Abstract
Metabolic abnormalities are common in patients with depressive disorders. However, the relationship between gout and depression is unclear. We explored the causal relationship among gout, antigout medication, and the associated risk of incidental depressive disorders.
In this nationwide cohort study, we sampled data from the National Health Insurance Research Database to recruit 34,050 patients with gout as the gout cohort and 68,100 controls (without gout) as the nongout cohort. Our primary endpoint was the diagnosis of depressive disorders during follow-up. The overall study population was followed up until depression diagnosis, withdrawal from the NHI program, or the end of the study. The differences in demographic and clinical characteristics between both cohorts were determined using the Chi-square test for categorical variables and the t-test for continuous variables. Cox proportional hazard regression models were used to examine the effect of gout on the risk of depression, represented using the hazard ratio with the 95% confidence interval.
Patients with gout exhibited a higher risk of depressive disorders than controls did. The risk of depressive disorders increased with age and was higher in female patients and those with hypertension, stroke, and coronary artery disease. Nonsteroidal antiinflammatory drug and prednisolone use was associated with a reduced risk of depression. Patients with gout who had received antigout medication exhibited a reduced risk of depressive disorders compared with nongout patients.
Our findings support that gout increases the risk of depressive disorders, and that antigout medication use reduces the risk.
INTRODUCTION
Metabolic abnormalities, including increased body mass index (BMI), central obesity, hyperlipidemia, hyperglycemia, and hyperuricemia, are closely associated with common mental disorders.1–6 Moreover, the prevalence of metabolic syndrome is higher in patients with mood disorders than in the general population, with a substantial impact on quality of life.7–9 However, the relationship between hyperuricemia (or gout) and mood disorders is unclear. Hyperuricemia is associated with obesity, insulin resistance, hypertension, and metabolic syndrome;10,11 therefore, the pathophysiological mechanisms of mood disorders and gout might be similar. Uric acid is a metabolite and downstream product of adenosine, a purine nucleoside. The purinergic system comprises signaling pathways involving the neurotransmitter adenosine triphosphate (ATP) and the neuromodulator adenosine. The system is present in many central nervous system (CNS) regions, such as the cerebral cortex, basal ganglia, and limbic system, and is responsible for neurotransmission, synapse formation, and neuronal plasticity.12,13 Moreover, this system interacts with other neurotransmitter systems including serotonin, dopamine, and gamma-aminobutyric acid. This implies that purinergic signaling dysfunction may disturb other neurotransmitter systems and cause neuroinflammation.14 Therefore, the purinergic system might be involved in mood regulation, sleep patterns, impulsivity, and locomotor activity.15,16
A nationwide study recently confirmed that patients with bipolar disorder were at a higher risk of gout than healthy controls.17 However, no study has explored the association of depressive disorders, the most common mood disorder, with gout. In this retrospective cohort study, we used a nationwide dataset to clarify whether gout is a predictor of depressive disorders. We also explored the association between antigout medication treatment and depressive disorders. We proposed the following hypotheses: first, patients with gout may have a higher risk of depressive disorders than the general population, after adjustment for other possible demographic and metabolic confounders. Second, antigout medication may lower the risk.
METHODS
Data Source
Since its establishment in 1995 in Taiwan, the National Health Insurance program has covered approximately 99% of Taiwan's population (23 million). The National Health Insurance Research Database (NHIRD) is a research database developed and managed by National Health Research Institute, and confidentiality is maintained according to the directives of the National Health Insurance Administration. For this retrospective cohort study, we used the dataset of Longitudinal Health Insurance Database 2000, which contains the longitudinally linked data of 1,000,000 enrollees randomly sampled from the NHIRD. To ensure patient privacy, patient identification numbers necessary to link files with identities are scrambled by the National Health Research Institute. Diagnoses are based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115). The IRB also specifically waived the consent requirement.
Sampled Population
We included patients aged 20 years and older with newly diagnosed gout (ICD-9-CM code 274) from 2000 to 2010 in the gout cohort. The date of gout diagnosis served as the index date. The nongout cohort consisted of randomly selected Longitudinal Health Insurance Database 2000 beneficiaries without gout, who were frequency matched to patients with gout at a ratio of 2:1 by age (every 5-year span), sex, and index year. Patients diagnosed with depression at baseline (ICD-9-CM codes 296.2–296.3, 300.4, and 311) or missing information for age or sex were excluded from this study.
Outcome, Comorbidity, and Medication
The overall study population was followed up from the index date to the date of depression diagnosis, withdrawal from the National Health Insurance program, or the end of 2011, whichever came first. Baseline comorbidities included hypertension (ICD-9-CM codes 401–405), diabetes (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), stroke (ICD-9-CM codes 430–438), and coronary artery disease (CAD) (ICD-9-CM codes 410–414), and medications included nonsteroidal antiinflammatory drug (NSAID) and prednisolone. We coded a patient with NSAID (or prednisolone) use if they started to use the medication after the index date and had used for at least 30 days. In addition, antigout medications including colchicine, xanthine oxidase inhibitors, and uricosuric agents were analyzed.
Statistical Analyses
Demographic characteristics, including sex and age, and baseline comorbidities were compared between the gout and nongout cohorts using the Chi-square test for categorical variables and the t-test for continuous variables. The cumulative incidence of depression in the gout and nongout cohorts was assessed using the Kaplan–Meier method, and the differences were assessed using the log-rank test. The sex-, age-, comorbidity-, and medication-specific incidence densities of depression were assessed for both cohorts. Univariate and multivariate Cox proportional hazard regression models were used to examine the effect of gout on the risk of depression, represented using the hazard ratio (HR) with the 95% confidence interval (CI). The multivariate models were adjusted for age, sex, and the comorbidities of hypertension, diabetes, hyperlipidemia, stroke, and CAD and the medications of NSAID and prednisolone. All statistical analyses were performed using the SAS statistical package (Version 9.4 for Windows; SAS Institute, Inc., Cary, NC); P < 0.05 was considered statistically significant.
RESULTS
Overall, the study population totaled 102,150 in this retrospective cohort study, including 34,050 patients with gout and 68,100 controls. The distribution of age and sex was similar in both cohorts. Most patients were men, and more than half were less than 49 years of age in our study. Compared with the nongout cohort, a higher prevalence of hypertension, diabetes, hyperlipidemia, stroke, and CAD was observed in the gout cohort (P < 0.001). NSAID and prednisolone use was more prevalent in the gout cohort at baseline than in the nongout cohort (P < 0.001) (Table 1). The cumulative incidence of depression was significantly higher in the gout cohort than in the nongout cohort (log-rank P < 0.001) (Figure 1).
TABLE 1.
Demographic Characteristics and Comorbidities in Patients With and Without Gout
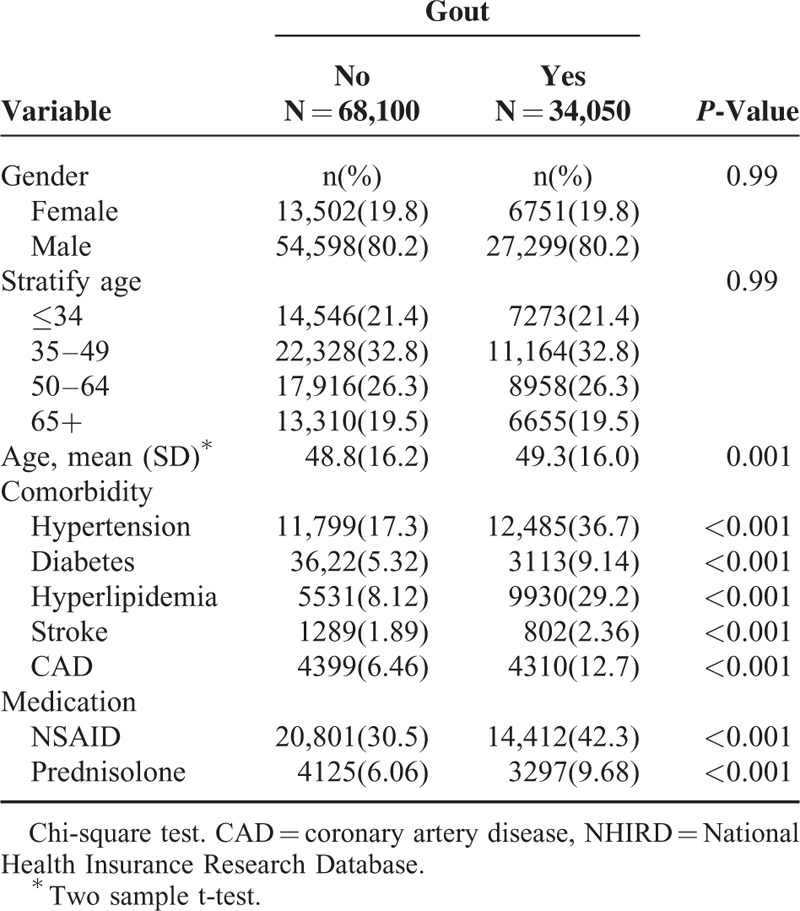
FIGURE 1.
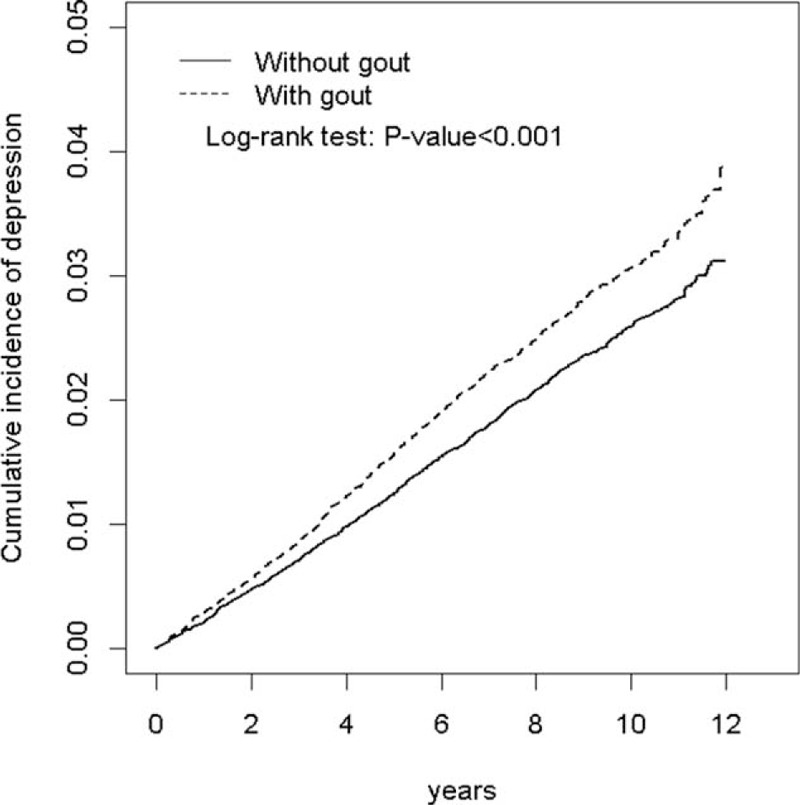
Cumulative incidence of depression in patients with and without gout.
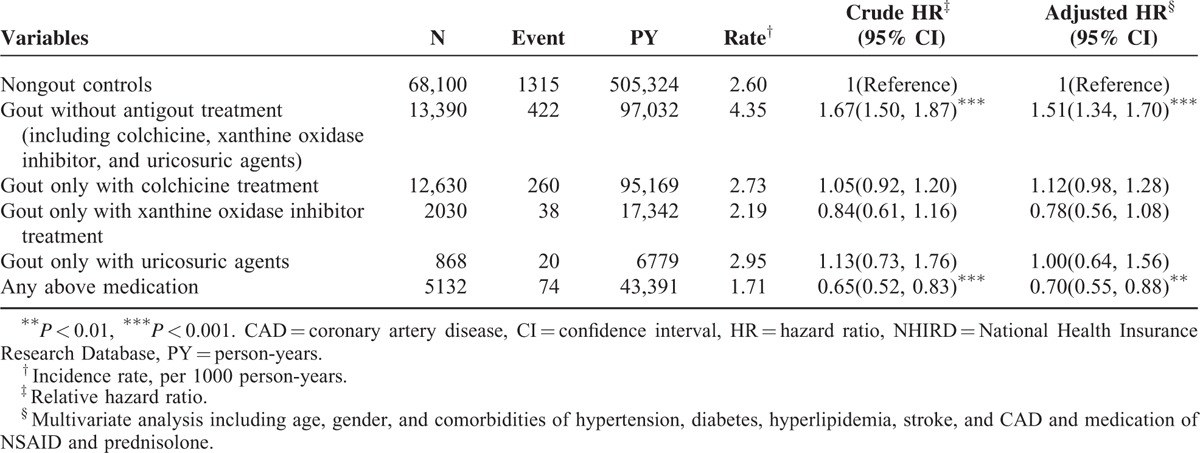
During the mean follow-up of 7.63 years for the gout cohort and 7.42 years for the nongout cohort, the overall incidence of depression (per 1000 person-year) was 3.13 and 2.60, respectively. After adjustment for age, sex, comorbidities, and medications, patients with gout had a 1.18-fold higher risk of depression compared with that in controls (95% CI = 1.07–1.29). The sex-specific gout cohort to nongout cohort adjusted HR of depression was significant for men (adjusted HR = 1.20, 95% CI = 1.07–1.34). The incidence of depression increased with age in both cohorts. The age-specific gout cohort to nongout cohort adjusted HR of depression was significant for the age groups of ≤34 (adjusted HR = 1.53, 95% CI = 1.23–1.91) and 35 to 49 (adjusted HR = 1.26, 95% CI = 1.05–1.50). The comorbidity-specific gout cohort to nongout cohort adjusted HR of depression was significant for patients without comorbidity (adjusted HR = 1.23, 95% CI = 1.08–1.39). Among patients without NSAID use, the risk of depression was 1.21-fold higher in the gout cohort than in the nongout cohort (95% CI = 1.08–1.36). Among patients without prednisolone use, the risk of depression was 1.21-fold higher in the gout cohort than in the nongout cohort (95% CI = 1.10–1.33) (Table 2). The risk of depression was 1.01-fold (95% CI = 1.00–1.01) increased with age (every 1 year) and was 1.54-fold higher in women than in men (95% CI = 1.39–1.69) (Table 3). A high risk of depression was observed in patients with hypertension (adjusted HR = 1.21, 95% CI = 1.08–1.36), stroke (adjusted HR = 1.43, 95% CI = 1.10–1.86), and CAD (adjusted HR = 1.17, 95% CI = 1.01–1.35). A low risk of depression was observed in patients with NSAID (adjusted HR = 0.70, 95% CI = 0.64–0.77) and prednisolone use (adjusted HR = 0.71, 95% CI = 0.59–0.84). Compared with controls, gout patients without antigout medication use exhibited a significantly higher risk of depression (adjusted HR = 1.51, 95% CI = 1.34–1.70) (Table 4). Compared with controls, patients with gout who took only 1 antigout drug, such as colchicine, a xanthine oxidase inhibitor, or uricosuric agent, did not exhibit a significantly higher risk of depression. Patients who took any one of above antigout drugs exhibited a significantly lower risk of depression (adjusted HR = 0.70, 95% CI = 0.55–0.88) compared with controls.
TABLE 2.
Comparison of Incidence and HRs of Depression Stratified by Sex, Age, and Comorbidities Between Patients With and Without Gout
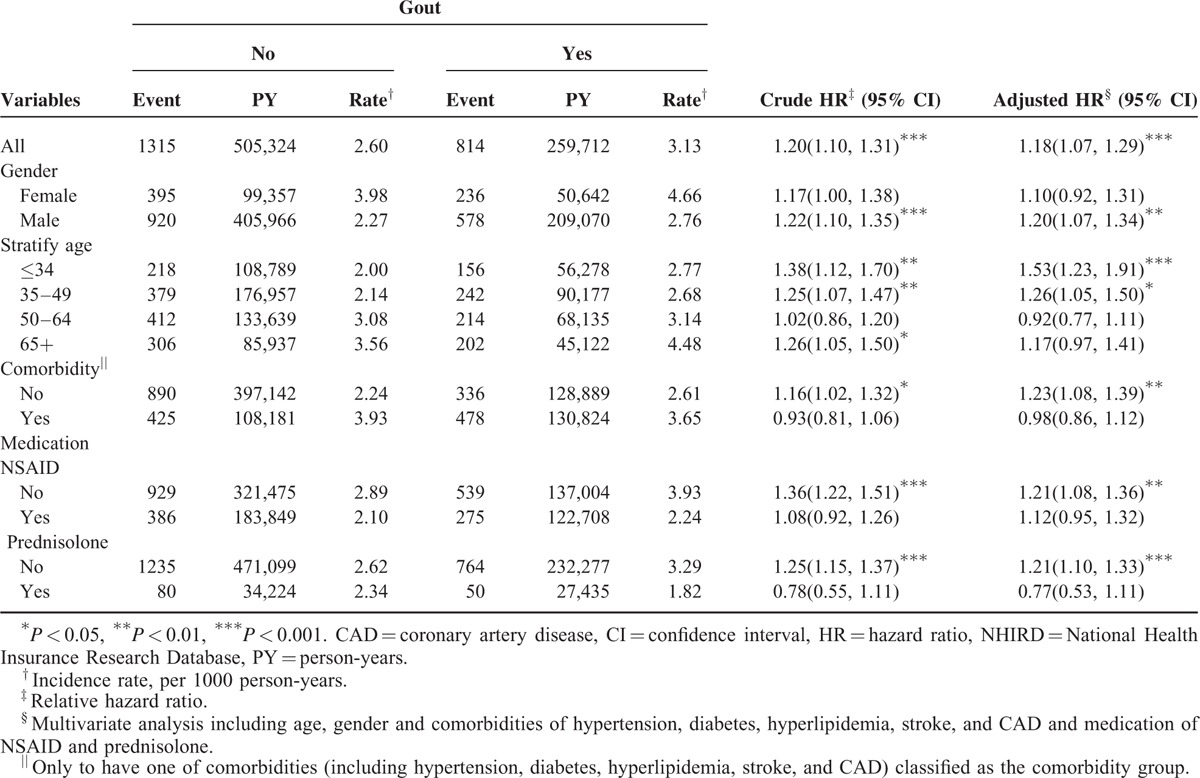
TABLE 3.
Comparison of HRs of Depression in Association With Age, Sex, Comorbidities, and Medication by Using Multivariate Cox Regression Models
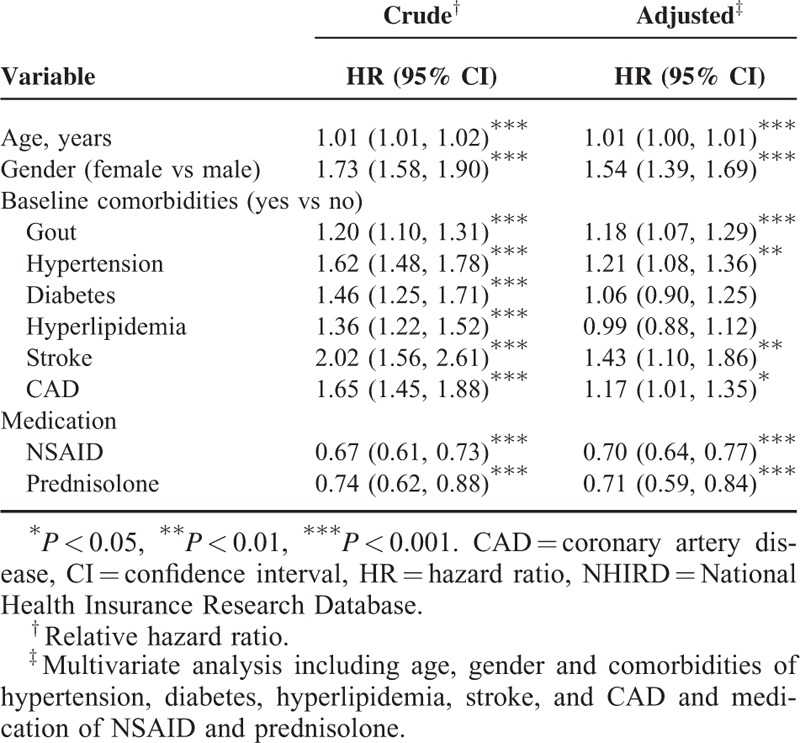
TABLE 4.
Comparison of Incidence and HRs of Depression Between Gout Patients With and Without Antigout Medication and Controls
DISCUSSION
In this study, patients with gout had a 1.18-fold higher risk of depressive disorders than controls (representing the general population) during the 10-year period. Moreover, compared with controls, gout patients without antigout medication use had a higher risk of depressive disorders, but antigout medication use was associated with a reduced risk of depression. These major findings support our hypotheses and were still statistically significant after adjustment for age, sex, and possible confounding variables such as comorbidities. Based on our research, the present study is the first to demonstrate the causal relationship among gout without antigout medication, gout with antigout medication, and depressive disorders by using a nationwide cohort dataset and a follow-up study design. Analyses stratified by sex, age, and comorbidities showed that men, patients aged ≤49 years, and those without comorbidities or NSAID or prednisolone use had a high risk of depression among patients with gout.
Studies have addressed the role of purinergic system dysfunction in mood disorders, including studies of genetic variations18 and peripheral purinergic biomarkers (such as uric acid levels),19 neuroimaging studies,20,21 and clinical trials (of antigout medication on adjunctive treatment of bipolar disorder).22,23 Ortiz et al14 reviewed the aforementioned studies to determine the involvement of purinergic signaling dysfunction in the pathophysiology and therapeutics of mood disorders. Sperlagh et al16 also reviewed available literature to explain the profound effects of a purine receptor, P2rx7, and its activation on mood-related behavior via glutamate release and neuronal plasticity in depressive disorders. However, few clinical studies have focused on the direct association of mood disorders with systematic purinergic dysfunction (medical comorbidities), particularly gout. A recent study with a matched retrospective cohort design in the United Kingdom failed to identify an association between gout and subsequent incident consultation for depression in primary care.24 However, compared with the current study that study had a smaller sample size (1689 patients with gout and 6756 controls), including older patients with gout (63 ± 16 year), and demonstrated a much higher incidence rate of depression (10.8 per 1000 person-year). Chung et al17 also used a nationwide population-based dataset to demonstrate the association between bipolar disorder and the risk of gout. They showed that the risk of gout was 1.19-fold higher in patients with bipolar disorder than in their matched controls during the 6-year follow-up period. They enrolled more female patients (60.7%) and found that those with renal disease, CAD, metabolic syndrome, and alcohol or substance dependence were all at risk of gout. Therefore, a bidirectional causal relationship seems to exist between mood disorders and gout. Another study demonstrated that most patients with gout in the United Kingdom have a higher burden of comorbidity at diagnosis, and that the risk of incident comorbidity continues to increase following diagnosis.25 Moreover, depression is one of the comorbidities potentially associated with gout.
Our findings that women and aging were both significantly associated with depressive disorders, was consistent with epidemiologic characteristics of depressive disorders. However, the further stratified analysis showed that men and patients aged ≤49 years had a high risk of depression among patients with gout. Some possible reasons may explain these inconsistencies. These patients may be representative of a specific subgroup with different pathophysiological mechanism of depression. Besides, accumulated evidence suggests that hormone fluctuations may play a role in the increased risk for depressive disorders in women, especially the menopausal transition.26 Hormonal effect seems to be a confounding factor to women, not men. But the patients recruited in our study are younger and male predominant. So, the preliminary results are limited to a subset of the cohort and should be interpreted cautiously.
An unexpected finding of our study is that NSAID use decreased the risk of depression; however, among patients without NSAID use, a significantly higher risk of depression was observed in the gout cohort than in the nongout cohort. This finding implies that gout and NSAID may have an interactive effect on depression, and that NSAID may even mediate the association of gout with depression. The immune inflammatory reaction is involved in the pathogenesis of gout, with increased C-reactive protein levels, blood neutrophil counts, and levels of cytokines, including interleukin 1 (IL-1), IL-2, IL-6, and tumor necrosis factor-alpha (TNF-α).27 NSAID is commonly used in the treatment of acute gout despite of limited evidence by a recent systematic review.28 On the other side, the inflammatory reaction and associated markers are also potential etiological factors for major psychiatric disorders. Depressive disorders have been demonstrated to be associated with an altered and dysregulated immune system, including changes in serum acute phase protein and cytokine levels.29 The cytokine hypothesis of depression has promising findings in connection with the effect on central serotonin levels, microglial activation, the hypothalamic–pituitary–adrenal (HPA) axis, and neuroplasticity.30 NSAIDs with antiinflammatory effects may have its role to support our finding. For example, acetyl-salicylic acid (ASA), one of the oldest known drugs in the world, irreversibly inhibits cyclooxygenase-1 and -2 (COX-1 and COX-2, respectively), thereby decreasing TNF-α and IL-6 levels.31 Berk et al32 reviewed and explored the potential of ASA as a therapeutic agent for depression, bipolar disorder, and schizophrenia. Eyre et al33 examined the efficacy of nonselective COX inhibitors (including ASA) for depressive symptoms in a critical review that included 1 randomized-controlled study, 4 retrospective cohort studies, and 1 open pilot study; their findings were inconsistent and negligible because of the heterogeneity in sample selection (age, sex), duration, antidepressant use, severity of depressive symptoms, and outcome measure. The underlying natures and clinical efficacy of antiinflammatory agents in the treatment of depression deserve further clinical research.
Many clinical trials of pharmacological agents targeting the purinergic system have been conducted;22,23,34 most of these trials have used the xanthine oxidase inhibitor allopurinol as adjunctive therapy in patients with bipolar disorder. A recent systematic review and meta-analysis demonstrated the efficacy of purinergic modulators (including allopurinol) as adjuvant therapy for bipolar mania in comparison with a placebo.35 However, no clinical trials of purinergic modulators have been conducted in patients with depressive disorders. Our preliminary finding that gout patients with antigout medication use have a low risk of depressive disorders may support the possible role of purinergic modulators in treating depressive disorders. Additional clinical trials are necessary to confirm the hypothesis.
Our study has some limitations. First, variables such as BMI, substance abuse history, and lifestyle factors, which may be associated with development of depression, were not included in our study because of the lack of data. Second, the serum uric acid levels are also not available in the NHIRD. The association between uric acid levels and depressive disorders cannot be confirmed. We may have underestimated the effect of purinergic dysfunction (particularly for hyperuricemia patients without gout) on mood symptoms. Third, because all definitions of variables were based on physicians’ diagnoses, misidentification may bias the study results. Finally, the use of NSAID in our sampled population might be for other reasons other than gout despite of our effort in inclusion criteria. And depressive disorders have heterogeneous symptoms, psychopathologies, and etiologies and can be the initial presentation of bipolar disorder. Therefore, the results should be interpreted with caution.
In conclusion, our study provides the first nationwide population-based evidence to support the increased risk of depressive disorders in patients with gout, particularly in those without antigout medication, and the reduced risk in gout patients with any antigout medication treatment, after controlling for demographic characteristics, comorbidities, and antiinflammatory agent use. We should pay more attention to the mental health in the patients with gout, especially in younger people, men, and those without any antigout treatment.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, NHIRD = National Health Insurance Research Database.
All authors have substantially contributed to the study and are in agreement with the content of the manuscript: Conception/design: T-CC and C-HK; Provision of study materials: C-HK; Collection and assembly of data: T-CC, C-LL, and C-HK; Data analysis and interpretation, Manuscript preparation, and Final approval of manuscript: all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
All authors have no conflicts of interest to disclose.
REFERENCES
- 1.Chuang CS, Yang TY, Muo CH, et al. Hyperlipidemia, statin use and the risk of developing depression: a nationwide retrospective cohort study. Gen Hosp Psychiatry 2014; 36:497–501. [DOI] [PubMed] [Google Scholar]
- 2.Detka J, Kurek A, Basta-Kaim A, et al. Neuroendocrine link between stress, depression and diabetes. Pharmacol Rep 2013; 65:1591–1600. [DOI] [PubMed] [Google Scholar]
- 3.Henderson DC, Vincenzi B, Andrea NV, et al. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry 2015; 2:452–464. [DOI] [PubMed] [Google Scholar]
- 4.Huang CJ, Lin CH, Lee MH, et al. Prevalence and incidence of diagnosed depression disorders in patients with diabetes: a national population-based cohort study. Gen Hosp Psychiatry 2012; 34:242–248. [DOI] [PubMed] [Google Scholar]
- 5.McElroy SL, Kotwal R, Malhotra S, et al. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry 2004; 65:634–651.quiz 730. [DOI] [PubMed] [Google Scholar]
- 6.Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Portilla MP, Saiz PA, Benabarre A, et al. The prevalence of metabolic syndrome in patients with bipolar disorder. J Affect Disord 2008; 106:197–201. [DOI] [PubMed] [Google Scholar]
- 8.Rethorst CD, Bernstein I, Trivedi MH. Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009–2010 National Health and Nutrition Examination Survey (NHANES). J Clin Psychiatry 2014; 75:e1428–e1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015; 14:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billiet L, Doaty S, Katz JD, et al. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol 2014; 2014:852954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol 2013; 25:210–216. [DOI] [PubMed] [Google Scholar]
- 12.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 2006; 7:423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich H, Abbracchio MP, Burnstock G. Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair. Stem Cell Rev 2012; 8:755–767. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz R, Ulrich H, Zarate CA, Jr, et al. Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog Neuropsychopharmacol Biol Psychiatry 2015; 57:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado-Vieira R, Lara DR, Souza DO, et al. Purinergic dysfunction in mania: an integrative model. Med Hypotheses 2002; 58:297–304. [DOI] [PubMed] [Google Scholar]
- 16.Sperlagh B, Csolle C, Ando RD, et al. The role of purinergic signaling in depressive disorders. Neuropsychopharmacol Hung 2012; 14:231–238. [PubMed] [Google Scholar]
- 17.Chung KH, Huang CC, Lin HC. Increased risk of gout among patients with bipolar disorder: a nationwide population-based study. Psychiatry Res 2010; 180:147–150. [DOI] [PubMed] [Google Scholar]
- 18.Halmai Z, Dome P, Vereczkei A, et al. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J Affect Disord 2013; 150:104–109. [DOI] [PubMed] [Google Scholar]
- 19.Kesebir S, Tatlıdil Yaylacı E, Süner O, et al. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: the role of affective temperament. J Affect Disord 2014; 165:131–134. [DOI] [PubMed] [Google Scholar]
- 20.Forester BP, Harper DG, Jensen JE, et al. 31Phosphorus magnetic resonance spectroscopy study of tissue specific changes in high energy phosphates before and after sertraline treatment of geriatric depression. Int J Geriatr Psychiatry 2009; 24:788–797. [DOI] [PubMed] [Google Scholar]
- 21.Renshaw PF, Parow AM, Hirashima F, et al. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry 2001; 158:2048–2055. [DOI] [PubMed] [Google Scholar]
- 22.Jahangard L, Soroush S, Haghighi M, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant allopurinol improved symptoms of mania in in-patients suffering from bipolar disorder. Eur Neuropsychopharmacol 2014; 24:1210–1221. [DOI] [PubMed] [Google Scholar]
- 23.Weiser M, Burshtein S, Gershon AA, et al. Allopurinol for mania: a randomized trial of allopurinol versus placebo as add-on treatment to mood stabilizers and/or antipsychotic agents in manic patients with bipolar disorder. Bipolar Disord 2014; 16:441–447. [DOI] [PubMed] [Google Scholar]
- 24.Prior JA, Ogollah R, Muller S, et al. Gout, anxiety, and depression in primary care: a matched retrospective cohort study. Scand J Rheumatol 2015; 44:257–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo CF, Grainge MJ, Mallen C, et al. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis 2016; 75:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares CN. Practical strategies for diagnosing and treating depression in women: menopausal transition. J Clin Psychiatry 2008; 69:e30. [DOI] [PubMed] [Google Scholar]
- 27.Cronstein BN, Sunkureddi P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. J Clin Rheumatol 2013; 19:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Durme CM, Wechalekar MD, Buchbinder R, et al. Non-steroidal anti-inflammatory drugs for acute gout. Cochrane Database Syst Rev 2014; 9:CD010120. [DOI] [PubMed] [Google Scholar]
- 29.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67:446–457. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblat JD, Cha DS, Mansur RB, et al. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2014; 53:23–34. [DOI] [PubMed] [Google Scholar]
- 31.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res 2003; 110:255–258. [DOI] [PubMed] [Google Scholar]
- 32.Berk M, Dean O, Drexhage H, et al. Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med 2013; 11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyre HA, Air T, Proctor S, et al. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuropsychopharmacol Biol Psychiatry 2015; 57:11–16. [DOI] [PubMed] [Google Scholar]
- 34.Akhondzadeh S, Milajerdi MR, Amini H, et al. Allopurinol as an adjunct to lithium and haloperidol for treatment of patients with acute mania: a double-blind, randomized, placebo-controlled trial. Bipolar Disord 2006; 8:485–489. [DOI] [PubMed] [Google Scholar]
- 35.Hirota T, Kishi T. Adenosine hypothesis in schizophrenia and bipolar disorder: a systematic review and meta-analysis of randomized controlled trial of adjuvant purinergic modulators. Schizophr Res 2013; 149:88–95. [DOI] [PubMed] [Google Scholar]


