Abstract
In Candida albicans, calcineurin is essential for virulence and survival during membrane perturbation by azoles. Crz1 is a proposed downstream target of calcineurin based on studies of Saccharomyces cerevisiae. However, the in vitro phenotypes of C. albicans crz1/crz1 and calcineurin mutants differ and Crz1 is not required for virulence.
Candida albicans is a commensal organism that resides on the vaginal mucosa, gastrointestinal tract, and skin of healthy individuals (14). However, this organism is also pathogenic due to its ability to cause disseminated infection in immunocompromised individuals and hospitalized patients (2, 14, 20). A majority of the antifungal drugs currently used to treat candidiasis disrupt the fungal cell membrane by targeting key enzymes in the ergosterol biosynthetic pathway. Drugs of the azole class have low toxicity and are generally effective, but these agents are fungistatic, not fungicidal, against Candida species. C. albicans cells exposed to fluconazole can overcome its antifungal activity and acquire drug resistance, hampering successful treatment of an infection. Alternative use of the fungicidal agent amphotericin B is limited by its severe nephrotoxicity. More recently developed drugs, such as caspofungin, appear safe and effective, but these agents are administered only intravenously (13). As a consequence, unique strategies must be employed to enhance the variety and improve the quality of the antifungal therapies available for treatment of invasive candidiasis.
Previous in vitro studies reveal that the antifungal properties of fluconazole, terbinafine, and fenpropimorph against C. albicans can be synergistically enhanced by the addition of FK506 or cyclosporine (6, 10, 11, 15). These agents inhibit calcineurin, a serine/threonine phosphatase that plays a central role in calcium signaling. C. albicans mutants lacking calcineurin are hypersensitive to serum and antifungal agents that target ergosterol biosynthesis in vitro (3, 6, 15, 16), and they are attenuated for virulence in a murine model of disseminated candidiasis (1, 3, 16). Given these findings, we sought to probe the C. albicans calcineurin signaling pathways to identify substrates that might contribute to virulence and/or modulate the antifungal properties of ergosterol biosynthesis inhibitors.
The zinc finger transcription factor Crz1 has been identified as a target of calcineurin in Saccharomyces cerevisiae (12, 17). In response to extracellular stress (high salt, high temperature, cell wall damage, or mating pheromone), calcineurin dephosphorylates Crz1, promoting nuclear translocation and induction of genes encoding biosynthetic cell wall enzymes and homeostatic ion machinery (FKS2, PMR2, and PMC1) (4, 12, 17, 18). We identified the C. albicans Crz1 homolog (orf19.7359) by a BLAST search, and crz1/crz1 mutants were created by using the UAU1 cassette gene disruption approach (8). The UAU1 cassette was amplified with primers JOHE9234 (ATTTTCCCCTTTTTATATCTAAATTTCATAAATCCCAATCGTTTTCCCAGTCACGACGTT) and JOHE9235 (AGGAATAACTATCGTGAATGACAACAACCTCAAAAAAAAATGTGGAATTGTGAGCGGATA), which are homologous to the 40-bp regions flanking the CRZ1 gene. Following PCR amplification, this crz1Δ::UAU1 disruption allele was introduced into an S. cerevisiae strain together with a linearized vector containing the CRZ1 gene with a flanking sequence to increase the length of flanking homology through in vivo homologous recombination. The resulting crz1Δ::UAU1 allele was rescued in an Escherichia coli strain, released by cleavage with the restriction enzyme NotI, and transformed into C. albicans auxotrophic strain BWP17 (21) with lithium acetate (19). Ura+ Arg+ transformants were selected. Before phenotypes were assessed, the remaining histidine auxotrophy was complemented by introducing the linearized pGEM-HIS1 vector (21). A fragment containing the CRZ1 open reading frame with 1,134 nucleotides of the 5′ noncoding region and 431 nucleotides of the 3′ noncoding region was inserted into the pGEM-HIS1 vector, and the resulting plasmid (pCOC7) was linearized with NruI and transformed into the crz1/crz1 mutant strain to complement the crz1/crz1 mutant with a single copy of CRZ1. All mutant strains were confirmed by Southern blot analysis (Fig. 1).
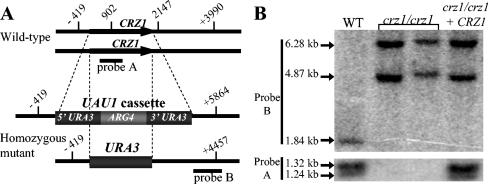
FIG. 1.
Homozygous disruption of the C. albicans CRZ1 gene. (A) Schematic illustration of wild-type and disrupted CRZ1 alleles. In homozygous mutants, each CRZ1 allele is replaced by the UAU1 cassette or the URA3 gene. Hatch marks and corresponding numbers designate SpeI restriction sites relative to the start of each allele. An internal probe (probe A) recognizes CRZ1-specific sequences, while a probe with a 3′-end-flanking sequence (probe B) distinguishes between the wild-type restriction pattern and the homozygous crz1/crz1 and reconstituted mutant restriction fragments, both of which contain UAU1 and URA3 alleles at the CRZ1 locus. (B) Confirmation of crz1/crz1 mutant strains. SpeI-digested genomic DNA from parent strain BWP17 (wild type [WT]), two independently derived crz1/crz1 mutant strains (OCC1.1 and OCC3.8), and the CRZ1-complemented mutant (OCC7) were analyzed by Southern blotting. Probe A recognizes 1.24- and 1.32-kb fragments derived from CRZ1 in the wild-type and reconstituted mutant strains, respectively. Probe B recognizes a 1.84-kb fragment in the wild type or 4.87- and 6.28-kb fragments in the crz1/crz1 homozygous and reconstituted mutant strains, respectively.
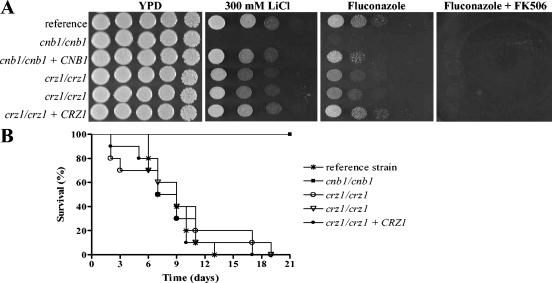
Two independently derived prototrophic crz1/crz1 mutants (OCC1.1 and OCC3.8), a previously described wild-type reference strain (DAY185) (7), a prototrophic cnb1/cnb1 mutant lacking the calcineurin B regulatory subunit (JRB64) (3), and a CRZ1-complemented crz1/crz1 mutant (OCC7) were each grown in liquid yeast extract-peptone-dextrose (YPD) medium overnight. Fivefold serial dilutions of each strain were prepared and spotted onto solid medium to compare their salt and drug sensitivities (Fig. 2A). Unlike calcineurin mutants, the crz1/crz1 mutants were not hypersensitive to lithium chloride, but they were hypersensitive to fluconazole, and this phenotype was complemented by reintroduction of the CRZ1 gene (Fig. 2A). Differences in fluconazole sensitivity were measured by the Etest according to the manufacturer's guidelines, with AB Biodisk strips and RPMI 1640 medium supplemented with 1.5% agar and 2% glucose and buffered to pH 7.0 with 0.165 M MOPS (morpholinepropanesulfonic acid) buffer (Remel, Lenexa, Kans.). Plates were incubated at 35°C and read after 48 h. Strain DAY185 is a derivative of strain BWP17 in which the auxotrophic mutations were complemented by reintroduction of the corresponding wild-type genes (7). Therefore, it most closely corresponds to the crz1/crz1 mutant genetic background and serves as a wild-type reference strain, and the MIC for it ranges from 0.75 to 1.0 μg/ml (Table 1). The crz1/crz1 mutants were slightly less sensitive to fluconazole than the cnb1/cnb1 mutants, and the Etest MICs for them were 0.38 and 0.19 to 0.25 μg/ml, respectively (Table 1).
FIG. 2.
crz1/crz1 mutant phenotypes. (A) Hypersensitivity profile. As indicated, solid YPD media contained no drug, 300 mM lithium chloride, fluconazole (50 μg/ml), or FK506 (1 μg/ml) overlaid with top agar containing 50 μg of fluconazole per ml. All plates were incubated at 30°C for 48 h. (B) Crz1 is not essential for the virulence of C. albicans. The reference strain (DAY185), the cnb1/cnb1 mutant (JRB64), the crz1/crz1 mutants (OCC1.1 and OCC3.8), and the CRZ1-complemented crz1/crz1 strain (OCC7) were used to infect groups of 10 mice each with an inoculum of 106 cells (7 mice for JRB64) by lateral tail vein injection, and survival was monitored over time. There was no significant difference in rates of survival between mice infected with the crz1/crz1 mutant strains and mice infected with the reference strain or the reconstituted mutant strain (P > 0.05). No significant difference was noted between results with the two crz1/crz1 mutant strains or between results with the reference and reconstituted strains (P > 0.05). The cnb1/cnb1 calcineurin strains were avirulent compared to all other strains (P < 0.001). Similar results were attained in a repeat experiment using an inoculum of 107 cells. Kaplan-Meier illustration and log rank statistical analysis of the survival data were performed using the PRISM 4.02 program (GraphPad Software, San Diego, Calif.).
TABLE 1.
crz1/crz1 mutants are less sensitive to fluconazole than cnb1/cnb1 mutants
| Strain | Genotype | MIC or range (μg/ml) |
|---|---|---|
| DAY185 | Prototrophic reference strain | 0.75-1.0 |
| JRB64 | cnb1/cnb1 | 0.19-0.25 |
| MCC85 | cnb/cnb1 CNB1 | 0.38-0.5 |
| OCC1.1 | crz1/crz1 | 0.38 |
| OCC3.8 | crz1/crz1 | 0.38 |
| OCC7 | crz1/crz1 CRZ1 | 0.75 |
These findings indicate that Crz1 is not the primary or sole target of calcineurin for responses to high salinity or azole-mediated membrane perturbation. To explore this hypothesis further, we examined the sensitivities of these mutants to drug combinations. If Crz1 were the sole target of calcineurin, then we would not expect FK506 to act synergistically with fluconazole against crz1/crz1 mutants. In contrast, we found that the addition of FK506 to fluconazole enhanced the inhibition of crz1/crz1 mutants to the level observed with cnb1/cnb1 calcineurin mutants exposed to fluconazole (Fig. 2A). C. albicans cells were not sensitive to FK506 alone (data not shown). We also performed fluconazole Etest assays on solid YPD media with and without 1 μg of FK506 per ml. On YPD medium, the MICs for all strains increased. Macrocolonies within the halo of inhibition were observed with the wild-type reference strain, the CRZ1-reconstituted mutant, and the crz1/crz1 mutants but not with the cnb1/cnb1 mutant. This trailing effect was abolished when FK506 was present in the medium (data not shown). Additionally, in contrast to calcineurin mutants, crz1/crz1 mutants do not exhibit in vitro sensitivity to serum (J. R. Blankenship, personal communication). These findings are consistent with models in which Crz1 functions downstream of calcineurin but is not the sole signaling effector.
Given the differences between the in vitro phenotypes of the calcineurin and crz1/crz1 mutant strains, we tested whether these mutants also behave differently in vivo. We established a murine model of disseminated candidiasis in which outbred ICR mice received 0.2 ml of phosphate-buffered saline containing ∼106 cells of the reference strain, a cnb1/cnb1 calcineurin mutant strain, one of two independently derived crz1/crz1 mutant strains, or a CRZ1-complemented crz1/crz1 mutant strain via tail vein injection. Animals were monitored for signs of infection over the course of 21 days, and moribund animals were sacrificed. Neither of the two crz1/crz1 strains was attenuated for virulence compared to the wild-type reference and complemented strains (Fig. 2B). As previously demonstrated, however, the cnb1/cnb1 mutant was avirulent, and all animals infected survived the entire course of the experiment (Fig. 2B). These findings demonstrate that, unlike calcineurin, Crz1 is not essential for virulence. Thus, Crz1 is not the exclusive or primary downstream target of the C. albicans calcineurin signaling cascade responsible for pathogenicity.
In C. albicans, calcineurin is essential for survival in serum, during membrane perturbation by ergosterol biosynthesis inhibitors in vitro, and for virulence in vivo (1, 3, 6, 10, 11, 15, 16). These are all traits relevant to the ability of C. albicans to establish an ongoing infection. Our goal is to determine the links among calcineurin, virulence, and membrane stability with the intent of exploiting important factors that can contribute to improved antifungal therapies. Here, we investigated the relevance of the C. albicans Crz1 transcription factor. In S. cerevisiae, Crz1 has been extensively characterized as a primary target of calcineurin signaling in response to a variety of environmental stresses (12, 17, 18). The S. cerevisiae Crz1 protein contains an N-terminal glutamine-rich region, a PxIxIQ motif (where x is any amino acid) that serves as a docking site for calcineurin, two C2H2-type zinc finger domains, and a serine-rich region that contains a nuclear export signal (4, 12, 17, 18). These C2H2-type zinc fingers are conserved among S. cerevisiae, C. albicans, and the putative Ashbya gossypii Crz1 homologs, and sequences resembling the other motifs are present in C. albicans but have not yet been characterized. Despite sharing limited protein sequence homology with its S. cerevisiae counterpart, C. albicans Crz1 has been shown to functionally complement the calcium hypersensitivity of an S. cerevisiae crz1 mutant (M. Karababa, E. Valentino, J. Bille, and D. Sanglard, Abstr. 7th Candida Candidiasis Conf., abstr. 191, 2003). Additionally, a crz1/crz1 mutation mirrors treatment with cyclosporine by reversing the fluconazole resistance phenotype of C. albicans cka2/cka2 mutant strains (5). Taken together, these findings provide additional evidence that the C. albicans Crz1 homolog plays a role in a calcineurin-related function(s).
We have analyzed the crz1/crz1 mutants on media containing high salt concentrations or fluconazole as well as in a murine model of candidiasis. The crz1/crz1 mutants are not hypersensitive to lithium chloride, they are less sensitive to fluconazole than cnb1/cnb1 calcineurin mutants, and they are not attenuated for virulence. The differences between these two mutants reveal that targets of calcineurin other than Crz1 play an equally or more important role in C. albicans. Calcineurin may act via multiple effector proteins that cooperate to respond to membrane perturbation and serum factors in C. albicans. Additionally, the calcineurin-dependent pathways for virulence and for responses to antifungal agents appear to be functionally distinct and may even be unrelated. The next challenge will be to identify calcineurin targets that act independently of or in concert with Crz1 to influence the cellular responses to stress and the host environment in the important human fungal pathogen C. albicans. Other calcineurin targets, such as Hph1 and Hph2, have been identified in S. cerevisiae since the discovery of Crz1 (9). However, as is evidenced by the present work, the functions of C. albicans homologs will need to be explored in C. albicans itself and not extrapolated from the model yeast system.
Acknowledgments
We are grateful to Alan Goldstein for assistance in generating the disruption construct and to Aaron Mitchell and members of his laboratory for discussions, guidance in creating mutant strains, and communication of unpublished results.
This study was supported by RO1 grants AI41937 and AI42159 from the NIH/NIAID to Joseph Heitman and in part by NIH supplement AI050438-04S1 from the NIAID to Chiatogu Onyewu. Chiatogu Onyewu is a 2004 UNCF/Merck Graduate Fellow. Joseph Heitman is an associate investigator of the Howard Hughes Medical Institute and a Burroughs Wellcome Scholar in molecular pathogenic mycology.
Editor: T. R. Kozel
REFERENCES
- 1.Bader, T., B. Bodendorfer, K. Schröppel, and J. Morschhäuser. 2003. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 71:5344-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck-Sague, C. M., W. R. Jarvis, et al. 1993. Secular trends in the epidemiology of nosocomial fungal injections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 3.Blankenship, J. R., F. L. Wormley, M. K. Boyce, W. A. Schell, S. G. Filler, J. R. Perfect, and J. Heitman. 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2:422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boustany, L. M., and M. S. Cyert. 2002. Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 16:608-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno, V. M., and A. P. Mitchell. Regulation of azole drug susceptibility by Candida albicans protein kinase CK2. Mol. Microbiol., in press. [DOI] [PubMed]
- 6.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enloe, B., A. Diamond, and A. P. Mitchell. 2000. A single-transformation gene function test in diploid Candida albicans. J. Bacteriol. 182:5730-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath, V. L., S. L. Shaw, S. Roy, and M. S. Cyert. 2004. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot. Cell 3:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. R. Perfect for the Caspofungin Invasive Candidiasis Study Group. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 14.Odds, F. C. 1988. Candida and candidiasis: a review and bibliography, 2nd ed. Baillière Tindall, London, United Kingdom.
- 15.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors in Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 489:59-76. [DOI] [PubMed] [Google Scholar]
- 17.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stathopoulos-Gerontides, A., J. J. Guo, and M. S. Cyert. 1999. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13:798-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther, A., and J. Wendland. 2003. An improved transformation protocol for the human fungal pathogen Candida albicans. Curr. Genet. 42:339-343. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 21.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]