Abstract
The zinc finger protein A20 is a newly identified negative regulator of immune response and mediates signal pathway of NF-κB in liver inflammation. However, the role of A20 in the natural history of patients with chronic hepatitis B (CHB) has not been demonstrated. In this present study, we aimed to investigate the dynamic expression of A20 and determine the potential association of A20 in the progression of chronic hepatitis B virus infection.
This retrospective study contained 136 patients with chronic hepatitis B and 30 healthy controls (HCs). The mRNA level of A20, TNF-α, NF-κB p65 and toll-like receptor (TLR) 4 in peripheral blood mononuclear cells (PBMCs) was determined using a relative quantitative real-time polymerase chain reaction. The hepatic A20 protein expression was determined by immunohistochemistry. Clinical and laboratory parameters were obtained.
In the present study, the relative expression of A20 mRNA was significantly increased in CHB patients compared with HCs and was positively associated with alanine aminotransferase, aspartate aminotransferase, and total bilirubin. In CHB patients, the levels of A20 mRNA in the immune clearance (IC) phase and hepatitis B negative (ENH) phase were significantly higher than that in immune tolerance (IT) phase and low-replicative (LR) phase (P < 0.001). Furthermore, the A20 mRNA level was significantly correlated with TNF-α/ NF-κB p65/TLR4 mRNA levels in CHB patients. Of note, we reported that cutoff values of 4.19 and 3.97 for the level of A20 mRNA have significant power in discriminating IC from IT, and ENH from LR in CHB patients respectively.
In conclusion, our results suggested that increased levels of A20 mRNA and protein contribute to disease progression of chronic hepatitis B virus infection.
INTRODUCTION
Hepatitis B virus (HBV) infection is a serious and prevalent public health problem in the world. Approximately 400 million persons worldwide are chronically infected with HBV and 10% to 30% of whom are at high risk of developing a progressive liver disease, complicated by fibrosis and cirrhosis.1,2 Chronic HBV infection is a dynamic process orchestrated by the complex interplay between virus replication and host immune response. Typically, the natural course of chronic HBV infection can be clinically categorized into 4 periods: an immune tolerant (IT) phase with the presence of hepatitis B e antigen (HBeAg) and active replication of HBV with normal alanine aminotransferase (ALT) levels; an immune clearance (IC) phase with positive HBeAg and high HBV DNA levels >2000 IU/mL associated with elevated ALT levels; an inactive or non/low-replicative (LR) phase with negative HBeAg and low viral replication (virus load < 2000 IU/mL) with persistently normal ALT; and an HBeAg negative hepatitis (ENH) phase with negative HBeAg and active viral replication (virus load >2000 IU/mL) associated with elevated ALT.3–5 It is accepted that the inefficient innate and adaptive immunity are the main reasons of chronic hepatitis B.6 However, the exact molecular mechanism underlying immune response malfunction in chronic HBV infection has not been completely understood.
A20, also known as tumor necrosis factor α-induced protein (TNFAIP) 3, is an immune negative regulatory molecule and has been characterized as an inhibitor of NF-κB signaling in immune response. Overexpression of A20 can terminate NF-κB signaling transduced from TNF receptors, toll-like receptors (TLRs), or nucleotide-binding oligomerization domain containing 2 receptors.7,8 A20-deficient mice can develop severe inflammation and tissue damage in multiple organs and die prematurely.9 Tumor necrosis factor (TNF) is demonstrated to stimulate excessive activation of NF-κB signaling of embryonic fibroblasts (MEFs) and thymocytes in A20-deficient mice.9 In addition, several reports suggested that A20 also functions to restrict innate antiviral signaling responses via regulation of interferon regulatory factor (IRF) pathway in response to pathogen recognition.10 Besides its crucial role for the regulation of innate immune response, A20 is also required for the adaptive immunity. Two independent studies showed that A20-silenced dendritic cells (DCs) had an enhanced T-cell stimulatory capacity.11,12 The former study demonstrated that A20-silenced DCs skewed naive CD4+T cells toward a T-helper (Th) 1 phenotype, but not a regulatory T-cells (Treg), Th2, or Th17 phenotype in vitro stimulations.11 Similarly, the latter report manifested that A20-silenced DCs inhibit Treg and hyperactivate cytotoxic T lymphocytes (CTLs) and T-helper cells that then overcome Treg-mediated immune suppression.12 These data indicate that A20 could negatively regulate inflammatory response and induce immunosuppress. Therefore, silencing A20 may provide a novel strategy to supersede Treg-mediated suppression in an antigen-specific manner and improve DC-based vaccines against cancer and infection.11-13
Emerging evidences suggested that A20 plays a crucial role in inflammatory and immunological diseases in human. A recent study showed the expression of A20 was decreased and negatively correlated with the disease activity of systemic lupus erythematosus patients.8 Additionally, the role of A20 has been implicated in infectious diseases such as influenza, measles, hepatitis C, and Leishmania donovani infection, where elevated levels of this protein in the host have been shown to be correlated with disease progression.14–17 However, the relationship between the expression level of A20 and HBV infection has not been reported.
In this study, we used quantitative real time-polymerase chain reaction (RT-qPCR) to investigate the mRNA expressions of A20 in peripheral blood mononuclear cells (PBMCs) from CHB patients with various immune phases and healthy controls (HCs). Then we used immunohistochemistry (IHC) to detect intrahepatic expression levels of hepatic A20 protein from HBV-infected patients with hepatic necroinflammatory grade and fibrosis stage. Finally, we examined the expression of TNF-α/p65 (NF-κB subunit)/TLR4 mRNA in PBMCs and analyzed the correlations with A20 mRNA in patients with chronic hepatitis B.
MATERIALS AND METHODS
Subjects
A total of 166 subjects containing 136 patients with CHB and 30 healthy volunteers as controls were enrolled in this present study. All the CHB patients were retrospectively collected at the Department of Hepatology, Qilu Hospital of Shandong University from July 2014 to February 2015. The diagnostic criteria for chronic hepatitis B were identified as persistent positive hepatitis B surface antigen (HBsAg) for no <6 months before the starting of patients enrollment.18 None of the patients had received antiviral and immune therapy within at least the preceding 6 months. The exclusive criteria was the following: infection with human immunodeficiency virus, hepatitis C virus or hepatitis D virus infection; pregnancy; autoimmune liver diseases; alcoholic liver diseases; nonalcoholic fatty liver diseases; drug-induced hepatitis; liver tumors; and other causes of chronic liver diseases. On the basis of the guidelines for the management of CHB, patients with chronic hepatitis B were classified into 4 groups with different immune stages4,19: 16 subjects were in the IT phase (HBeAg positive and HBV DNA load >5 × 107 IU/mL as well as persistently normal ALT); 80 subjects were in the IC phase (HBeAg positive and moderate to high level of viral load >2000 IU/mL as well as elevated serum ALT); 15 were in the LR phase (HBeAg negative and HBV DNA load < 2000 IU/mL as well as persistently normal ALT); and 25 were in the ENH phase (HBeAg negative and HBV DNA load >2000 IU/mL as well as elevated ALT). The upper limit of normal of ALT was set as 40 U/L. Of these patients, 26 subjects underwent liver biopsy. In addition, the liver specimens of 6 healthy liver transplant donors without detectable liver diseases comprised a normal control (NC) group. The Scheuer scoring system was used to evaluated hepatic necroinflammatory grade and fibrosis stage.20 Written informed consent was obtained from each participant according to the Declaration of Helsinki and the present study was approved by the Medical Ethical Committee of Qilu Hospital of Shandong University.
Isolation of Peripheral Blood Mononuclear Cells
Five milliliters of venous peripheral blood were extracted from each subject. PBMCs were separated by density gradient centrifugation from the peripheral blood anticoagulated with ethylene diamine tetraacetic acid via Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) and stored at −20°C until use.
RNA Extraction and Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction
Total RNA was extracted from PBMCs using the TRIzol reagent according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA). Two micrograms of total RNA were reversely transcribed into cDNAs using RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). The expression of A20 was examined in triplicate by qPCR using the Lightcycler 480 (Roche Diagnostics, Mannheim, Germany). Real-time PCR was performed using an SYBR Premix Ex TaqTM (Takara, Shiga, Japan).
The PCR cycle profile included an initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, 60°C for 30 s, and 72°C for 30 s. The primer sequences were described as Table 1. The data were analyzed by the Lightcycler 480 Software (Roche Diagnostics, Mannheim, Germany) using the comparative (2-ΔΔCt) method.
TABLE 1.
Primer Sequence Used for real-time RT-PCR
Immunohistochemistry for Detection of Intrahepatic A20 Expression
Immunohistochemistry analysis of the A20 expression in human liver tissue was conducted by indirect detection via the Polink-2 plus® Polymer HRP Detection System (Zhongshan Goldenbridge Biotechnology, China). Formalin-fixed paraffin-embedded liver biopsy specimens were baked and deparaffinized, followed by antigen retrieval with the use of All-purpose Powerful Antigen Retrieval Solution (Beyotime, China) at 99°C to 100°C for 30 min. Endogenous peroxidase was abolished by immersing the tissue array in 3% H2O2 for 20 min. Primary rabbit anti-TNFAIP3 monoclonal antibody at a dilution of 1:75 (Abcam, Cambridge, MA) was then applied and sections were incubated overnight at 4°C. The next day, sections were washed thrice with PBS and then subjected to Polymer Helper (Zhongshan Goldenbridge Biotechnology, China) for 20 min. After washing, the next incubation was with polyperoxidase-anti-rabbit IgG (Zhongshan Goldenbridge Biotechnology, China) for 20 min. Finally, the samples were stained with a 3-3′-diaminobenzidine kit and counterstained with hematoxylin. Otherwise, samples were incubated with PBS instead of A20-monolyclonal antibody as a negative control. The results were observed using an Olympus microscope connected to a CCD camera (Olympus IX83, Tokyo, Japan).
Image Analysis
Intensity of positive staining was measured using the mean density (integrated optical density sum/positive area sum) by the Image-Pro Plus 6.0 software (National Institutes of Health, Bethesda, MD). Data were expressed as relative mean density.
Clinical and Lab Parameters
Serological markers for HBV infection including hepatitis B surface antigen (HBsAg) and HBeAg were determined using an automatic analyzer (Cobas 6000 analyzer series, Roche Diagnostics, Basel, Switzerland). The serum biochemical markers including ALT, aspartate aminotransferase (AST), total bilirubin (TBIL), and albumin (ALB) were determined using standard methodologies in the Department of Laboratory Medicine, Qilu Hospital of Shandong University. Serum HBV DNA load was quantified using a PCR System (ABI 7300, Applied Biosystems, Foster City, CA), whereas the HBV DNA kit was purchased from Bioer corporation limit (Bioer, Hangzhou, China) with a detection sensitivity of 500 IU/mL and the detectable range was 500 to 108IU/mL.
Statistical Analysis
The Kolmogorov–Smirnov test was performed to identify whether the data was appropriate for normal distribution. Continuous variables were expressed as median (centile 25; centile 75). Categorical variables were expressed as relative frequencies. Comparisons between or among different groups were performed using the Mann–Whitney U test or Kruskal–Wallis test. The chi-square test was performed to compare the differences within categorical variables. Spearman's rank test was used to determine possible correlations coefficient. The sensitivity, specificity of A20 in identify different immune stages of patients was determined using receiver operating characteristic (ROC) curves. The diagnostic accuracy was performed using the areas under the ROC (AUROC) curve. All statistical analyses were 2-sided, and P value <0.05 was set as statistical significance. All of the statistical analyses were performed using the IBM SPSS 19.0 software (SPSS Inc, Chicago, IL).
RESULTS
Clinical Characteristics of All the Subjects Included in the Study
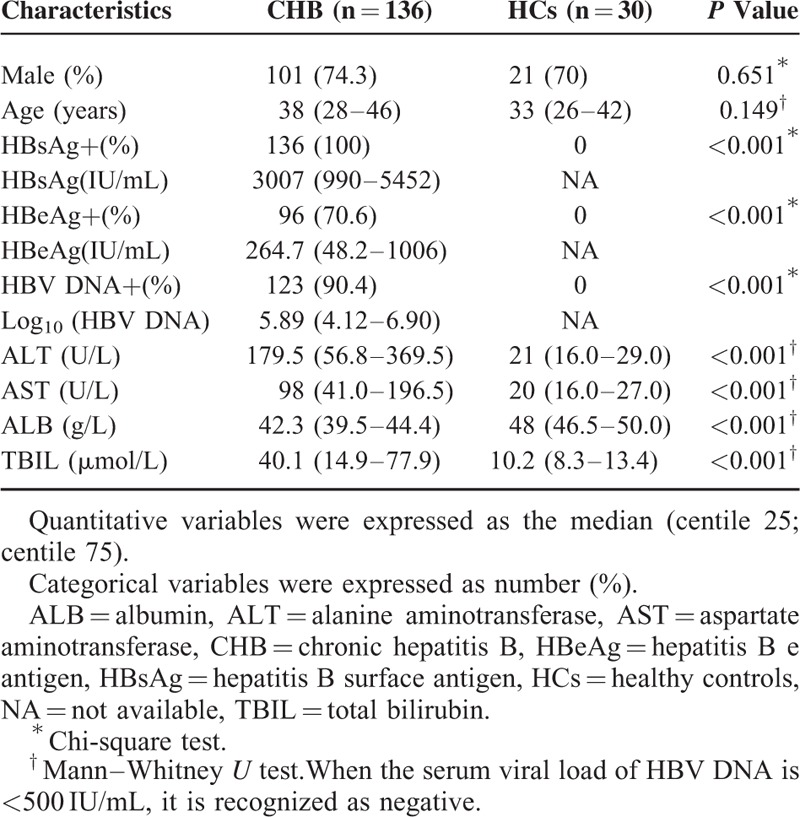
In this present study, we retrospectively enrolled a total of 166 subjects including 136 patients with CHB and 30 age- and gender-matched healthy controls at the Department of Hepatology, Qilu Hospital of Shandong University. The clinical characteristics of all the subjects were showed in Table 2. There were 96 patients (70.6%) with positive HBeAg level and 123 patients (90.4%) with positive HBV DNA level in all the 136 CHB patients. Patients with CHB showed significantly higher levels of HBsAg, HBeAg, Log10 [HBV-DNA], ALT, AST, and TBIL compared with HCs.
TABLE 2.
General Characteristics of the Studied Subjects Including CHB and HCs
Expression of A20 mRNA in Peripheral Blood Mononuclear Cells of CHB Patients and HCs
As chronic hepatitis B infection is characterized by persistent virus inflammation and A20 plays an essential role in limiting inflammatory response, the aberrant level of A20 expression may have a close relationship with the development of CHB. Therefore, we examined the expression of A20 mRNA in PBMCs from 136 patients with CHB and 30 healthy controls by using RT-PCR. The A20 mRNA level in patients with CHB was significantly higher than that in healthy controls (median (centile 25; centile 75), 5.80 [3.18, 9.68] vs 0.31 [0.21, 1.36], P < 0.001) (Figure 1A). To further identify whether A20 mRNA expression is contributed to the severity of CHB, we evaluated the correlations between A20 mRNA and clinical parameters in patients with CHB, including serum ALT, AST, ALB, TBIL, HBsAg, HBeAg, and HBV-DNA load. The results showed that A20 mRNA expression levels were positively correlated with ALT (r = 0.679, P < 0.001), AST (r = 0.656, P < 0.001), and TBIL (r = 0.326, P < 0.001), respectively (Figure 1D–F). No statistically significant relationships were found between A20 mRNA expression levels and other clinical manifestations for the patients with CHB (Figure 1 B, C, G, H). These results suggest the possibility that aberrant expression of A20 might be involved in the development of CHB and closely associated with the disease severity of CHB.
FIGURE 1.
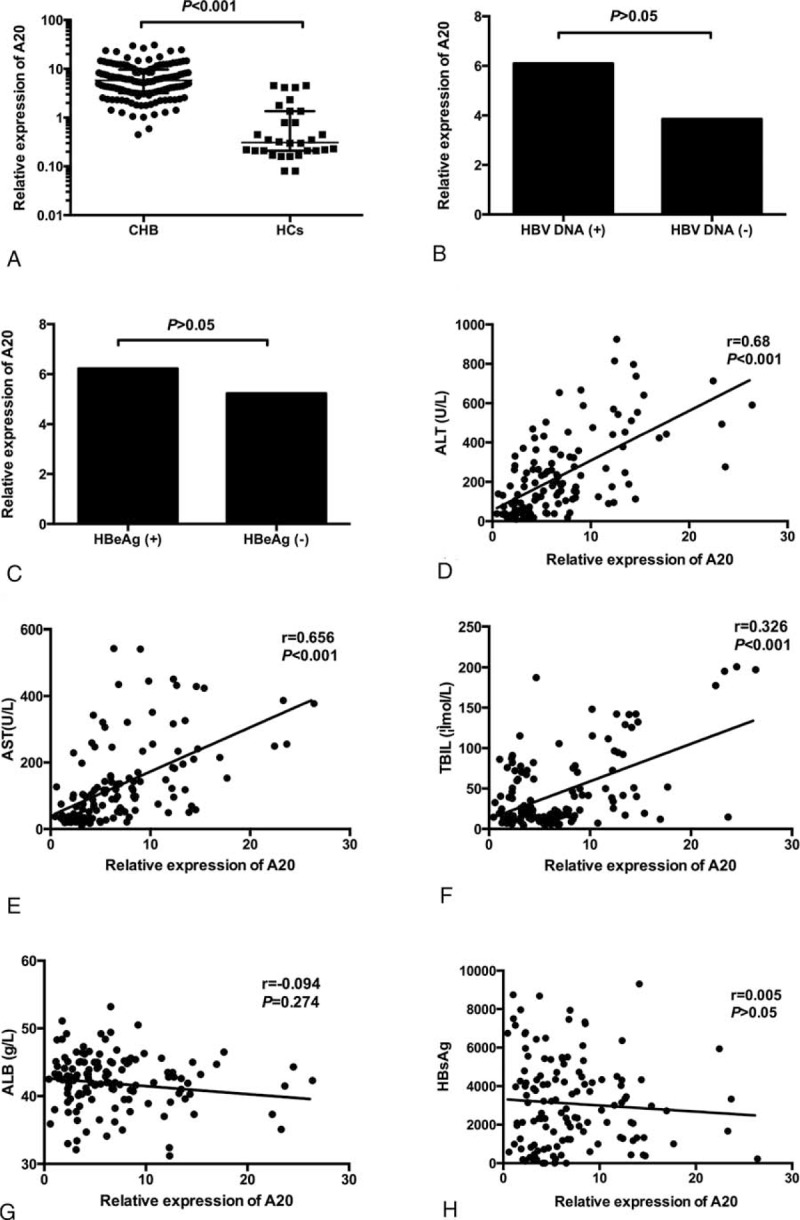
A20 mRNA expression levels in PBMCs from patients with CHB and healthy controls. There was a significant increase in A20 expression in CHB patients compared with healthy controls (A). No significant difference was found between HBV DNA (+) and HBV DNA (−) groups as well as between HBeAg(+) and HBeAg (−) groups (B,C). The expression of A20 was significantly positively correlated with alanine aminotransferase (ALT) (D), aspartate aminotransferase (AST) (E), and serum total bilirubin (TBIL) (F). No significant correlations were found between the A20 mRNA level and albumin (ALB) (G), hepatitis B surface antigen (HBsAg) (H). ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CHB = chronic hepatitis B, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, PBMCs = peripheral blood mononuclear cells, TBIL = total bilirubin.
Expression of PBMCs A20 mRNA in Different Immune Phases of Chronic Hepatitis B
According to the characteristics of the host immune response, HBeAg status, and the virus load, the natural course of chronic HBV infection can be classified into 4 phases. Of all the CHB patients, 4 patients had a serum virus load < 2000 IU/mL but with minor elevated ALT were included in the LR group when the subjects had no subsequent ALT elevation within the next 12 months. Five subjects had a serum virus load < 500 IU/mL but intrahepatic virus load >2000 IU/mL with persistently elevated ALT were included in ENH group. All the 5 patients have undergone liver biopsy and the clinic data for individual case 17, 21, 23, 25, and 26 were shown in Table 4. In these patients, there were no evidences for other obvious causes for liver injury, whereas both the increased elevated levels of intrahepatic HBV-DNA load and inflammation G ≥ 2 also support the statue of ENH. Therefore, we included the 5 patients in the ENH group. Finally, there are ∼16 patients for IT and 80 for IC in HBeAg-positive patients, 15 for LR and 25 for ENH in HBeAg-negative patients. Herein, we determined the differences of A20 mRNA levels in the 4 phases of chronic hepatitis B patients. The baseline characteristics of CHB patients with different immune phases were shown in Table 3.
TABLE 4.
The Basic Characteristics of the Subjects Receiving Liver Biopsy
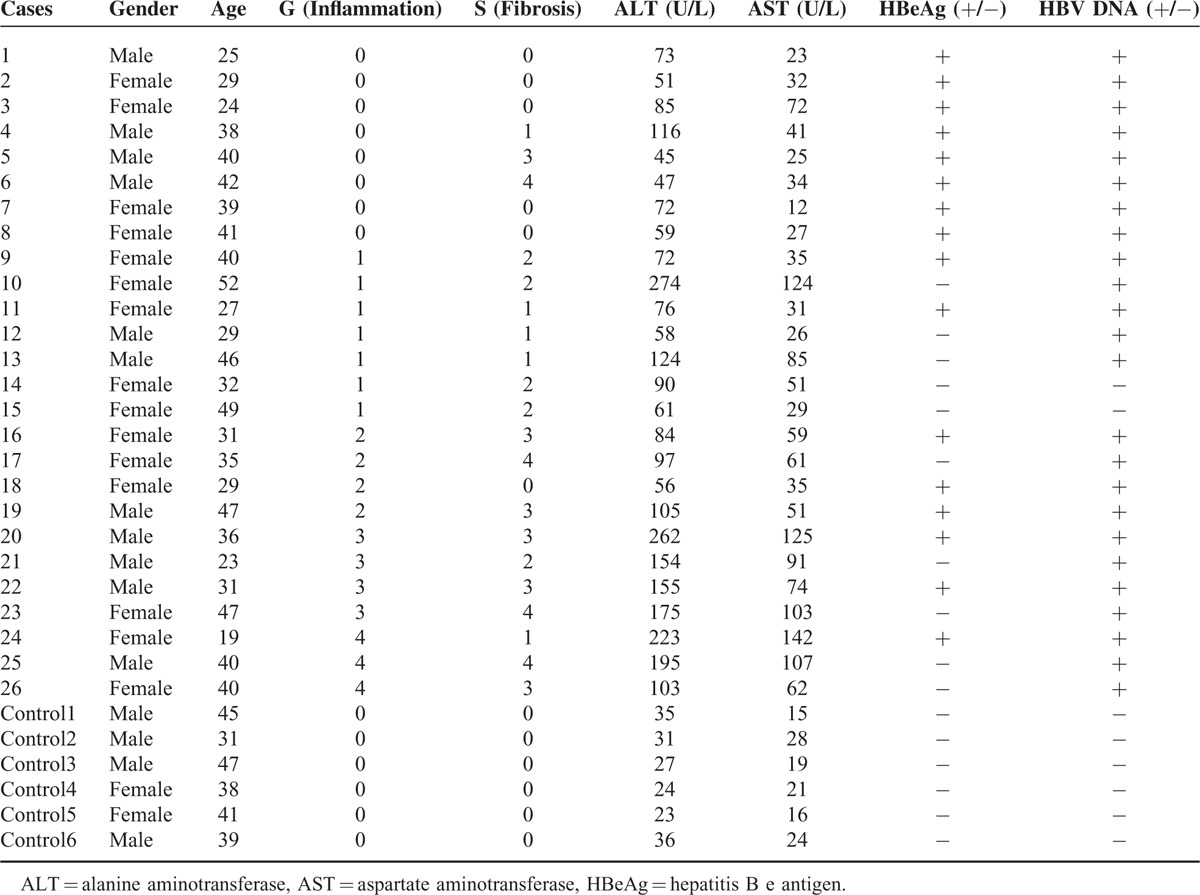
TABLE 3.
Baseline Characteristics of Immune Phases of Chronic Hepatitis B
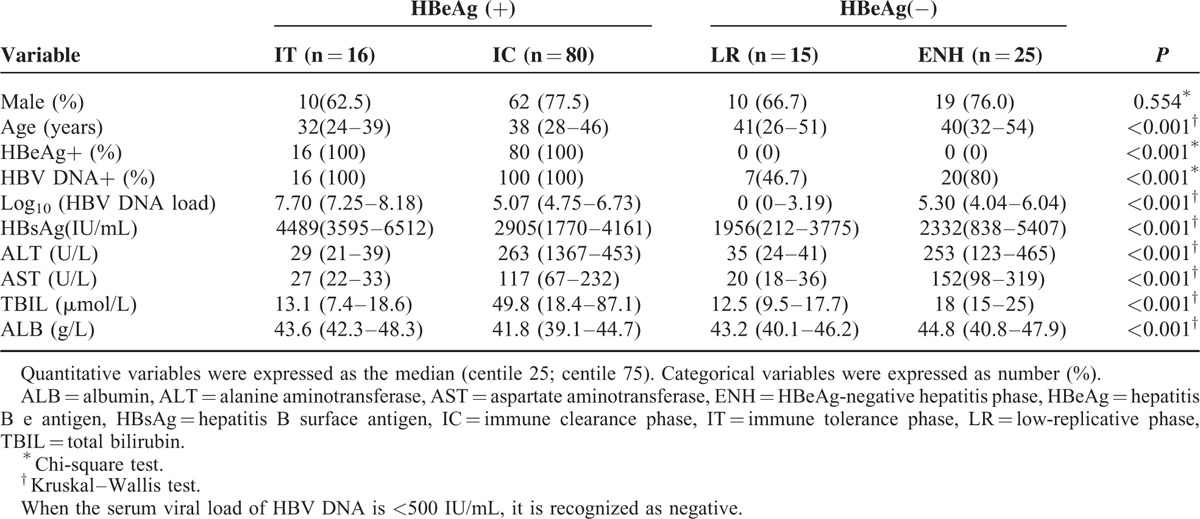
The median and interquartile ranges of A20 were 2.29 (1.32–3.99) for IT patients, 6.59 (3.85–12.32) for IC patients, 3.35 (2.27–3.94) for LR patients, and 8.33 (5.62–12.23) for ENH patients. In Figure 2A, we reported that the relative expression of A20 mRNA in IC/ENH immune phases were significantly higher than healthy controls (0.31 [0.21–1.36]; both P < 0.001, respectively). Within the 4 immune phases, we found that relative expression of A20 mRNA in IC/ENH phases shared the higher levels of median and interquartile ranges compared with those in LR/IT phases (all P < 0.05, respectively). Furthermore, we did not find significant differences of A20 mRNA in ENH and IC phases (P > 0.05), as well as LR and IT phases (P > 0.05).
FIGURE 2.
Expression of A20 mRNA from peripheral blood mononuclear cells in different immune phases of chronic hepatitis B. (A) Expression of A20 mRNA from peripheral blood mononuclear cells in healthy controls and CHB patients with IT, IC, LR, and ENH. (B) The receiver operating characteristic curve for predicative accuracy of A20 mRNA in discriminating IC from IT. (C) The receiver operating characteristic curve for predicative accuracy of A20 mRNA in discriminating ENH from LR. CHB = chronic hepatitis B, ENH = negative hepatitis, IC = immune clearance, IT = immune tolerance, LR = low-replicative.
Furthermore, ROC analysis was performed to identify whether A20 mRNA could discriminate the IC phase from the IT phase, and the ENH phase from the LR phase. Figure 2B showed the AUROC of A20 mRNA for the diagnosis of IC from IT individuals was 0.831(95% confidence interval 0.741–0.900, P < 0.001), and the optimal cutoff was 4.19 with a sensitivity of 71.25% and a specificity of 81.25%. Meanwhile, the AUROC of A20 mRNA for the diagnosis of ENH from LR individuals was 0.927 (95% confidence interval 0.798 –0.985, P < 0.001), and the optimal cutoff was 3.97 with a sensitivity of 92.0% and a specificity of 86.67% in Figure 2C.
Expression of Intrahepatic A20 Protein in Patients With Chronic Hepatitis B
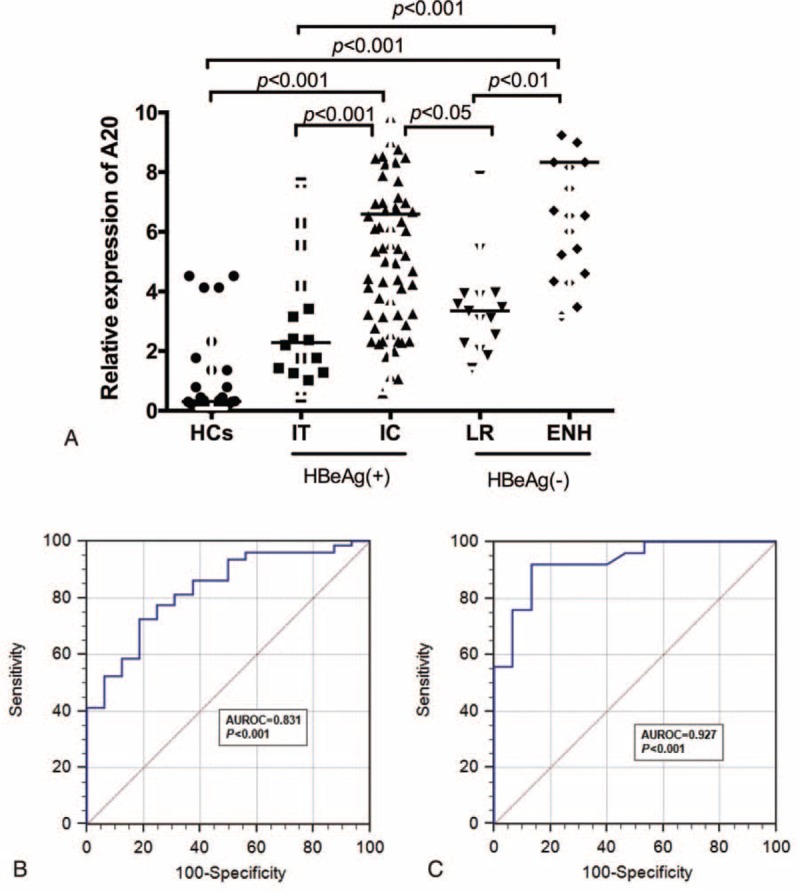
Furthermore, we have determined the intrahepatic expression of A20 protein in 26 patients with chronic hepatitis B and 6 normal controls. Table 4 illustrated the clinical characteristics of all the patients and normal controls. In Figure 3B–D, we demonstrated apparent intrahepatic A20 expression in the cytoplasm from patients with chronic hepatitis B. As presented in Figure 3A–E, the intrahepatic expression of A20 protein was significantly increased in the CHB patients compared with normal controls. Furthermore, the intrahepatic A20 expression was gradually increased in accordance with the raised grade of liver inflammation (Figure 3B–D). In detail, the intrahepatic A20 expression in patients with inflammation grade 3/4 was significantly higher than those with inflammation grade 1/2(P < 0.05) (Figure 3F). In Figure 3G, we also demonstrated that the intrahepatic A20 expression in patients with fibrosis stage 0 was significantly decreased compared to fibrosis stage 1/2 or 3/4(P < 0.05, respectively). However, there were no significant differences between fibrosis stage 1/2 and fibrosis stage 3/4 (P > 0.05).
FIGURE 3.
Expression of intrahepatic A20 protein in liver tissues using the immunohistochemical method. A20 positivity was found nearly in all hepatocytes (B,C,D) (200×). The intensity of staining of A20 was correlated with the severity of liver inflammation (B,C,D) (200×). Relative mean density analysis showed the difference in hepatic A20 staining between the CHB group and healthy controls (E). (F) The difference in hepatic A20 staining between inflammation grade (1–2) and grade (3–4). (G) Hepatic A20 staining in fibrosis stage (3–4), stage (1–2), and fibrosis stage 0. CHB = chronic hepatitis B.
Relationship Between A20 mRNA and TNF-α/p65/TLR4 mRNA Levels in PBMCs From CHB Patients
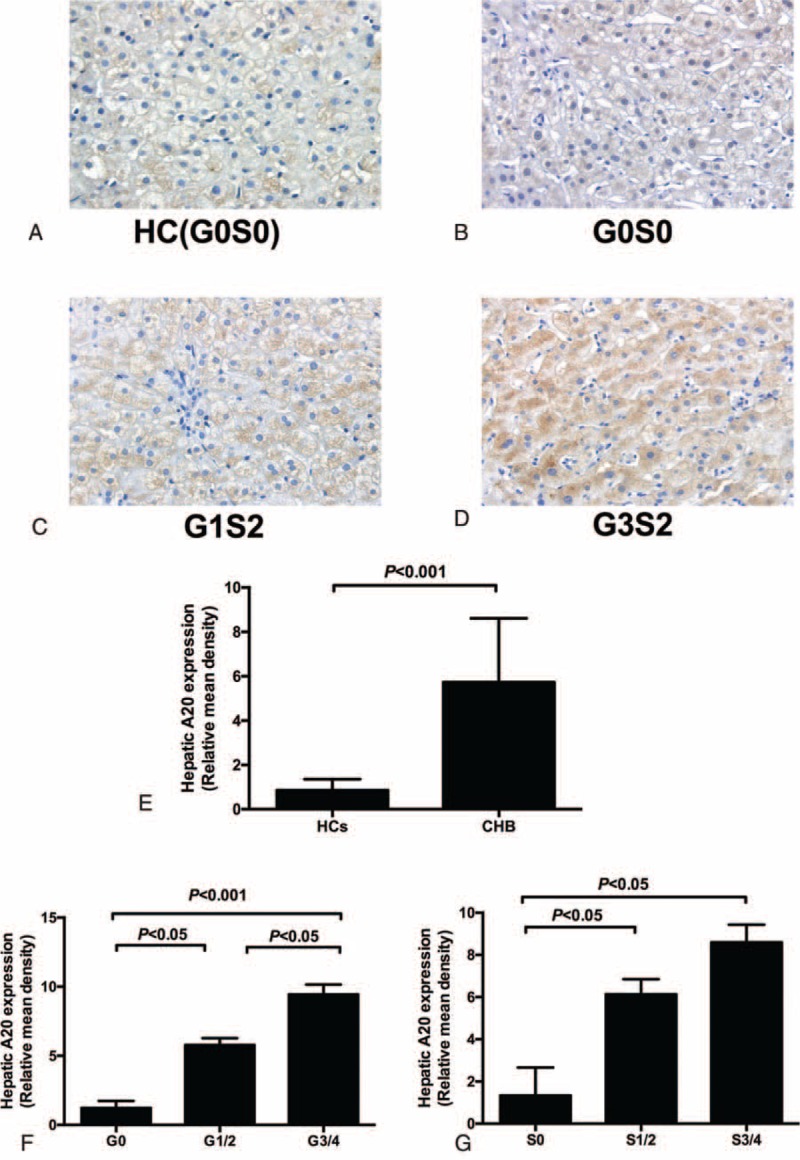
TNF-α is a key cytokine that mediates host immune response to HBV and viral clearance.21 As A20 is identified as a TNF-α-induced protein and negatively modulates NF-κB activation through a variety of receptors including TLR4, we therefore detected the expression of TNF-α/p65 (NF-κB subunit)/TLR4 mRNA and analyzed the relationship between the A20 mRNA level and the TNF-α/ p65/TLR4 mRNA level. The mRNA levels of TNF-α and p65 were upregulated in CHB patients compared with healthy controls (TNF-α: CHB 6.06 [4.92–7.52], healthy controls 1.04 [0.63–3.17], P < 0.001; p65: CHB 8.0 [6.29–11.05], healthy controls 0.68 [0.32–1.38], P < 0.001) (Figure 4A). Inversely, the expression of TLR4 mRNA was significantly reduced in CHB patients than HCs (7.64 [3.21–8.77] vs 16.17 [10.15–19.01], P < 0.001) (Figure 4A). Further analysis indicated significantly positive correlations between A20 and TNF-α, p65 mRNA levels (r = 0.245, P < 0.05; r = 0.446, P < 0.001, respectively) (Figure 4 B and C). A significantly negative correlation was also found between A20 and TLR4 mRNA levels (r = −0.191, P < 0.05) (Figure 4D).
FIGURE 4.
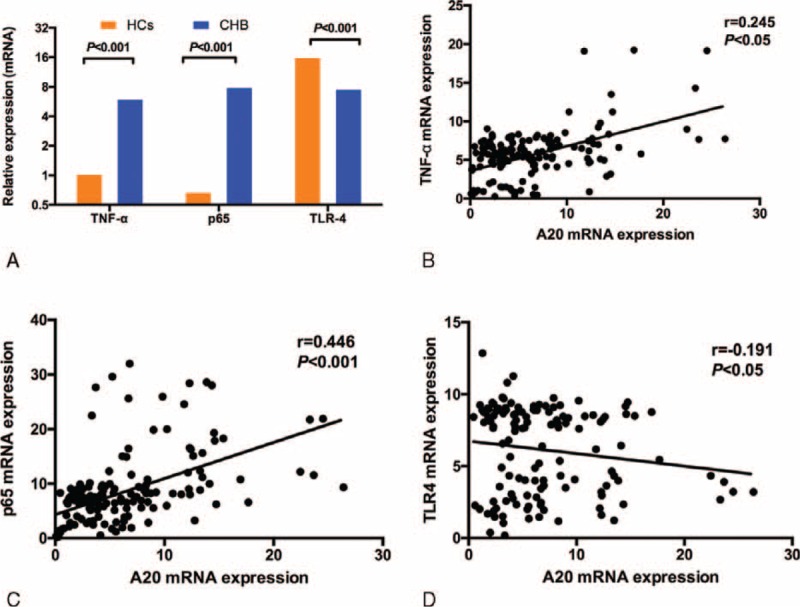
TNF-α, p65, and TLR4 mRNA expression in peripheral blood mononuclear cells (PBMCs) from chronic hepatitis B (CHB) patients and healthy controls (HCs). (A) the significant difference of TNF-α/p65/TLR4 mRNA level between the CHB group and HCs. (B,C,D) The correlation between the A20 mRNA level and the TNF-α/p65/TLR4 mRNA level. CHB = chronic hepatitis B, HC = healthy controls, PBMCs = peripheral blood mononuclear cells, TLR = toll-like receptor.
DISCUSSION
A20 is an immune negative regulatory molecule and mediates the regulation of NF-κB signaling in inflammation and immunity. Zhang et al reported hepatitis B virus X protein sensitizes TRAIL-induced hepatocyte apoptosis by inhibiting the E3 ubiquitin ligase A20.22 However, there was no data for the expression and potential role of A20 in patients with chronic hepatitis B up to date. In our manuscript, we demonstrated the expression of A20 mRNA in different immune phases of chronic hepatitis B and explored the intrahepatic expression of A20 protein in a relatively small number of patients. To our knowledge, this is first study to report the role of A20 in patients with chronic hepatitis B.
In this present study, we first demonstrated that the level of A20 mRNA in CHB patients was significantly higher than that in healthy controls. Intrahepatic A20 protein expression in CHB patients was also increased compared with healthy controls. Immunostaining method also showed that hepatic A20 protein expression is closely related to the degree of liver inflammation. Furthermore, ALT, AST are the major biomarkers for liver inflammation and abnormal TBIL level usually accompanied with progressive liver diseases.23 Our results showed that A20 mRNA was significantly associated with ALT, AST, and TBIL. Therefore, it is plausible to foresee that A20 might contribute to the severity of liver injury in patients with chronic hepatitis B. To identify the close associations of A20 with immune phases in the clinical condition, we also determined the expression of PBMCs A20 mRNA in different immune phases of chronic hepatitis B. The higher expression of A20 in IC and ENH phases suggested that the A20 gene is involved in the immune-inflammatory process of chronic HBV infection. Of importance, the predictive value of A20 in discriminating IC/ENH phases from IT/LR phases has been determined and these results are mainly to highlight the importance of A20 in immune phases of chronic hepatitis B.
Proinflammatory cytokines secreted by immune cells are involved in the aggravating of inflammatory injury in liver. NF-κB functions as a transcription factor that triggers innate and adaptive immunity and induces inflammatory cytokines. Persistent inflammation in CHB is characterized by the elevated serum levels of multiple proinflammatory cytokines, including TNF-α, IL-6, IL-8, and IL-1β24–26. In this study, we showed a dramatic enhanced TNF-α and p65 mRNA levels in patients with CHB compared with healthy controls and demonstrated a positive correlation between A20 mRNA and TNF-α as well as p65 mRNA levels. Some inflammatory cytokines, such as TNF-α, can activate NF-κB transcription. A20 has been described previously to be an NF-κB target gene and contains 2 NF-κB binding sequences in its promoter region.27 Induced by TNF-α, the activated NF-κB is translocated into the nucleus to bind to 2 specific NF-κB elements on the A20 promoter, enhancing transcription of the A20 gene.28 Induced by NF-κB-dependent signals, A20 in turn restricts the duration and intensity of signaling by several molecules involved in the NF-κB pathway.7 Therefore, the increased TNF-α-mediated NF-κB activation accounts for the increased A20 expression in PBMCs of patients with CHB. A reduced TLR4 mRNA expression in CHB patients and a reverse association between A20 and TLR mRNA levels were also found in our study. NF-κB is a common transcription factor involved in several pathways, including TLRs, TNFR, T-cell receptor and B-cell receptor signaling pathways, cytokine/cytokine receptor interaction. Remarkably, specific HBV proteins have an immune-modulating ability to initiate molecular mechanisms that “evade” host immune surveillance.2 For example, the HBV X protein (HBX) was shown to be able to escape innate immunity through the degradation of TLR-domain-containing adaptor inducing interferon-beta (TRIF), which is an adaptor protein in the TLR3 signaling.29 Chen et al demonstrated that TLR2 and TLR4 mRNA levels were decreased in CHB patients.30 We speculated that HBV might induce A20 expression to suppress TLR-mediated immune response and escape immune recognition. However, the process of HBV infection is complex, whether the hepatitis B virus induces elevated A20 expression to escape immune response, or persistent inflammation stimulates A20 expression to avoid severe liver injury by limiting excessive inflammatory response is worth investigating.
There are also some limitations in the present study. First, we determined the A20 mRNA and protein levels in the small number of patients from a single unit. This issue is also agreeable with the clinical fact that only patients with elevated ALT level usually ask to doctors and would agree to undergo liver biopsy. However, we believe that a community-based population or large cohort dataset would be of help. Second, other diseases during the progression of HBV infection including cirrhosis, acute hepatitis, and liver failure should be included to explore the comprehensive role of A20 in the complete progression of HBV infection. In fact, there should be a series studies on this issue, we have recently reported the expression of A20 in acute-on-chronic hepatitis B liver failure.31 Third, our study is a cross-sectional cohort but not a perspective study; therefore we did not include resolved HBV cases and patients with/after treatment. However, the role of A20 in resolved HBV cases and their A20 level of patients on treatments should be extensively studied in the future. Finally, silence of A20 gene in cell lines and HBV transgenic mice should be comprehensively investigated for the interplay of A20 and HBV in our future study.
In conclusion, our finding revealed that the expression of A20 is up-regulated and positively correlated with disease severity in CHB patients. The results suggested that A20 might contribute to the pathogenesis of CHB disease and A20 gene may be a candidate target for immune therapy of HBV infection. Furthermore, we reported that a cutoff values of 4.19 and 3.97 for the level of A20 mRNA have significant power in discriminating IC from IT, and ENH from LR in CHB patients respectively. These results might highlight the importance of A20 in immune phases of chronic hepatitis B.
Footnotes
Abbreviations: ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUROC = areas under the ROC curves, CHB = chronic hepatitis B, ENH = HBeAg negative hepatitis, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCs = healthy controls, IC = immune clearance, IT = immune tolerance, LR = low-replicative, NF-κB = nuclear factor-κB, PBMCs = peripheral blood mononuclear cells, PCR = polymerase chain reaction, ROC = receiver operating characteristic, TBIL = total bilirubin, TLR = toll-like receptor, TNF = tumor necrosis factor.
Funding source: this work is supported by the grants from National Natural Science Foundation of China (81201287, 81171579, and 81371832), Key Project of Chinese Ministry of Science and Technology (2012ZX10002007 and 2013ZX10002001), and Science and Technology Development Plan of Shandong Province (2014GSF118068).
Author contributions: Y-YS, Y-CF, and NW contributed to the majority of the experiments and data collection. Y-CF and Y-YS contributed to data analysis. Y-YS, Y-CF, and KW contributed to the study design and drafted the manuscript. Y-CF and HH-XX contributed to revising the manuscript. Y-CF and KW conceived the study. KW was responsible for study supervision and obtaining funds.
Y-YS and Y-CF contributed equally to this study and should be regarded as co-first authors.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lai CL, Yuen MF. Chronic hepatitis B—new goals, new treatment. N Engl J Med 2008; 359:2488–2491. [DOI] [PubMed] [Google Scholar]
- 2.Balmasova IP, Yushchuk ND, Mynbaev OA, et al. Immunopathogenesis of chronic hepatitis B. World J Gastroenterol 2014; 20:14156–14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet 2014; 384:2053–2063. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 5.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009; 49 (5 Suppl):S45–55. [DOI] [PubMed] [Google Scholar]
- 6.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 2012; 61:1754–1764. [DOI] [PubMed] [Google Scholar]
- 7.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 2012; 12:774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Wang L, Fan Y, et al. Down-regulation of A20 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. J Clin Immunol 2012; 32:1287–1291. [DOI] [PubMed] [Google Scholar]
- 9.Lee EG. Failure to regulate TNF-induced NF-kappa B and cell death responses in A20-deficient mice. Science 2000; 289:2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catrysse L, Vereecke L, Beyaert R, et al. A20 in inflammation and autoimmunity. Trends Immunol 2014; 35:22–31. [DOI] [PubMed] [Google Scholar]
- 11.Breckpot K, Aerts-Toegaert C, Heirman C, et al. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J Immunol 2009; 182:860–870. [DOI] [PubMed] [Google Scholar]
- 12.Song XT, Evel-Kabler K, Shen L, et al. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med 2008; 14:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong B, Song XT, Rollins L, et al. Mucosal and systemic anti-HIV immunity controlled by A20 in mouse dendritic cells. J Clin Invest 2011; 121:739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onose A, Hashimoto S, Hayashi S, et al. An inhibitory effect of A20 on NF-kappaB activation in airway epithelium upon influenza virus infection. Eur J Pharmacol 2006; 541:198–204. [DOI] [PubMed] [Google Scholar]
- 15.Yokota S, Okabayashi T, Yokosawa N, et al. Measles virus P protein suppresses Toll-like receptor signal through up-regulation of ubiquitin-modifying enzyme A20. FASEB J 2008; 22:74–83. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Zhou Y, Zhang Y, et al. Role of A20 in interferon-alpha-mediated functional restoration of myeloid dendritic cells in patients with chronic hepatitis C. Immunology 2014; 143:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastav S, Kar S, Chande AG, et al. Leishmania donovani exploits host deubiquitinating enzyme A20, a negative regulator of TLR signaling, to subvert host immune response. J Immunol 2012; 189:924–934. [DOI] [PubMed] [Google Scholar]
- 18.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–662. [DOI] [PubMed] [Google Scholar]
- 19.Song LW, Liu PG, Liu CJ, et al. Quantitative hepatitis B core antibody levels in the natural history of hepatitis B virus infection. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2015; 21:197–203. [DOI] [PubMed] [Google Scholar]
- 20.Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994; 19:1513–1520. [PubMed] [Google Scholar]
- 21.Tuncbilek S. Relationship between cytokine gene polymorphisms and chronic hepatitis B virus infection. World J Gastroenterol 2014; 20:6226–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Huang C, Wang Y, et al. Hepatitis B virus X protein sensitizes TRAIL-induced hepatocyte apoptosis by inhibiting the E3 ubiquitin ligase A20. PloS One 2015; 10:e0127329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang TJ. Hepatitis B: the virus and disease. Hepatology 2009; 49 (5 Suppl):S13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Yue B, Wang GQ, et al. Serum and ascites levels of macrophage migration inhibitory factor, TNF-alpha and IL-6 in patients with chronic virus hepatitis B and hepatitis cirrhosis. Hepatobiliary Pancreat Dis Int 2002; 1:577–580. [PubMed] [Google Scholar]
- 25.Zhang G, Li Z, Han Q, et al. Altered TNF-alpha and IFN-gamma levels associated with PD1 but not TNFA polymorphisms in patients with chronic HBV infection. Infect Genet Evol 2011; 11:1624–1630. [DOI] [PubMed] [Google Scholar]
- 26.Bortolami M, Kotsafti A, Cardin R, et al. Fas /FasL system, IL-1beta expression and apoptosis in chronic HBV and HCV liver disease. J Viral Hepatitis 2008; 15:515–522. [DOI] [PubMed] [Google Scholar]
- 27.Liuwantara D, Elliot M, Smith MW, et al. Nuclear factor-kappaB regulates beta-cell death: a critical role for A20 in beta-cell protection. Diabetes 2006; 55:2491–2501. [DOI] [PubMed] [Google Scholar]
- 28.Krikos A, Laherty CD, Dixit VM. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem 1992; 267:17971–17976. [PubMed] [Google Scholar]
- 29.Hong Y, Zhou L, Xie H, et al. Innate immune evasion by hepatitis B virus-mediated downregulation of TRIF. Biochem Biophys Res Commun 2015; 463:719–725. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Cheng Y, Xu Y, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol 2008; 128:400–408. [DOI] [PubMed] [Google Scholar]
- 31.Fan YC, Sun YY, Wang N, et al. Up-regulation of A20 gene expression in peripheral blood mononuclear cells is associated with acute-on-chronic hepatitis B liver failure. J Viral Hepat 2015; doi: 10.1111/jvh.12478. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


