Abstract
Several studies investigating the relationship between body mass index (BMI), waist circumference (WC), and/or body fat (BF) with macronutrient composition of the diet have suggested that dietary composition may play an important role to overweight/obesity in childhood, but its relation remains inconclusive. The aim was to assess the association between energy intake (EI) and macronutrient diet composition with overweight/obesity among children.
Nonrandomized cohort study including 396 Italian children and preadolescents (9–13 years old), 200 overweight/obese and 196 normal-weight. The children's weight, height, WC, and food intake were measured.
Reported EI was higher in overweight/obese than in nonoverweight children; however, after body weight was considered, the overweight/obese children had less EI than their leaner counterparts. Percentages of EI from proteins, SFA, MUFA and PUFA (in males), and dietary fiber (g/1000 kcal) were higher in the overweight/obese children than in the leaner ones. EI from carbohydrates and fats was lower in overweight/obese males and females, respectively. Positive correlations between BMI and waist-to-height ratio with EI from proteins were found in males (r = 0.296, P < 0.01 and r = 0.326, P < 0.01; respectively) and females (r = 0.374, P < 0.01 and r = 0.405, P < 0.01; respectively), but negative correlations with fats were found in females (r = −0.240, P < 0.01 and r = −0.188, P < 0.05; respectively). Using binary logistic regression, the highest EI from proteins were associated with higher odds ratio for overweight/obesity, while the lowest EI from carbohydrates was associated with higher odds ratio for overweight/obesity in males.
Reported EI of overweight/obese children was higher than nonoverweight peers. Overweight/obese children had higher intakes of proteins compared with nonoverweight ones. Overweight/obese males and females showed lower EI from carbohydrates and fats, respectively, than their leaner counterparts.
INTRODUCTION
Last decades, children and adolescent worldwide population showed high prevalence of overweight, which has been converted in an important public health problem not only in these segments of the population but also in their future adulthood.1,2 Children and adolescents overweight and obesity are multifactorial, and caused by interactions between genetic, socio-demographic, behavioral, and environmental factors.
It has been pointed out that obesity grows after energy intake (EI) exceeds energy expenditure, but sometimes overweight and/or obese children did not show higher EIs,3–7 which may have been due to methodological errors, or to the fact that children at risk of overweight could have eaten more than nonobese children at a previous stage but not at the present, or may also be influenced by characteristic psychological and dieting aspects that appear after obesity has been already arisen.8 Nevertheless, it has been pointed out that body mass index (BMI), waist circumference (WC), and/or body fat (BF) are related with EI, which suggested that the proportion of dietary protein, carbohydrate, and fat (the macronutrient diet composition) may play an important role on childhood overweight and/or obesity similarly to the findings in adults.2,3–7,9–11
Elliott et al2 pointed out that few studies specifically addressed fat, protein, and carbohydrate intake in relation to BMI, WC, and/or BF in children. Several cross-sectional studies reported a direct association between fat intake and adiposity in children3,4,10,12 but others did not.2,5–7,11,12 In several studies, an inverse relationship between total BMI and EI as carbohydrates have been demonstrated, and overweight children and adolescents consumed less energy as carbohydrates compared with their lean counterparts.3,5,6,9 It has been proposed that dietary protein intake may also modulate BF contents; however, the results on the associations of protein intake with overweight and/or obesity are controversial.3–7,11,12 Therefore, the relationships between dietary composition and the development of childhood obesity demands further work.12
To test the hypothesis that diet composition is associated with the presence of overweight/obesity in children, we assessed diet composition in a group of overweight/obese children before treatment and normal weight age-matched controls and we estimated the relationship between diet composition and the presence of overweight/obesity in this sample.
MATERIALS AND METHODS
Subjects
The study was a nonrandomized, case–control study including 396 Italian children and preadolescents: 200 overweight and obese recruited from 2008 onward at the Obesity Clinic of the Regional Center for Pediatric Diabetes of Verona, Italy, where they were addressed by their public health system-provided general pediatrician because of overweight/obesity, before starting a multidimensional treatment program for obesity,13 and 196 age- and gender-matched control subjects of Caucasian ethnicity who were recruited from a survey conducted in the same geographical area by the same research team to assess the prevalence of cardiovascular risk factors, as it was previously described.14,15 In this design, control sample was larger than the case sample (about 700 children 8–13 years old), and the matched control was automatically selected for each case, within the proper age and gender group, by random extraction function of Microsoft Office Excel. None of the children had any overt disease other than obesity or was taking medication. To exclude obese children with potential impaired glucose tolerance or type 2 diabetes, which have a 6.9% and a 0.1% prevalence, respectively, among those from the study area, children underwent an oral glucose tolerance test (1.75 g glucose/kg body weight, maximum 75 kg). None had impaired glucose tolerance or diabetes.15,16
The characteristics of the present study have been previously described elsewhere.13,15 Inclusion criteria were age (9–13 years old), and ethnic group (Caucasian). Obesity secondary to other diseases, chronic diseases and chronic use of drugs, dieting at the time of the study, or EI < 110% of their basal metabolic rate predicted by age, weight, and gender17 were considered as exclusion criteria. The Ethical Committee of the University of Verona approved the study. Informed consents from all the participants and parents were obtained.
All the participants underwent a physical examination, comprising height, weight, WC, and blood pressure measurements, as well as assessment of Tanner stages (pubertal development).13 Height was determined using an anthropometer (SECA 217, Hamburg, Germany) to the nearest millimeter, with the subject's head in the Frankfurt plane. Body weight was determined to the nearest 100 g using a digital scale (Tanita BC-418, Tanita Corporation, Arlington Heights, IL). The subjects were weighed in bare feet and light underwear. The BMI was calculated. Subjects were defined as overweight or obese on the basis of their BMI, using the International Obesity Task Force BMI cut-offs for overweight and obesity, corresponding to a BMI of 25 and 30, respectively, at the age of 18 years.18 WC was measured halfway between the iliac crest and lower rib using a nonstretching tape with attached spring balance pulled to a tension of 250 g. Waist-to-height ratio (WHtR) was also calculated.
Physical activity was evaluated translated Italian version of the International Physical Activity Questionnaire (IPAQ), which is available upon request. In order to improve validity, the questionnaire was interviewer-administered and short version was adopted19 asking participant (or his/her parents, according to age and maturity) frequency and overall duration of sedentary activity, walking, moderate and vigorous physical activity during the last 7 days. Interview was performed by the same trained dietician in charge of dietary assessment.
Dietary Assessment
As it has been previously described elsewhere,13 weekly meal and snack intake was determined through 1 interview (diet history) with mothers and children conducted by a trained dietician. Information regarding portion size, frequency of eating, food preparation, and place of consumption was also recorded. To define food portions and the amount of consumed food, pictures included in an atlas specially designed for this purpose, and approved by the ANDID (National Association of Dieticians)20 were used. Computerized food composition tables of the Italian Institute of Nutrition (Metadieta, Meteda, S. Benedetto del Tronto, Italy) were used to calculate the daily food energy values.
Biochemical Parameters
Blood samples were collected from antecubital vein after ≥12-hour overnight fast. Triglycerides (TG), total cholesterol (TC), and HDL-cholesterol (HDL-c) levels were assessed by means of enzyme-based assays (Cobas integra, Roche Diagnostics, Indianapolis, IL). LDL-cholesterol (LDL-c) level was calculated using the Friedewald equation.15
Plasma glucose was analyzed by using the glucose oxidase method. Serum insulin level was measured using the specific chemiluminescence method with an Insulin Bridge kit (Adaltis Inc., Montreal, QC, Canada). Quantitative insulin sensitivity check index (QUICKI),21 the homeostatic model assessment (HOMA),22 and HOMA-β23 were also calculated.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics 21.0 software (SPSS, Chicago, IL). All analyses were stratified by gender.
Minimal sample size of each of the 4 samples used to compare diet composition of obese versus normal weight children/preadolescents (ie, obese and normal weight boys, obese and normal weight girls), was calculated in order to have a 90% statistical power to detect a minimal difference of 2%, 5%, and 3% in the mean intakes of protein, carbohydrates, and fat as percentage of total energy, respectively, with a 5% α error. Formula used was: n ≥ 2 ∗ [variable VARIANCE]/[minimal difference]2 ∗ (tα + tβ)2.24
Variable variances were issued from previous local data.13 Minimal differences to detect were arbitrarily decided as the 10% of the recommended intake based on Italian RDIs.25 tα and tβ were set at 2.0 and 1.3, respectively, that are acceptable proxy t values guessing that an n sample size of 50 to 200 individuals would be needed, in order to avoid solving equation by iterative steps. Sixty individuals was the minimal sample size needed for each sample, to be 90% powerful to detect all of the 3 above-cited minimal differences. Based on the final sizes of children/preadolescents samples we were able to include in the analyses, the study was 99%, 100%, and 95% powerful to detect the minimal differences set for proteins, carbohydrates, and fat, respectively, in both boys and girls.
The Shapiro–Wilk test was used to assess normality of distribution of variables. While differences in means were tested by an unpaired Student t test when variables were normally distributed, difference of medians was tested by the Mann–Whitney U test when variables were not normally distributed. Spearman's correlation analysis between nutritional variables and BMI z-score and WHtR were performed. Multiple-linear regression analysis to explore the relationship with nutritional variables separately and the dependent variable (BMI and WHtR) were also performed. Prior to linear regression analysis, both dependent and independent variables were log transformed. To evaluate the relation between overweight/obesity and nutritional variables, logistic regression analysis were used, with the prevalence odds ratio (OR) and confidence interval (CI) of overweight/obesity as the outcome, and each of the nutrient categories as predictors. The multivariable analyses were adjusted for age (years) and physical activity level (PAL). A P value < 0.05 was considered as significant.
RESULTS
Overall, 20 normal-weight children (10 males and 10 females) and 29 overweight/obese children (13 males and 16 females) were excluded. Therefore, the analyses were conducted on 347 children, 171 overweight/obese and 176 nonobese. No statistical significant differences in mean age and BMI were obtained between excluded and included children.
Table 1 shows physical characteristics and biochemical variables of normal-weight and overweight/obese children, by gender. The samples studied were homogeneous in terms of puberty. In both genders, there were significant differences in weight, BMI, BMI z-score, WC, WHtR, diastolic and systolic blood pressures, HDL-c and triglycerides levels between normal-weight and overweight/obese children, but not in height, total cholesterol levels, FI, HOMA, and QUICKY. Differences in LDL-c levels, FPG, and HOMA-β were found between normal-weight and overweight/obese subjects in males but not in females. The mean PAL was not significant different between normal-weight and overweight/obese children in either gender.
TABLE 1.
Physical Characteristics and Biochemical Variables of Normal-Weight and Overweight/Obese Children by Gender
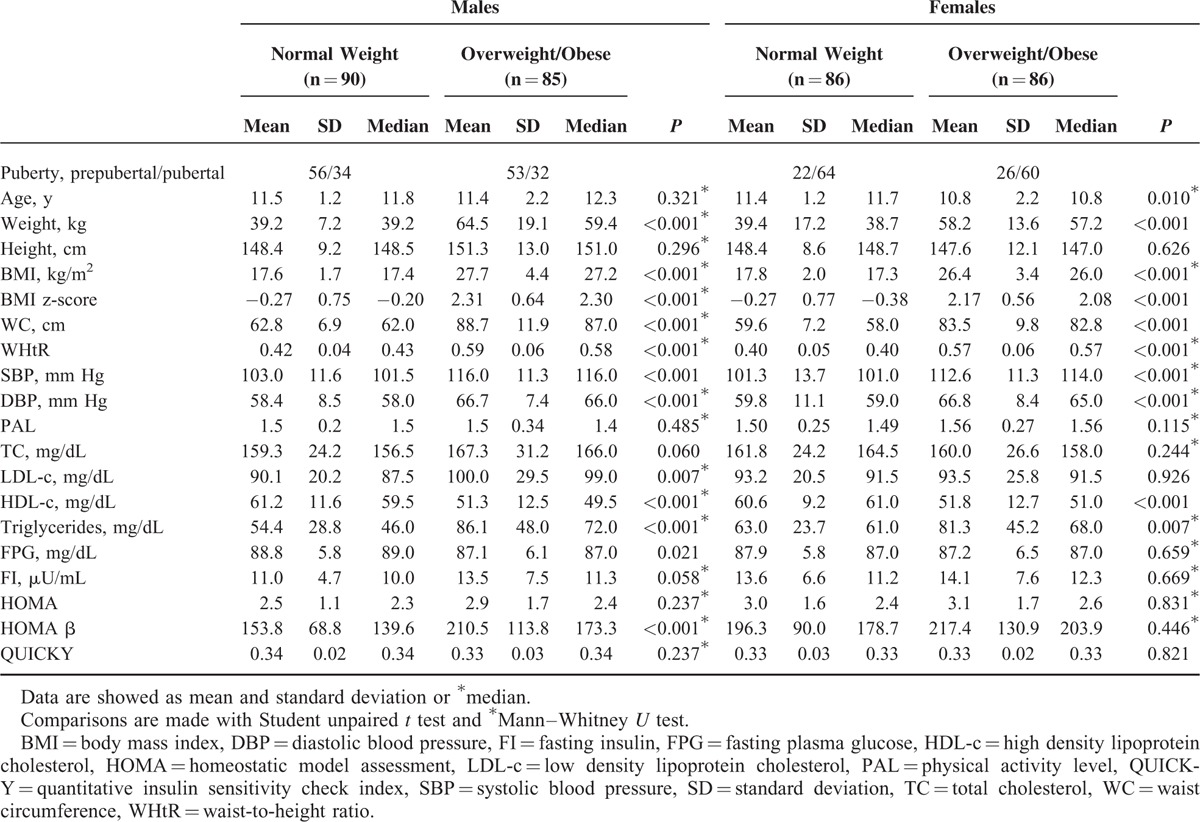
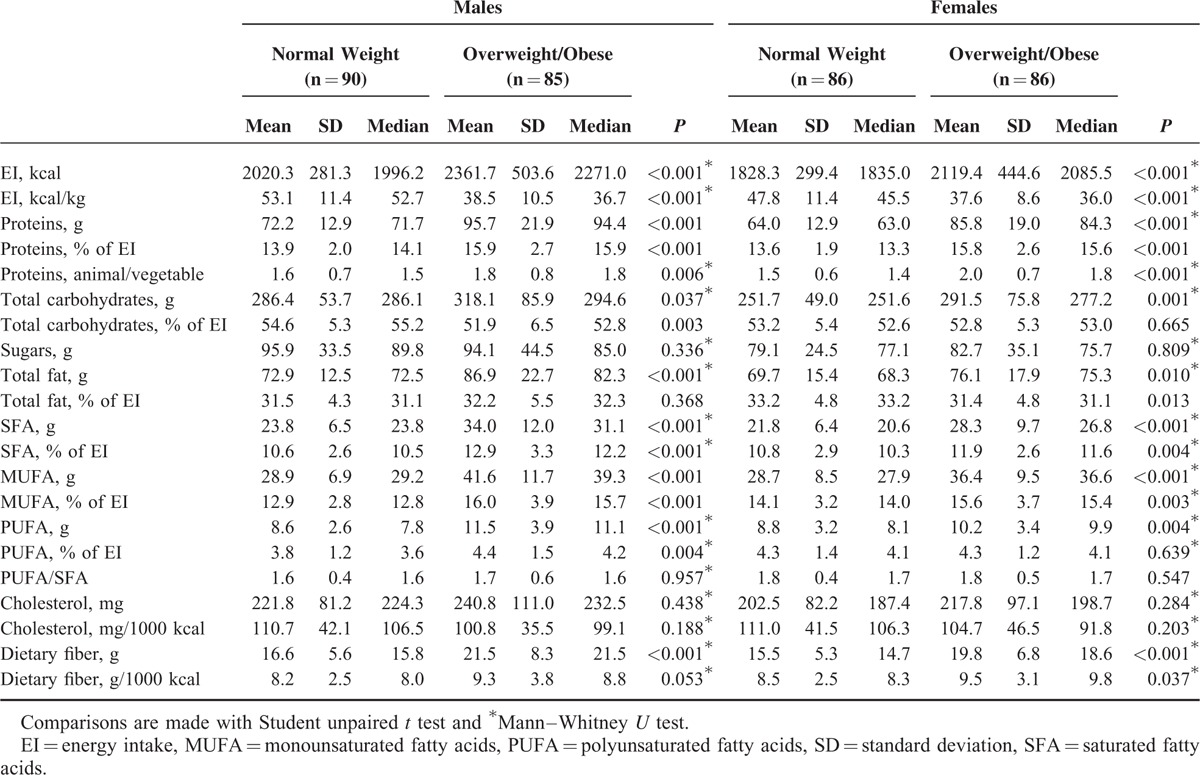
Table 2 shows EI and macronutrient composition of the diet of normal-weight and overweight/obese children, by gender. In both genders, there were significant differences in EI (kcal/d and kcal/kg): overweight/obese children had significantly greater crude daily EI than did nonoverweight children; however, after body weight was considered, the overweight/obese children had less EI than did their leaner counterparts. Overweight/obese children had significantly higher carbohydrate, protein, total fat, SFA, MUFA, PUFA, and dietary fiber intakes expressed in total grams than their leaner counterparts. The differences between the means, when expressed as percentage of EI, were statistically significant for proteins, SFA, and MUFA (and PUFA was significantly only for males), and also dietary fiber expressed as g/1000 kcal; but EI from carbohydrates and fats was significantly lower in our population of overweight/obese males and females, respectively. Overweight/obese children also showed a higher ratio of dietary animal to vegetable protein than nonoverweight ones.
TABLE 2.
Energy and Nutrient Intake, and Physical Activity Level of Normal-Weight and Overweight/Obese Children by Gender
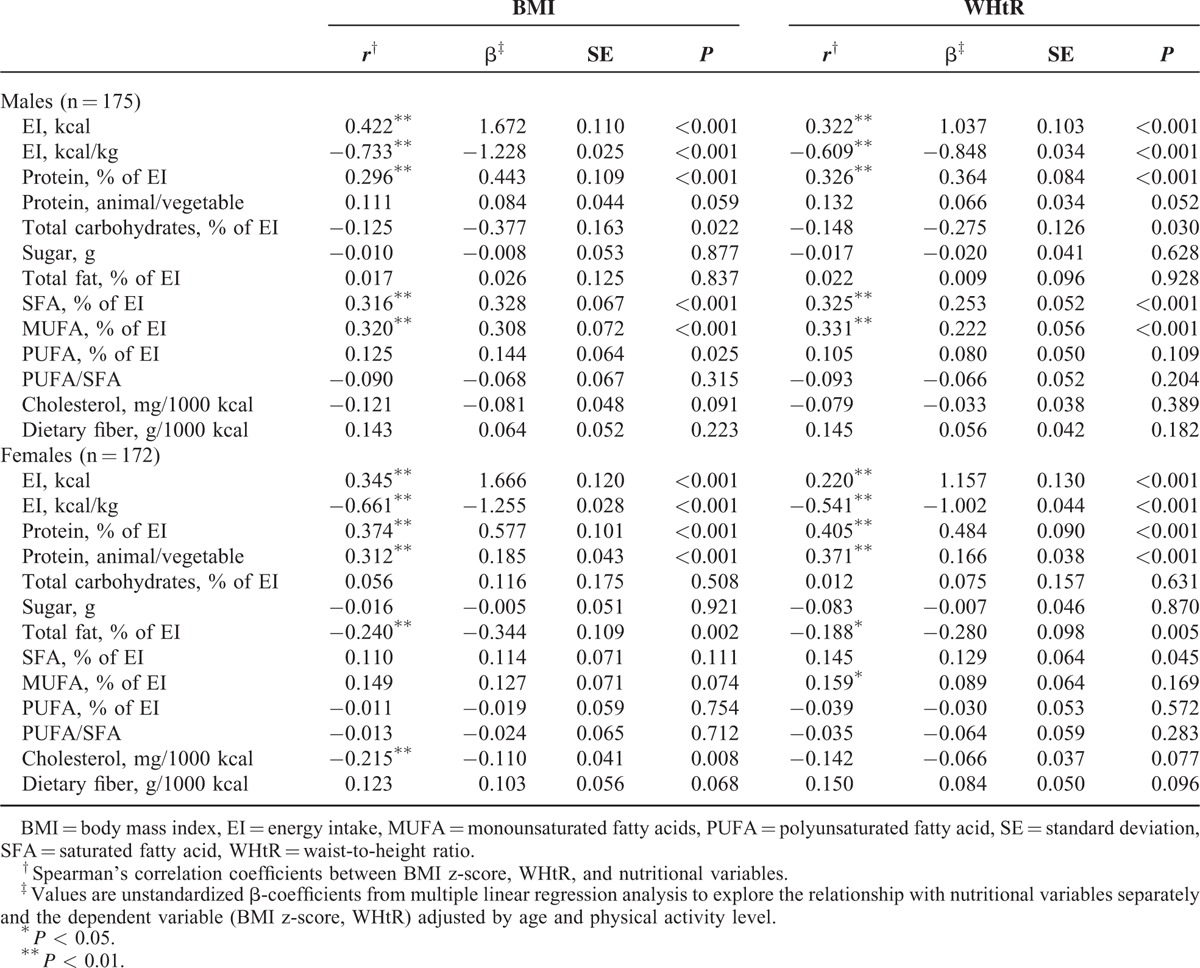
Spearman's correlation and linear regression were used to evaluate the association between BMI, WHtR, and nutritional variables are shown in Table 3. BMI was strongly correlated with WHtR (r = 0.914, P < 0.001). In both genders, total energy and percentage of energy from proteins were positively related with BMI and WHtR, while EI per kilogram of body weight (kcal/kg) correlated negatively with BMI and WHtR. Dietary ratio of animal to vegetable protein was also significantly positively correlated with BMI (in females) and WHtR. Total fat (% of EI) was significantly negatively correlated with BMI and WHtR in females, while energy from SFA and MUFA and PUFA was directly associated with BMI and WHtR in males (except PUFA for WHtR). A negative association between percentage of energy from carbohydrates and BMI was also found in boys. A weak but significant negative association between cholesterol intake (expressed as g/1000 kcal) and BMI was also found in females.
TABLE 3.
Associations With BMI and WHtR and Nutritional Variables
For the 175 males and 172 females, we examined nutritional determinants of overweight plus obesity (Table 4). The relationship between BMI and protein intake based on quartiles shows a significant difference among the groups, suggesting that overweight/obese children consumed more energy from proteins than their leaner peers. The proportion of the overweight/obese children was tenfold higher in quartile 4 of protein intake compared with quartile 1 in males and females.
TABLE 4.
Odds Ratios (95% CI) for Dietary Intake Determinants of Overweight/Obesity vs Normal-Weight by Gender
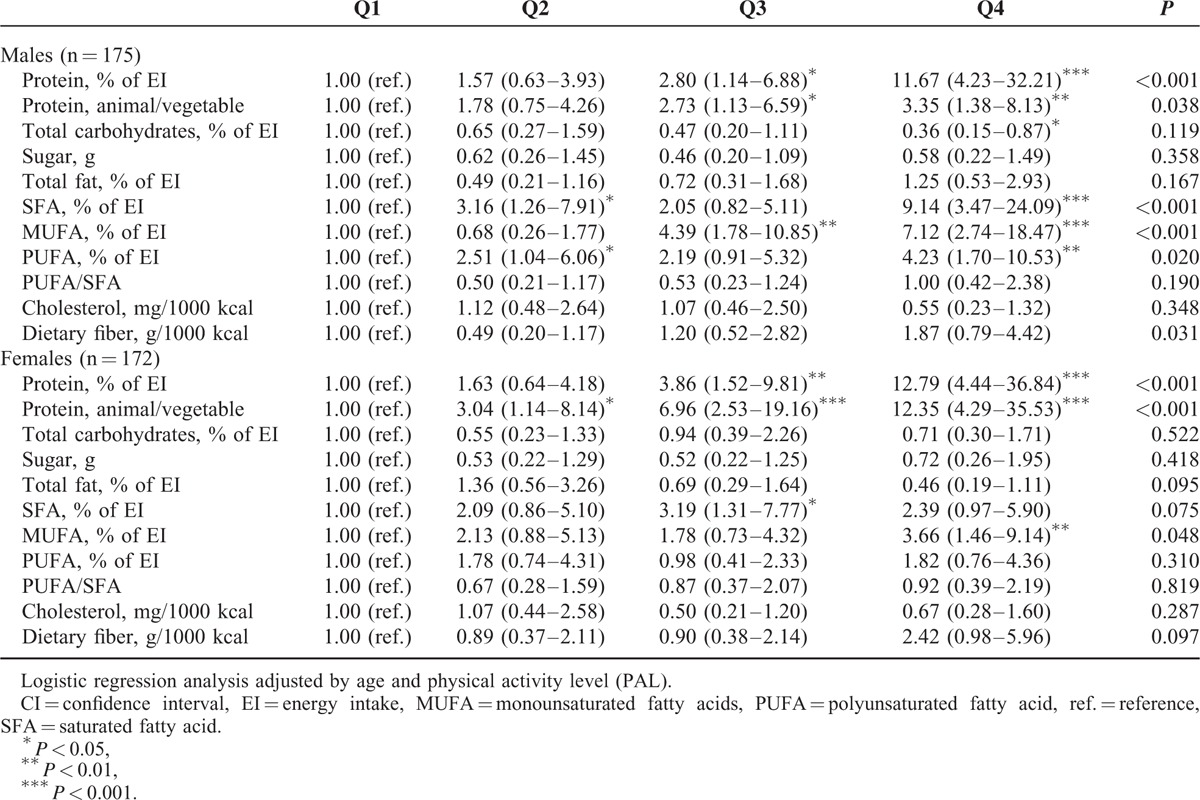
The highest carbohydrate intake (quartile 4) showed 64% lower OR (CI: 15–87%) for overweight/obesity among males, but it was not significantly related to BMI among females. Total fat and PUFA/SFA ratio were no associated with the OR for overweight/obesity, although, a higher proportion of overweight/obese children was found in quartile 4 of EI from SFA, MUFA, and PUFA compared to quartile 1 in males and in quartile 4 of EI from MUFA compared to quartile 1 in females. OR for overweight/obesity increased across the quartile categories of dietary fiber variable in males. None of the other nutritional determinants (sugars and cholesterol) were associated with the risk for overweight/obesity among children.
DISCUSSION
The main finding of this study is that EI and the macronutrient composition of children's diet, particularly higher protein intake (including a high ratio of dietary animal to vegetable protein), are related to adiposity. Our findings further support the idea that the diet macronutrient composition may play a contributing role to obesity in childhood, although data from children and adolescents researching this relationship are scarce and have produced inconsistent results.3,5–7,9,11,12
Several studies evaluating the relation between the EI and overweight and/or obesity, have failed to show that fatter children have higher reported EIs. Several authors4–6 reported an EI in overweight and/or obese children significantly lower than lean ones. Other authors3,7,11 detected no significant difference between normal-weight and overweight children regarding the EI. However, several researchers12 found a modest but significant correlation between EI and BMI z-score and WC in a study performed on 1352 children ages 5 to 17 years old, after screening out misreporters. These researchers12 also reported a significantly higher EI in subjects with the highest BMI z-score (quartile 4) than in their leaner counterparts. The findings of the present study are consistent with previous results,12 suggesting that our overweight/obese population consume more energy than their leaner counterparts. However, in agreement with previous findings,9 even if overweight/obese children had significantly greater daily EI than did nonoverweight children, the overweight/obese children showed less EI/kg of body weight than did their leaner counterparts. This is not surprising, because obese children have large amounts of fat that is less metabolically active than fat-free tissues, contributing less to the overall energy expenditure, thus they have lower resting energy expenditure/kilograms of body weight than their lean counterparts.26
With respect to differences in diet composition, we found that protein intake was directly associated with BMI and WHtR, and our overweight/obese children consumed more proteins than their leaner counterparts. Although our results differ from some published studies,4–6,12 which detected no difference in protein intake between overweight and nonoverweight children and adolescents, they are consistent with previous authors11 who found a direct association between EI from proteins and BF percentage, WC and hip circumference among Finnish children ages 6 to 8 years old. Several authors3,7 also reported a greater contribution of protein to the total EI in overweight children and adolescents. Aeberli et al7 reported that among Swiss children (n = 156) ages 6 to 14 years old, after excluding under-reporters, overweight children consumed 7.7 g more protein or 1.2% more energy as protein than normal-weight children. It has been pointed out that high BMI and high BF percentages in late childhood may be predicted by a high protein intake during the weaning period and the first years of life.8,11,27–29 However, the assessment of the association of protein intake after weaning period with childhood overweight showed controversial results.11 One explanation for the high protein intake observed in our overweight/obese population could be attributed to unhealthy dietary choices such as replacing vegetables with meat, as suggest the differences found in the ratio of dietary animal to vegetable protein when groups were compared.
EI from carbohydrates was lower in our population of overweight/obese males. Again, using binary logistic regression, a higher EI from carbohydrates (quartile 4) was associated with a lower OR for prevalence of overweight/obesity in males. This inverse association between dietary carbohydrate intake and adiposity has also been reported by previous studies.3,5,6,9
Several studies reported a positive association between the lack of dietary fiber in pediatrics’ diet and the degree of body fatness in children30 and adolescents31 while others did not.10–12,32 Contrary to expected findings, results of the present study are consistent with those of previous works,33 which found that higher fiber density was associated with increased risk for overweight/obesity in German children. Moreover, Brauchla et al31 previously examined the food sources of dietary fiber in US children (2- to 11-year-old) and adolescents (12- to 18-year-old), and found that the foods providing the highest proportion of dietary fiber to the plausible diets of children and adolescents were not high-fiber foods (ie, French fries, pizza, or white bread/rolls). These authors31 also found differences in the main sources of fiber in the healthy weight 2- to 11-year-old children compared with their overweight/obese peers (ie, the healthy weight children consumed more high-fiber foods such as peanut butter or popcorn); however, there was a not significant trend of decreasing risk for overweight/obesity with increasing fiber density among them. Accordingly, data on dietary fiber intake and body weight in the pediatric population are not consistent yet.31
Regarding fat intake, overweight/obese children have significantly higher intakes of total fat than their leaner counterparts. However, EI from fats was lower in our population of overweight/obese females. A negative association between BMI and WHtR and energy from fats was also found in females. These findings seem to be consistent with a previous study12 in which an inverse relationship was observed between fat intake and BMI z-score, although no significant difference between groups when subjects were split into quartiles based on BMI z-score and WC were observed. To the contrary, there were not found significant differences between the groups in boys. This finding accords with several other studies which showed no significant difference in percentage fat intake in overweight and nonoverweight children and adolescents.5–7,11 However, our results also conflict with other studies that have demonstrated a positive association between adiposity and dietary fat.3,4,9,10,12
When dietary fat composition is concerned, it appears that the overweight/obese children in this study consumed a significantly greater proportion of their overall energy in the form of both saturated and unsaturated, than did their leaner counterparts. Few studies with children and adolescents have published data on differences in actual dietary fatty acid consumption between overweight and nonoverweight people.7,9,11 However, the results of this study are similar with results previously reported,9 in which SFA, MUFA, and PUFA were significantly greater in obese children ages 9 to 11 years old than in their nonobese counterparts, and significantly and positively correlated with %BF. With respect to cholesterol intake, in the present study a significant negative association with BMI was found in females. Unfortunately, few studies with children or adolescents subjects have published data on differences in cholesterol intake between overweight and nonoverweight people, which showed that obese children12 and overweight adolescents3 consumed greater amounts of cholesterol than their leaner counterparts.
LIMITATIONS AND STRENGTHS
This study has several limitations. The difficulties to assess food intake in humans and especially in young people are well known.34 It is possible that unknown confounder factors may have influenced the results of this study, such as parental educational or professional level. Moreover, we cannot infer causality because of the cross-sectional design of the study and hence the study provides information on the obese state only, not on the development of obesity.
This study also has several strengths. First, the present study is based on objectively measured weight, height, and WC instead of reported values, and on rigorous methods for assessing diet. Second, overweight/obese was defined by internationally accepted age and specific BMI cut offs,18 which enables the comparison of results with studies conducted in the same age group in other countries. And finally, this study presents vital information on the relationship between the EI, diet composition, and adiposity in children.
CONCLUSIONS
In summary, the findings of this study support that EI and the macronutrient composition of children's diet may play an important contributing role to adiposity. In fact, we found a higher daily EI in the overweight/obese group when several studies have failed to demonstrate it. It has also been suggested that diets high in fat3,4,9,10,12 and protein3,7,11 and low in carbohydrate3,5,6,9 may cause an accumulation of excessive BF even when total EI is not in excess. In the present study, the overweight/obese children were consuming a greater proportion of their overall energy in the form of protein than nonoverweight ones. Moreover, the overweight/obese males and females were consuming a lower proportion of their overall energy in the form of carbohydrates and fats, respectively, than their nonoverweight counterparts. It also appears that the overweight and obese children consumed a significantly greater proportion of their overall energy in the form of both saturated and unsaturated fat, than did their leaner counterparts. However, robust longitudinal studies are needed to elucidate the relationship linking obesity and dietary intake in children.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, EI = energy intake, FI = fasting insulin, FPG = fasting plasma glucose, HDL-c = high density lipoprotein cholesterol, HOMA = homeostatic model assessment, LDL-c = low density lipoprotein cholesterol, MUFA = monounsaturated fatty acids, OR = odds ratio, PAL = physical activity level, PUFA = polyunsaturated fatty acids, QUICKY = quantitative insulin sensitivity check index, ref. = reference, SBP = systolic blood pressure, SD = standard deviation, SFA = saturated fatty acids, TC = total cholesterol, WC = waist circumference, WHtR = waist-to-height ratio.
The authors’ contributions were as follows: MdMB, JAT, and CM conceived, designed, and devised the study, AM, MT, FT, and CM collected and supervised the samples, and MdMB, JAT, and CM analyzed the data and wrote the manuscript.
Spanish Ministry of Health and Consumption Affairs (Projects 14/00636, Red Predimed-RETIC RD06/0045/1004, and CIBEROBN CB12/03/30038), Grant of support to research groups no. 35/2011 (Balearic Islands Gov.), EU FEDER funds.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet 2002; 360:473–482. [DOI] [PubMed] [Google Scholar]
- 2.Elliott SA, Truby H, Lee A, et al. Associations of body mass index and waist circumference with: energy intake and percentage energy from macronutrients, in a cohort of Australian children. Nutr J 2011; 10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortega RM, Requejo AM, Andrés P, et al. Relationship between diet composition and body mass index in a group of Spanish adolescents. Br J Nutr 1995; 74:765–773. [DOI] [PubMed] [Google Scholar]
- 4.Maffeis C, Pinelli L, Schutz Y. Fat intake and adiposity in 8 to 11-year-old obese children. Int J Obes Relat Metab Disord 1996; 20:170–174. [PubMed] [Google Scholar]
- 5.Rocandio AM, Ansotegui L, Arroyo M. Comparison of dietary intake among overweight and non-overweight schoolchildren. Int J Obes Relat Metab Disord 2001; 25:1651–1655. [DOI] [PubMed] [Google Scholar]
- 6.Hassapidou M, Fotiadou E, Maglara E, et al. Energy intake, diet composition, energy expenditure, and body fatness of adolescents in northern Greece. Obesity (Silver Spring) 2006; 14:855–862. [DOI] [PubMed] [Google Scholar]
- 7.Aeberli I, Kaspar M, Zimmermann MB. Dietary intake and physical activity of normal weight and overweight 6 to 14 year old Swiss children. Swiss Med Wkly 2007; 137:424–430. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez G, Moreno LA. Is dietary intake able to explain differences in body fatness in children and adolescents? Nutr Metab Cardiovasc Dis 2006; 16:294–301. [DOI] [PubMed] [Google Scholar]
- 9.Gazzaniga JM, Burns TL. Relationship between diet composition and body fatness, with adjustment for resting energy expenditure and physical activity, in preadolescent children. Am J Clin Nutr 1993; 58:21–28. [DOI] [PubMed] [Google Scholar]
- 10.Tucker LA, Seljaas GT, Hager RL. Body fat percentage of children varies according to their diet composition. J Am Diet Assoc 1997; 97:981–986. [DOI] [PubMed] [Google Scholar]
- 11.Eloranta AM, Lindi V, Schwab U, et al. Dietary factors associated with overweight and body adiposity in Finnish children aged 6–8 years: the PANIC Study. Int J Obes (Lond) 2012; 36:950–955. [DOI] [PubMed] [Google Scholar]
- 12.Saker M, Merzouk H, Merzouk SA, et al. Predictive factors of obesity and their relationships to dietary intake in schoolchildren in Western Algeria. Maedica (Buchar) 2011; 6:90–99. [PMC free article] [PubMed] [Google Scholar]
- 13.Maffeis C, Maschio M, Costanzi S, et al. Diet macronutrient composition reported before treatment predicts BMI change in obese children: the role of lipids. Eur J Clin Nutr 2012; 66:1066–1068. [DOI] [PubMed] [Google Scholar]
- 14.Takemoto K, Deckelbaum RJ, Saito I, et al. Adiponectin/resistin levels and insulin resistance in children: a four country comparison study. Int J Pediatr Endocrinol 2015; 2015:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maffeis C, Morandi A, Ventura E, et al. Diet, physical, and biochemical characteristics of children and adolescents with type 1 diabetes: relationship between dietary fat and glucose control. Pediatr Diabetes 2012; 13:137–146. [DOI] [PubMed] [Google Scholar]
- 16.Maffeis C, Pinelli L, Brambilla P, et al. Fasting plasma glucose (FPG) and the risk of impaired glucose tolerance in obese children and adolescents. Obesity (Silver Spring) 2010; 18:1437–1442. [DOI] [PubMed] [Google Scholar]
- 17.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr 2003; 133:895S–920S. [DOI] [PubMed] [Google Scholar]
- 18.Cole TJ, Flegal KM, Nicholls D, et al. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007; 335:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Park I, Kang M. Convergent validity of the International Physical Activity Questionnaire (IPAQ): meta-analysis. Pub Health Nutr 2013; 16:440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Istituto Scotti Bassani. Atlante Fotografico delle porzioni e degli Alimenti. Milano: Istituto Scotti Bassani, 2005. [Google Scholar]
- 21.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 23.Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of (-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care 2013; 36:2324–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zar JH. Biostatistical Analysis. 4th edNew Jersey: Prentice-Hall; 1999. [Google Scholar]
- 25.SINU, Società Italiana di Nutrizione Umana. Livelli di Assunzione di Riferimento di Nutrienti ed energia. Milano: SICS Editore, 2014. [Google Scholar]
- 26.Maffeis C, Schutz Y, Micciolo R, et al. Resting metabolic rate in six- to ten-year-old obese and nonobese children. J Pediatr 1993; 122:556–562. [DOI] [PubMed] [Google Scholar]
- 27.Günther AL, Buyken AE, Kroke A. The influence of habitual protein intake in early childhood on BMI and age at adiposity rebound: results from the DONALD Study. Int J Obes (Lond) 2006; 30:1072–1079. [DOI] [PubMed] [Google Scholar]
- 28.Günther AL, Remer T, Kroke A, et al. Early protein intake and later obesity risk: which protein sources at which time points throughout infancy and childhood are important for body mass index and body fat percentage at 7 y of age? Am J Clin Nutr 2007; 86:1765–1772. [DOI] [PubMed] [Google Scholar]
- 29.Garden FL, Marks GB, Almqvist C, et al. Infant and early childhood dietary predictors of overweight at age 8 years in the CAPS population. Eur J Clin Nutr 2011; 65:454–462. [DOI] [PubMed] [Google Scholar]
- 30.Johnson L, Mander AP, Jones LR, et al. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am J Clin Nutr 2008; 87:846–854. [DOI] [PubMed] [Google Scholar]
- 31.Brauchla M, Juan W, Story J, et al. Sources of dietary fiber and the association of fiber intake with childhood obesity risk (in 2–18 year olds) and diabetes risk of adolescents 12–18 year olds: NHANES 2003–2006. J Nutr Metab 2012; 2012:736258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis JN, Alexander KE, Ventura EE, et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am J Clin Nutr 2007; 86:1331–1338. [DOI] [PubMed] [Google Scholar]
- 33.Cheng G, Karaolis-Danckert N, Libuda L, et al. Relation of dietary glycemic index, glycemic load, and fiber and whole-grain intakes during puberty to the concurrent development of percent body fat and body mass index. Am J Epidemiol 2009; 169:667–677. [DOI] [PubMed] [Google Scholar]
- 34.Wärnberg J, Nova E, Romeo J, et al. Lifestyle-related determinants of inflammation in adolescence. Br J Nutr 2007; 98 Suppl 1:S116–S120. [DOI] [PubMed] [Google Scholar]


