Abstract
Nontypeable Haemophilus influenzae is a major causative agent of bacterial otitis media in children. H. influenzae Hap autotransporter protein is an adhesin composed of an outer membrane Hapβ region and a moiety of an extracellular internal 110-kDa passenger domain called HapS. The HapS moiety promotes adherence to human epithelial cells and extracellular matrix proteins, and it also mediates bacterial aggregation and microcolony formation. A recent work (D. L. Fink, A. Z. Buscher, B. A. Green, P. Fernsten, and J. W. St. Geme, Cell. Microbiol. 5:175-186, 2003) demonstrated that HapS adhesive activity resides within the C-terminal 311 amino acids (the cell binding domain) of the protein. In this study, we immunized mice subcutaneously with recombinant proteins corresponding to the C-terminal region of HapS from H. influenzae strains N187, P860295, and TN106 and examined the resulting immune response. Antisera against the recombinant proteins from all three strains not only recognized native HapS purified from strain P860295 but also inhibited H. influenzae Hap-mediated adherence to Chang epithelial cells. Furthermore, when mice immunized intranasally with recombinant protein plus mutant cholera toxin CT-E29H were challenged with strain TN106, they were protected against nasopharyngeal colonization. These observations demonstrate that the C-terminal region of HapS is capable of eliciting cross-reacting antibodies that reduce nasopharyngeal colonization, suggesting utility as a vaccine antigen for the prevention of nontypeable H. influenzae diseases.
Nontypeable Haemophilus influenzae (NTHi), a nonencapsulated gram-negative bacterium, is the cause of a number of human respiratory tract diseases, such as otitis media, sinusitis, bronchitis, and pneumonia (15, 16). Otitis media is among the most common infections in young children. By 3 years of age, approximately 80% of children have had at least one episode of acute otitis media (25). Recurring bouts of otitis media may lead to significant hearing loss, which in turn may result in developmental delay. A vaccine that prevents nontypeable H. influenzae disease would provide major benefits to the health of children and the general population.
The pathogenesis of H. influenzae disease begins with colonization of the nasopharynx. Subsequently, organisms spread to other sites in the respiratory tract, including the middle ear, sinuses, and lower airways (21). Based on in vitro and animal studies, a number of factors appear to influence the process of colonization. One such factor is the Hap adhesin, which promotes bacterial interaction with human respiratory epithelial cells and extracellular matrix proteins as well as mediates bacterial aggregation and microcolony formation (10, 23).
Hap belongs to the autotransporter family of proteins common among gram-negative pathogens (9). It is synthesized as a 155-kDa precursor protein, which consists of an N-terminal 25-amino-acid signal peptide, an internal 110-kDa passenger domain called HapS, and a C-terminal 45-kDa outer membrane domain called Hapβ (9). HapS has serine protease activity and is released from the precursor protein via autoproteolysis. Of note, autoproteolysis is inhibited by secretory leukocyte protease inhibitor, which is a natural component of respiratory secretions. The HapS domain is responsible for all the adhesive properties of Hap (10, 23). Furthermore, purified HapS is immunogenic in mice, eliciting significant anti-HapS antibody titers. In a mouse intranasal challenge model, animals immunized with purified HapS from NTHi strain P860295 or N187 in the presence of mutant cholera toxin CT-E29H as an adjuvant are protected against nasopharyngeal colonization (5). These findings suggest that HapS has potential as a vaccine antigen against NTHi. However, the development of a HapS-based vaccine has been hindered by difficulties in purifying adequate quantities of HapS from the bacterium and the tendency of this protein to self associate.
Fink et al. recently reported that the domain in Hap responsible for promoting adherence to epithelial cells resides in the C-terminal 311 amino acids of HapS (6). Additional work revealed that this region mediates bacterial aggregation via HapS-HapS interaction between molecules on neighboring organisms and is a part of the C-terminal 511 amino acids required for adherence to selected extracellular matrix proteins, including fibronectin, laminin, and collagen IV (7). To address whether the C-terminal 311 amino acids of HapS (the cell binding domain [CBD]) are capable of eliciting a protective immune response, we prepared recombinant CBD (rCBD) either from glutathione S-transferase (GST) fusion proteins or from urea-solubilized inclusion bodies. Using the mouse intranasal challenge model, we found that immunization with rCBD derived from three different NTHi strains leads to significant reduction in nasopharyngeal colonization. These results should facilitate efforts to develop a HapS-based vaccine to prevent nontypeable H. influenzae disease.
MATERIALS AND METHODS
Bacterial strains and plasmids.
NTHi strains N187 (obtained from Eric Hansen, University of Texas), P861454, P860295 (obtained from Charles Brinton, University of Pittsburgh), and SR7332 (11) were isolated from middle ear fluid of children with acute otitis media. NTHi strain TN106 (obtained from Eric Hansen) was isolated from a patient with pneumonia (19, 23). TN106.P2 is a streptomycin-resistant derivative of TN106 described previously (5). DB117 is an unencapsulated, recombination-deficient derivative of a serotype d H. influenzae strain that contains a mutated hap gene and fails to express Hap (20). Strain DB117/HapP860295 produces on its surface plasmid-encoded wild-type HapP860295, and strain DB117/HapN187 produces plasmid-encoded wild-type HapN187. DB117/pGJB103 contains the plasmid vector pGBJ103 and does not express Hap (23). Escherichia coli strain Top10 was purchased from Invitrogen (Carlsbad, Calif.), and strain BL21(DE3)/pLysS was purchased from Novagen (Madison, Wis.). Plasmids pET17b and pGEX-6P-1 were purchased from Novagen and Amersham Pharmacia Biotech (Piscataway, N.J.), respectively. Plasmid pGJB103 is an H. influenzae-E. coli shuttle vector described previously (26).
Bacterial cultures.
NTHi cells were grown on brain-heart infusion agar plates supplemented with hemin and NAD (BHI-XV agar), on BBL CHOC II agar plates (Becton Dickinson & Co., Cockeysville, Md.), or in brain-heart infusion broth supplemented with hemin and NAD (BHI) as described previously (8). E. coli cells were grown on SOB agar or in SOB broth (14) containing the appropriate antibiotic.
PCR primers, PCR amplification, and DNA sequencing.
PCR primer oligonucleotides were synthesized on a PerSeptive Biosystems oligonucleotide synthesizer (Applied Biosystems, Foster City, Calif.) using β-cyanoethylphosphoramidite chemistry. PCRs were performed in ReddyMix PCR Master Mix (ABgene House, Surrey, United Kingdom) on a Perkin-Elmer GeneAmp 2400 unit. A hot start of 5 min at 95°C prior to amplification was followed by 30 cycles of 1 min at 95°C, 1 min at 55°C, and 2 min at 72°C. DNA sequencing was carried out using BigDye terminator chemistry with specifically designed primers on an Applied Biosystems 377 automated DNA sequencer. Sequencher 4.0.5 software (Gene Codes, Corp., Ann Arbor, Mich.) was used to analyze the DNA sequences.
Construction of plasmids for the expression of GST-rCBD fusion proteins.
GST-rCBD fusion proteins were made with plasmids that contain the hap gene from NTHi strain N187, 860295, or TN106 (5). PCR was performed to amplify the appropriate segment of the gene. The DNA segments include sequences encoding residues 726 to 1036 from HapN187, residues 734 to 1040 from HapP860295, and residues 728 to 1032 from HapTN106. PCR primers were designed to incorporate a BamHI site at the 5′ end and an XhoI site at the 3′ end of each segment. Following amplification, PCR products were digested with BamHI and XhoI and then were ligated into BamHI-XhoI-digested pGEX-6P-1 (Amersham Pharmacia Biotech), juxtaposing the hap fragments downstream of the coding sequence for glutathione S-transferase and a PreScission protease cleavage site. The resulting plasmids pGEXN187C′311, pGEXP860295C′307, and pGEXTN106C′305 were transformed into E. coli BL21(DE3)pLysS by electroporation for expression of GST-rCBDN187, GST-rCBDP860295, and GST-rCBDTN106 fusion proteins.
Construction of a CBDN187 expression vector.
Plasmid pJS106 (23), containing the hap gene from strain N187, was used as a template for cloning the DNA sequence encoding CBDN187. The PCR primers for amplifying the CBD coding region are oligonucleotides 5′GATCGCTTAAGCTTCCCAAAAACACAAATCAAT3′ and 5′CCTTGTCAGCGTCGACTTATAACAGGCTTTGATCAGGCAGGGTAT3′. The PCR products were purified and ligated to the T7 tag in the expression vector pET17b. The ligation reaction was transformed into TOP10 cells, and transformants were screened by PCR for the correct insert, yielding pWV1024. This plasmid was verified to have the correct nucleotide sequence and was then transformed into E. coli BL21(DE3)pLysS for expression of rCBDN187.
Isolation of rCBD from GST fusion protein.
E. coli BL21(DE23)/pLysS containing plasmid pGEX-N187C′311, pGEX-P860295C′307, or pGEX-TN106C′305 was grown overnight at 37°C with shaking in SOB broth supplemented with 1% glucose and ampicillin, and it was then diluted into fresh broth to an optical density at 600 nm (OD600) of 0.1. The culture was incubated at 37°C with shaking to an OD600 of 1. To induce the cells, the temperature was shifted down to 28°C and 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added to the culture. Two hours later, cells were harvested by low-speed centrifugation. Cells from 1 liter of induced culture were resuspended in phosphate-buffered saline (PBS) containing 0.2% Triton X-100, 2 mM pefabloc (Boehringer Mannheim, Indianapolis, Ind.), and 1 mM EDTA, and then they were lysed by passing through a Microfluidizer (Microfluidics International Corp., Newton, Mass.). Cell lysate was centrifuged at 10,000 × g for 20 min to remove cell debris. The portion of GST fusion protein that remained in the supernatant was absorbed onto affinity matrix glutathione Sepharose 4B and cleaved with PreScission protease according to the manufacturer's guide (Amersham Pharmacia Biotech). Bound rCBD was eluted with 50 mM Tris buffer at pH 7.5 containing 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol (DTT), and 0.2% Triton X-100. Eluted rCBD was concentrated to ∼1 mg/ml with Triton X-100 adjusted to 0.5%. The resulting rCBD preparation was stored at −20°C. Protein concentrations were determined by using the modified method of Lowry (17).
As a negative control, E. coli BL21(DE23)/pLysS containing the plasmid vector pGEX-6P-1 was grown and induced and a cell lysate was prepared as described above. This induced E. coli cell lysate was subsequently used to generate a mouse polyclonal antiserum (see below).
Isolation of rCBDN187 from inclusion bodies.
E. coli BL21(DE23)/pLysS+pWV1024 was incubated at 37°C on SOB agar supplemented with 1% glucose and ampicillin. Cells were then grown in SOB broth overnight and were diluted into fresh SOB broth to an OD600 of 0.1. When the culture reached an OD600 of 1 to 1.5, 1 mM IPTG was added. Two hours after induction with IPTG, cells were harvested by low-speed centrifugation. Cells from 1 liter of induced culture were resuspended in PBS containing 1% Triton X-100, 5 mM pefabloc, 4 mM EDTA, 0.2 mg of lysozyme/ml, and 5-μg/ml concentrations of each DNase and RNase. After incubation at 37°C for 1 h with gentle rocking, cells were lysed by passing through a Microfluidizer, and the resulting cell lysate was centrifuged at 10,000 × g for 20 min. The pellet composed mainly of rCBDN187 inclusion bodies was resuspended in 0.1 M Tris buffer at pH 8 containing 6 M urea, 2 mM EDTA, and 5 mM pefabloc. The inclusion bodies were extracted with urea at 4°C overnight with rocking. The extract was then centrifuged at 36,000 × g for 20 min to remove insoluble material. The supernatant containing urea-solubilized rCBDN187 was adjusted with the same buffer to ∼6 mg/ml and was stored at −70°C.
Renaturation of urea-solubilized rCBDN187 involves diluting the denatured protein into a buffer containing a high concentration of l-arginine (1, 2, 18). Briefly, urea-solubilized rCBDN187 was first reduced with 0.3 M DTT for 2 h at room temperature. The reduced protein was diluted 100-fold into a buffer consisting of 0.1 M Tris at pH 8, 2 mM EDTA, 3 mM DTT, 5 mM oxidized glutathione (GSSG), 0.4 M l-arginine, 150 mM NaCl, 0.015% Triton X-100, and 2 mM pefabloc. Protein renaturation proceeded in this diluted state at 10°C for 4 days. Renatured rCBDN187 was concentrated to 1 mg/ml in ∼0.5% Triton X-100. Dialysis was carried out stepwise to reduce l-arginine from 0.4 M to 0.3 M to 0.2 M, and finally down to 0.1 M in a buffer consisting of 0.1 M Tris at pH 8, 150 mM NaCl, and 0.5% Triton X-100. The dialyzed protein solution was centrifuged at 36,000 × g for 20 min to remove insoluble material. The final renatured rCBDN187 was stored at −20°C.
Purification of native Haps from H. influenzae strain P860295.
Native HapS (nHapS-P860295) was purified from the culture supernatant as described previously (5), with minor modifications. Briefly, bacteria were grown in 10 liters of BHI broth for 18 h at 35°C with aeration. All the following steps were performed at 4°C. Bacterial cells were removed by centrifugation at 10,000 × g. The culture supernatant was concentrated 20-fold by using an Amicon stir cell and was fractionated overnight with ammonium sulfate at 60% saturation. After centrifugation at 17,000 × g for 1 h, the precipitate was dissolved in 20 mM Tris buffer at pH 7.4 containing 50 mM NaCl and 1 mM EDTA, dialyzed against the same buffer, and then centrifuged at 100,000 × g for 1 h to remove insoluble material. The resulting supernatant was loaded at a flow rate of 2 ml/min onto a 20-ml SP Sepharose column (Amersham Pharmacia Biotech) equilibrated with the same buffer. The column was washed until the OD280 reached the baseline, and nHapS-P860295 was eluted at a flow rate of 3 ml/min with a linear gradient of NaCl (from 55 to 500 mM) in 20 mM Tris at pH 7.5 with 1 mM EDTA. Based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, fractions containing nHapS-P860295 were pooled.
SDS-PAGE and Western blot analysis.
SDS-PAGE was performed with 12% gels according to the method of Laemmli (13). For Western blot analysis, proteins were transferred to nitrocellulose (Schleicher & Schull, Keene, N.H.) (27) and probed with anti-GST antibody or MAb 314, a murine monoclonal antibody that recognizes an epitope located in the C-terminal one-third of HapS (6). Bound antibodies were detected with alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) and IgM antibodies (Biosource, Camarillo, Calif.).
ELISA.
The antibody response of mice to rCBD was measured by using a standard enzyme-linked immunosorbent assay (ELISA) procedure. MaxiSorp surface Nunc-Immuno 96-well plates (Nalge Nunc International, Naperville, Ill.) were coated overnight at 37°C with purified nHapS-P860295 diluted to 5 μg/ml in PBS. Antibodies bound to nHapS-P860295 were detected with alkaline phosphatase-conjugated goat anti-mouse IgG and IgM antibodies. Antibody titers were expressed as the reciprocal of serum dilution with an absorbance of 0.1 extrapolated from a linear plot of the logarithm of absorbance versus the logarithm of serum sample dilution.
Antibody inhibition of adherence.
Mouse antisera against rCBD were tested for their ability to block H. influenzae Hap-mediated adherence to human epithelial cells. In performing these assays, bacteria were grown in BHI broth to late log phase, pelleted in a microcentrifuge, and resuspended in plain BHI broth containing an antiserum with a 1:100 dilution. After incubation for 30 min at room temperature, 1 × 107 to 2 × 107 CFU were inoculated onto monolayers of Chang conjunctival epithelial cells (Wong-Kilbourne derivative, clone 1-5c-4 [human conjunctiva]), and adherence was measured as described previously (22). Percent adherence was calculated by dividing the number of adherent CFU per monolayer by the number of inoculated CFU. Preimmune serum and antiserum against an induced E. coli BL21(DE23)/pLysS/pGEX-6P-1 cell lysate were tested as negative controls.
Subcutaneous immunization.
Groups of 10 female, 6- to 8-week-old Swiss Webster or BALB/c mice (Taconic Farms, Germantown, N.Y.) were immunized subcutaneously at weeks 0 and 4 with protein antigens. To prepare the protein antigens for vaccination, 10 μg of rCBD or 2 μg of an induced E. coli BL21(DE23)/pLysS/pGEX-6P-1 cell lysate was absorbed onto 100 μg of aluminum phosphate at 37° for 1 h, and then 50 μg of 3-O-deacylated monophosphoryl lipid A (MPL; Corixa Corp., Hamilton, Mont.) was added. Sera collected at weeks 0, 4, and 6 were pooled for analysis.
Intranasal immunization.
Groups of 10 female, 6-week-old BALB/c mice were immunized intranasally with protein antigens as described previously (5) at weeks 0, 1, 3, and 5. To prepare the protein antigens for vaccination, 15 μg of rCBD was diluted in Dulbecco's PBS (D-PBS) to a final volume of 20 to 40 μl with or without 0.1 μg of CT-E29H (a mutant cholera toxin) as an adjuvant (24). Control mice received D-PBS alone or D-PBS with 0.1 μg of CT-E29H. Sera collected at weeks 0, 3, 5, and 8 were pooled for analysis. Prior to immunization, mice were anesthetized with a mixture of ketamine (80 mg per kg of body weight) and xylazine (7 mg per kg of body weight), a dosage that maintains a state of anesthesia for 15 to 20 min. Vaccines were delivered by pipette in a volume of 20 μl per nostril. The pipette was positioned so that the tip touched the opening of the nostril, allowing the liquid to be drawn into the nasopharynx with breathing. Immediately following immunization, mice were placed in a supine position for 3 to 5 min.
Intranasal challenge.
Three weeks after the final immunization, mice were challenged intranasally with approximately ×106 CFU of strain TN106.P2 as described previously (5). To determine the actual bacterial count of the inoculum, an aliquot of the bacterial suspension was diluted in D-PBS and plated on BHI-XV plates. Three days after challenge, mice were sacrificed and the nasal tissue was harvested, weighed, homogenized, and plated on BHI-XV plates containing 100 μg of streptomycin/ml. The plates were incubated overnight, and the colonies were counted. The data were expressed as log CFU per gram of nasal tissue. The variability of the standard errors of the means between the groups in the present study violates the assumption of equal variance between groups, making it more appropriate to first rank the data and then use an analysis of variance. Accordingly, statistical differences among groups were analyzed by use of the Tukey-Kramer test, a nonparametric method applied when data points are not normally distributed (3, 4).
RESULTS
Protein sequence alignment of CBD of H. influenzae.
In an earlier work, Fink et al. (6) established that the C-terminal 311 amino acids of HapS-N187 harbor the adhesive activity of the protein. To determine the level of conservation of this region, we used the AlignX program of VectorNTI, version 6.0, software to align the C-terminal end of HapS from three NTHi strains. The analysis indicates that the CBD fragments from strains P860295, TN106, and N187 are 97.1% similar and 81% identical.
Antibody response elicited by rCBD generated from GST fusion protein.
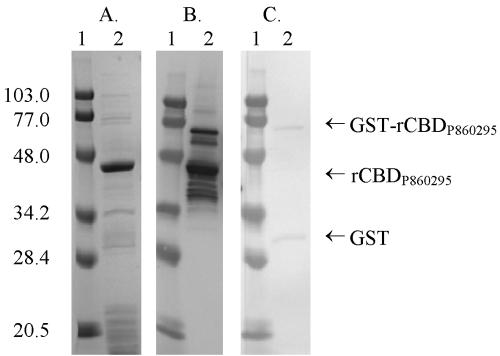
rCBDP860295, rCBDTN106, and rCBDN187 were generated from the soluble fraction of the respective GST-rCBD fusion proteins. As shown in Fig. 1A, rCBDP860295 is the major protein in the affinity-purified preparation, with a purity of ∼60%. In Western blot analysis, rCBDP860295 reacts with MAb 314 (Fig. 1B) but not with anti-GST antibody (Fig. 1C). MAb 314 also detects small amounts of uncleaved GST-rCBDP860295, fusion protein cleaved at alternate sites, and degradation products of rCBDP860295 (Fig. 1B). Some of the minor bands in Fig. 1A not recognized by MAb 314 or anti-GST antibody probably represent contaminating E. coli proteins. Similar patterns of reactivity are observed with rCBDTN106 and rCBDN187 preparations derived from GST fusions (data not shown).
FIG. 1.
SDS-PAGE and Western blot analysis of rCBDP860295 generated from GST-rCBDP860295 fusion protein. (A) Coomassie-stained SDS gel loaded with 5 μg of rCBDP860295 (lane 2); lane 1 shows Bio-Rad prestained low-molecular-mass markers (in kilodaltons). (B) Western blot probed with MAb 314, showing GST-rCBDP860295 fusion protein and rCBDP860295. (C) Western blot probed with anti-GST antibody, showing GST and GST-rCBDP860295 fusion protein.
Mouse antisera against the three rCBD fragments derived from soluble GST fusion proteins were tested for antibody response to nHapS purified from strain P860295. After one immunization, the antisera had low titers toward nHapS-P860295 (data not shown). After a second immunization, high antibody titers were detected in mice that received rCBDN187 and rCBDP860295 and a relatively high titer was detected in mice that received rCBDTN106 (Table 1), suggesting that rCBDN187 and rCBDTN106 are able to elicit antibodies that cross-react with nHapS-P860295. Preimmune sera had antibody titers to nHapS-P860295 of less than 100. Because the rCBD preparations obtained from GST fusion proteins contain small amounts of GST and possibly E. coli proteins, we generated a control antiserum against an induced E. coli BL21(DE3)/pLysS/pGEX-6P-1 cell lysate. Like the preimmune sera, this control antiserum exhibited an antibody titer toward nHapS-P860295 of less than 100.
TABLE 1.
Antibody responsea elicited by rCBD via subcutaneous immunization
| Antiserum against rCBDb derived from | Antibody titer to nHaps-P860295 |
|---|---|
| GST-rCBDN187 FP | 58,050 |
| GST-rCBDP860295 FP | 97,739 |
| GST-rCBDTN106 FP | 14,833 |
| rCBDN187 IB | 32,440 |
| Preimmune sera | <100 |
| Control antiserum against E. coli lysatec | <100 |
Mice were immunized subcutaneously with rCBD mixed with MPL and aluminum phosphate. Preimmune era and week 6 sera pooled from 10 mice were analyzed for antibody titer to purified nHapS-P860295.
rCBD was generated from soluble GST-rCBD fusion protein (FP) or urea-solubilized inclusion bodies (IB).
Control antiserum against an induced BL21 (DE3)/pLysS/pGEX-6P-1 cell lysate.
Antibody inhibition of adherence to Chang epithelial cells.
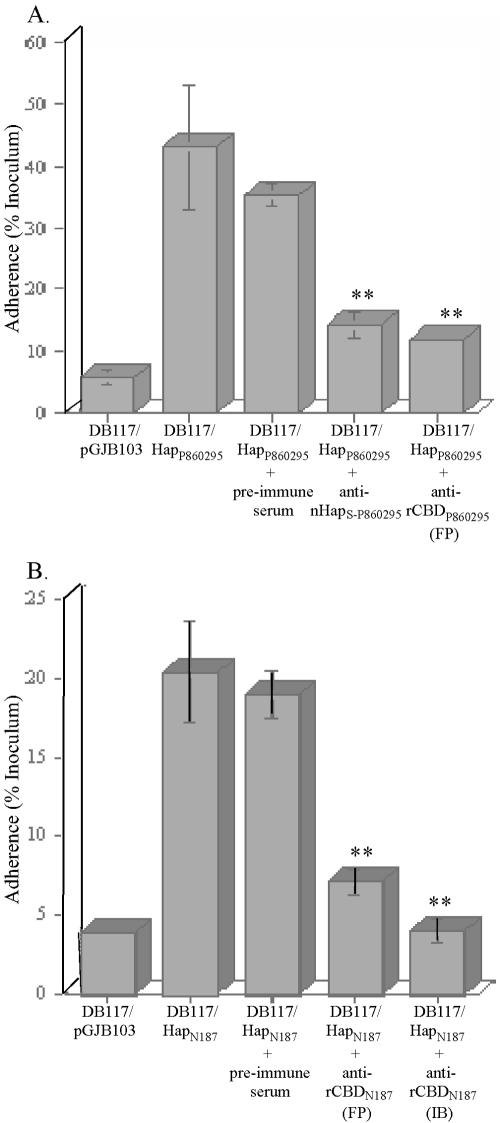
To assess functional characteristics of antisera raised against rCBD derived from GST fusion protein, we examined their effect on H. influenzae Hap-mediated adherence. Both strains DB117/HapP860295 (Fig. 2A) and DB117/HapN187 (Fig. 2B) exhibited appreciable adherence to Chang cells, well above the background level of adherence by the DB117/pGJB103 control. Adherence by DB117/HapP860295 was reduced significantly by preincubation with anti-nHapS-P860295 serum (Fig. 2A). Similar decreases in adherence were observed with DB117/HapP860295 preincubated with anti-rCBDP860295 serum (Fig. 2A) and with DB117/HapN187 preincubated with anti-rCBDN187 serum (Fig. 2B). In contrast, adherence by DB117/HapP860295 or DB117/HapN187 was unaffected by preincubation with preimmune serum (Fig. 2A and B). Additional control experiments using antiserum against an induced E. coli BL21(DE3)/pLysS/pGEX-6P-1 cell lysate also showed no effects on adherence of DB117/HapP860295 or DB117/HapN187 (data not shown). These findings suggest that nHapS-P860295, rCBDP860295, and rCBDN187 elicit antibodies capable of blocking Hap-mediated adherence to human epithelial cells.
FIG. 2.
(A) Adherence to monolayers of Chang conjunctival epithelial cells by strains DB117/pGJB103 (vector), DB117/HapP860295, and DB117/HapP860295 that had been preincubated with either preimmune serum, antiserum against nHapS-P860295, or antiserum against rCBDP860295 derived from GST fusion protein (FP). **, P < 0.05 compared to DB117/HapP860295 plus preimmune serum. (B) Adherence to monolayers of Chang conjunctival epithelial cells by strains DB117/pGJB103, DB117/HapN187, and DB117/HapN187 that had been preincubated with either preimmune serum, antiserum against rCBDN187 derived from GST fusion protein (FP), or antiserum against rCBDN187 isolated from inclusion bodies (IB). **, P < 0.05 compared to DB117/HapN187 plus preimmune serum. In both panels, each bar represents the means ± standard error of the means of measurements made in triplicate from a representative experiment.
Effect of immunization with rCBD on nasopharyngeal colonization.
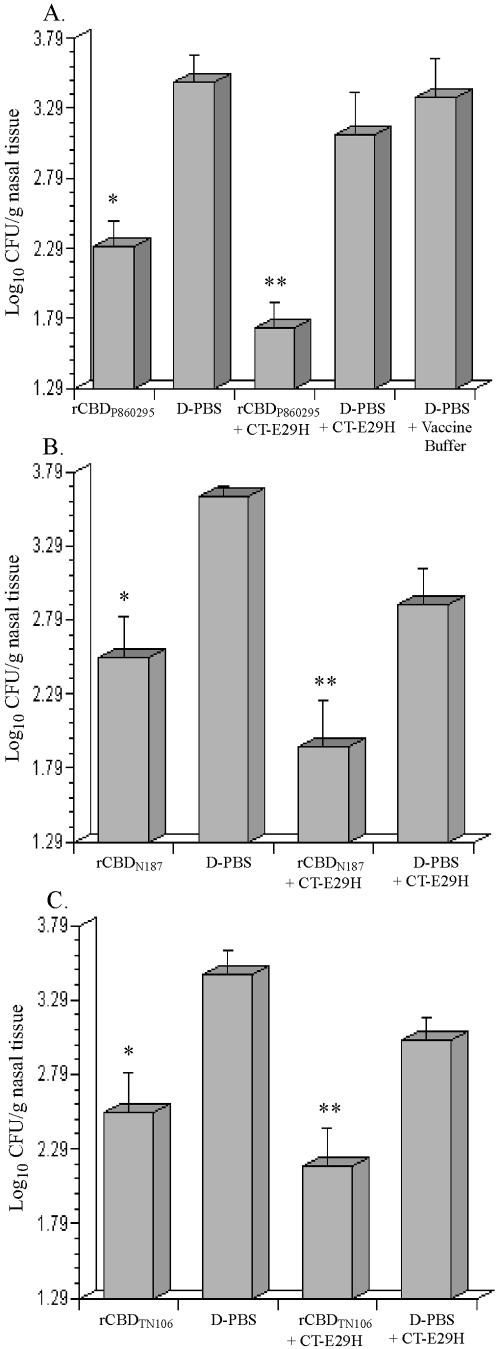
To investigate if immunization with rCBD would affect nasopharyngeal colonization by H. influenzae, rCBDN187, rCBDP860295, and rCBDTN106 generated from GST fusion proteins were administered intranasally to mice. Upon challenge with strain TN106, a significant reduction in nasal colonization was observed in mice immunized with rCBDP860295 compared to that of the control group D-PBS (Fig. 3A). Further reduction in nasal colonization was detected in mice immunized with rCBDP860295 together with CT-E29H adjuvant compared to that of the control groups D-PBS plus CT-E29H and D-PBS plus vaccine buffer. The same pattern was noted for mice immunized with either rCBDN187 (Fig. 3B) or rCBDTN106 (Fig. 3C). These results demonstrate that rCBD derived from strains P860295, N187, and TN106 can elicit antibodies capable of reducing nasopharyngeal colonization by strain TN106.
FIG. 3.
Nasopharyngeal colonization by strain TN106.P2 in BALB/c mice immunized with rCBD derived from GST fusion proteins. (A) Bacterial colonization in mice vaccinated with rCBDP860295. (B) Bacterial colonization in mice vaccinated with rCBDN187. (C) Bacterial colonization in mice vaccinated with rCBDTN106. In panel A, an asterisk indicates significant difference from D-PBS control and two asterisks indicates significant difference from D-PBS plus CT-E29H and D-PBS plus vaccine buffer controls. In panels B and C, an asterisk indicates significant difference from D-PBS control and two asterisks indicates significant difference from D-PBS plus CT-E29H control. The x axis represents the detection limit of the assay, and each bar represents the standard error of the means.
Antisera from intranasally immunized mice were tested for antibody response to nHapS purified from strain P860295. After four immunizations, mice that received either rCBDN187 or rCBDP860295 had high antibody titers to nHapS-P860295 (Table 2). The anti-nHapS-P860295 antibody titer was increased by threefold when rCBDN187 was delivered with CT-E29H adjuvant. In contrast, no such adjuvant effect was observed with rCBDP860295. These results demonstrate that intranasal immunization with rCBD elicits antibodies that cross-react with nHapS-P860295.
TABLE 2.
Antibody responsea elicited by rCBD via intranasal immunization
| Antiserum against rCBDb derived from | Buffer/adjuvant | Antibody titer to nHapS-P860295 |
|---|---|---|
| GST-rCBDN187 FP | D-PBS/none | 8,120 |
| D-PBS/CT-E29H | 27,240 | |
| GST-rCBDP860295 FP | D-PBS/none | 21,175 |
| D-PBS/CT-E29H | 20,659 | |
| None | D-PBS/none | <100 |
| None | D-PBS/CT-E29H | <100 |
| Preimmune sera | NAc | <100 |
Mice were immunized intranasally with rCBD mixed with D-PBS or adjuvant CT-E29H. Preimmune sera and week 8 sera pooled from 15 mice were analyzed for antibody titer toward purified nHapS-P860295.
rCBD was generated from soluble GST-rCBD fusion protein (FP).
NA, not applicable.
Antibody response elicited by rCBDN187 derived from inclusion bodies.
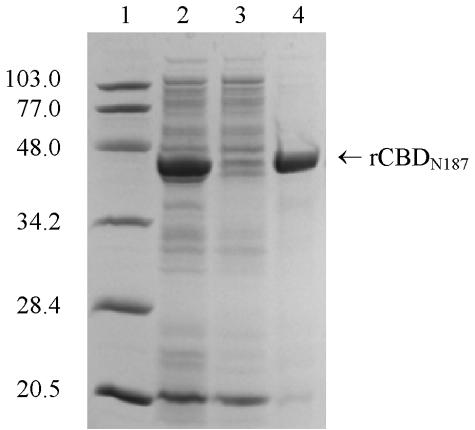
Because it is impractical to produce large quantities of pure rCBD from GST fusion protein, we constructed an E. coli strain that overproduces rCBDN187 under the control of an IPTG-inducible T7 expression system. The amount of rCBDN187 represented 43% of the total protein of induced cells (Fig. 4, lane 2). The bulk of rCBDN187 formed inclusion bodies with a purity of greater than 92% (Fig. 4, lane 4). The inclusion bodies were extracted with urea, and urea-solubilized rCBDN187 was renatured in a dilute state at 10°C in the presence of 0.4 M l-arginine. Renatured rCBDN187 required 0.1 M l-arginine and 0.5% of Triton X-100 to remain soluble upon repeated cycles of freeze and thaw from storage at −20°C.
FIG. 4.
Subcellular location of rCBDN187 produced by E. coli BL21(DE3)/pWV1024. Coomassie-stained gel showing cell lysate (lane 2) of an IPTG-induced culture, cytosolic and membrane proteins (lane 3), and urea-solubilized rCBDN187 inclusion bodies (lane 4). The amount of sample loaded in each lane is equivalent to 20 μl of culture. Lane 1 shows Bio-Rad prestained low-molecular-mass markers (in kilodaltons).
Mice immunized once with rCBDN187 derived from inclusion bodies had an anti-nHapS-P860295 antibody titer of 2,053, which was boosted almost 16-fold by a second immunization (Table 1). This antibody response demonstrates that renatured rCBDN187 resembles rCBDN187 generated from soluble GST fusion protein, capable of eliciting antibodies that cross-react with nHapS-P860295. The antiserum against renatured rCBDN187 also exhibited moderate titers toward whole cells of NTHi strains N187, P860295, TN106, SR7332, and P861454 while the preimmune sera showed titers of less than 100 (data not shown), suggesting that antibodies elicited by renatured rCBDN187 are cross-reactive with surface-exposed Hap of heterologous strains.
Inhibition of adherence to Chang epithelial cells by antibodies against rCBDN187 derived from inclusion bodies.
The antiserum raised against rCBDN187 isolated from inclusion bodies was tested in the in vitro adherence assay. As shown in Fig. 2B, strain DB117/HapN187 readily adhered to Chang cells, and this adherence was markedly reduced by preincubation with anti-rCBDN187 serum, indicating that, similar to rCBD generated from soluble GST fusion protein, rCBDN187 derived from inclusion bodies was also able to elicit functional antibodies that block HapN187-mediated adherence of H. influenzae to human epithelial cells.
Effect of immunization with rCBDN187 derived from inclusion bodies on nasopharyngeal colonization.
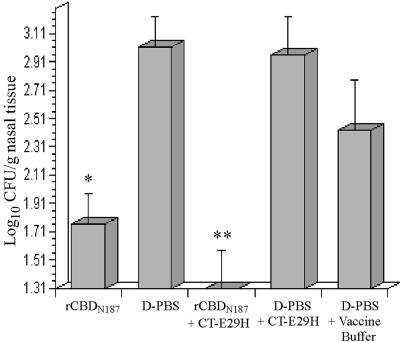
Mice immunized intranasally with rCBDN187 isolated from inclusion bodies were challenged with strain TN106 as described in Materials and Methods. A significant reduction in nasal colonization was observed in mice that received renatured rCBDN187 compared to that of the control group D-PBS (Fig. 5). This level of colonization was further reduced to the limit of detection when renatured rCBDN187 was administered with CT-E29H compared to that of the control groups D-PBS plus CT-E29H and D-PBS plus vaccine buffer. These results demonstrate that rCBDN187 derived from inclusion bodies, just like rCBD generated from soluble GST fusion protein elicits cross-reacting antibodies capable of reducing nasopharyngeal colonization by strain TN106.
FIG. 5.
Nasopharyngeal colonization by strain TN106.P2 in BALB/c mice immunized with rCBDN187 renatured from urea-solubilized inclusion bodies. *, significant difference from D-PBS control; **, significant difference from D-PBS plus CT-E29H and D-PBS plus vaccine buffer controls. The x axis represents the detection limit of assay, and each bar represents the standard error of the means.
DISCUSSION
To establish infection, H. influenzae utilizes adhesive molecules to interact with tissues of the respiratory tract, a process that facilitates bacterial colonization. The Hap protein is one such adhesin, promoting bacterial aggregation, microcolony formation, and adherence to human respiratory epithelial cells and extracellular matrix proteins. The HapS portion of Hap harbors all adhesive activities, making HapS an attractive vaccine candidate. Furthermore, antibodies against HapS have been shown to impede colonization by H. influenzae in an animal model (5). The recent finding that the C-terminal 311 amino acid residues of HapS retain the functional domain essential for adherence to human epithelial cells and formation of microcolonies (6) prompted us to examine this fragment for its vaccine potential.
While many surface-exposed proteins of NTHi are highly variable, HapS appears to be similar among strains, based on the homology of available hap gene sequences (5). Protein sequence alignment of the C-terminal binding domain of HapS from strains P860295, N187, and TN106 indicates that the CBD region is highly conserved. However, it was unclear whether this degree of homology would be sufficient for CBD from one strain to elicit functional antibodies that are cross-reactive with HapS of other strains. To determine if CBD derived from more than one strain would be required for generating protective immunity against multiple strains, we examined the immune response of mice to rCBD from these three strains.
Initially we constructed E. coli strains to express CBD as GST fusion protein, taking advantage of the commercially available protease and affinity matrix for purifying target proteins from their GST fusion counterparts. To avoid complications due to insolubility, rCBD was isolated from GST fusion protein that remained soluble. Although rCBD is the major species in these affinity-purified preparations, high purity was not achieved. Nevertheless, the small amounts of GST and presumed E. coli proteins did not contribute to the immune response elicited by rCBD, because the antiserum against an induced E. coli BL21(DE3)/pLysS/pGEX-6P-1 cell lysate, resembling the preimmune sera, exhibited an antibody titer of less than 100 toward nHapS-P860295. Therefore, the significant titers observed with the antisera against rCBDP860295, rCBDN187, and rCBDTN106 derived from soluble GST fusion protein demonstrate that, indeed, anti-rCBD antibodies are cross-reactive with nHapS-P860295.
Using in-frame deletion derivatives of Hap expressed by DB117, Fink et al. established that the C-terminal 311 amino acids of HapS mediate H. influenzae adherence to human epithelial cells (6). In addition, they reported that MAb 314 inhibits HapS-mediated bacterial adherence to both A549 cells and Chang cells, suggesting that the epitope recognized by MAb 314 is located nearby the adhesive domain. In this study we found that MAb 314 is reactive with rCDB from strains P860295 (Fig. 1B), N187, and TN106 (data not shown), indicating that the 314 epitope is in a conserved region of CBD. More importantly, antibodies elicited by rCBDP860295 and rCBDN187 block Hap-mediated adherence of DB117/Hap to Chang cells (Fig. 2), while antiserum against an induced E. coli cell lysate had no effects on adherence. These results imply that the adherence-blocking activity of anti-nHap sera previously reported (7) is attributed to anti-rCBD antibodies.
Intranasal immunization has been utilized to demonstrate the potential of vaccine candidates against H. influenzae infections (11, 12). Because otitis media is a mucosal surface disease, intranasal immunization may be the preferred route for humans. While mice immunized intranasally with native HapS purified from strain P860295 or N187 showed significant reductions in nasopharyngeal colonization by strain TN106 (5), it was unclear which portion(s) of HapS was responsible for generating such cross-reactive immune responses. In this investigation, we used the same murine model to test rCBD. Intranasal immunization with either rCBDN187 or rCBDP860295 elicited antibodies that were reactive with purified nHapS-P860295 (Table 2). Furthermore, mice immunized intranasally with rCBDP860295, rCBDN187, or rCBDTN106 exhibited significant decreases in nasopharyngeal colonization by TN106, and the reduction was lowered still further when rCBD was administered with CT-E29H (Fig. 3). These results suggest that an rCBD generated from soluble GST fusion protein, regardless of the strain, assumes a proper conformation to elicit antibodies that are cross-reactive and functional in the in vitro adherence assay as well as in the in vivo challenge model.
Obstacles to developing a HapS-based vaccine are twofold. At the cellular level, H. influenzae does not produce large amounts of HapS. Thus, it is impractical to purify native HapS for vaccine use. At the protein level, HapS tends to form aggregates at even moderate concentrations, making handling of the protein a challenge. Although protein solubility is substantially improved by expressing only the N-terminal 311 amino acids of HapS as a GST fusion protein, producing rCBD from GST fusion protein as a vaccine component is not a desirable approach because of processing issues. Moreover, rCBD purified by affinity chromatography contains small amounts of uncleaved fusion protein, rCBD breakdown products, GST, and possibly E. coli proteins. Therefore, we sought to generate a non-fusion-derived rCBD of much greater purity.
CBD from HapN187 was selected for further investigation based on good expression levels in pilot experiments. rCBDN187 under the control of T7 promoter was overproduced as inclusion bodies. The inclusion bodies were readily solubilized by urea with a purity of greater than 92% (Fig. 4). Utilizing l-arginine to assist protein renaturation, renatured rCBDN187 remained soluble above 1 mg/ml. Just like rCBD derived from GST-fusion proteins, renatured rCBDN187 elicited antibodies that are not only cross-reactive with purified nHapS-P860295 but also are capable of blocking Hap-mediated adherence of strain DB117/HapN187 to Chang cells (Fig. 2B). Most importantly, intranasal immunization of mice with renatured rCBDN187 significantly reduced nasopharyngeal colonization by strain TN106 (Fig. 5). The high purity of rCBDN187 preparations shows that the immune response to rCBD is responsible for these functional activities both in vitro and in vivo. These results clearly demonstrate that rCBDN187 solubilized and renatured from inclusion bodies is a potential vaccine candidate against H. influenzae infection.
Editor: J. N. Weiser
REFERENCES
- 1.Anderson, M., D. Blowers, N. Hewitt, P. Hedge, A. Breeze, I. Hampton, and I. Taylor. 1999. Refolding, purification, and characterization of a loop deletion mutant of human Bcl-2 from bacterial inclusion bodies. Protein Expr. Purif. 15:162-170. [DOI] [PubMed] [Google Scholar]
- 2.Buchner, J., and R. Rudolph. 1991. Renaturation, purification and characterization of recombinant Fab-fragments produced in Escherichia coli. Bio/Technology 9:157-162. [DOI] [PubMed] [Google Scholar]
- 3.Conover, W. J., and R. L. Iman. 1975. On some alternative procedures using ranks of experimental designs. Commun. Stat. A. 5:1345-1368. [Google Scholar]
- 4.Conover, W. J., and R. L. Iman. 1981. Rank transformation as a bridge between parametric and non-parametric statistics. Am. Stat. 35:124-133. [Google Scholar]
- 5.Cutter, D., K. W. Mason, A. P. Howell, D. L. Fink, B. A. Green, and J. W. St. Geme III. 2002. Immunization with the Haemophilus influenzae Hap adhesin protects against nasopharyngeal colonization in experimental mice. J. Infect. Dis. 186:1115-1121. [DOI] [PubMed] [Google Scholar]
- 6.Fink, D. L., A. Z. Buscher, B. A. Green, P. Fernsten, and J. W. St. Geme III. 2003. The Haemophilus influenzae Hap autotransporter mediates microcolony formation and adherence to epithelial cells and extracellular matrix via binding regions in the C-terminal end of the passenger domain. Cell. Microbiol. 5:175-186. [DOI] [PubMed] [Google Scholar]
- 7.Fink, D. L., B. A. Green, and J. W. St. Geme III. 2002. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 70:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green, B. A., J. E. Farley, T. Quinn-Dey, R. A. Deich, and G. W. Zlotnick. 1991. The e (P4) outer membrane protein of Haemophilus influenzae: Biologic activity of anti-e serum and cloning and sequencing of the structural gene. Infect. Immun. 59:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrixson, D. R., M. L. de la Morena, C. Stathopoulos, and J. W. St. Geme III. 1997. Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol. Microbiol. 26:505-518. [DOI] [PubMed] [Google Scholar]
- 10.Hendrixson, D. R., and J. W. St. Geme III. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841-850. [DOI] [PubMed] [Google Scholar]
- 11.Hotomi, M., S. Saito, and N. Yamanaka. 1998. Specific mucosal immunity and enhanced nasopharyngeal clearance of nontypeable Haemophilus influenzae after intranasal immunization with outer membrane protein P6 and cholera toxin. Vaccine 16:1950-1956. [DOI] [PubMed] [Google Scholar]
- 12.Kurono, Y., M. Suzuki, G. Mogi, M. Yamamoto, K. Fujihashi, J. R. McGhee, and H. Kiyono. 1999. Effects of intranasal immunization on protective immunity against otitis media. Int. J. Pediatr. Otorhinolaryngol. 49:S227—S229. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Murphy, T. F., and M. A. Apicella. 1987. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev. Infect. Dis. 9:1-15. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, T. F., and S. Sethi. 1992. Bacterial infection in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 146:1067-1083. [DOI] [PubMed] [Google Scholar]
- 17.Peterson, G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83:346-356. [DOI] [PubMed] [Google Scholar]
- 18.Rudolph, R., and H. Lilie. 1996. In vitro folding of inclusion body proteins. FASEB J. 10:49-56. [PubMed] [Google Scholar]
- 19.Sanders, J. D., L. D. Cope, G. P. Jarosik, I. Maciver, J. L. Latimer, G. B. Toews, and E. J. Hansen. 1993. Reconstitution of a porin-deficient mutant of Haemophilus influenzae type b with a porin gene from nontypeable H. influenzae. Infect. Immun. 61:3966-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setlow, J. K., D. C. Brown, M. E. Boling, A. Mattingly, and M. P. Gordon. 1968. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J. Bacteriol. 95:546-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St. Geme, J. W., III. 1997. Insights into the mechanism of respiratory tract colonization by nontypable Haemophilus influenzae. Pediatr. Infect. Dis. J. 16:931-935. [DOI] [PubMed] [Google Scholar]
- 22.St. Geme, J. W., III, S. Falkow, and S. J. Barenkamp. 1993. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc. Natl. Acad. Sci. USA 90:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St. Geme, J. W., III, M. L. de la Morena, and S. Falkow. 1994. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol. Microbiol. 14:217-233. [DOI] [PubMed] [Google Scholar]
- 24.Tebbey, P. W., C. A. Unczur, J. A. Peek, D. Zhu, N. A. LaPierre, E. D. Phillips, A. R. Ibraghimov, B. A. Green, J. H. Eldridge, and G. E. Hancock. 2000. Effective mucosal immunization against respiratory syncytial virus using a genetically detoxified cholera holotoxin, CT-E29H. Vaccine 18:2723-2734. [DOI] [PubMed] [Google Scholar]
- 25.Teele, D. W., J. O. Klein, B. Rosner, and G. B. O. M. S. Group. 1989. Epidemiology of otitis media during the first five years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 160:83-94. [DOI] [PubMed] [Google Scholar]
- 26.Tomb, J. F., G. J. Barcak, M. S. Chandler, R. J. Redfield, and H. O. Smith. 1989. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J. Bacteriol. 171:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]