Abstract
Background
Substance use is a leading cause of morbidity and mortality that is under-identified in medical practice.
Objective
The Tobacco, Alcohol, Prescription medication, and other Substance use (TAPS) Tool was developed to address the need for a brief screening and assessment instrument that includes all commonly used substances, and fits into clinical workflows. The goal of this study was to assess the performance of the TAPS Tool in primary care patients.
Design
Multi-site study conducted within the National Drug Abuse Treatment Clinical Trials Network, comparing the TAPS Tool against a reference standard measure.
Setting
Five adult primary care clinics.
Participants
2,000 adult patients were consecutively recruited from clinic waiting areas.
Measurements
Interviewer- and self-administered versions of the TAPS Tool were compared to the reference standard modified Composite International Diagnostic Interview (CIDI), which measures problem use and substance use disorders (SUD).
Results
Interviewer- and self-administered versions of the TAPS Tool had similar diagnostic characteristics. For identifying problem use (at a cutoff of 1+), the TAPS Tool had sensitivity 0.93 (95% CI 0.90–0.95) and specificity 0.87 (95% CI 0.85–0.89) for tobacco, and sensitivity 0.74 (95% CI 0.70–0.78), specificity 0.79 (95% CI 0.76–0.81) for alcohol. For problem use of illicit and prescription drugs, sensitivity ranged from 0.82 (95% CI 0.76–0.87) for marijuana to 0.63 (95% CI 0.47–0.78) for sedatives, and specificity was 0.93 or higher. For identifying any SUD, sensitivity was lower, but a score of 2+ greatly increased the likelihood of having a SUD.
Limitations
Low prevalence of some drug classes led to poor precision in some estimates. Research assistants were not blinded to the participant’s TAPS Tool responses when they administered the CIDI.
Conclusions
In a diverse population of adult primary care patients, the TAPS Tool detected clinically relevant problem substance use. While it may also detect tobacco, alcohol, and marijuana use disorders, further refinement is needed before the TAPS Tool can be broadly recommended as a screener for SUD.
Introduction
Tobacco and alcohol use are among the leading causes of preventable death in the US, (1, 2) and illicit substance use is a significant contributor to the HIV and opioid overdose epidemics(3, 4). Health care settings offer an opportunity to identify substance use and related problems, provide timely interventions, and link patients to treatment. Tobacco screening and treatment is a core clinical quality measure for primary care(5), and screening followed by brief intervention (BI) for unhealthy alcohol use is recommended by the United States Preventive Services Task Force(6–9). While the efficacy of BI for reducing drug use has not been clearly established(10–13), screening for substance use in medical settings may be clinically justified by the impact of substance use on the prevention and treatment of other medical conditions(14–16), and the potential for drug-medication interactions(17, 18) and overdose(19).
Primary care settings require a screening and assessment approach that is efficient, accurate, and informs clinical care(20–23). Very brief screening tools to efficiently identify alcohol and drug use have been developed(24–27), but they do not provide enough information about the specific substances used, or the patient’s risk level, to guide clinical actions. A structured substance use assessment can provide this information, but current options are either too lengthy(28), or do not provide sufficient detail(29, 30) to meet the needs of medical providers.
We developed the Tobacco, Alcohol, Prescription medication, and other Substance use (TAPS) Tool as a more optimal instrument for substance use screening in primary care settings. The TAPS Tool consists of a 4-item screen for tobacco, alcohol, illicit drugs, and non-medical use of prescription drugs, followed by a substance-specific assessment of risk level for individuals who screen positive. The TAPS Tool has the flexibility to be administered face-to-face or self-administered using a tablet computer, to accommodate a variety of clinical workflows. The current paper presents the results from a large multi-site study conducted within the National Institute on Drug Abuse (NIDA) National Drug Abuse Treatment Clinical Trials Network (CTN) to assess the performance of the TAPS Tool in comparison to a reference standard measure in adult primary care patients.
Methods
Participants & Recruitment
Participants were recruited from five primary care clinics located in Baltimore, MD; New York, NY; Richmond, VA; and two sites in Kannapolis, NC. Clinics were selected to provide a geographically diverse sample of urban and suburban participants, which would be expected to vary in their substance use patterns. A sample size of 2,000 was set prior to initiating recruitment, and sites enrolled competitively from August, 2014 to April, 2015, until the total sample was achieved. Sample size was determined through computer simulations to calculate the precision of the estimates of sensitivity and specificity as a function of the number of participants and substance use prevalence. We determined that a sample size of 2,000 allowed us to estimate sensitivity with precision (defined as 95th percentile of the half-width of the 95% confidence interval) of 12.5% for substances with a prevalence of 5%, up to 2.5% for substances with a prevalence of 50%.
Eligibility Criteria
Adults 18 years and older who were in clinic for a medical visit and able to provide informed consent were eligible to participate. Individuals were excluded if they were unable to comprehend spoken English, physically unable to complete the self-administered TAPS Tool, or previously enrolled in this study.
Recruitment
Research assistants (RAs) consecutively approached each individual in the waiting area. Interested patients were screened for eligibility and provided verbal consent. Participants were given the option of having the study visit before their appointment if they had more than a 1-hour wait time, otherwise the study visit was done after the medical visit.
Study Procedures
Study visits were completed in a private room. Participants were assigned a unique identifier and informed that responses were confidential, and would not be shared with anyone in the clinic. The electronic data capture system randomly assigned half of the participants to begin with the self-administered TAPS Tool, and half to begin with the interviewer-administered TAPS Tool. After completing the TAPS Tool in the first format (e.g. self-administered), they completed it in the alternate format (e.g. interviewer). The self-administered version was delivered on a tablet computer (iPad®) that gave participants the option of hearing the questions and response options read verbatim by a recorded female voice. For the interviewer version, all questions and response options were read aloud by the RA. Following completion of both versions, the RA administered a questionnaire about the feasibility and acceptability of the TAPS Tool. This was followed by additional substance use measures that were collected for comparison purposes. RAs were not blinded to participant responses on the interviewer-administered TAPS Tool when they administered the reference standard measure (CIDI). After completing self-reported assessments, all participants were asked to provide verbal consent to participate in oral fluid testing for drugs. Individuals received $20 for the main study and an additional $10 for oral fluid testing. All study procedures were approved by local Institutional Review Boards (Duke University Health System, Friends Research Institute, New York University School of Medicine, and Virginia Commonwealth University).
Measures
Experimental instrument: TAPS Tool
The TAPS Tool was developed as a two-step screening and brief assessment tool from instruments that had not been validated. TAPS-1, the screening component, was adapted from the National Institute on Drug Abuse (NIDA) Quick Screen V1.0.(31) TAPS-2, the brief assessment component, is a modified version of the Alcohol, Smoking, and Substance Involvement Screening Test-Lite (ASSIST-Lite).(32) A prototype of the TAPS Tool was evaluated using cognitive interviewing (33–35) with 30 adult primary care patients from 3 of the study sites. Minor modifications to the wording of the TAPS Tool items were made based on these interviews, prior to finalizing the instrument that was used in this study.
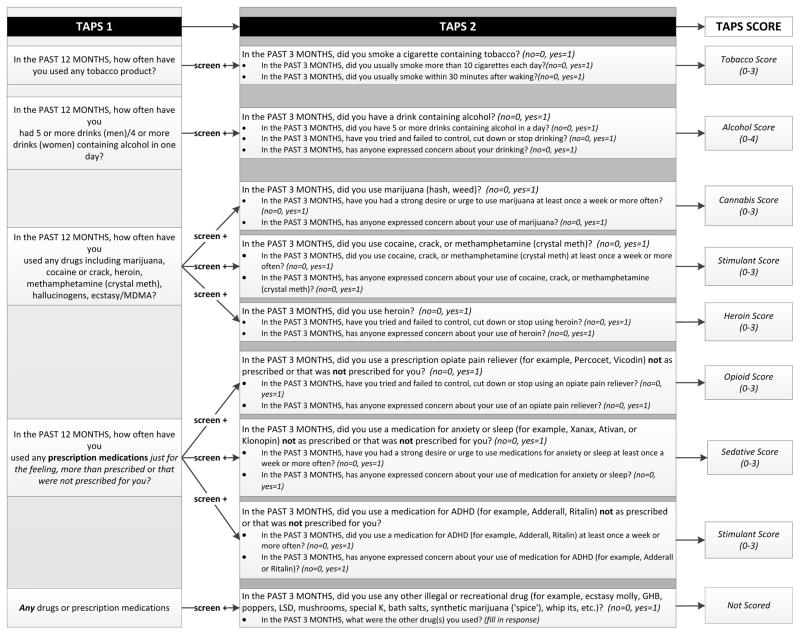
The TAPS Tool is shown in Figure 1. The TAPS-1 asks about frequency of use in the past 12 months of tobacco, alcohol above guideline-recommended daily limits (>5 drinks/day for men, >4 drinks/day for women),(36) illicit drugs, and non-medical use of prescription medications (sedatives, opioids, and stimulants). Respondents choose from five response options ranging from ‘never’ to ‘daily or almost daily.’ The TAPS-2 assesses use in the past three months of tobacco, alcohol, six different classes of illicit drugs, and ‘other’ drugs using a yes/no format. When the answer is ‘yes’ to any use, the participant receives 2–3 follow-up items specific to that substance class.
Figure 1.
TAPS Tool Part 1 and Part 2, showing items, skip pattern, and scoring system
For the TAPS-1, any response other than ‘never’ constitutes a positive screen. Those with a positive screen complete the corresponding items on the TAPS-2, and responses are summed within each substance class to generate a substance-specific risk score. TAPS Tool scores have a potential range of 0–3 for tobacco and other drugs, and 0–4 for alcohol. In clinical practice the TAPS-2 items would only be administered to those with a positive screen, but for the purposes of the study, participants completed all TAPS-2 items, regardless of their TAPS-1 responses.
Reference measures
The modified Composite International Diagnostic Interview, Second Edition, Substance Abuse Module (CIDI) was the ‘gold standard’ reference measure(37–41). The CIDI has been widely used in epidemiological and clinical research to assess substance use disorders (SUDs) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM), 4th edition. As in our previous research(42), we used the existing CIDI items that mapped onto the DSM-5 SUD classifications (by omiting the item on legal problems and including the CIDI item on craving).
Problem use was defined as past-year use with endorsement of one or more items on the CIDI. This approach has been used in prior screening tool studies to identify clinically important substance use that may not be severe enough to meet criteria for a SUD(42, 43). SUDs were defined using the standard diagnostic threshold of meeting two or more DSM criteria on the CIDI.
Oral Fluid Testing
The Intercept™ immunoassay (OraSure Technologies) provided an objective measure of point prevalence for the following drug classes: marijuana, cocaine, PCP, opiates, amphetamines, opiates, benzodiazepines, and barbiturates. It has a window of detection of up to 3 days for most drugs(44–46). To assist in interpretation of results, participants were assessed for medical use of medications that would be detected by the test. Oral fluid test results were not shared with participants, and were linked to the self-report data by the participant’s unique identifier.
Statistical Analysis
Concurrent validity of the interviewer-administered and self-administered versions of the TAPS Tool, in comparison to the reference standard CIDI, was assessed for the risk categories of problem use and SUD for each substance class. We calculated sensitivity, specificity, and positive and negative diagnostic likelihood ratios (LRs)(47), with exact 95% confidence intervals (CIs). There were 5 participants who did not complete the entire CIDI, and one participant who did not complete the entire interviewer-administered TAPS Tool. When the score for the CIDI or TAPS Tool was missing for a given substance, these cases were excluded from the analyses. For identification of problem use, we selected cutoffs that maximized sensitivity. For SUD, cutoffs were selected that were both higher than the cutoff for problem use and had highest sensitivity.
To assess for differences based on order of administration of the TAPS Tool, we examined differences in prevalence for each substance related to order of administration and instrument (self-administered vs interviewer-administered) using a generalized estimating equation (GEE) approach.(48, 49) A two-way crossed model was fit, and to account for the crossover nature of the data, an unstructured working correlation matrix was used under the GEE framework. The most important parameter of interest was the interaction between order and instrument. This variable was highly significant for alcohol, suggesting there is a difference in the participant responses to self-administered and interviewer-administered TAPS tool due to order of administration. Reporting of alcohol use was higher (38.6%) among participants who had the self-administered tool first, compared to participants who had the self-administered tool second (30.7%). For substances other than alcohol, analyses of diagnostic accuracy were conducted without regard to order of administration. For alcohol, analyses were also conducted separately, restricted to the first version received (see Supplementary Tables 3a and 3b). All analyses were conducted in SAS® version 9.3 or 9.4.
Role of the funding source
The study was supported by a National Institute on Drug Abuse (NIDA) Center for Clinical Trials Network (CCTN) Cooperative Agreement. Co-authors include NIDA CCTN staff (GS and CC), who contributed to protocol development including study design and methods, conduct, and the preparation of the manuscript.
RESULTS
Participants
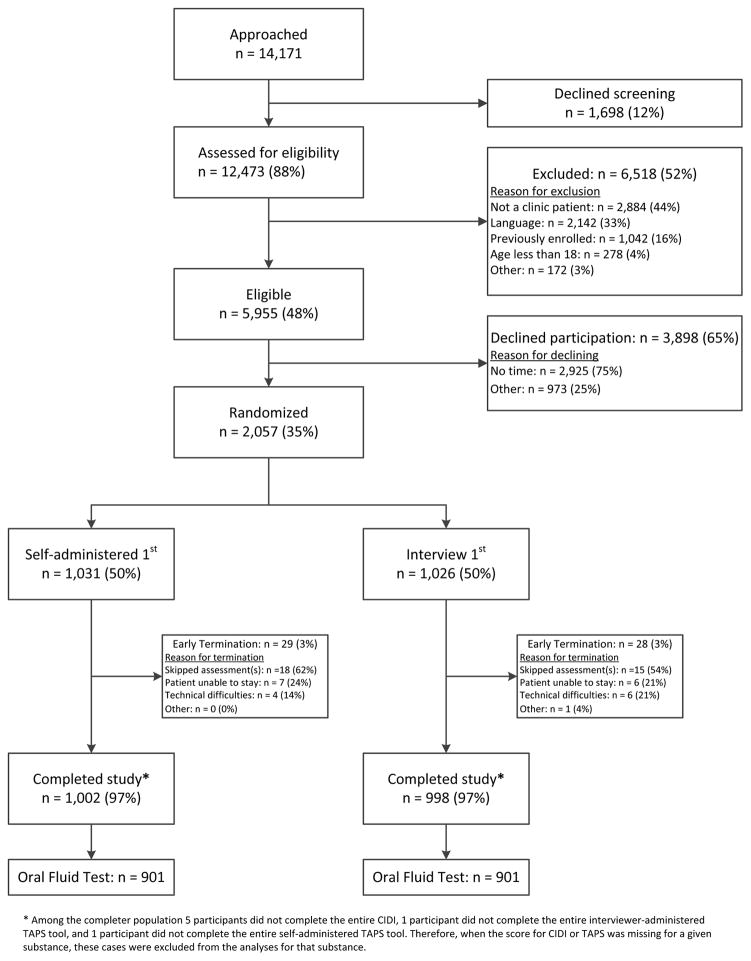
A total of 12,473 individuals were assessed for eligibility, of which 48% were eligible for participation (Figure 2). Among those eligible, 35% agreed to participate and were randomized. Of the 2,000 participants who completed the measures, 1,802 (90%) subsequently agreed to oral fluid testing.
Figure 2.
Recruitment summary
As shown in Table 1, the mean (SD) age of the participants was 46.0 (14.7) and more than half (56.2%) were women. The prevalence of past-year use, as reported on the modified CIDI, is shown in Table 2. Among the participants who provided a sample for oral fluid testing, point prevalence was 11.3% for illicit substances, and 5.0% for non-medical use of prescription medications.
Table 1.
Demographic characteristics of participants (N=2000)
| Characteristic | N (%) |
|---|---|
| Gender | |
| Male | 874 (43.7%) |
| Female | 1124 (56.2%) |
| Other/Refused | 2 (0.1%) |
| Age (years) at Enrollment | |
| Mean (SD) | 46.0 (14.7) |
| Range | 18–94 |
| Ethnicity | |
| Hispanic | 233 (11.7%) |
| Non-Hispanic | 1761 (88.1%) |
| Other/Refused | 6 (0.3%) |
| Race | |
| White | 667 (33.4%) |
| Black/African American | 1112 (55.6%) |
| Asian | 35 (1.8%) |
| Multiracial | 66 (3.3%) |
| Other* | 113 (5.7%) |
| Refused | 7 (0.4%) |
| Highest Completed Education Level | |
| Less than high school | 383 (19.2%) |
| High school graduate or GED | 578 (28.9%) |
| Some college | 426 (21.3%) |
| Associate’s degree | 224 (11.2%) |
| Bachelor’s degree | 279 (14.0%) |
| Graduate degree | 109 (5.5%) |
| Don’t know | 1 (0.1%) |
| Employment | |
| Employed | 712 (35.6%) |
| Unemployed | 419 (21.0%) |
| Disability | 472 (23.6%) |
| Retired | 172 (8.6% |
| Student | 118 (5.9%) |
| Other† | 107 (5.4%) |
Race-Other category refers to American Indian or Alaska Native (N=12), Unknown (N=32), and Other/not specified (N=69).
Employment-Other category refers to ‘keeping house’ (N=66) and Other/not specified (N=41).
Table 2.
Substance use prevalence, based on reference standard measures
| Past Year Use (from CIDI) N=2,000 N (%) |
Point Prevalence (from Oral Fluid Test) N=1802 N (%) |
|
|---|---|---|
| Substance | ||
| Tobacco | 882 (44.1%) | N/A |
| Alcohol | 1239 (62.0%) | N/A |
| Illicit substance(s)* | 511 (25.6%) | 204 (11.3%) |
| Prescription Medication(s)† | 147 (7.4%) | 91 (5.0%) |
| Substance Class | ||
| Marijuana | 416 (20.8%) | 103 (5.7%) |
| Cocaine | 145 (7.3%) | 85 (4.7%) |
| Methamphetamine | 14 (0.7%) | 4 (0.2%) |
| Heroin | 78 (3.9%) | 20 (1.1%) |
| Prescription Opioids | 96 (4.8%) | 90 (5.0%) |
| Sedatives | 82 (4.1%) | 19 (1.1%) |
| Prescription Stimulants | 23 (1.2%) | 2 (0.1%) |
| Combined Substance Categories | ||
| Opioids (Heroin or Prescription Opioids) | 145 (7.3%) | 93 (5.2%) |
| Stimulants (Cocaine, Methamphetamine, or Prescription Stimulants) | 161 (8.1%) | 90 (5.0%) |
| Drugs Other than Marijuana‡ | 265 (13.3%) | 181 (10.0%) |
Notes to Table 2
N=2,000 for the CIDI, and N=1802 for point prevalence because some participants declined to participate in the optional oral fluid test.
Includes marijuana, cocaine/crack, heroin, methamphetamine, hallucinogens, and inhalants
Includes prescription opioids, amphetamine-containing medications, and sedatives.
Includes all drug classes with the exception of marijuana, alcohol, and tobacco
Acceptability of the TAPS Tool
The vast majority (99%) of participants said they felt comfortable answering the TAPS Tool questions, and said they would be comfortable sharing the results with their doctor (95%). Participants differed in their preferences for interviewer-versus self-administered screening: 24% preferred the iPad, 31% preferred the interview, and 45% had no preference.
Comparison of Interviewer and Self-administered versions
Overall, the interviewer-administered and self-administered versions of the TAPS Tool performed similarly, and generated the same cutoffs for problem use and SUD. Because they generated similar results, only the interviewer-administered version is presented in Table 3, while data for the self-administered version is in Supplemental Table 2. Small differences in sensitivity were observed, but they did not follow a consistent pattern. Specificity was almost uniformly high across substance classes, for both versions.
Table 3.
Diagnostic accuracy of the interviewer-administered TAPS tool for identifying unhealthy use and substance use disorder (N=2000)
| True Positive (n) | False Negative (n) | True Negative (n) | False Positive (n) | Sensitivity (95% CI) | Specificity (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Risk Level: Problem use (1 or more items positive on CIDI) | ||||||||
| Tobacco (Cutoff=1) | 599 | 47 | 1174 | 179 | 0.93 (0.90, 0.95) | 0.87 (0.85, 0.89) | 7.01 (6.10, 8.05) | 0.08 (0.06, 0.11) |
| Alcohol (Cutoff=1) | 352 | 122 | 1198 | 327 | 0.74 (0.70, 0.78) | 0.79 (0.76, 0.81) | 3.46 (3.10, 3.86) | 0.33 (0.28, 0.38) |
| Marijuana (Cutoff=1) | 189 | 42 | 1637 | 128 | 0.82 (0.76, 0.87) | 0.93 (0.91, 0.94) | 11.28 (9.45, 13.47) | 0.20 (0.15, 0.26) |
| Cocaine, meth* (Cutoff=1) | 82 | 38 | 1855 | 20 | 0.68 (0.59, 0.77) | 0.99 (0.98, 0.99) | 64.06 (40.74, 100.73) | 0.32 (0.25, 0.42) |
| Heroin (Cutoff=1) | 54 | 15 | 1919 | 6 | 0.78 (0.67, 0.87) | 1.00 (0.99, 1.00) | 251.09 (111.86, 563.60) | 0.22 (0.14, 0.34) |
| Rx opiate† (Cutoff=1) | 42 | 17 | 1908 | 28 | 0.71 (0.58, 0.82) | 0.99 (0.98, 0.99) | 49.22 (32.93, 73.57) | 0.29 (0.20, 0.44) |
| Sedative‡ (Cutoff=1) | 26 | 15 | 1926 | 28 | 0.63 (0.47, 0.78) | 0.99 (0.98, 0.99) | 44.25 (28.64, 68.38) | 0.37 (0.25, 0.56) |
| Risk Level: Substance use disorder (2 or more items positive on CIDI) | ||||||||
| Tobacco (Cutoff=2) | 372 | 134 | 1332 | 161 | 0.74 (0.69, 0.77) | 0.89 (0.88, 0.91) | 6.82 (5.84, 7.96) | 0.30 (0.26, 0.34) |
| Alcohol (Cutoff=2) | 194 | 84 | 1466 | 255 | 0.70 (0.64, 0.75) | 0.85 (0.83, 0.87) | 4.71 (4.11, 5.40) | 0.35 (0.30, 0.42) |
| Marijuana (Cutoff=2) | 105 | 42 | 1764 | 85 | 0.71 (0.63, 0.79) | 0.95 (0.94, 0.96) | 15.54 (12.33, 19.58) | 0.30 (0.23, 0.39) |
| Cocaine, meth* (Cutoff=2) | 61 | 46 | 1873 | 15 | 0.57 (0.47, 0.67) | 0.99 (0.99, 1.00) | 71.76 (42.23, 121.94) | 0.43 (0.35, 0.54) |
| Heroin (Cutoff=2) | 43 | 22 | 1926 | 3 | 0.66 (0.53, 0.77) | 1.00 (1.00, 1.00) | 425.37 (135.50, 1335.39) | 0.34 (0.24, 0.48) |
| Rx opiate† (Cutoff=2) | 23 | 25 | 1941 | 6 | 0.48 (0.33, 0.63) | 1.00 (0.99, 1.00) | 155.49 (66.35, 364.39) | 0.52 (0.40, 0.69) |
| Sedative‡ (Cutoff=2) | 15 | 13 | 1947 | 20 | 0.54 (0.34, 0.72) | 0.99 (0.98, 0.99) | 52.69 (30.22, 91.86) | 0.47 (0.32, 0.70) |
Notes to Table 3. CI = Confidence Interval; LR=Likelihood Ratio; CIDI = Composite International Diagnostic Interview Composite Index; TAPS = Tobacco, Alcohol, Prescription medication, and other Substance use
There were 5 participants who did not complete the entire CIDI, and one participant who did not complete the entire interviewer-administered TAPS Tool. Therefore, when the score for CIDI or TAPS Tool is missing for a given drug, these cases were excluded from the analyses.
Described in TAPS as ‘Cocaine (crack) or methamphetamine’
Described in TAPS as ‘Prescription opiate pain reliever’
Described in TAPS as ‘Medication for anxiety or sleep’
Identification of problem use (Table 3)
The optimal cutoff score for problem use was 1+. At this cutoff, the interviewer-administered TAPS Tool had good sensitivity and specificity for identifying any problem use of tobacco (0.93 and 0.87, respectively) and alcohol (0.74 and 0.79, respectively). For illicit drugs, sensitivity ranged from 0.82 (marijuana) to 0.68 (cocaine). For non-medical use of prescription drugs, sensitivity was 0.71 for opiates and 0.63 for sedatives. The 95% confidence intervals were broad for substances with lower prevalence in the study population. Specificity for identifying problem use was high (0.93 or greater) for all illicit and prescription drug classes. Positive LRs ranged from 3.5 for tobacco to over 250 for heroin, while negative LRs ranged from 0.08 for tobacco to 0.37 for sedatives.
Identification of substance use disorder (Table 3)
The optimal cutoff score on the TAPS Tool for identifying SUD was 2+. A lower cutoff of 1+ had higher sensitivity for identifying SUD, but because this was the optimal cutoff for problem use, it was not selected. Conversely, a higher cutoff of 3 demonstrated high specificity for identifying SUDs, but was not selected due to unacceptably low sensitivity (<0.50). At a score of 2+, sensitivities were lower for identification of SUD than for problem use (although they were 0.70 or greater for tobacco, alcohol and marijuana), and specificities were no lower than 0.85. Positive LRs ranged from 4.7 to over 990, and negative LRs ranged from 0.30 to 0.52. The small number of participants with prescription stimulant use, (7 with problem use and 4 with SUD were identified on the CIDI), did not allow us to make meaningful estimates for this substance class.
DISCUSSION
This multi-site study of a substance use screening instrument found that for those substances that are most commonly used by primary care patients (i.e. tobacco, alcohol, and marijuana), the TAPS Tool has good sensitivity and specificity for identifying problem use. For substances that are less frequently encountered in primary care, sensitivity and specificity estimates were lower and less precise, and sensitivity for detection of SUD was unacceptably low. Thus, while the TAPS Tool cutoffs of 1+ for problem use and 2+ for SUD can be applied for alcohol, tobacco, and marijuana, for other drugs, any patient with a score of 1+ should be further assessed for presence of a SUD.
In comparison to other substance use screening instruments, the TAPS Tool has several characteristics that make it attractive for primary care. First, the TAPS Tool screens and assesses tobacco, alcohol, and all major drug classes in a single instrument. It has the potential to be easily integrated into regular clinical workflows, which in most settings have already been designed to screen for tobacco use (required under Meaningful Use Stage 2(50)). Second, the TAPS Tool gives substance-specific risk information, which is essential for ensuring patient safety, providing feedback and education, and guiding treatment decisions. Neither the widely-used Drug Abuse Screening Test (DAST-10)(25, 51) nor the newer Screen of Drug Use (SoDU)(29) provides this level of detail. Third, the TAPS Tool provides the option of a patient self-administered format, which can facilitate more accurate reporting of stigmatized behavior(52, 53), ensure fidelity of administration(54, 55), increase patient comfort(56), and reduce the burden on staff. It has the potential to be completed through a web-based patient portal, or on a kiosk or tablet computer in the clinic, that would upload screening results into the electronic health record.
In some practice settings, there may still be a role for very brief screeners (e.g. Substance Use Brief Screen (SUBS)(27), or single-item screening questions (SISQs) for alcohol and drugs(24–26)) to quickly identify patients with any unhealthy substance use. The four-item TAPS-1 screener could potentially accomplish this, and future reports will examine its performance as a stand-alone instrument. Yet, for the significant proportion of patients who are positive on a brief screener (28–29% in some studies(26, 27)), it is essential to have an efficient follow-up assessment that can risk-stratify patients and guide care. For alcohol, this can be accomplished with the AUDIT or AUDIT-C, which have been widely adopted(36, 43, 57, 58). There has been no similarly brief structured assessment tool for other drugs. Until now, the WHO ASSIST has been the only screening tool that provides substance-specific risk stratification for drugs, but its length and complexity has hindered its implementation in primary care settings(32, 59), (though a computer self-administered version could be more feasible(60)). The TAPS Tool streamlines the ASSIST to perform this assessment relatively quickly. Future research may explore whether the TAPS-2 could be simplified even further, either by reducing either the number of substances queried or the number of items.
The TAPS Tool has some shortcomings. Sensitivity was low for detecting problem use of some substances. It is possible that this reflects differences between the timeframe of our reference standard measure and the TAPS Tool. Although the TAPS Tool screens for use in the past 12 months (TAPS-1), the final score is based on use in the past 3 months (TAPS-2). As a result, the TAPS Tool could fail to identify individuals who had problem use in the past year that had not continued into the most recent 3-month period. However, by focusing on current use, the TAPS Tool identifies those patients who are most in need of clinical intervention, which is important in primary care settings where providers have multiple demands on their time. We have limited ability to draw comparisons with other instruments that screen for illicit and prescription drug use, because only the ASSIST, (and its shortened version the ASSIST-Lite), provide substance-specific results. Validation studies of the ASSIST have not reported its diagnostic accuracy at the WHO-recommended cutoffs, and were conducted in samples that included drug treatment and psychiatric patients(28, 61, 62). The ASSIST-Lite (on which the TAPS-2 is based) was developed from secondary analysis of data from the validation study of the full ASSIST(32), and its performance has not been previously assessed.
Performance of the TAPS Tool for detecting problem use and SUD was lowest for prescription medications, particularly on the self-administered version, but still compares favorably to an existing brief screening tool that specifically queries non-medical use of prescription drugs(27). The relatively low sensitivity of screening for this substance class could be due to confusion among participants about what constitutes non-medical use(63), poor comprehension, or question fatigue due to the length and complexity of these items on the TAPS-1. The variability in how non-medical use is described in the TAPS-1 versus the TAPS-2 could have confused some respondents. In practice, performance of the TAPS and similar screening tools may be further compromised by patients’ reluctance to disclose misuse of a medication to the physician who is prescribing it.
Interviewer-administered screening approaches can be challenging to implement in practice because they require staff time and training, and interviewers may modify the screening language in ways that compromise the tool’s accuracy(54, 64). Self-administered tools could help to promote disclosure of substance use(52, 53, 65–67), but this format may not be feasible in all practice settings. Self-administration on an iPad could be problematic in patients with low literacy or poor vision, though the audio guidance can help to address these barriers. Elderly patients may have difficulty using an iPad. Tablet computers are currently uncommon in primary care settings, and their use would require considerations for workflow, security, and hygiene.
Our study design has several limitations. Although our sample was large, for most drug classes we did not have enough participants with problem use or SUD to develop and then test the cutoffs in separate samples. The low prevalence of certain drugs also led to poor precision in some estimates, particularly with respect to identifying a SUD. RAs administering the CIDI were not blinded to participant responses on the interviewer-administered TAPS Tool, which could potentially bias the CIDI responses. Our analyses relied on self-reported substance use, which has consistently shown good accuracy in research studies(68–71), but nonetheless depends on accurate and truthful disclosure of use. Data were collected with an assurance of confidentiality and anonymity, as they have been for all prior studies examining the concurrent validity of substance use screening tools, to increase the accuracy of self-report. In clinical practice, where patients are aware that medical providers will view their screening results, the diagnostic accuracy of the TAPS Tool might be expected to differ(54, 56, 69, 72).
The TAPS Tool was developed and evaluated only in English, which may limit its application in some settings. Though we recruited from clinics that had geographic and demographic diversity, our participants may not be representative of patients seen in all primary care settings. In particular, we had a high proportion of African-Americans and individuals with lower levels of education in our sample. Many eligible patients declined to participate in the study, and we are unable to assess how their inclusion would have impacted the performance of the TAPS Tool. Our study also has notable strengths, including enrollment of a large and diverse sample of adult primary care patients, high rates of completion of the reference standard measures, and a rigorous approach to testing both a self-administered and interviewer-administered version of the TAPS Tool.
Conclusion
Having information about a patient’s substance use is essential for ensuring the quality and safety of medical care. This study supports the use of the TAPS Tool (at a cutoff of 1+) in screening primary care patients for problem substance use. It may also detect alcohol, tobacco, and marijuana use disorders, although further refinement is needed before it can be broadly recommended as a screener for SUD. Because it identifies problem use of all commonly used substances with a limited number of questions, and has the flexibility to be either self-administered or completed as an interview, the TAPS Tool has the potential to ease barriers to incorporating substance screening into busy clinical environments.
Supplementary Material
Acknowledgments
Funding Source: National Institute on Drug Abuse cooperative grant awards: UG1DA013034; U10DA013727 and UG1DA040317; UG1DA013035.
The authors would like to acknowledge the contributions of Robert Ali and John Marsden, who developed the ASSIST-Lite instrument. We also express our appreciation for the work done by members of the study team: Saima Mili, Phoebe Gauthier, Sarah Farkas, Patsy Novo, Laura McElherne, Kimberly Micki Roseman, Carla Kingsbury, Melissa Johnston, Eve Jelstrom, Patrice Yohannes, Jack Chally, Paul Van Veldhuisen, Anne Hoehn, Lauren Yesko, and Alex Borbely.
Footnotes
Reproducible Research Statement: Available from Dr. McNeely (Jennifer.McNeely@nyumc.org)
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Disclosures: The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest to disclose.
Author contributions:
J. McNeely was Co-Lead Investigator and made substantial contributions to study conception and design, data collection, analysis, and interpretation, and led the preparation and writing of the manuscript.
L.T. Wu was Co-Lead Investigator and made substantial contributions to study conception and design, data collection, analysis, and interpretation, as well as participating in the writing of the manuscript.
G.A. Subramaniam was the Scientific Officer at the NIDA CCTN and contributed to study conception and design, data collection, analysis, and interpretation, as well as participating in the writing of the manuscript.
G. Sharma was the study statistician and led the data analysis, contributed to study design, and participated in the writing of the manuscript.
L. Cathers and D. Svikis led the study at the Virginia Commonwealth University site, and assisted with writing and reviewing the manuscript.
L. Sleiter participated in drafting and revising the manuscript, and assisted in data collection.
L. Russell, C. Nordeck, A. Sharma, and L. Bouk made significant contributions to data collection and reviewed and suggested edits to the manuscript.
K.E. O’Grady contributed to study design, analysis, and drafting and revision of the manuscript.
C. Cushing was Co-NIDA protocol coordinator and contributed to study conception and design, and revision of the manuscript.
J. King and A. Wahle contributed to the data analysis, and reviewed and contributed to the manuscript.
R.P. Schwartz was Lead Investigator and made substantial contributions to study conception and design, data collection, analysis, and interpretation, and led the writing of the manuscript.
All authors approved of the final version of the manuscript and agree to be accountable for all aspects of the work.
Registration: ClinicalTrials.gov # NCT 02110693
References
- 1.U. S. Department of Health and Human Services; U. S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health, editor. A report of the Surgeon General. Atlanta, GA: 2014. The health consequences of smoking: 50 years of progress. [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.De Cock KM, Jaffe HW, Curran JW. The evolving epidemiology of HIV/AIDS. AIDS. 2012;26(10):1205–13. doi: 10.1097/QAD.0b013e328354622a. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention; Prevention CfDCa, editor. Morbidity and Mortality Weekly Report. Atlanta, GA: U. S. Department of Health and Human Services; 2012. CDC grand rounds: Prescription drug overdoses - a U.S. epidemic; pp. 10–3. [PubMed] [Google Scholar]
- 5.Centers for Medicare and Medicaid Services (CMS) 2014 Clinical Quality Measures (CQMs): Adult Recommended Core Measures. 2014. [Google Scholar]
- 6.Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31(1):52–61. doi: 10.1016/j.amepre.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;159(3):210–18. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- 8.Solberg LI, Maciosek MV, Edwards NM. Primary care intervention to reduce alcohol misuse ranking its health impact and cost effectiveness. Am J Prev Med. 2008;34(2):143–52. doi: 10.1016/j.amepre.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(7):557–68. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- 10.Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312(5):502–13. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitz R, Alford DP, Bernstein J, Cheng DM, Samet J, Palfai T. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4(3):123–30. doi: 10.1097/ADM.0b013e3181db6b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Preventive Services Task Force. Screening for Illicit Drug Use: U.S. Preventive Services Task Force Recommendation Statement. 2008. [Google Scholar]
- 14.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103(8):1242–57. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 16.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598–613. doi: 10.1345/aph.1A447. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey WT, Stewart D, Childress D. Drug Interactions between Common Illicit Drugs and Prescription Therapies. American Journal of Drug and Alcohol Abuse. 2012;38(4):334–43. doi: 10.3109/00952990.2011.643997. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Unintentional poisoning deaths--United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2007;56(5):93–6. [PubMed] [Google Scholar]
- 20.Lanier D, Ko S. Screening in Primary Care Settings for Illicit Drug Use: Assessment of Screening Instruments: A Supplemental Evidence Update for the U.S. Preventive Services Task Force. Rockville (MD): 2008. [PubMed] [Google Scholar]
- 21.Wu LT, Blazer DG, Woody GE, Burchett B, Yang C, Pan JJ, et al. Alcohol and drug dependence symptom items as brief screeners for substance use disorders: results from the Clinical Trials Network. J Psychiatr Res. 2012;46(3):360–9. doi: 10.1016/j.jpsychires.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu LT, Swartz MS, Pan JJ, Burchett B, Mannelli P, Yang C, et al. Evaluating brief screeners to discriminate between drug use disorders in a sample of treatment-seeking adults. Gen Hosp Psychiatry. 2013;35(1):74–82. doi: 10.1016/j.genhosppsych.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghitza UE, Wu LT, Tai B. Integrating substance abuse care with community diabetes care: implications for research and clinical practice. Subst Abuse Rehabil. 2013;4:3–10. doi: 10.2147/SAR.S39982. Epub 2013 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24(7):783–8. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A Single-Question Screening Test for Drug Use in Primary Care. Archives of Internal Medicine. 2010;170(13):1155–60. doi: 10.1001/archinternmed.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeely J, Cleland CM, Strauss SM, Palamar JJ, Rotrosen J, Saitz R. Validation of Self-Administered Single-Item Screening Questions (SISQs) for Unhealthy Alcohol and Drug Use in Primary Care Patients. J Gen Intern Med. 2015 doi: 10.1007/s11606-015-3391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeely J, Strauss SM, Saitz R, Cleland CM, Palamar JJ, Rotrosen J, et al. A Brief Patient Self-administered Substance Use Screening Tool for Primary Care: Two-site Validation Study of the Substance Use Brief Screen (SUBS) Am J Med. 2015 doi: 10.1016/j.amjmed.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103(6):1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 29.Tiet QQ, Leyva YE, Moos RH, Frayne SM, Osterberg L, Smith B. Screen of Drug Use: Diagnostic Accuracy of a New Brief Tool for Primary Care. JAMA Intern Med. 2015 doi: 10.1001/jamainternmed.2015.2438. [DOI] [PubMed] [Google Scholar]
- 30.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32(2):189–98. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 31.National Institute on Drug Abuse (NIDA) Screening for Drug Use in Medical Settings. National Institutes of Health; 2010. [Google Scholar]
- 32.Ali R, Meena S, Eastwood B, Richards I, Marsden J. Ultra-rapid screening for substance-use disorders: The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST-Lite) Drug and Alcohol Dependence. 2013;132(1–2):352–61. doi: 10.1016/j.drugalcdep.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Campanelli P. Testing survey questions: New directions in cognitive interviewing. Bulletin de Methodologie Sociologique. 1997;55:5–17. [Google Scholar]
- 34.Willis G, Royston P, Bercini D. The use of verbal report methods in the development and testing of survey questionnaires. Applied Cognitive Psychology. 1991;5:251–67. [Google Scholar]
- 35.Willis G, Schechter S. Evaluation of cognitive interviewing techniques: do the results generalize to the field? Bulletin de Methodologie Sociologique. 1997;55:40–66. [Google Scholar]
- 36.National Institute on Alcohol Abuse and Alcoholism (NIAAA) Helping patients who drink too much: A clinician’s guide. 2005. National Institute on Alcohol Abuse and Alcoholism (NIAAA); [Google Scholar]
- 37.Forman RF, Svikis D, Montoya ID, Blaine J. Selection of a substance use disorder diagnostic instrument by the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2004;27(1):1–8. doi: 10.1016/j.jsat.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compton WM, Cottler LB, Dorsey KB, Spitznagel EL, Mager DE. Comparing assessments of DSM-IV substance dependence disorders using CIDI-SAM and SCAN. Drug Alcohol Depend. 1996;41(3):179–87. doi: 10.1016/0376-8716(96)01249-5. [DOI] [PubMed] [Google Scholar]
- 39.Cottler LB. Composite International Diagnostic Interview - Substance Abuse Module (SAM) St. Louis, MO: Department of Psychiatry, Washington University School of Medicine; 2000. [Google Scholar]
- 40.Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br J Addict. 1989;84(7):801–14. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- 41.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45(12):1069–77. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 42.Gryczynski J, Kelly SM, Mitchell SG, Kirk A, O’Grady KE, Schwartz RP. Validation and performance of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) among adolescent primary care patients. Addiction. 2015;110(2):240–7. doi: 10.1111/add.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 44.Cone EJ, Presley L, Lehrer M, Seiter W, Smith M, Kardos KW, et al. Oral fluid testing for drugs of abuse: positive prevalence rates by Intercept immunoassay screening and GC-MS-MS confirmation and suggested cutoff concentrations. J Anal Toxicol. 2002;26(8):541–6. doi: 10.1093/jat/26.8.541. [DOI] [PubMed] [Google Scholar]
- 45.Heltsley R, DePriest A, Black DL, Robert T, Marshall L, Meadors VM, et al. Oral fluid drug testing of chronic pain patients. I. Positive prevalence rates of licit and illicit drugs. J Anal Toxicol. 2011;35(8):529–40. doi: 10.1093/anatox/35.8.529. [DOI] [PubMed] [Google Scholar]
- 46.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10(4):607–12. doi: 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- 47.Pepe MS. The statistical evaluation of medical tests for classification and prediction. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 48.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 49.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 50.Department of Health and Human Services. Medicare and Medicaid Programs; Electronic Health Record Incentive Program - Stage 2. Federal Register. 2012;77(171) [PubMed] [Google Scholar]
- 51.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 52.Tourangeau R, Smith TW. Asking sensitive questions - The impact of data collection mode, question format, and question context. Public Opinion Quarterly. 1996;60(2):275–304. [Google Scholar]
- 53.Wight RG, Rotheram-Borus MJ, Klosinski L, Ramos B, Calabro M, Smith R. Screening for transmission behaviors among HIV-infected adults. Aids Education and Prevention. 2000;12(5):431–41. [PubMed] [Google Scholar]
- 54.Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, et al. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med. 2011;26(3):299–306. doi: 10.1007/s11606-010-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams EC, Achtmeyer CE, Rittmueller SE, Lapham GT, Chavez LJ, Thomas RM, et al. Factors underlying quality problems with alcohol screening in routine care. Addiction Science & Clinical Practice. 2013;8(Suppl 1):A85. [Google Scholar]
- 56.Spear SESM, Gilberti B, Fiellin M, McNeely J. Feasibility and acceptability of an audio computer-assisted self-interview version of the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) in primary care patients. Subst Abus. 2015 doi: 10.1080/08897077.2015.1062460. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, et al. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163(7):821–9. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 58.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31(2):185–99. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 59.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 60.McNeely J, Strauss SM, Rotrosen J, Ramautar A, Gourevitch MN. Validation of an audio computer assisted self interview (ACASI) version of the alcohol, smoking and substance involvement screening test (ASSIST) in primary care patients. Addiction. 2015 doi: 10.1111/add.13165. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hides L, Cotton SM, Berger G, Gleeson J, O’Donnell C, Proffitt T, et al. The reliability and validity of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in first-episode psychosis. Addict Behav. 2009;34(10):821–5. doi: 10.1016/j.addbeh.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24(3):217–26. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- 63.McNeely J, Halkitis PN, Horton A, Khan R, Gourevitch MN. How patients understand the term “nonmedical use” of prescription drugs: insights from cognitive interviews. Subst Abus. 2014;35(1):12–20. doi: 10.1080/08897077.2013.789463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams EC, Achtmeyer CE, Rittmueller SE, Lapham GT, Chavez LJ, Thomas RM, et al. Factors underlying quality problems with alcohol screening in routine care. Addiction Science & Clinical Practice. 2013;8(Suppl 1):A85. [Google Scholar]
- 65.Des Jarlais DC, Paone D, Milliken J, Turner CF, Miller H, Gribble J, et al. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: a quasi-randomised trial. Lancet. 1999;353(9165):1657–61. doi: 10.1016/s0140-6736(98)07026-3. [DOI] [PubMed] [Google Scholar]
- 66.Rogers SM, Miller HG, Turner CF. Effects of interview mode on bias in survey measurements of drug use: do respondent characteristics make a difference? Subst Use Misuse. 1998;33(10):2179–200. doi: 10.3109/10826089809069820. [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Dubowitz H, Hudson-Martin E, Lane W. Comparison of 3 data collection methods for gathering sensitive and less sensitive information. Ambul Pediatr. 2008;8(4):255–60. doi: 10.1016/j.ambp.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 68.Babor TF, Brown J, Delboca FK. Validity of Self-Reports in Applied-Research on Addictive Behaviors - Fact or Fiction. Behavioral Assessment. 1990;12(1):5–31. [Google Scholar]
- 69.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 70.Hser YI. Self-reported drug use: results of selected empirical investigations of validity. NIDA Res Monogr. 1997;167:320–43. [PubMed] [Google Scholar]
- 71.Secades-Villa R, Fernandez-Hermida JR. The validity of self-reports in a follow-up study with drug addicts. Addict Behav. 2003;28(6):1175–82. doi: 10.1016/s0306-4603(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 72.Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327–33. doi: 10.1046/j.1525-1497.2002.10625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.