Abstract
Recent studies have investigated the most efficacious dose of intravenous tissue plasminogen activator (IV-tPA) for acute ischemic stroke (AIS) patients. There remains no definitive consensus concerning the superior efficacious IV-tPA dose (standard- vs. low-dose), prompting us to perform a meta-analysis comparing the efficacy and safety profile of standard- versus low-dose IV-tPA.
We identified relevant studies pertaining to the specific aim of our meta-analysis by searching PubMed and EMBASE (January 1990–September 2015) Either a fixed- or random-effects model was employed (dependent upon data heterogeneity) to analyze the efficacy and safety outcome.
Ten cohort studies involving 4389 sum patients were included in the meta-analysis. By using the random-effects model, the meta-analysis indicated no statistically significant difference in favorable functional outcome (modified Rankin scale 0–1) at 3 months (heterogeneity: χ2 = 17.45, P = 0.04, I2 = 48%; OR: 0.88 [95% CI: 0.71–1.11]; P = 0.28) and incidence of symptomatic intracranial hemorrhage (SICH) (heterogeneity: χ2 = 14.41, P = 0.11, I2 = 38%; OR: 1.19 [95% CI: 0.76 to 1.87]; P = 0.45) between the standard- and low-dose groups. The fixed-effects model demonstrated no significant difference in mortality within 3 months (heterogeneity: χ2 = 6.73, P = 0.57, I2 = 0%; OR: 0.91 [95% CI: 0.73–1.12]; P = 0.37) between the standard- and low-dose groups.
Low-dose IV-tPA is comparable to standard-dose IV-tPA in both efficacy (favorable functional outcome) and safety (SICH and mortality). Confirmation of these findings through randomized trials is warranted.
INTRODUCTION
Since its approval by the United States FDA in 1996, intravenously administered tissue plasminogen activator (IV-tPA) remains the only established pharmaceutical therapy for acute ischemic stroke (AIS).1 Per the National Institute of Neurological Disorders and Stroke (NINDS) trial, the recommended dose of IV-tPA is 0.9 mg/kg (maximum 90 mg).1 In consideration of racial differences in blood coagulation–fibrinolysis factors, cost of treatment, and risk of symptomatic intracranial hemorrhage (SICH), a low-dose (or even variable-dose) IV-tPA regimen is frequently administered in many Asian hospitals. Thrombolytic therapy experience in Japanese AIS patients has demonstrated a low-dose IV-tPA regiment (0.6 mg/kg) might be more suitable for the Asian population.2–7 However, all the Japanese clinical studies were single-arm observational studies, and no other studies offer high-level evidence in support of the low-dose IV-tPA regimen. The optimal IV-tPA dose for AIS remains unknown in the absence of randomized validation. We have conducted a meta-analysis of controlled cohort studies comparing the efficacy and safety of low-dose versus standard-dose IV-tPA treatment in AIS patients.
MATERIALS AND METHODS
Ethics Statement
The MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines were followed in performing the present meta-analysis.8 This study did not involve patients, so ethical approval was not required.
Search Strategy
To identify all potential studies related to the efficacy and safety of differential dose IV-tPA without bias, a systematic literature search was conducted using PubMed and EMBASE, within the time parameters January 1990 to September 2015. The following search terms were used: “cerebrovascular accident,” “stroke,” “ischemia,” “cerebral infarction,” “cerebral artery occlusion,” “thrombosis,” “AIS,” “thrombolytic treatment,” “thrombolysis,” “tissue plasminogen activator,” “tPA,” “alteplase,” “dose.” No restrictions were imposed. Additionally, reference lists of all retrieved papers and recent reviews were reviewed.
Study Selection
An initial screening of titles/abstracts was performed. A second screening reviewed full-text. Studies were considered eligible if the following criteria were met: The study design was a cohort study; enrolled ischemic stroke patients were treated with low- or standard-dose IV-tPA; functional outcome 3 months after treatment was recorded; and safety data (SICH and mortality) were recorded.
Data Extraction
Data extraction was performed via standardized data-collection form, which included: publication reference, country, dose of IV-tPA administered, number of participants, age, gender, baseline National Institute of Health Stroke Scale (NIHSS) score, time from symptom onset to treatment initiation with IV-tPA, favorable functional outcome at 3 months [defined as modified Rankin scale (mRS) score 0–1], mortality within 3 months, and incidence of SICH [defined as intracranial hemorrhage within 36 hours resulting in neurological deterioration (increased NIHSS score by ≥4 points) unless otherwise specified]. Low-dose IV-tPA was defined as <0.85 mg/kg, and doses 0.85 to 0.95 mg/kg were defined as standard-dose. 0.85 mg/kg was chosen as the cut-off point, because 0.85 mg/kg has been demonstrated to have similar efficacy as 0.95 mg/kg.9 Two investigators (M.D.L. and W.D.N.) independently extracted the data and graded the methodological quality of each eligible study using the Newcastle–Ottawa Scale (NOS).10 Discrepancies were resolved by discussion with a third investigator (Y.Q.), or referencing the original publication.
Statistical Analysis
Dichotomous data (eg, SICH incidence, percentage of favorable functional outcome, mortality) were analyzed via odds ratio (OR). The Mantel–Haenszel (MH) approach was implemented by either fixed- or random-effects models, based upon included study heterogeneity.
The heterogeneities of the studies were assessed using the χ2 test and I2 statistic, with Pheterogeneity < 0.1 or I2 > 50% considered to be statistically significant.
Publication biases were assessed by visual examination of funnel plots, and were confirmed by analytic methods such as the Begg rank correlation test and Egger linear regression test.11,12 A P-value less than 0.05 indicated significant publication bias.
All analyses were performed via Review Manager (version 5.2, The Cochrane Collaboration, Oxford, UK) and Stata (version 12.1, Stata Corporation, College Station, USA).
RESULTS
Literature Search
One thousand two hundred eleven citations were initially retrieved from PubMed and EMBASE. The majority were excluded based upon abstracts or titles, due to being reviews, case reports, animal trials, or irrelevancy to our analytic aim. After full-text review of 21 papers, 11 articles were excluded, because: 7 studies utilized low-dose IV-tPA treatment without a standard-dose IV-tPA treatment group,2–7 1 study employed low-dose IV-tPA with urokinase,13 and 3 studies did not report SICH incidence.14–16 Ten studies were ultimately included in our meta-analysis.17–26 Study selection workflow is schematically shown in Figure 1.
FIGURE 1.
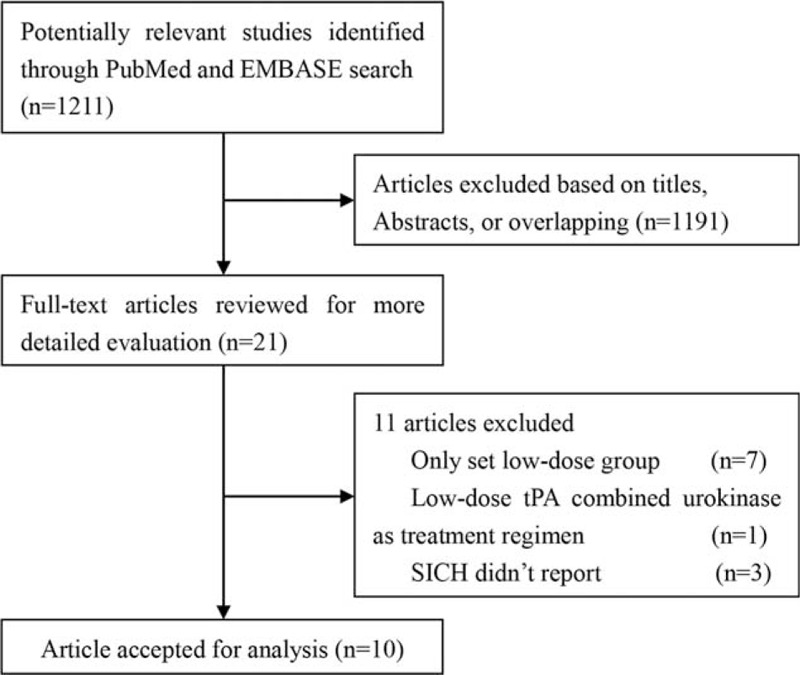
Study selection workflow schematic.
Study Characteristics and Quality
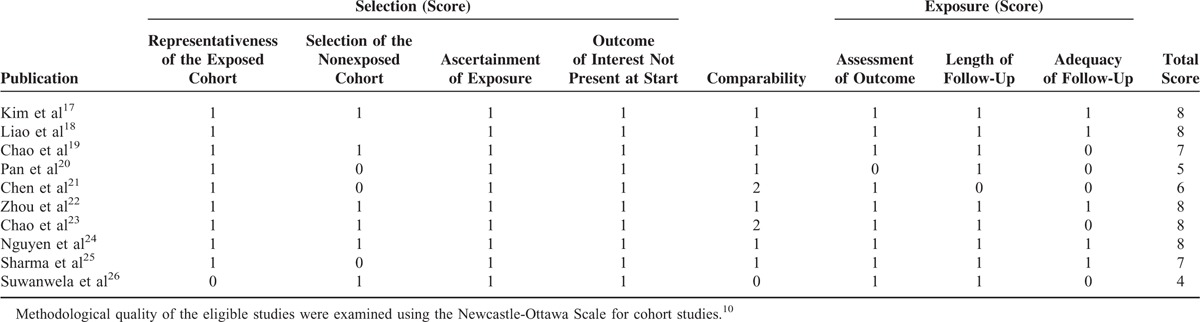
Table 1 lists the characteristics of the 10 included cohort studies, all published between 2006 and 2015. All studies were conducted in Asia (6 in China, 1 in Korea, 1 in Vietnam, 1 in Singapore, and 1 in Thailand). Individual study cohort size ranged from 34 to 1526 (total 4389). Across all studies, the dose of administered IV-tPA ranged from 0.5 to 0.95 mg/kg (doses ranging from 0.5 to 0.85 mg/kg were included in the low-dose group). There was no significant difference in any study's reported time between symptom onset and IV-tPA treatment initiation. Two studies reported significant patient age differences,19,25 and significant differences in gender were reported in 5 studies.17,18,20,22,24Table 2 lists the quality assessment for all included studies.
TABLE 1.
Characteristics of the Nine Included Cohort Studies
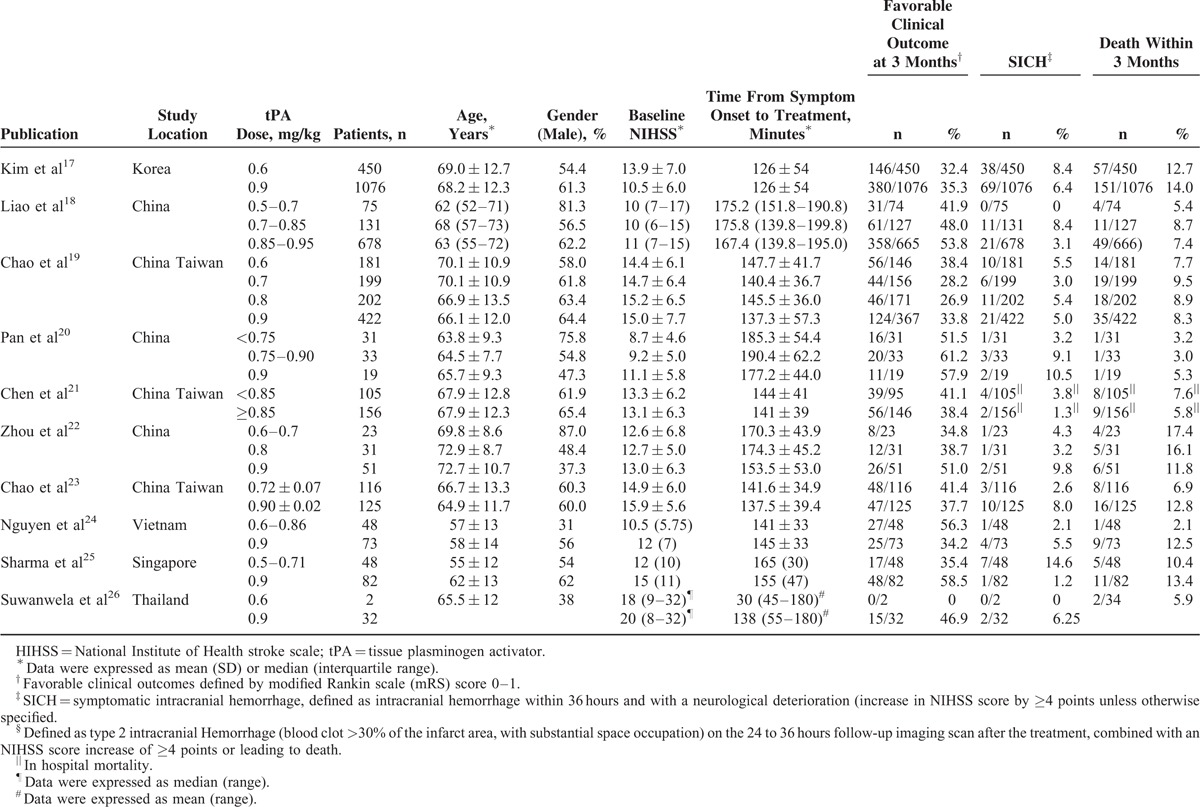
TABLE 2.
Quality Assessment of the Nine Included Cohort Studies
Functional Outcome
Several studies included in our analysis reported distinctly divergent outcomes from differential dosing of IV-tPA. However, when employing a random-effect model, our meta-analysis revealed no statistically significant difference in favorable functional outcome at 3 months between the standard- and low-dose IV-tPA groups (heterogeneity: χ2 = 17.45, P = 0.03, I2 = 48%; OR: 0.88 [95% CI: 0.71–1.11]; P = 0.28) (Figure 2).
FIGURE 2.
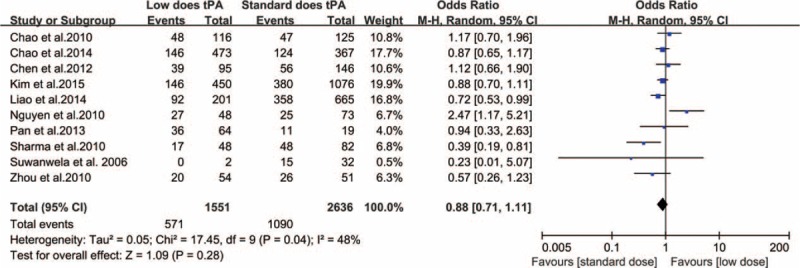
Relationship between IV-tPA dose and favorable functional outcome (0–1) at 3 months.
Safety Outcome
Only 1 study in our analysis reported significant relationship between low-dose IV-tPA and SICH [SICH occurred more frequently with low-dose IV-tPA (14.5%, 7 out of 48 patients) compared to standard dose (1.2%, 1 out of 82 patients), P = 0.004].25 A random-effects model demonstrated no significant difference in SICH incidence between the standard- and low-dose IV-tPA groups (heterogeneity: χ2 = 14.41, P = 0.11, I2 = 38%; OR: 1.19 [95% CI: 0.76–1.87]; P = 0.45) (Figure 3). Nine of the 10 studies included in our analysis described mortality data in each dosing group arm. Analysis by a fixed-effects model demonstrated no significant mortality difference within 3 months between the standard- and low-dose IV-tPA groups (heterogeneity: χ2 = 6.73, P = 0.57, I2 = 0%; OR: 0.91 [95% CI: 0.73–1.12]; P = 0.37) (Figure 4). Notably, 2 included studies demonstrated patients aged 70 years and older experienced increased functional and safety outcomes when receiving lose-dose IV-tPA compared to standard-dose (Table 3).
FIGURE 3.
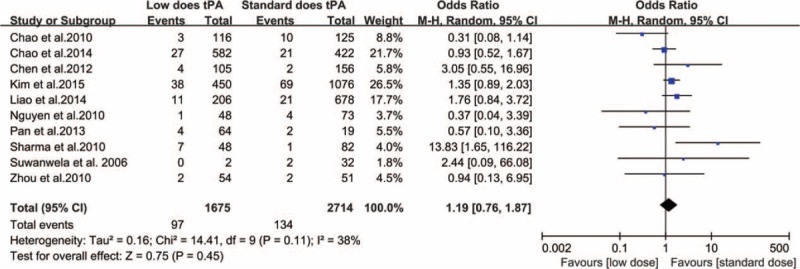
Relationship between IV-tPA dose and symptomatic intracranial hemorrhage.
FIGURE 4.
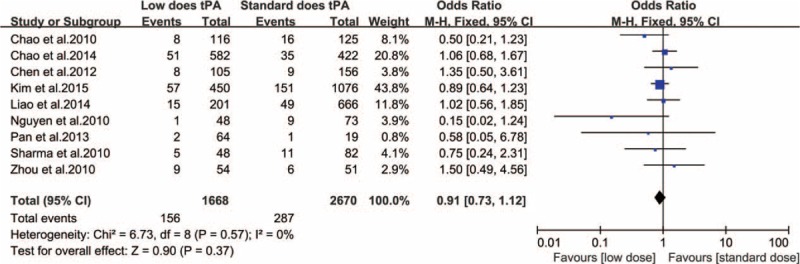
Relationship between IV-tPA dose and mortality within 3 months.
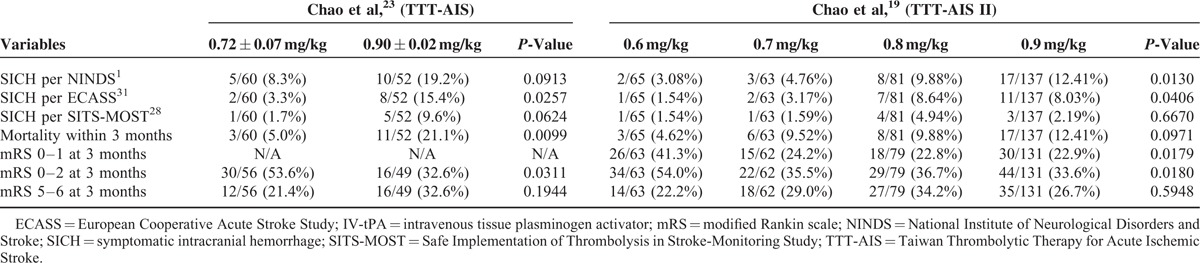
TABLE 3.
Comparison of Treatment Results Between Patients Aged ≥70 Years Receiving Standard (0.9 mg/kg) or Low-Dose IV-tPA
Publication Bias
Visual inspection of the funnel plot did not identify substantial asymmetry (Figure 5). Neither the Begg rank correlation test, nor the Egger linear regression test, indicated evidence of publication bias among studies concerning favorable functional outcome (Begg, P = 1.00; Egger, P = 0.93), incidence of SICH (Begg, P = 0.72; Egger, P = 1.00) or mortality (Begg, P = 0.47; Egger, P = 0.37).
FIGURE 5.
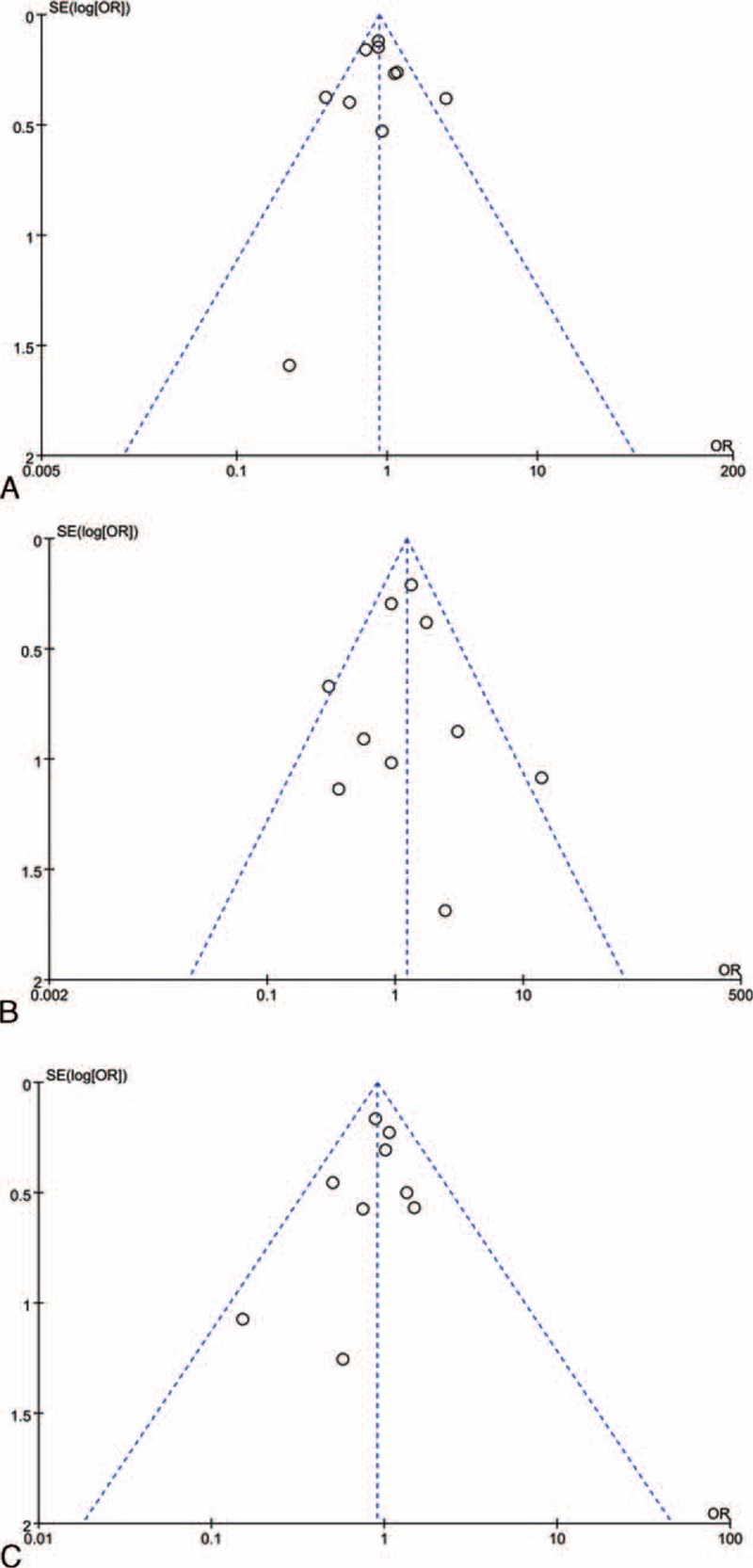
Funnel plot assessing publication bias. (A) Funnel plot of favorable functional outcome at 3 months. (B) Funnel plot of symptomatic intracranial hemorrhage. (C) Funnel plot of mortality within 3 months.
DISCUSSION
The basis for standard IV-tPA dosing for AIS patients is derived primarily from 2 pilot dose escalation studies.9,27 Nevertheless, even these 2 studies conducted by the NINDS investigators yielded no conclusive results concerning the optimal dosage for AIS.7 In the recent years, several cohort studies have investigated whether standard-dose IV-tPA was superior to low-dose IV-tPA, but no consistent results have been reported. We therefore conducted a meta-analysis to provide a quantitative assessment of available data.
Administration of low-dose IV-tPA for AIS first began in 2006. Due to concerns the Asian population experienced increased rates of tPA-related intracranial hemorrhage from racial differences in blood coagulation fibrinolysis factors, the Japan Alteplase Clinical Trial (J-ACT) tested the efficacy and safety of low-dose IV-tPA regimen (0.6 mg/kg).7 A prospective cohort study that evaluated 103 patients presenting within 3 hours of AIS, the J-ACT demonstrated low-dose IV-tPA provided comparable benefits to the IV-tPA dose employed in the NINDS trial.7 Encouraged by the J-ACT, a series of clinical studies employing low-dose IV-tPA (0.6 mg/kg) were subsequently conducted in Japan, and demonstrated further low-dose IV-tPA provided similar, or even superior efficacy and safety, in comparison to standard-dose therapy in Western patients (Table 4).1–7,28,29 These studies suggest low-dose IV-tPA may be associated with decreased SICH incidence.30
TABLE 4.
Results of the NINDS, SITS-MOST, SITS-NEW, and Japanese Low-Dose IV-tPA Studies
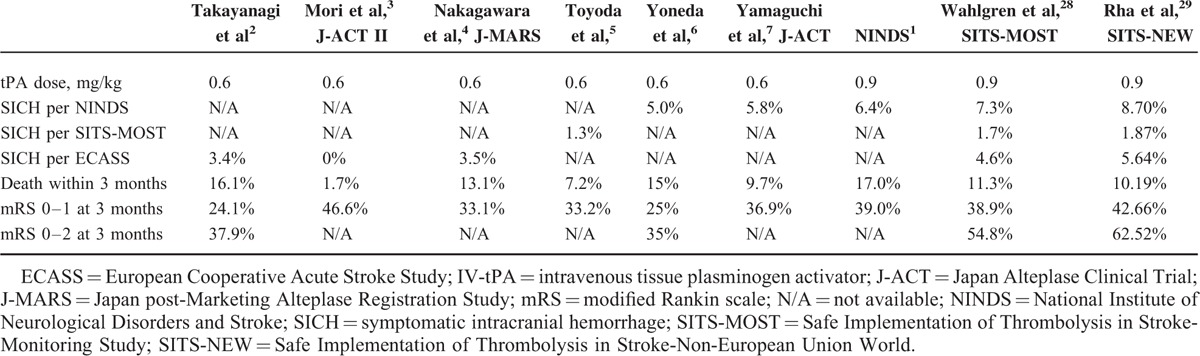
Most strokes occur in elderly people. Investigations of the relationship between age and outcome from IV-tPA therapy concluded older patients experienced greater risk of severe hemorrhagic transformation post-tPA treatment.31,32 Chao et al conducted the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) and TTT-AIS II, which were prospective observational studies directly comparing the efficacy and safety outcomes of different IV-tPA regimens in Taiwanese patients aged 70 years and older.19,23 The TTT-AIS reported significantly decreased SICH rates (3.3% vs. 15.4%, P = 0.0257), mortality (5.0% vs. 21.1%, P = 0.0099), and significantly increased rates of patient independence (mRS score 0–2, 53.6% vs. 23.6%, P = 0.0311) in patients 70 years and older receiving low-dose versus standard-dose IV-tPA (SICH in the TTT-AIS was determined per the European Cooperative Acute Stroke Study criteria).23 In patients aged 71 to 80 years, the TTT-AIS II revealed significantly increased symptomatic intracerebral hemorrhage (P = 0.0130), and less favorable functional outcome (P = 0.0179), with increasing IV-tPA doses.19 The TTT-AIS II excluded patients aged greater than 80 years, due to fears of excess SICH risk. Takayanagi et al2 compared the safety of low-dose IV-tPA (0.6 mg/kg) between a younger (70 patients, less than 80 years old) and older patient group (17 patients, 80 years and older). In this study, the authors report no significant difference in SICH incidence between the younger (4.3%) and older (0%) groups (P = 0.61), concluding low-dose tPA therapy appears as safe and feasible for AIS patients young and old (greater than 80 years) alike.2 All the aforementioned studies suggest low-dose IV-tPA may be more suitable than standard-dose IV-tPA for the elderly AIS population.
While 85% of strokes worldwide occur in developing countries, the number of patients receiving IV-tPA in such countries is extremely low.33,34 Financial constraints remain a primary reason for low utilization of thrombolytic therapy in developing countries. The cost of standard-dose IV-tPA in developing countries is $1400 (USD) per patient, a notably heavy burden for most patients.35 In Iran, only 30% of stroke patients can afford to pay for IV-tPA out of pocket.36 A study from Northwest India reported that, among 22 patients eligible for thrombolysis, only 5 actually received IV-tPA, because the remaining patients were unable to afford the high cost of treatment.37 If the efficacy and safety of low-dose IV-tPA could be confirmed by randomized controlled studies, reductions of pharmaceutical cost could be passed downstream, alleviating the financial burden faced by prospective patients.
Our study carries several limitations. Firstly, “low-dose” IV-tPA in various included studies ranged from 0.5 to 0.85 mg/kg. This wide dose range reduced the accuracy of comparison results. Secondly, the sample sizes of some included studies were limited. In one study, only 2 patients treated with low-dose IV-tPA.26 A limited patient population limits result reliability. Thirdly, all included cohort studies were conducted in Asian populations. The resultant meta-analytical conclusions cannot be broadly applicable to all world population groups due to racial genetic differences. However, 1 study from Czech Republic, which was excluded for lacked data on incidence of SICH, also concluded that in clinical practice, the actual dose of t-PA often differed from the recommended dose of 0.9 mg/kg, but this had no significant impact on the outcome after t-PA treatment.15 The optimal IV-tPA dose for AIS might need reassessment, not just in Asian populations but also in other population groups around the world.
The current meta-analysis of nine selected cohort studies suggests low-dose IV-tPA is comparable to standard-dose IV-tPA in terms of safety (defined by SICH incidence and mortality) and efficacy (defined by favorable functional outcome). Additionally, low-dose IV-tPA may be more suitable than standard-dose IV-tPA for patients aged 70 years and older. Moreover, the reduced cost of low-dose IV-tPA will be of financial benefit, which may promote applicability of thrombolytic therapy in developing countries. However, randomized and controlled trials are necessary to confirm these derived conclusions. Physicians must remain cautious in clinical practice when considering low-dose IV-tPA treatment of AIS patients. In summary, our analysis demonstrated comparable efficacy and safety between standard- and low-dose IV-tPA.
Footnotes
Abbreviations: AIS = acute ischemic stroke, IV-tPA = intravenous tissue plasminogen activator, J-ACT = Japan Alteplase Clinical Trial, MOOSE = Meta-Analysis of Observational Studies in Epidemiology, mRS = modified Rankin scale, NIHSS = National Institute of Health Stroke Scale, NINDS = National Institute of Neurological Disorders and Stroke, OR = odds ratio, SICH = symptomatic intracranial hemorrhage, TTT-AIS = Taiwan Thrombolytic Therapy for Acute Ischemic Stroke.
Meng-Dong Liu, Wei-Dong Ning Ren-Cong Wang, and Wei Chen contributed equally to this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 2.Takayanagi S, Ochi T, Hanakita S, et al. The safety and effectiveness of low-dose recombinant tissue plasminogen activator (0.6 mg/kg) therapy for elderly acute ischemic stroke patients (>/=80 years old) in the pre-endovascular era. Neurol Med Chir 2014; 54:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori E, Minematsu K, Nakagawara J, et al. Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke 2010; 41:461–465. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawara J, Minematsu K, Okada Y, et al. Thrombolysis with 0.6 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post-Marketing Alteplase Registration Study (J-MARS). Stroke 2010; 41:1984–1989. [DOI] [PubMed] [Google Scholar]
- 5.Toyoda K, Koga M, Naganuma M, et al. Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke 2009; 40:3591–3595. [DOI] [PubMed] [Google Scholar]
- 6.Yoneda Y, Yamamoto S, Hara Y, et al. Post-licensed 1-year experience of systemic thrombolysis with tissue plasminogen activator for ischemic stroke in a Japanese neuro-unit. Clin Neurol Neurosurg 2007; 109:567–570. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi T, Mori E, Minematsu K, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke 2006; 37:1810–1815. [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 9.Brott TG, Haley EC, Jr, Levy DE, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke 1992; 23:632–640. [DOI] [PubMed] [Google Scholar]
- 10.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 11.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Zhu G, Liu N, et al. Low-dose tissue plasminogen activator is as effective as standard tissue plasminogen activator administration for the treatment of acute ischemic stroke. Curr Neurovasc Res 2014; 11:62–67. [DOI] [PubMed] [Google Scholar]
- 14.Ross AM, Gao R, Coyne KS, et al. A randomized trial confirming the efficacy of reduced dose recombinant tissue plasminogen activator in a Chinese myocardial infarction population and demonstrating superiority to usual dose urokinase: the TUCC trial. Am Heart J 2001; 142:244–247. [DOI] [PubMed] [Google Scholar]
- 15.Aulicky P, Rabinstein A, Seet RC, et al. Dosing of tissue plasminogen activator often differs from 0.9 mg/kg, but does not affect the outcome. J Stroke Cerebrovasc Dis 2013; 22:1293–1297. [DOI] [PubMed] [Google Scholar]
- 16.Salam KA, Ummer K, Kumar VG, et al. Intravenous thrombolysis for acute ischemic stroke: the Malabar experience 2003 to 2008. J Clin Neurosci 2009; 16:1276–1278. [DOI] [PubMed] [Google Scholar]
- 17.Kim BJ, Han MK, Park TH, et al. Low-versus standard-dose alteplase for ischemic strokes within 4.5 hours: a comparative effectiveness and safety study. Stroke 2015; 46:2541–2548. [DOI] [PubMed] [Google Scholar]
- 18.Liao X, Wang Y, Pan Y, et al. Standard-dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke 2014; 45:2354–2358. [DOI] [PubMed] [Google Scholar]
- 19.Chao AC, Liu CK, Chen CH, et al. Different doses of recombinant tissue-type plasminogen activator for acute stroke in Chinese patients. Stroke 2014; 45:2359–2365. [DOI] [PubMed] [Google Scholar]
- 20.Pan SM, Liu JF, Liu M, et al. Efficacy and safety of a modified intravenous recombinant tissue plasminogen activator regimen in Chinese patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2013; 22:690–693. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Hsieh CY, Lai TB, et al. Optimal dose for stroke thrombolysis in Asians: low dose may have similar safety and efficacy as standard dose. J Thromb Haemost 2012; 10:1270–1275. [DOI] [PubMed] [Google Scholar]
- 22.Zhou XY, Wang SS, Collins ML, et al. Efficacy and safety of different doses of intravenous tissue plasminogen activator in Chinese patients with ischemic stroke. J Clin Neurosci 2010; 17:988–992. [DOI] [PubMed] [Google Scholar]
- 23.Chao AC, Hsu HY, Chung CP, et al. Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) study. Stroke 2010; 41:885–890. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TH, Truong AL, Ngo MB, et al. Patients with thrombolysed stroke in Vietnam have an excellent outcome: results from the Vietnam Thrombolysis Registry. Eur J Neurol 2010; 17:1188–1192. [DOI] [PubMed] [Google Scholar]
- 25.Sharma VK, Tsivgoulis G, Tan JH, et al. Feasibility and safety of intravenous thrombolysis in multiethnic Asian stroke patients in Singapore. J Stroke Cerebrovasc Dis 2010; 19:424–430. [DOI] [PubMed] [Google Scholar]
- 26.Suwanwela NC, Phanthumchinda K, Likitjaroen Y. Thrombolytic therapy in acute ischemic stroke in Asia: the first prospective evaluation. Clin Neurol Neurosurg 2006; 108:549–552. [DOI] [PubMed] [Google Scholar]
- 27.Haley EC, Jr, Levy DE, Brott TG, et al. Urgent therapy for stroke. Part II. Pilot study of tissue plasminogen activator administered 91–180 minutes from onset. Stroke 1992; 23:641–645. [DOI] [PubMed] [Google Scholar]
- 28.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369:275–282. [DOI] [PubMed] [Google Scholar]
- 29.Rha JH, Shrivastava VP, Wang Y, et al. Thrombolysis for acute ischaemic stroke with alteplase in an Asian population: results of the multicenter, multinational Safe Implementation of Thrombolysis in Stroke-Non-European Union World (SITS-NEW). Int J Stroke 2014; 9 Suppl. A100:93–101. [DOI] [PubMed] [Google Scholar]
- 30.Ramaiah SS, Yan B. Low-dose tissue plasminogen activator and standard-dose tissue plasminogen activator in acute ischemic stroke in Asian populations: a review. Cerebrovasc Dis 2013; 36:161–166. [DOI] [PubMed] [Google Scholar]
- 31.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274:1017–1025. [PubMed] [Google Scholar]
- 32.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 33.Feigin VL. Stroke in developing countries: can the epidemic be stopped and outcomes improved? Lancet Neurol 2007; 6:94–97. [DOI] [PubMed] [Google Scholar]
- 34.Durai Pandian J, Padma V, Vijaya P, et al. Stroke and thrombolysis in developing countries. Int J Stroke 2007; 2:17–26. [DOI] [PubMed] [Google Scholar]
- 35.Ghandehari K. Barriers of thrombolysis therapy in developing countries. Stroke Res Treat 2011; 2011:686797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghandehari K, Zahed AP, Taheri M, et al. Estimation of Iranian stroke patients eligible for intravenous thrombolysis with tPA. Int J Stroke 2009; 4:236. [DOI] [PubMed] [Google Scholar]
- 37.Pandian JD, Sethi V, Dhillon R, et al. Is intravenous thrombolysis feasible in a developing country? Cerebrovasc Dis 2005; 20:134–136. [DOI] [PubMed] [Google Scholar]


