The results showed that the UACR was positively correlated with body weight (r = 0.245, P < 0.01), plasma homocysteine (r = 0.228, P < 0.01), systolic pressure (r = 0.171, P < 0.01), diastolic pressure (r = 0.114, P < 0.05), urea nitrogen (r = 0.212, P < 0.01), serum creatinine (r = 0.230, P < 0.01), cystatin C (r = 0.171, P < 0.01), uric acid (r = 0.213, P < 0.01), and aldosterone (r = 0.131, P < 0.05) and was negatively correlated with glomerular filtration rate (r = −0.237, P < 0.01). The other indicators were not correlated with the UACR (P > 0.05) (Table 3).
TABLE 3.
Pearson's Correlation Analysis
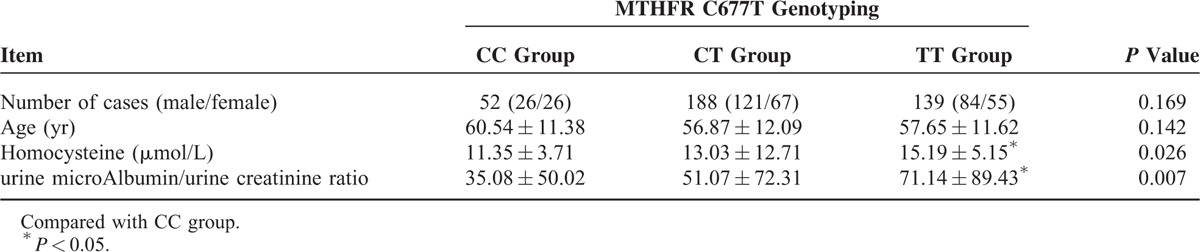
MTHFR C677T Genotyping
MTHFR C677T had 3 genotypes. There were 52 cases with the CC genotype, 188 cases with the CT genotype, and 139 cases with the TT genotype. Sex and age were similar among these 3 genotypes, and any differences were not statistically significant (P > 0.05). Homocysteine and the UACR of these 3 genotypes were used for one-way analysis of variance. The homocysteine level (11.35 ± 3.71 μmol/L, 15.19 ± 5.15 μmol/L, P < 0.05) and the UACR (35.08 ± 50.02 mg/g, 71.14 ± 89.43 mg/g, P < 0.05) in the TT genotype group were higher than those in the CC genotype group, whereas the CT genotype group was not significantly different from the CC and TT genotype groups (P > 0.05) (Table 4).
TABLE 4.
MTHFR C677T Genotyping
Analysis of the Association Between the MTHFR C677T Gene Polymorphism and Early Renal Damage in Hypertension
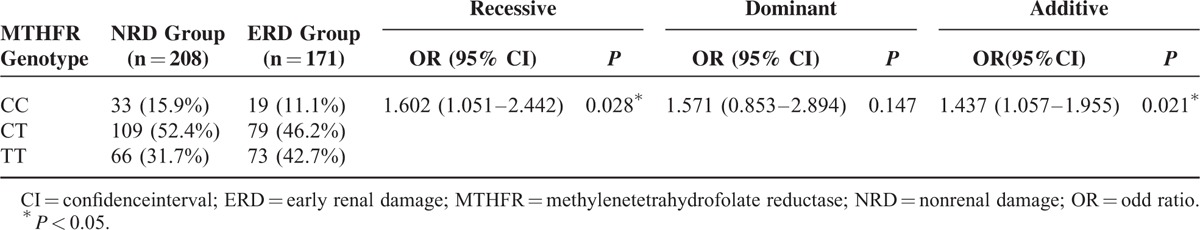
The gene distribution between the NRD group and the ERD group conformed to the Hardy–Weinberg equilibrium testing (P > 0.05) (Table 5). The binary logistic regression analysis results showed that after sex and age were adjusted, the MTHFR C677T gene polymorphism was correlated with early renal damage in hypertension in both the recessive model and in the additive model (P < 0.05). In the dominant model, the MTHFR C677T gene polymorphism was not correlated with early renal damage in hypertension (P > 0.05) (Table 6).
TABLE 5.
Hardy–Weinberg Equilibrium Testing
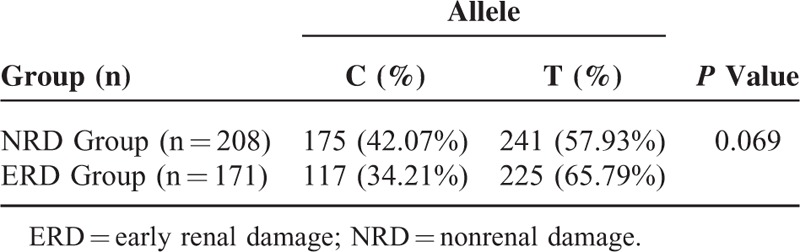
TABLE 6.
Analysis of the Association Between the MTHFR C677T Gene Polymorphism and Early Renal Damage in Hypertension
DISCUSSION
Renal damage in hypertension is always a focus in the prevention of cardiovascular diseases. As a sensitive indicator of early renal damage in hypertension, urine microalbumin has been recognized by the hypertension guidelines.4 Urine microalbumin is a common indicator of subclinical target organ damage in hypertension and is an independent predictive factor of cardiovascular events.12 This study chose the UACR to define early renal damage in hypertension, because that the ratio was more stable in an individual and the sample collection was convenient. Factors such as age, body weight, and smoking can affect the production of urine microalbumin. This study compared enrolled hypertensive patients with or without early renal damage, and the results showed that sex, age, body weight, smoking history, drinking history, and family history of hypertension between the populations in these 2 groups were similar and that there was no significant difference between the groups. Therefore, the influences of the above factors were excluded to ensure the reliability of the results in this study.
The occurrence of plasma homocysteine levels greater than 10 μmol/L is called hyperhomocysteinemia.13 Hypertension patients with combined hyperhomocysteinemia are called H-type hypertensive. As previously mentioned, H-type hypertension is a feature of the Chinese hypertensive population. The hypertensive population enrolled in this study had an average plasma homocysteine level greater than 10 μmol/L. The total incidence of hyperhomocysteinemia was 72.03% (75.32% in men and 66.89% in women). The incidence was 67.79% in the group without renal damage and 77.19% in the group with combined early renal damage. After the increase of plasma homocysteine, the development and progression of some cardiovascular and cerebrovascular diseases might be affected. Hyperhomocysteinemia can affect vascular endothelial functions through a variety of mechanisms, such as causing oxidative damage,14,15 affecting the proliferation of vascular smooth muscle cells,16 and accelerating the progression of atherosclerosis.17 As we know, endothelial dysfunction plays a critical role in early renal damage in hypertension. Therefore, hyperhomocysteinemia has certain associations with early renal damage in hypertension. This study showed that patients with hypertension combined with early renal damage had higher levels of plasma homocysteine than hypertensive patients without renal damage (15.37 ± 13.21 μmol/L, 12.13 ± 4.70 μmol/L, P < 0.01); moreover, the UACR showed a positive correlation with plasma homocysteine (r = 0.228, P < 0.01), which showed that with the increase of plasma homocysteine, urine microalbumin also showed an increasing trend. These results were similar to those of Sabanayagam and Shankar.18 Therefore, plasma homocysteine was correlated with urine microalbumin and was an influencing factor of early renal damage in hypertension.
In recent years, increased interest has focused on the effect of the gene polymorphism of MTHFR, a critical enzyme in folate metabolism, on the metabolism of homocysteine. In 1988, Kang19 et al first discovered a low activity and heat-labile MTHFR mutant, MTHFR C677T. This mutation is located on the 677th amino acid residue in exon 4 of the MTHFR gene, causing a mutation from C to T and resulting in the C677T mutation. The C677T mutation can cause the replacement of Ala with Val at position 223 of the encoded protein molecule, thus producing the labile change of MTHFR. Normal MTHFR activity will decrease by 60% after 5 minutes at 46°C, whereas the activity of the MTHFR mutant encoded by the C677T mutation will decrease by approximately 80% to 90%. This heat-labile change causes the decrease of MTHFR activity in the human body, thus causing the increase of the concentration of plasma homocysteine.
The C677T mutation selected in this study is the most common missense mutation of MTFHR. In normal populations, the frequency of the TT type homozygote is approximately 1.45% in African black people, 12% in Indians,20 and 23% in Italians in Europe.21 The frequency of the mutant T allele of the MTHFR C677T gene in the Chinese population is 41%,7 which is much higher. The frequency of the T allele in the hypertensive population enrolled in this study was 61.5%, which was even higher than the above reports. This result indicated that the hypertensive patients enrolled in this study had a higher T allele mutation rate, and the C677T mutation might be associated with essential hypertension, which was similar to the results of Niu et al.22
We performed MTHFR C677T genotyping on the enrolled hypertensive patients. We detected 52 cases of the CC genotype, 188 cases of the CT genotype, and 139 cases of the TT genotype. A comparison of plasma homocysteine and the UACR among the different genotypes showed that homocysteine levels (11.35 ± 3.71 μmol/L, 15.19 ± 5.15 μmol/L, P < 0.05) and the UACR (35.08 ± 50.02 mg/g, 71.14 ± 89.43 mg/g, P < 0.05) of the mutant homozygous TT genotype group were higher than those of the wild-type homozygous CC genotype group. The binary logistic regression analysis targeting the relationship between the gene polymorphism and early renal damage in hypertension showed that in both the recessive model (OR = 1.602, 95% CI: 1.051–2.442, P < 0.05) and the additive model (OR = 1.437, 95% CI: 1.057–1.955, P < 0.05), the MTHFR C677T gene polymorphism was associated with early renal damage in hypertension. These results indicated that the T allele mutation might be an independent risk factor causing early renal damage in hypertension. Therefore, we speculated that the MTHFR gene polymorphism affected the metabolic process of homocysteine. The frequency of the T allele mutation in MTHFR C677T was higher in the Chinese hypertensive population, which caused the higher percentage of H-type hypertensive patients. The increased homocysteine produced urine microalbumin through the damage of glomerular capillary endothelial cells and finally caused the development of early renal damage.
In addition to the association between homocysteine and the MTHFR gene polymorphism and early renal damage, we also performed correlation analyses on other clinical indicators. The results showed that the UACR was positively correlated with body weight, blood pressure, and some renal function indicators. The clinical indicators above could cause changes in the structure and function of glomerular intrinsic cells, damaging the glomerular vascular endothelial cells, and increasing the secretion of urine microalbumin.23 The routine and regular detection of the levels of renal function indicators can dramatically increase our understanding of the renal function of patients.
However, this study did not confirm whether the reduction in plasma homocysteine was helpful for the reduction in urine microalbumin in hypertensive patients. Future studies will attempt to supplement folic acid to reduce plasma homocysteine levels based on the metabolic features of homocysteine to further confirm the association between plasma homocysteine and early renal damage in hypertension to provide a theoretical basis for preventing early renal damage in hypertension.
In summary, because plasma homocysteine levels and the MTHFR gene polymorphism are independent risk factors of early renal damage in the hypertensive Chinese Han population, the MTHFR gene polymorphism should be selectively detected in hypertensive patients simultaneously with a reasonable reduction of blood pressure; in addition, plasma homocysteine levels should be controlled within the normal range, which may be helpful for the early discovery, prevention or delayed development and progression of early renal damage in the hypertensive Chinese Han population. Furthermore, the regular detection of indicators such as blood pressure, urea nitrogen, serum creatinine, uric acid, and cystatin C can dramatically increase the understanding of early renal damage in hypertensive patients.
CONCLUSION
Early renal damage in the Chinese hypertensive population was associated with body weight, systolic pressure, diastolic pressure, urea nitrogen, serum creatinine, cystatin C, uric acid, aldosterone, and glomerular filtration rate. Plasma homocysteine and the C677T gene polymorphism of its key metabolic enzyme MTHFR might be independent risk factors of early renal damage in the hypertensive Chinese Han population.
Footnotes
Abbreviations: ANOVA = one-way analysis of variance, BMI = body mass index, ERD group = hypertension combined with early renal damage group, MTHFR = methylenetetrahydrofolate reductase, NRD group = hypertension without renal damage group, UACR = urine microalbumin/urine creatinine ratio.
LY acquired, analyzed and interpreted data, wrote the manuscript. GL, YY, JL, DC, XX, LZ acquired and analyzed data, reviewed/edited the manuscript. RX designed the study, acquired, analyzed and interpreted the data, wrote the manuscript. All authors read and approved the final manuscript.
The local ethics committee approved the study, and all patients gave written informed consent for participating in the study.
This study was supported by Outstanding Young Scientist Research Award Fund of Shandong, China (Grant No. BS2010YY062), projects of medical and health technology development program of Shandong, China (Grant No. 2013WS0130), and Natural Science Foundation of Shandong, China (Grant No. ZR2014HP046).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223. [DOI] [PubMed] [Google Scholar]
- 2.Zou HQ. Pay attention to early management of hypertensive renal injury. Chin J Nephrol 2005; 21:575–576. [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease and end stage renal disease in the United States. Am J Kidney Dis 2012; 59 (1 Suppl 1):7. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). J Hypertens 2007; 25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 5.Li JP, Huo Y, Liu P, et al. Efficacy and safety of Enalapril-Folate acid tablets in lowering blood pressure and plasma homocysteine. J Peking University (Health Sciences) 2007; 39:614–618. [PubMed] [Google Scholar]
- 6.Bottiglieri T. Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:1103–1112. [DOI] [PubMed] [Google Scholar]
- 7.Miao X, Xing D, Tan W, et al. Susceptibility to gastric cardia adenocarcinoma and genetic polymorphism in methylenetetrahydrofolate reductase in an at-risk Chinese population. Cancer Epidemiol Biomarkers Prev 2002; 11:1454–1458. [PubMed] [Google Scholar]
- 8.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 2002; 288:2015–2022. [DOI] [PubMed] [Google Scholar]
- 9.Wang HL, Tan S, Song B, et al. Correlation of h-type hypertension and prognosis of ischemic stroke. Zhonghua Yi Xue Za Zhi 2012; 92:1183–1186. [PubMed] [Google Scholar]
- 10.Liu LS. Writing Group of 2010 Chinese Guidelines for the Management of Hypertension. 2010 Chinese guidelines for the management of hypertension. Chin J Cardiol 2011; 579–616. [PubMed] [Google Scholar]
- 11.Minoo F, Mahdavi-Mazdeh M, Abbasi MR, et al. Impact of the severity of obesity on microalbuminuria in obese normotensive nondiabetic individuals. J Renal Inj Prev 2015; 4:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarraya F, Lakhdar R, Kammoun K, et al. Microalbuminuria: a useful marker of cardiovascular disease. Iran J Kidney Dis 2013; 7:178–186. [PubMed] [Google Scholar]
- 13.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 2006; 113:e409–e449. [PubMed] [Google Scholar]
- 14.Durand P, Prost M, Loreau N, et al. Impaired homocysteine metabolism and atherothrombotic disease. Lab Invest 2001; 81:645–672. [DOI] [PubMed] [Google Scholar]
- 15.Dayal S, Aming E, Bottiglieri T, et al. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 2004; 35:1957–1962. [DOI] [PubMed] [Google Scholar]
- 16.Tawakol A, Omland T, Ge rhard M, et al. Hyperhomocyst(e)inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation 1997; 95:1119–1121. [DOI] [PubMed] [Google Scholar]
- 17.McCully KS. Hperhomocysteinemia and arteriosclerosis: historical perspectives. Clin Chem Lab Med 2005; 43:980–986. [DOI] [PubMed] [Google Scholar]
- 18.Sabanayagam C, Shankar A. Association between plasma homocysteine and microalbuminuria in persons without hypertension, diabetes mellitus, and cardiovascular disease. Clin Exp Nephrol 2011; 15:92–99. [DOI] [PubMed] [Google Scholar]
- 19.Kang SS, Wong PW, Zhou JM, et al. Thermolabile methylenetetrahydrofolate reductase in patients with coronary artery disease. Metabolism 1988; 37:611–613. [DOI] [PubMed] [Google Scholar]
- 20.Markan S, Schdeva M, Sehrauat BS, et al. MTHFR 677 CT/MTHFR 1298 CC genotypes are associated with increase risk of hypertension in Indians. Mol Cell Biochern 2007; 302:125–131. [DOI] [PubMed] [Google Scholar]
- 21.Giusti B, Gori AM, Marcucci R, et al. Role of C677T and A 1298C MTHFR, A2756G MTR and-786 C/T eNOS gene polymorphisms in atrial fibrillation susceptibility. PLoS One 2007; 2:e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu WQ, You YG, Qi Y. Strong association of methylenetetrahydrofolate reductase gene C677T polymorphism with hypertension and hypertension-in-pregnancy in Chinese: a meta-analysis. J Hum Hypertens 2012; 26:259–267. [DOI] [PubMed] [Google Scholar]
- 23.Aronson S, Fontes ML, Miao Y, et al. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115:733–742. [DOI] [PubMed] [Google Scholar]


