Abstract
Nafamostat mesilate (NM), a synthetic serine protease inhibitor, has been used increasingly as an anticoagulant during continuous renal replacement therapy (CRRT). However, there, are limited data from randomized studies on NM use in patients with a bleeding tendency. This prospective study evaluated the efficacy and safety of NM use during CRRT in patients with acute kidney injury (AKI) patients at high risk of bleeding.
Patients with AKI at high risk of bleeding were randomized into the NM and no anticoagulant (NA) groups. The primary outcome was the treatment efficacy represented by the filter lifespan. Several parameters, including safety and patient survival rates at 30 and 90 days, were analyzed as secondary outcomes.
Fifty-five patients were included in this study (NM group = 31, NA group = 24). The baseline characteristics did not significantly differ between the groups. The mean filter lifespan was significantly longer in the NM group than in the NA group (31.7 ± 24.1 versus 19.5 ± 14.9 hours; P = 0.035). The most common cause of filter failure was filter clotting, which was significantly more frequent in the NA group than in the NM group (59.6% versus 37.7%, P = 0.024). The Cox proportional hazards model showed a 42.2% longer filter lifespan in the NM group compared with the NA group (hazard ratio, 0.578; 95% confidence interval, 0.362–0.923; P = 0.022). There were no significant differences in the frequencies of transfusions and major bleeding between the groups. Patient survival rates at 30 and 90 days after CRRT initiation were comparable between the groups.
Nafamostat mesilate is a safe and effective anticoagulant for CRRT and allows sufficient filter survival without increasing the risk of bleeding in critically ill patients with AKI and bleeding tendencies.
INTRODUCTION
Continuous renal replacement therapy (CRRT) is widely used to treat acute kidney injury (AKI) in critically ill patients. Continuous renal replacement therapy has several advantages, such as improved hemodynamic stability, the possibility of unlimited alimentation, optimal fluid balance, and gradual urea removal without fluctuation.1 This therapy is favored over intermittent hemodialysis in hemodynamically unstable and critically ill patients with multiple organ dysfunction syndrome, and for those in a hypercatabolic state.2
The main disadvantage of CRRT is the need for continuous anticoagulation to prevent clotting of the extracorporeal circuit.3 Heparin is the most frequently used anticoagulant, but it is associated with a risk of bleeding in approximately 4% to 25% of patients.4,5 Although anticoagulation is needed to optimize the filter lifespan, most patients requiring CRRT are at risk of bleeding. Thus, alternative anticoagulation modalities, such as low-molecular weight heparin, prostacyclin, and regional citrate anticoagulation, have been proposed for patients with hemorrhagic risk.6
Nafamostat mesilate (NM), a synthetic serine protease inhibitor, has been used in hemodialysis patients at a high risk of bleeding because of its short half-life. Although a few studies have reported the effectiveness of NM as a substitute to heparin in CRRT patients with a high risk of bleeding, there are limited clinical experiences with NM and few published reports of its safety and efficacy. Moreover, there is limited clinical data from randomized trials on NM use in patients with a bleeding tendency. Therefore, we performed this randomized study to evaluate the efficacy and safety of NM use during CRRT in patients with AKI at high risk of bleeding (NCT02478242).
MATERIALS AND METHODS
Study Design and Population
Between July 2010 and June 2013, 60 patients with AKI who were at high risk of bleeding and required CRRT were assessed for eligibility for this study at Kyungpook National University Hospital, Daegu, South Korea. Patients who were >18 years old, were admitted to the intensive care unit and required CRRT were enrolled if they met the following criteria for high bleeding risk: active bleeding such as gastrointestinal bleeding and intracranial hemorrhage, activated partial thromboplastin time >60 seconds, prothrombin time–international normalized ratio >2.0, thrombocytopenia (<100,000/μL), and surgery within 48 hours before CRRT. Pregnant or possibly pregnant women were excluded from the study. Patients who were allergic to NM or were hypercoagulable were also excluded. Ethics approval was obtained from the regional ethics boards of Kyungpook National University Hospital. Written informed consent was obtained from all patients or their families before randomization.
Randomization and Intervention
Patients were randomly assigned to the NM group or the no anticoagulant (NA) group by using a random number table with a randomization ratio, as previously designed. In patients assigned to the NM group, filters were primed with 500 mL of normal saline containing 20 mg of NM (Futhan®, SK chemicals, Seoul, South Korea) dissolved in 1 mL of 5% dextrose water. For maintenance anticoagulation, the initial dose of NM was 20 mg/h, and the NM dose was regulated to 10 to 30 mg/h according to the physicians’ decision. In patients assigned to the NA group, filters were primed with 2 L of normal saline containing 5000 IU of heparin, and then, heparin was washed out using 500 mL of normal saline before use in patients. During CRRT, normal saline (2 mL/h) was infused in the NA group patients.
Continuous renal replacement therapy was performed using multiFiltrate® (Fresenius Medical Care, Bad Homburg, Germany) with a standard polysulfone membrane hemofilter (Ultraflux® AV 600S, Bad Homburg, Germany). Blood flow rates were maintained between 150 and 200 mL/h. The dialysate flow rate, replacement flow rate, and fluid removal rate were modified at the discretion of the attending physician to achieve optimal hemodynamic balance and dialysis.
Outcome Measurements and Safety Assessment
Illness severity and organ failure were scored using the acute physiology and chronic health evaluation (APACHE) II systems upon admission to the intensive care unit. Vital signs, including blood pressure, pulse rate, and body temperature were checked every hour during CRRT. Laboratory data including the complete blood count, blood urea nitrogen level, serum creatinine concentration, electrolyte levels, prothrombin time, and activated partial thromboplastin time were prospectively collected at CRRT initiation and then at 24 hours intervals thereafter till cessation of CRRT.
The primary outcome of this study was to assess the efficacy of NM in CRRT by measuring the filter lifespan, number of filters used per hour, filter survival rate, and proportion of filter clotting between the NM and NA groups. To measure the filter lifespan, number of filters used per hour, filter survival rate, and the time at initiation and cessation of CRRT were recorded when the filters were changed. Simultaneously, the causes of filter failure were recorded. Filter failure was categorized as follows: filter clotting, persistent transmembrane pressure >200 mm Hg, malfunction of vascular access, dysfunction of extracorporeal circuits, change in CRRT schedule (ie, interruptions in treatment for radiologic assessment), and cessation of CRRT owing to other causes. Filter clotting was defined as visible clot formation at the inlet and outlet of the filter or in the lines with a more than 50% decrease in ultrafiltration rate, as in a previous study.4 The number and duration of filters used were included in the efficacy analysis, only for filter failure reasons such as filter clotting and persistent transmembrane pressure >200 mm Hg. In addition, mortality rates were compared between the groups at 30 and 90 days after CRRT initiation.
To assess the safety of NM, we investigated the frequency of transfusions [ie, ≥2 units of packed red blood cells (RBCs)]. Blood transfusions had administered for hemoglobin levels were <8.0 g/dL or medical needs such as acute blood loss. Any gastrointestinal bleeding or anaphylaxis after NM infusion was also documented when present.
Statistical Analysis
It was estimated that 30 patients per group would have 90% power to reveal a difference of at least 15% (margin of error, 0.150) with an α error rate of <0.05. Data are expressed as means ± standard deviations or frequencies (percentages). We assessed the overall differences between the groups using independent samples t tests for continuous variables and the χ2 or Fisher exact test for categorical variables, as appropriate. Filter survival and patient survival were analyzed using the Kaplan–Meier method. The Cox proportional hazards model was used to compare the risk factors for filter lifespan between the 2 groups. SPSS version 19.0 (SPSS, Chicago, IL) was used for the statistical analyses. A P value of <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of the Patients
Sixty AKI patients who were at high risk of bleeding and had met the inclusion criteria were randomized to receive NM or NA during CRRT. Of these, 55 patients completed the study. Thirty-one patients were allocated to the NM group and 29 patients were allocated to the NA group. Five NA group patients, however, dropped out because of withdrawal of informed consent. The baseline characteristics of the 55 patients are summarized in Table 1. The mean age of the patients was 63.6 ± 11.5 years in the NM group and 58.6 ± 18.0 years in the NA group. The serum creatinine concentrations before randomization were 4.2 mg/dL and 3.9 mg/dL in the NM and NA groups, respectively. Twenty-three patients (74.2%) and 16 patients (66.7%) were on mechanical ventilation, and the APACHE II scores were 23.5 and 25.9 in the NM and NA groups, respectively. The most common etiology of AKI was sepsis, and there were no significant differences between groups in the etiology of AKI. The baseline characteristics did not differ significantly between the groups.
TABLE 1.
Baseline Characteristics of Patients
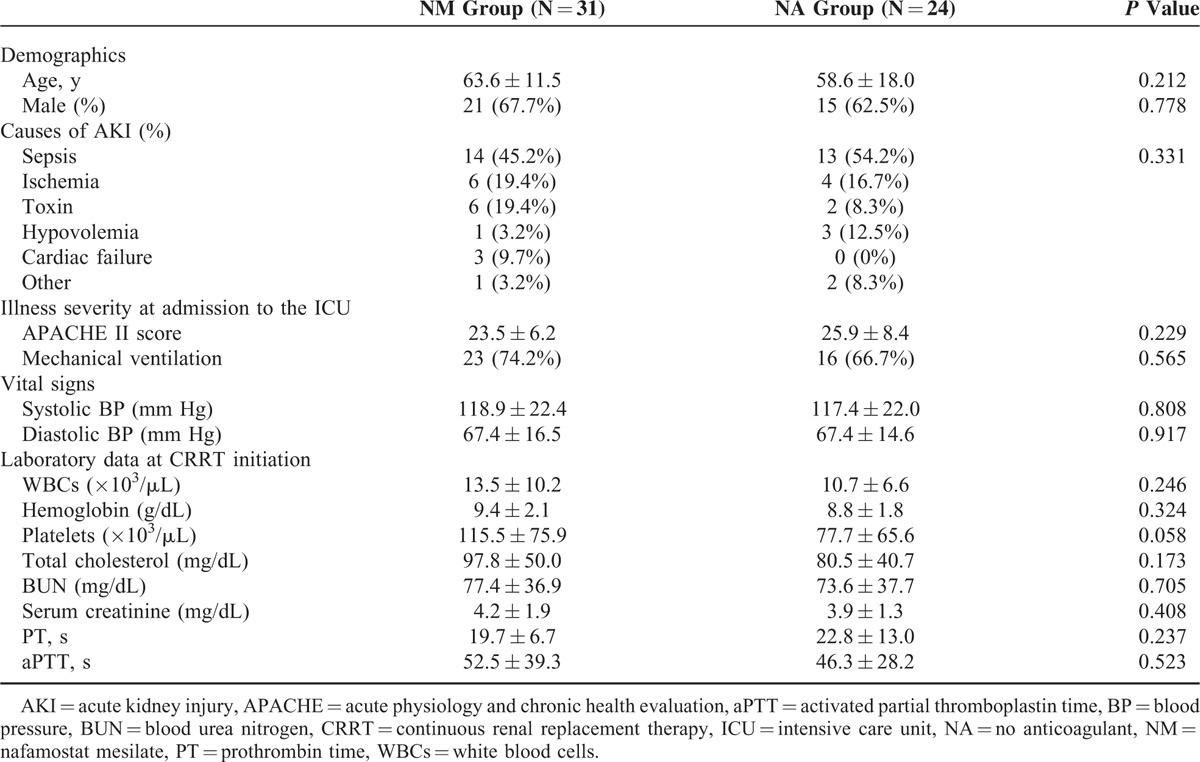
Comparison of Filter Efficacy and the Causes of Filter Failure
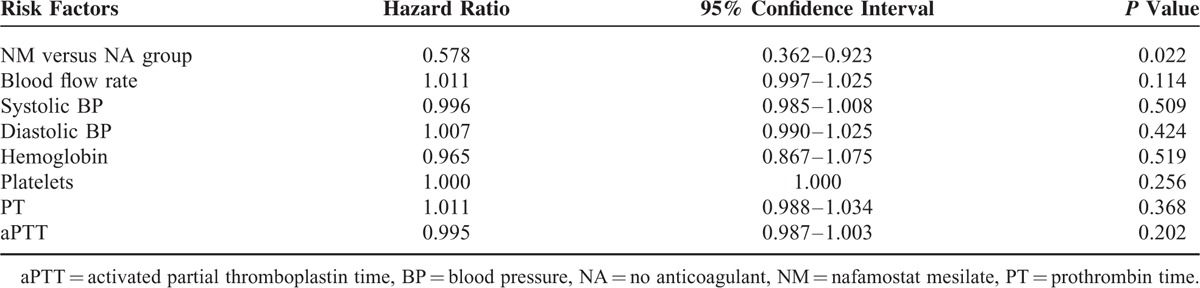
Filter efficacy and the causes of filter failure were compared between the NM and NA groups (Table 2). The mean filter lifespan was significantly longer in the NM group than in the NA group (31.7 ± 24.1 versus 19.5 ± 14.9 hours; P = 0.035). Moreover, the filter survival rate was higher in the NM group than in the NA group (P = 0.001), as shown in Kaplan–Meier survival analysis (Figure 1). The number of filters used every 24 hours during CRRT was lower in the NM group than in the NA group (1.4 ± 1.2 versus 2.4 ± 2.2), although this difference did not reach statistical significance (P = 0.061). A total of 110 events of filter failure occurred, 53 (48.2%) in the NM group and 57 (51.8%) in the NA group. The most common cause of filter failure was filter clotting, which was significantly lower in the NM group than in the NA group (37.7% versus 59.6%, P = 0.024). In addition, cessation of CRRT owing to conversion to conventional intermittent hemodialysis was significantly higher in the NM group than in the NA group (20.8% versus 1.8%; P = 0.002). The multivariate Cox proportional hazards model showed a 42.2% longer filter lifespan in the NM group compared with the NA group (hazard ratio, 0.578; 95% confidence interval, 0.362–0.923; P = 0.022; Table 3).
TABLE 2.
Comparison of Filter Efficacy and Causes of Filter Failure Between Groups
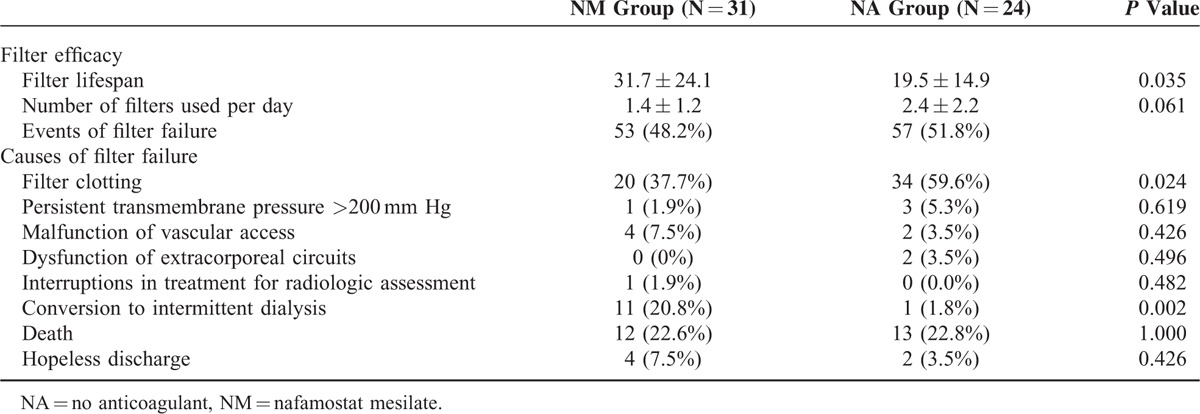
FIGURE 1.
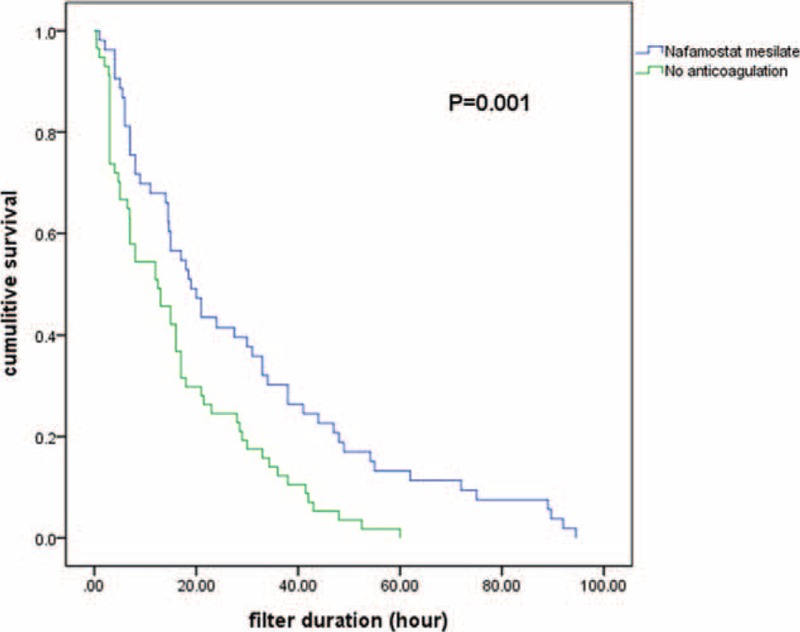
Kaplan–Meier curves comparing duration of hemofilter survival between the nafamostat mesilate and no anticoagulant groups.
TABLE 3.
Cox Proportional Hazards Model Predicting Filter Lifespan
Comparison of Patients’ Clinical Outcomes
Comparisons of the clinical outcomes between the 2 groups are shown in Table 4. The blood flow rates during CRRT were similar between the NM and NA groups (155 ± 15.7 versus 157 ± 15.9 mL/min; P = 0.588). Moreover, the total duration of CRRT per patient did not differ significantly between the NM and NA groups (50.8 ± 39.3 versus 41.5 ± 37.8 hours, P = 0.381). The urea reduction ratio at 6 hours from CRRT initiation was 16.4% ± 17.7% in the NM group and 18.7% ± 16.9% in the NA group (P = 0.691). In addition, the creatinine reduction ratio at 6 hours from CRRT initiation was similar between the NM and NA groups (18.7% ± 18.1% versus 16.9% ± 12.2%, P = 0.691). There was no significant difference in in-hospital mortality between the NM and NA groups (47.2% versus 52.8%, P = 0.054). The patient survival rate at 30 days was 46% in the NM group and 16.7% in the NA group (P = 0.075, Figure 2A). The patient survival rate at 90 days was 42.2% in the NM group and 11.2% in the NA group (P = 0.063, Figure 2B).
TABLE 4.
Clinical Outcomes and Safety
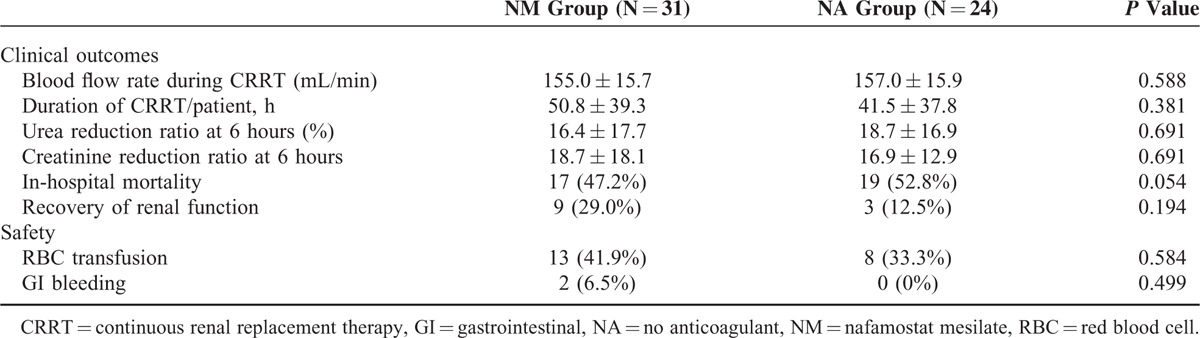
FIGURE 2.
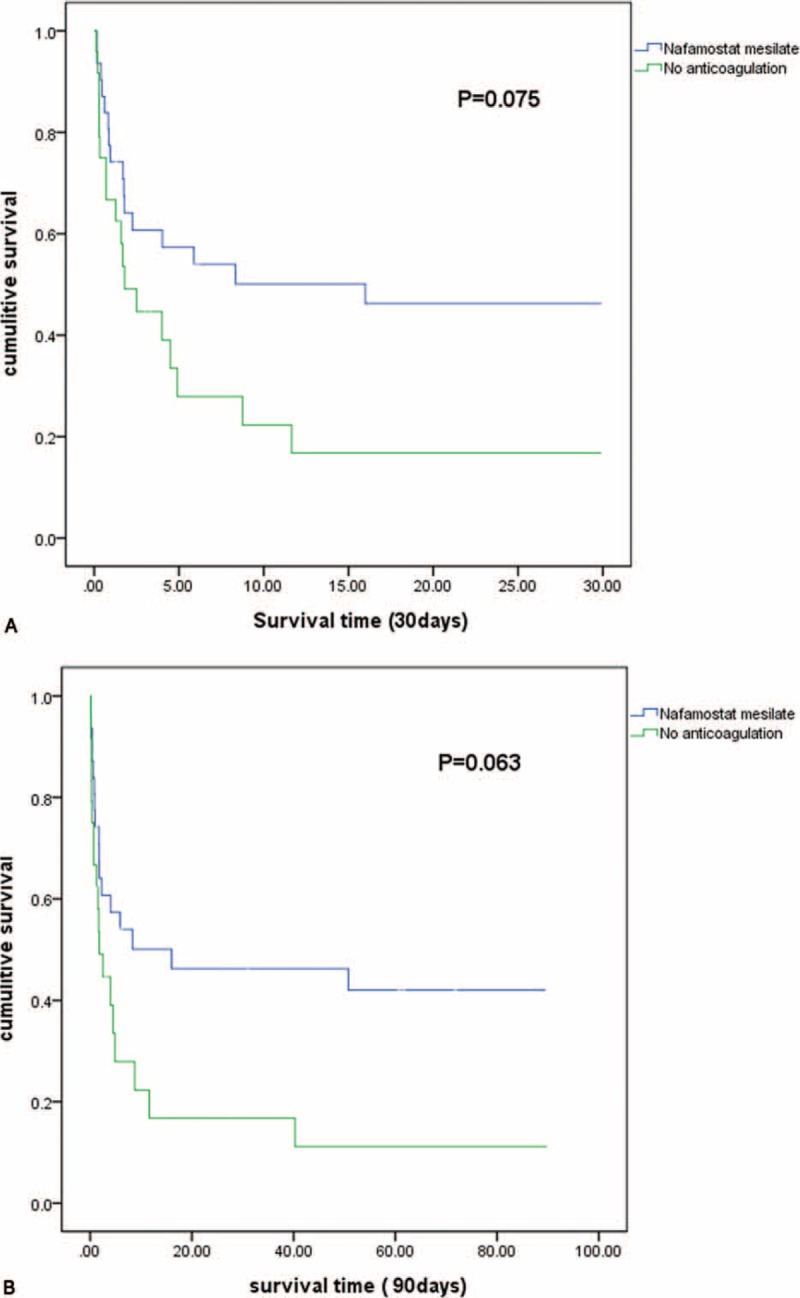
Kaplan–Meier survival curves for the nafamostat mesilate and no anticoagulant groups [(A) at 30 days; (B) at 90 days].
Safety
Thirteen patients (41.9%) in the NM group and 8 patients (33.3%) in the NA group (P = 0.584) received ≥2 units of packed RBCs. There were no significant differences in the incidence of gastrointestinal bleeding between the NM and NA groups (6.5% versus 0%, P = 0.499; Table 4). Adverse reactions associated with NM infusion, such as anaphylactic reaction, hyperkalemia, and agranulocytosis, did not occur during CRRT.
DISCUSSION
In this randomized prospective trial, compared with anticoagulant-free treatment, NM treatment reduced the frequency of filter clotting and prolonged filter lifespan without producing adverse reactions in patients with a high bleeding tendency. These results suggest that anticoagulation with NM in the extracorporeal circuit for CRRT could be more effective than an anticoagulant-free regimen in patients with contraindications for traditional anticoagulants such as heparin.
Systemic anticoagulation with heparin is contraindicated in various conditions, such as in patients with high bleeding risk, heparin-induced thrombocytopenia, or certain specific conditions including gastrointestinal bleeding and aortic dissection. In those cases, several alternative strategies for anticoagulation during CRRT have been recommended: regional heparinization,7 low-molecular weight heparin,8,9 saline flushes,10 prostacyclin,11,12 serine protease inhibitors (eg, NM),13 hirudin,14 and regional citrate anticoagulation.15,16 The half-life of low-molecular weight heparin and hirudin can be prolonged in patients with renal impairment. In addition, of the reported anticoagulants, only citrate has been proven to be superior to heparin in randomized controlled trials.17–19 Moreover, in the 2012 Kidney Disease Improving Global Outcome Clinical Practice Guidelines, citrate was recommended for AKI patients with bleeding tendencies.20 Citrate use, however, causes several side effects such as hypocalcemia and metabolic alkalosis. Although a study in Korea demonstrated that regional anticoagulation with citrate provided better filter survival than systemic anticoagulation with heparin in patients undergoing CRRT without severe adverse effects,21 further studies with larger numbers of patients with high bleeding risk are required to accurately define the relative benefits and risks of citrate anticoagulation.
Nafamostat mesilate is a strong protease inhibitor, and its inhibitory action affects various enzyme systems including the coagulation, and fibrinolytic and complement systems.22–24 Nafamostat mesilate has a low-molecular weight and short half-life of only 8 minutes, which makes it suitable for use as an anticoagulant in extracorporeal circuits during CRRT in patients with a high risk of bleeding. Although several retrospective studies have shown that NM is effective for CRRT in patients with high bleeding risk,25–27 to the best of our knowledge, only 1 randomized controlled trial compared NM with anticoagulant-free CRRT. In that study, CRRT with NM prolonged filter lifespan without any adverse events.28 Similarly, our study also found that the NM group had better outcomes in terms of filter efficacy than the NA group. In our study, the mean filter survival in the NM group was 31.7 hours, which is longer than that reported in a study by Lee et al28 (ie, 26.6 hours)and that reported in a study by Maruyama et al26 (ie, 22.5 hours). Filter clotting was significantly lower in the NM group than in the NA group in our study. Thus, the NM regimen led to a 42.2% longer filter survival time than the anticoagulant-free regimen, suggesting that CRRT with NM provides a better filter lifespan than the anticoagulant-free regimen in patients with bleeding tendencies.
Previous studies of NM's effects on patient survival reported that NM use did not affect patient mortality.28,29 In our study, the patient survival rates at 30 and 90 days after CRRT initiation were better in the NM group than in the NA group, although this difference did not reach statistical significance. Considering the significantly lower frequency of filter clotting and higher rate of conversion to intermittent dialysis in the NM group, NM use led to a longer filter lifespan, which resulted in effective fluid removal and clearance in NM group patients. Taking into consideration, the difference observed and the trend in the P values in daily filter use (P = 0.061) and in-hospital mortality (P = 0.054), a study including more participants might have proven statistically significant. Future multicenter studies with larger sample sizes are needed to confirm the beneficial effects of NM on patient survival.
The number of patients receiving packed RBC transfusions did not differ significantly between the groups. In our study, 41.9% of the NM group patients and 33.3% of the NA group patients received packed RBC transfusions; these percentages are lower than those reported in a previous retrospective study, in which 71% of the NM group patients and 70% of the anticoagulant-free group patients received packed RBC transfusions.25 In this study, 2 patients in the NM group and no patients in the NA group showed gastrointestinal bleeding. Although there were no statistical differences in the incidence of gastrointestinal bleeding between 2 groups, the 6% difference could have clinical significance. In our study, NM has proven extremely valuable as anticoagulant in CRRT in patients with bleeding risk, but the possibility of increased gastrointestinal bleeding is needed to be considered. A future study with a larger study population can elucidate that point.
No antidote is available for NM, and its use is reported to be associated with several side effects, including agranulocytosis, hyperkalemia, and anaphylactoid reactions.24,30,31 In this study, adverse events associated with NM use, however, did not occur. Our study demonstrated that NM could be used safely as an anticoagulant for CRRT in patients with bleeding tendencies.
This study has several limitations. First, this was a single-center study with a small sample size. Second, the NA group had a higher than expected dropout rate. Twenty-nine patients were enrolled in the NA group, of which 5 patients dropped out; there were no dropouts in the NM group. Nevertheless, a significantly longer filter lifespan and a higher filter efficacy were observed in the NM group compared with the NA group in this randomized prospective study.
In conclusion, NM is a safe and effective anticoagulant for CRRT and allows sufficient filter survival without the additional risk of bleeding in critically ill AKI patients with bleeding tendencies.
Footnotes
Abbreviations: AKI = acute kidney injury, APACHE = acute physiology and chronic health evaluation, CRRT = continuous renal replacement therapy, KDIGO = Kidney Disease Improving Global Outcome, NA = no anticoagulant, NM = nafamostat mesilate, RBC = red blood cells.
This work was supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) that is funded by the Ministry of Health and Welfare, Republic of Korea (HI15C0001) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2014R1A5A2009242).
J-YC and Y-JK contributed equally to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ronco C. Continuous renal replacement therapies for the treatment of acute renal failure in intensive care patients. Clin Nephrol 1993; 40:187–198. [PubMed] [Google Scholar]
- 2.Manns M, Sigler MH, Teehan BP. Continuous renal replacement therapies: an update. Am J Kidney Dis 1998; 32:185–207. [DOI] [PubMed] [Google Scholar]
- 3.Tolwani AJ, Wille KM. Anticoagulation for continuous renal replacement therapy. Semin Dial 2009; 22:141–145. [DOI] [PubMed] [Google Scholar]
- 4.van de Wetering J, Westendorp RG, van der Hoeven JG, et al. Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol 1996; 7:145–150. [DOI] [PubMed] [Google Scholar]
- 5.Ward DM, Mehta RL. Extracorporeal management of acute renal failure patients at high risk of bleeding. Kidney Int Suppl 1993; 41:S237–S244. [PubMed] [Google Scholar]
- 6.Cointault O, Kamar N, Bories P, et al. Regional citrate anticoagulation in continuous venovenous haemodiafiltration using commercial solutions. Nephrol Dial Transplant 2004; 19:171–178. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan AA, Petrillo R. Regional heparinization for continuous arterio-venous hemofiltration (CAVH). ASAIO Trans 1987; 33:312–315. [PubMed] [Google Scholar]
- 8.Jeffrey RF, Khan AA, Douglas JT, et al. Anticoagulation with low molecular weight heparin (Fragmin) during continuous hemodialysis in the intensive care unit. Artif Organs 1993; 17:717–720. [DOI] [PubMed] [Google Scholar]
- 9.Reeves JH, Cumming AR, Gallagher L, et al. A controlled trial of low-molecular-weight heparin (dalteparin) versus unfractionated heparin as anticoagulant during continuous venovenous hemodialysis with filtration. Crit Care Med 1999; 27:2224–2228. [DOI] [PubMed] [Google Scholar]
- 10.Paganini EP. Slow continuous hemofiltration and slow continuous ultrafiltration. ASAIO Trans 1988; 34:63–66. [PubMed] [Google Scholar]
- 11.Kozek-Langenecker SA, Kettner SC, Oismueller C, et al. Anticoagulation with prostaglandin E1 and unfractionated heparin during continuous venovenous hemofiltration. Crit Care Med 1998; 26:1208–1212. [DOI] [PubMed] [Google Scholar]
- 12.Ponikvar R, Kandus A, Buturovic J, et al. Use of prostacyclin as the only anticoagulant during continuous venovenous hemofiltration. Contrib Nephrol 1991; 93:218–220. [DOI] [PubMed] [Google Scholar]
- 13.Ohtake Y, Hirasawa H, Sugai T, et al. Nafamostat mesylate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol 1991; 93:215–217. [DOI] [PubMed] [Google Scholar]
- 14.Vargas Hein O, von Heymann C, Lipps M, et al. Hirudin versus heparin for anticoagulation in continuous renal replacement therapy. Intensive Care Med 2001; 27:673–679. [DOI] [PubMed] [Google Scholar]
- 15.Gabutti L, Marone C, Colucci G, et al. Citrate anticoagulation in continuous venovenous hemodiafiltration: a metabolic challenge. Intensive Care Med 2002; 28:1419–1425. [DOI] [PubMed] [Google Scholar]
- 16.Oudemans-van Straaten HM. Citrate anticoagulation for continuous renal replacement therapy in the critically ill. Blood Purif 2010; 29:191–196. [DOI] [PubMed] [Google Scholar]
- 17.Kutsogiannis DJ, Gibney RT, Stollery D, et al. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int 2005; 67:2361–2367. [DOI] [PubMed] [Google Scholar]
- 18.Monchi M, Berghmans D, Ledoux D, et al. Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med 2004; 30:260–265. [DOI] [PubMed] [Google Scholar]
- 19.Oudemans-van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Critical Care Med 2009; 37:545–552. [DOI] [PubMed] [Google Scholar]
- 20.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Kim GH, Kang CM, et al. Regional anticoagulation with citrate is superior to systemic anticoagulation with heparin in critically ill patients undergoing continuous venovenous hemodiafiltration. Korean J Intern Med 2011; 26:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitomi Y, Ikari N, Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis 1985; 15:164–168. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta 1981; 661:342–345. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama H, Miyakawa Y, Gejyo F, et al. Anaphylactoid reaction induced by nafamostat mesilate in a hemodialysis patient. Nephron 1996; 74:468–469. [DOI] [PubMed] [Google Scholar]
- 25.Baek NN, Jang HR, Huh W, et al. The role of nafamostat mesylate in continuous renal replacement therapy among patients at high risk of bleeding. Ren Fail 2012; 34:279–285. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama Y, Yoshida H, Uchino S, et al. Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: a three-year retrospective cohort study. Int J Artif Organs 2011; 34:571–576. [DOI] [PubMed] [Google Scholar]
- 27.Hwang SD, Hyun YK, Moon SJ, et al. Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs 2013; 36:208–216. [DOI] [PubMed] [Google Scholar]
- 28.Lee YK, Lee HW, Choi KH, et al. Ability of nafamostat mesilate to prolong filter patency during continuous renal replacement therapy in patients at high risk of bleeding: a randomized controlled study. PLoS One 2014; 9:e108737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park II, Choi MJ, Yoon JW, et al. Saline versus nafamostat mesilate anticoagulation for continuous veno-venous hemofiltration (CVVH) in patients at high risk of bleeding: a prospective study. Korean J Nephrol 2009; 28:205–210. [Google Scholar]
- 30.Muto S, Imai M, Asano Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen Pharmacol 1995; 26:1627–1632. [DOI] [PubMed] [Google Scholar]
- 31.Okada H, Suzuki H, Deguchi N, et al. Agranulocytosis in a haemodialysed patient induced by a proteinase inhibitor, nafamostate mesilate. Nephrol Dial Transplant 1992; 7:980. [DOI] [PubMed] [Google Scholar]


