Abstract
A better knowledge on how immune responses are initiated in mucosal tissues would facilitate the design of new mucosal vaccines, as well as improve our understanding on host defense against infection. We investigated the mechanisms of adjuvanticity of the Mycoplasma-derived macrophage-activating 2-kDa lipopeptide (MALP-2), which binds to the heterodimer formed by the Toll-like receptors 2 and 6 (TLR2 and -6), at the level of the murine nasal mucosa-associated lymphoid tissues (NALT). TLR2 expression analysis demonstrated that several cell types from the nasal cavity were able to overexpress this receptor, either constitutively (such as B cells) or after stimulation (i.e., T cells). MALP-2 stimulated a strong B-cell activation. In addition, the antigen presentation capacity of dendritic cells was improved after in vivo loading with antigen in the presence of MALP-2. We also observed an up-regulated expression of activation markers and adhesion molecules on T cells, suggesting that they have enhanced responsiveness and interaction potential. Quantitative reverse transcription-PCR analysis showed that MALP-2 administration resulted in the stimulation of a proinflammatory cascade. We observed an early up-regulated expression of IP-10, MCP-1, MCP-3, MIP-1α, MIP-2, and CCR-2 which was reversed within 36 h. The obtained results demonstrated that MALP-2 creates a reversible local microenvironment which promotes effective priming of T and B cells in the NALT.
The ability of a vaccine to induce protective immunity requires not only an efficient presentation of antigenic epitopes, but also the capacity to promote an adequate immune stimulation. In this context, the improved understanding of the interactions between the innate and adaptive immune systems is having a major impact in the field of vaccinology. As a matter of fact, the innate immune system does not only provide the first line of defense against infection, but also determines the nature of acquired immune responses (14). Danger signals (e.g., microorganisms) are recognized, leading to the recruitment and activation of immune cells. The stimulation of local mucosal immune responses after vaccination is thought to facilitate infection control by preventing microbial colonization. However, since the mucosal epithelium constitutes a natural barrier, antigens administered by this route are usually poorly immunogenic. One of the potential strategies to overcome this problem is their coadministration with mucosal adjuvants. On the other hand, the body is also confronted with potentially harmful antigens from the environment and/or food at the mucosal barrier. Thus, the local immune system plays a critical role in the tight balance between tolerance and immune responsiveness.
The use of a particular adjuvant can strongly influence the quality of the immune response elicited. However, it is difficult to predict the type of immune response that will be induced by a given adjuvant, since not all adjuvants behave in a similar manner in a given mucosal site. Thus, it is crucial to understand the underlying mechanisms to the adjuvanticity of these molecules. The designation as mucosal adjuvant has been quite largely attributed to and covers a variety of structural molecules (35). The definition of the chemical nature of these immune stimulants is an essential parameter for the identification of their potential receptors, as well as for the better understanding of their mechanism of action. Recently, our investigators have demonstrated that the Mycoplasma-derived macrophage-activating lipopeptide of 2 kDa (MALP-2) is able to enhance mucosal and systemic immune responses against coadministered antigens (4, 30). MALP-2 was shown to induce leukocyte infiltration and activation of macrophages (7, 15) upon engagement of the heterodimer formed by the Toll-like receptors 2 and 6 (TLR2 and -6) (24, 28).
Thus, MALP-2 is an attractive immune stimulatory molecule to unravel the cascade leading to an improved antigen-specific response through activation of the innate immune system. Here, we evaluated the influence of MALP-2 in the activation of immune effector cells at the level of an antigen-exposed and tolerogenic inductive site, namely, the nasal mucosa-associated lymphoid tissues (NALT) (1, 38). The obtained results demonstrated that MALP-2 acts in vivo as a proinflammatory stimulus, leading to synergistic effects on dendritic cells, macrophages, B cells, and T cells.
MATERIALS AND METHODS
Animals and cell cultures.
Six- to 8-week-old female BALB/c (H-2d) mice were purchased from Harlan-Winkelmann (Germany), whereas DO11.10 (26), TCR-HA, and Ins-HA (16, 34) mice were bred at the GBF animal facility. The animals were maintained in accordance with local and European Community guidelines. DO11.10 mice are transgenic for a T-cell receptor (TCR) recognizing the ovalbumin (OVA)-derived peptide 323-339 (SQAVHAAHAEINEAGR) (26). TCR-HA transgenic mice express an α/β (Vβ6.5) TCR specific for the peptide 111-119 from the hemagglutinin of the influenza virus (A/PR8/34) in the context of I-Ed class II molecules (16). TCR-HA mice were back-crossed onto Ins-HA mice, which express the hemagglutinin (HA) protein in pancreatic β cells under the control of the rat insulin promoter (2). Transgene expression was determined by analyzing tail DNA by PCR using TCR-Vβ- and HA-specific primers, as already described (34). The mice used were heterozygous for both transgenes. All cells were grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 100 U of penicillin/ml, 50 mg of streptomycin/ml, 5 × 10−5 M 2-mercaptoethanol, and 1 mM l-glutamine (GIBCO-BRL, Karlsruhe, Germany) and maintained at 37°C in a humidified 5% CO2 atmosphere.
Intranasal administration of MALP-2 and NALT preparation.
BALB/c mice (n = 12) received 20 μl of synthetic MALP-2 (0.5 μg) in phosphate-buffered saline (PBS) or PBS only by intranasal route (25). Animals were sacrificed immediately following the administration or after 2, 6, 16, 24, or 36 h. Then, cells from NALT were recovered for RNA isolation, antigen presentation assays, or flow cytometry as previously described (11, 12, 41). Briefly, after exsanguination of the mice and isolation of cervical lymph nodes and spleen, the head and the foreteeth were cut off. The facial skin, lower jaw, and cheek muscles were removed, and the NALT were exposed by carefully peeling away the palate. Individual NALT were removed by microsurgical tweezers under a stereoscopic microscope and placed in ice-cold RPMI-10% FCS. The lymphoid cells were dissociated by teasing with a syringe plunger through a 100-μm nylon mesh. Red blood cells were lysed in ACK buffer, and cellular suspensions were filtered through a 40-μm mesh. Cell suspensions from cervical lymph nodes and spleens were obtained following the same process.
Cell division assays.
Total spleen cells were labeled with 0.5 or 2 μM 5-(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Molecular Probes, Eugene, Oreg.) for 5 min at 37°C, as described by Lyons et al. (19). Stained cells (2 × 106 cells per well in 800 μl of RPMI) were then cultured in the presence of concanavalin A (ConA; 0.15 to 10 μg/ml), lipopolysaccharide (LPS; 0.31 to 20 μg/ml), or MALP-2 (0.015 to 1 μg/ml) in a 24-well plate. Alternatively, peritoneal macrophages (105 per well) were incubated overnight and washed twice from the nonadherent cells prior to culture with CFSE-labeled spleen cells. After 4 days of culture, cells were harvested and cell division was analyzed by flow cytometry. Cells were stained with phycoerythrin (PE)-conjugated anti-CD3 and anti-CD19-peridinin-chlorophyll a-protein complex (PerCP) antibodies (BD PharMingen, San Diego, Calif.) prior to acquisition for identification of dividing cells.
Analysis by flow cytometry.
Spleen (5 × 105) or NALT (2 × 105) cells were first incubated with mouse Fc block (BD PharMingen) in PBS-1% FCS for 1 h at 4°C. Then, cells were stained for 30 min at 4°C with specific antibodies conjugated with fluorescein isothiocyanate, PE, or PerCP from BD PharMingen. TLR2 expression was detected after cell permeabilization by using a biotinylated anti-TLR2 monoclonal antibody from HyCult Biotechnology (clone 6C2). Irrelevant labeled antibodies were used as isotype controls in all experiments. For intracellular staining, cells were first fixed in 2% paraformaldehyde at 4°C for 30 min, washed in PBS, and permeabilized for 30 min at 4°C with 0.5% saponin in PBS-1% FCS. Antibodies and streptavidin-allophycocyanin were further incubated in PBS-1% FCS-0.5% saponin. The cells were phenotypically characterized using a FACSort or FACSCalibur flow cytometer and the CellQuest-Pro software (Becton Dickinson, Mountain View, Calif.). NALT lymphocytes were gated according to the physical characteristics and forward and side scatters of splenic lymphocytes. A minimum of 20,000 gated events were used for the analysis.
Quantitative RT-PCR.
Total RNA was extracted from nasal tissues (12 mice per time point), using the TRIzol reagent according to the manufacturer's protocol (Gibco BRL, Life Technologies, Gaithersburg, Md.). RNA samples were subsequently treated with 2 μl of DNase (Promega) for 30 min at 37°C. A one-step reverse transcription-PCR (RT-PCR) was performed using LightCycler-RNA Master SYBR Green I apparatus, following the recommendations of the producer (Roche). PCRs without RT were performed to control RNA quality for all samples by using the LightCycler-DNA SYBR Green I. Each RT-PCR run included a negative control (water) and amplification of β-actin as housekeeping gene. The primers used are indicated in Table 1. Data analysis was performed using the LightCycler software (version 3.5). The specificity of the RT-PCR was controlled by analysis of the melting curves in comparison to the water sample. To ensure that the amplified fragments had the correct size, all products were run in a 2% agarose gel containing ethidium bromide and visualized under UV illumination. A preliminary relative quantification of the specific mRNA of the tested products was obtained by conversion of the fluorescence signal, using a standard curve based on β-actin amplification. Then, the specific mRNA level was standardized on the β-actin amount for each sample, thereby giving a final relative quantification. Quantifiable specific products were expressed over a range of at least 4 orders of magnitude, between 0.01 and 20% of the β-actin level. Where possible, the results were finally expressed as relative amount with respect to the untreated control group (nonactivated cells).
TABLE 1.
Oligonucleotide primers used for RT-PCR analysis
| Target | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product length (bp) |
|---|---|---|---|
| β-Actin | TGGAATCCTGTGGCATCCATGAAAC | TAAAACGCAGCTCAGTAACAGTCCG | 348 |
| IP-10 | CTCTCCATCACTCCCCTTTACCC | GCTTCGGCAGTTACTTTTGTCTCA | 150 |
| MIP-1α | CTGCCCTTGCTGTTCTTCTCTGTA | GATCTGCCGGTTTCTCTTAGTCA | 200 |
| MIP-2 | ACCCTGCCAAGGGTTGACTTC | GGCACATCAGGTACGATCCAG | 285 |
| MCP-1 | CTCACCTGCTGCTACTCATTC | GCTTGAGGTGGTTGTGGAAAA | 317 |
| MCP-3 | GTGCCTGAACAGAAACCAACCT | CATTCCTTAGGCGTGACCATT | 146 |
| CCR2 | TGTTACCTCAGTTCATCCACGG | CAGAATGGTAATGTGAGCAGGAAG | 316 |
| CCR5 | GGAGAACGCCCCCAATAAT | CAACACTGCTCCGAAACTGC | 179 |
| CCR6 | GGAGCTGCAGCACCGAGAAAGT | AGTAAACCCGGGCACAGAGGAAG | 201 |
| CCR7 | TCCCGGAGATTCAAGACAGA | ATTCCCATCATAGAGACCCAAACT | 210 |
| CCR9 | GAGGGCAGGAAAAACAAAACAAAC | CTGCTGCGCTTCTGGAGTGC | 111 |
Cell purification.
CD4+ T cells were purified from the spleens of BALB/c or DO.11 mice by using the CD4+ T-cell isolation kit from Miltenyi, as previously described (10). T-cell preparations demonstrated a purity of >96% by fluorescence-activated cell sorter analysis. Positive selection of dendritic and B cells from NALT was conducted using CD11c microbeads (Miltenyi Biotech) and B220 dynabeads (Dynal, Oslo, Norway), in accordance with the manufacturers' instructions.
Antigen presentation assays.
To investigate the effects of MALP-2 on antigen presentation, peritoneal macrophages seeded at a density of 2 × 104 cells/well in flat-bottom 96-well microtiter plates (Nunc) were incubated with or without MALP-2 (0.5 μg/ml) in the presence of the OVA323-339 peptide (SQAVHAAHAEINEAGR) for 16 h. Each concentration of peptide was tested in triplicate. Then, cells were washed twice with medium before adding OVA-TCR-specific CD4+ T cells prepared from the spleens of DO11.10 mice (2 × 105/well). To investigate the in vivo-induced effect of MALP-2, NALT B cells and dendritic cells isolated from mice that received 200 μg of the OVA peptide or 10 mg of OVA protein (purity, ≥98%; Scripps Laboratories) coadministered with either PBS or 0.5 μg of MALP-2 were cocultured with the OVA-specific T cells obtained from DO11.10 mice. Enriched dendritic cells were washed and seeded at an estimated concentration of 104 cells per well in quadruplicate, and B cells were seeded at 105 per well. T-cell proliferation was measured after pulsing with 1 μCi of [3H]thymidine for 16 h. The results are expressed as the arithmetic mean of [3H]thymidine uptake, in counts per minute.
Measurement of glucose levels.
Glucose levels in mouse urine were determined by using Diabur-Test 5000 strips (Roche, Mannheim, Germany) and confirmed by blood glucose measurements using Haemo Glukotest 200-800R (Roche). Mice were considered diabetic when the blood glucose level was >200 mg/dl for three consecutive measurements.
Statistical analysis.
Comparisons between two experimental groups were performed by using a double-sided Student's t test. A P value of <0.05 was considered significant. The statistical comparison of the data generated by flow cytometric analysis and displayed as histograms was performed using the Kolmogorov-Smirnov test from the CellQuest-Pro software (Becton-Dickinson).
RESULTS
MALP-2 leads to strong B-cell activation in vitro.
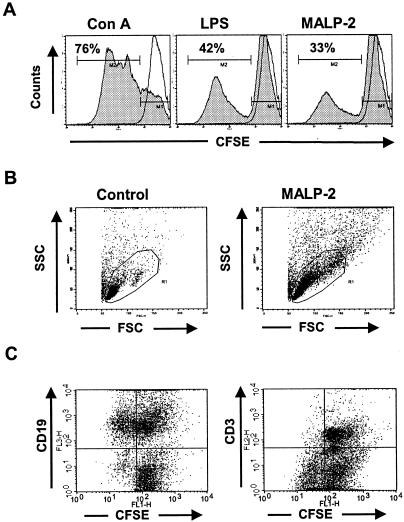
The stimulatory capacity of MALP-2 was initially evaluated on spleen cells in comparison to that of ConA and LPS, as T-cell and B-cell reference activators, respectively. When CFSE-labeled cells were stimulated, a dose-dependent response was observed for all three activators (data not shown). After 4 days of culture, the highest percentage of cells undergoing division was reached at a concentration of 5, 10, and 0.5 μg/ml for ConA, LPS, and MALP-2, respectively (Fig. 1A). In comparison to ConA (76%), fewer dividing cells were observed upon LPS (42%) or MALP-2 (33%) stimulation. However, high division levels were observed upon LPS or MALP-2 stimulation, as measured by the CFSE intensity of dividing cells. The side scatter and forward scatter analysis showed cells with increased granularity and size, which correspond to activated lymphocytes (Fig. 1B). Specific staining using anti-CD3- and anti-CD19-conjugated antibodies demonstrated that only B cells divided upon MALP-2 stimulation (Fig. 1C). Similar results were obtained with the addition of peritoneal macrophages (data not shown), suggesting that MALP-2 exerts a direct mitogenic effect on B cells, thereby leading to a larger pool of B cells at the site of administration.
FIG. 1.
Cell division analysis of CFSE-labeled splenic cells by flow cytometry. (A) Division profile showing the decrease of FL1 fluorescence resulting from the repartition of the CFSE dye after 4 days in culture in the presence of ConA (5 μg/ml), LPS (10 μg/ml), or MALP-2 (0.5 μg/ml) in comparison to that in nonactivated cells (solid line). (B) Morphological changes (side scatter [SSC]granularity and foreward scatter [FSC] size) of splenic cells after 16 h in culture in the presence of MALP-2 (0.5 μg/ml) compared to control cells. (C) CFSE-labeled dividing cells were analyzed by three-color staining after MALP-2 stimulation, using anti-CD3-PE and anti-CD19-PerCP antibodies. Results are representative of three independent experiments.
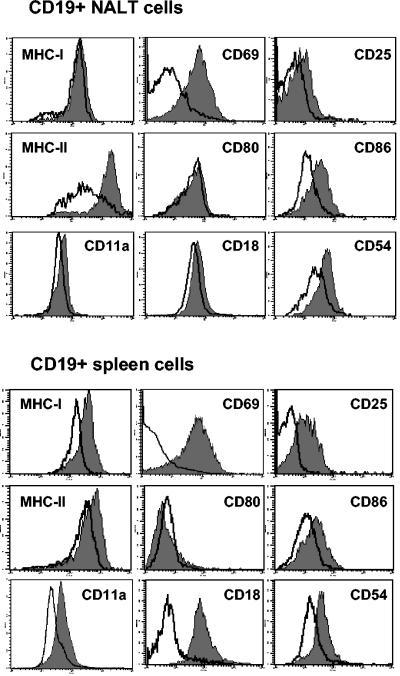
Interestingly, the analysis of surface expression on CD19+ B cells from NALT cultured in the presence of MALP-2 demonstrated an up-regulation of several activation markers (Fig. 2): major histocompatibility complex (MHC) class II (mean fluorescence intensity [MFI], 1,644 versus 585), CD69 (MFI, 69 versus 14), CD25 (MFI, 20 versus 9), CD86 (MFI, 34 versus 15), CD11a (MFI, 52 versus 38), CD18 (MFI, 55 versus 44), CD54 (MFI, 61 versus 28), and CD40 (MFI, 32 versus 18). A similar analysis performed with splenocytes showed a comparable activation of B cells in terms of surface markers expressed (Fig. 2). Some differences were observed in basal expression levels and activation intensity (Fig. 2). However, these results clearly demonstrated that B cells from NALT and spleens are both responsive to activation with MALP-2. A kinetic analysis performed with splenic cells demonstrated that CD40, CD86, and CD54 were already up-regulated after 6 h of culture in the presence of MALP-2 (data not shown). LPS activation led to a similar up-regulation of activation markers on cultured spleen cells after 16 h (data not shown).
FIG. 2.
Phenotypic characterization of B cells upon MALP-2 activation. NALT cells (upper panels) or splenic cells (lower panels) cultured in the presence of MALP-2 (0.5 μg/ml) during 16 h were analyzed by flow cytometry gating on the CD19+ cells. The surface expression of activation markers was compared in MALP-2-treated cells (gray histogram) and nonstimulated cells (solid line). Results are representative of three independent experiments.
In vivo activation of lymphocytes upon MALP-2 stimulation.
To evaluate B- and T-cell in situ activation upon MALP-2 administration, a fluorescence-activated cell sorter analysis was performed using NALT cells isolated from BALB/c mice 16 to 18 h after nasal administration of 0.5 μg of MALP-2. The cell preparations from NALT of naïve BALB/c mice contained 46.2% ± 9.8% mature B cells and 16.1% ± 3.5% mature T cells (with a CD4/CD8 ratio of 4:1), according to the forward- and side-scatter setting used for spleen lymphocytes and the specific staining for CD19, CD4, and CD8. No significant expansion of T or B cells was observed 16 to 18 h after administration of MALP-2. However, an increment in cell size was evident at this early time point, as already observed in in vitro studies (data not shown).
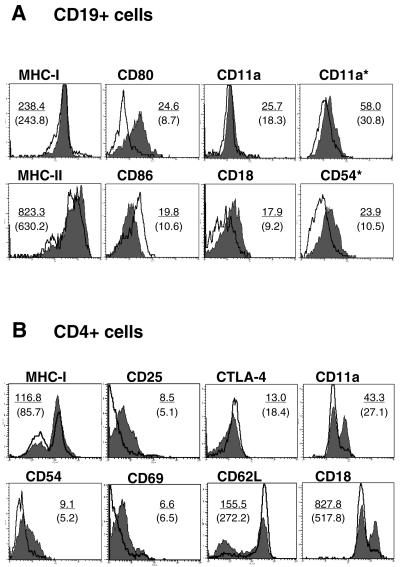
Then, surface markers were evaluated by flow cytometry gating on CD19+ B cells from NALT (Fig. 3A). In contrast to the in vitro data, no remarkable changes were observed in the surface expression of MHC class I, CD40, CD25, and CD54 after nasal administration of MALP-2. However, the expression of the costimulatory molecule CD80 showed a significant increment on cells isolated from NALT of MALP-2-treated animals (Fig. 3A). This is very important, since B7 molecules, which include B7-1 (CD80) and B7-2 (CD86), play an important role in antigen presentation by providing critical costimulatory signals for T-cell activation (22). Concerning the adhesion molecules, an increment in the surface expression of CD18 was noticed (Fig. 3A). In contrast, the increment in CD11a and CD54 expression was only evident at an intracellular level (Fig. 3A).
FIG. 3.
Phenotypic characterization of B and T cells from NALT after intranasal administration of MALP-2. In vivo-activated CD19+ B cells (upper panel) and CD4+ T cells (lower panel) isolated from NALT of mice 16 to 18 h after administration of MALP-2 (0.5 μg) were analyzed by flow cytometry. The surface expression of activation markers was compared in treated mice (gray histogram) and untreated controls (solid line). For each marker, the MFI is given for the treated group (underlined MFI) versus the control group (MFI in brackets). Results are representative of three independent experiments. *, intracellular staining.
The expression of surface markers was also analyzed on CD4+ T cells isolated from NALT 16 to 18 h after nasal administration of MALP-2. Surface expression of CD45RB showed that the majority of the CD4+ T cells from NALT of control animals corresponded to naïve T cells (CD45RBhigh) (data not shown). In addition, T cells from naïve animals did not express the interleukin-2 receptor (CD25) or the activation marker CD69 (Fig. 3B). In contrast, MALP-2-treated animals demonstrated an increase of T cells expressing at their surface CD25 (17.7%) and CD69 (5%). A slightly increased expression of MHC class I molecules was also observed on T cells from MALP-2-treated mice (MFI, 117 versus 86) (Fig. 3), giving some evidence of cellular activation. The interaction of the molecules CD80 or CD86 with CD28 provides a potent costimulatory signal for T-cell activation, whereas it is negatively regulated by the engagement of CTLA-4 (33). No significant increase of CD28 expression could be observed on T cells from NALT 16 to 18 h after MALP-2 administration, whereas the expression of CTLA-4 was decreased (MFI, 13.0 versus 18.4 in the control group) (Fig. 3B). In addition, a substantial reduction in the expression of the L-selectin CD62L, which is a marker for naïve T cells, was noted after MALP-2 activation (58 versus 84% CD62Lhigh) (Fig. 3B).
The expression of adhesion molecules is critical for cellular interactions, cell migration, and homing. As observed for the NALT-derived B cells, the expression of the α-subunit CD11a of the LFA-1 antigen, as well as that of the β2 integrin subunit CD18, were substantially up-regulated in T cells isolated from NALT of MALP-2-treated mice (Fig. 3B). Interestingly, while NALT T cells exhibited an activated phenotype after in situ stimulation by intranasal administration of MALP-2, no activation of T cells could be observed after in vitro culture in the presence of MALP-2 (data not shown).
TLR2 expression.
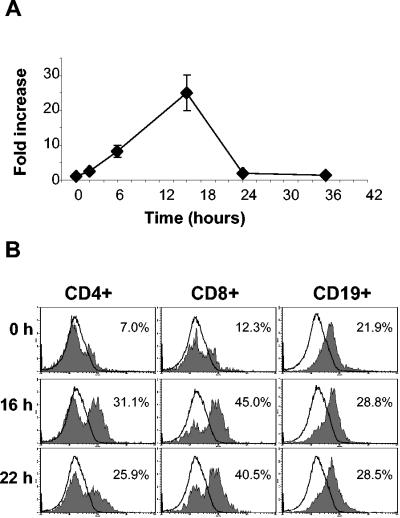
In order to understand the responsiveness of NALT cells to MALP-2, TLR2 expression was analyzed by RT-PCR and flow cytometry, after gating on CD4+, CD8+, or CD19+ cells (Fig. 4B). A high constitutive expression of TLR2 was detected in the B-cell population, which was not further affected by MALP-2 treatment. In contrast, T cells showed a weaker expression of TLR2, which was up-regulated after activation with MALP-2. After exclusion of B and T cells, TLR2 expression could be also measured in the remaining non-phenotypically characterized cells from NALT (data not shown). These results suggest that various cell types are involved in the global activation of NALT promoted by MALP-2. In accordance with the mRNA levels, the peak of TLR2 expression was detected about 16 h after MALP-2 application. According to the RT-PCR and flow cytometry analysis, TLR2 expression was rapidly down-regulated to basal levels after 36 h.
FIG. 4.
TLR2 expression in murine cells from NALT. (A) TLR2 mRNA levels were measured by real-time RT-PCR with RNA isolated from total cells from NALT of mice treated with MALP-2 (0.5 μg/ml). (B) NALT cells isolated from mice treated with MALP-2 were analyzed for the intracellular expression of TLR2 by flow cytometry 16 and 22 h after nasal administration of MALP-2 and compared to responses in untreated mice (time zero). Cells were gated on the basis of the staining with PE-conjugated anti-CD4, fluorescein isothiocyanate-conjugated anti-CD8, and PerCP-Cy5-conjugated anti-CD19, and the expression of TLR2 was evaluated with biotinylated TLR2 plus allophycocyanin-conjugated streptavidin (filled area). The percentage of TLR2-positive cells with respect to control cells (solid lines) is indicated in each histogram.
Characterization of the NALT microenvironment in MALP-2-treated mice.
To further investigate the local mechanisms of immune induction and recruitment of effector cells in NALT, the mRNA expression profile was analyzed by quantitative real-time RT-PCR. RNA was isolated from NALT of mice 2, 6, 16, 24, and 36 h after nasal administration of MALP-2 (0.5 μg). Templates containing 0.5 to 1 μg of total RNA/μl allowed the generation of strong PCR signals and their quantification by RT-PCR, for products expressed up to a minimum of 0.01% of the housekeeping gene β-actin. The earliest and strongest change upon MALP-2 activation corresponded to an increment (>300-fold) in the transcription of the IFN-γ-inducible protein 10 (IP-10) with respect to controls (Fig. 5A). The activation peak was reached 6 h after administration and rapidly decreased to basal levels after 36 h. IP-10 induction was accompanied by an up-regulation of MIP-1α, MIP-2, MCP-1, and MCP-3 (Fig. 5A and Table 2). Transcripts from MIP-1α were detectable 6 h after MALP-2 activation, whereas those from MCP-1 and MIP-2 were detectable only after 16 and 24 h, respectively. These chemokines peaked after 24 h and returned to basal levels after 36 h (Fig. 5A and Table 2). Among the screened chemokine receptors (CCR2, CCR5, CCR6, CCR7, and CCR9), significant signals were only obtained for CCR2. The basal level of transcription for CCR2 (control group) corresponded to approximately 0.2% of the β-actin gene. A rapid increment in its expression was evident already 2 h after MALP-2 administration, which was maintained for at least 36 h. However, when the profile of CCR expression was analyzed with mRNA isolated from MALP-2-activated J774A.1 macrophages, an up-regulation of CCR2, as well as CCR5 and CCR6, was observed in comparison to nonactivated J774 cells (Fig. 5B and data not shown). CCR7 and CCR9 were up-regulated only on LPS-treated control cells (Fig. 5B). Flow cytometric analysis of J774A.1 cells further confirmed that activation with MALP-2 resulted in an up-regulated expression of CCR5 and CCR6 (Fig. 5C).
FIG. 5.
RT-PCR analysis of RNA extracts obtained from NALT after MALP-2 treatment. (A) Kinetic transcriptional analysis of the genes coding for IP-10, MCP-3, and CCR2. Results are expressed as the fold increase with respect to the untreated controls (mice which received only PBS). (B) Electrophoretic analysis of the β-actin, CCR5, CCR6, CCR7, and CCR9 products obtained by RT-PCR. For these specific products the profile of the melting curves obtained by real-time RT-PCR did not allow exact quantification. The gel shows the results obtained with mRNA isolated from the macrophage-like cell line J774A.1 (ATCC TIB 67) after activation with LPS (10 μg/ml) or MALP-2 (0.5 μg/ml) during 16 h in comparison to results in nontreated control cells. Twenty microliters of each PCR mixture was loaded per lane. The relative amount of mRNA for β-actin was 304, 189, and 142 ng/μl for nonactivated, LPS-activated, and MALP-2-activated cells, respectively. (C) The surface expression of CCR5 and CCR6 was evaluated on J774A.1 cells after 18 h of treatment with MALP-2 by flow cytometry, using anti-CCR5-PE and anti-CCR6-Alexa Fluor 647 antibodies (BD Pharmingen). The percentages of CCR5- and CCR6-positive cells are indicated in each quadrant for MALP-2-activated and nonactivated control cells (in brackets).
TABLE 2.
Kinetic analysis of mRNA expression in NALT after MALP-2 administrationa
| Timeb (h) | Relative expression (%)
|
||
|---|---|---|---|
| MIP-1αc | MIP-2c | MCP-1c | |
| 0 | NDd | ND | ND |
| 2 | ND | ND | ND |
| 6 | 0.95 | ND | ND |
| 16 | 1.10 | ND | 0.15 |
| 24 | 11.14 | 0.25 | 12.19 |
| 36 | ND | ND | ND |
MIP-1α, MIP-2, and MCP-1 were either not constitutively expressed in NALT or the basal transcription levels were below the detection limit.
Time after intranasal administration of MALP-2 (0.5 μg).
Results are expressed as the percentage with respect to the expression of the housekeeping gene β-actin. Values are means of at least two independent measurements.
ND, not detectable RT-PCR product.
MALP-2 improves the antigen presentation capacity of professional antigen-presenting cells.
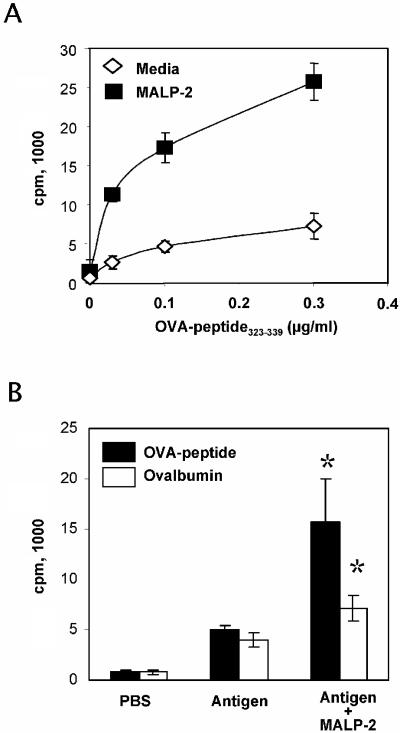
Studies were performed to evaluate the activity of MALP-2 on the antigen presentation capacity of macrophages, B cells, and dendritic cells cocultured with splenic T cells from TCR transgenic DO11.10 mice (Fig. 6). In vitro pretreatment of peritoneal macrophages with MALP-2 in the presence of the OVA323-339 peptide resulted in an improved proliferation of DO11.10 CD4+ T cells, demonstrating an increased antigen presentation capacity of pretreated macrophages (Fig. 6A). Similar results were obtained by using the OVA protein as antigen (data not shown). Then, we assessed the effect of MALP-2 on the antigen presentation capacity of B cells and dendritic cells from nasal tissues. Mice received the OVA-peptide323-339 or the OVA protein by intranasal route in the presence or absence of MALP-2. After 3 h, the animals were sacrificed, and enriched B220+ or CD11c+ cells from NALT were cocultured with DO11.10 CD4+ T cells. As shown in Fig. 6B, stronger T-cell proliferation was observed with dendritic cells isolated from MALP-2-treated animals than in controls, with either the OVA peptide or OVA protein. In contrast, the antigen presentation capacity of B cells was not improved by MALP-2 treatment and was restricted to preloading with the OVA peptide (data not shown).
FIG. 6.
Effect of MALP-2 treatment on antigen presentation. (A) OVA-specific CD4+ T cells purified from DO11.10 mice were cocultured with macrophages loaded in vitro with serial dilutions of the OVA peptide in the presence or absence of MALP-2 (0.25 μg/ml). (B) Dendritic cells, which were isolated by magnetic sorting for CD11c+ cells from NALT of mice that received OVA peptide (200 μg) or OVA protein (10 mg), in the presence or absence of MALP-2 (0.5 μg) by the intranasal route. Cells were cocultured during 4 days, and T-cell proliferation was assessed by [3H]thymidine incorporation. Results are expressed as the mean counts per minute values from triplicate wells; standard deviations are indicated by vertical lines. *, statistically significant at a P level of <0.05 by Student's t test.
Nasal administration of MALP-2 does not break peripheral anergy.
We investigated the possibility that immune activation triggered by MALP-2 could favor autoimmune reactions by using the experimental model of diabetes based on TCR-HA × Ins-HA double transgenic mice (34). Although the pancreatic islets of all double transgenic mice are heavily infiltrated (i.e., insulitis) by the age of 4 weeks, only 30 to 40% of them develop overt diabetes and the rest remain clinically healthy over a period of up to 24 weeks (2). These animals carry approximately 22 to 25% CD4+ T cells expressing the 6.5 transgenic TCR in spleen, lymph nodes, and NALT (data not shown). Four weekly nasal administrations of MALP-2 to clinically healthy double transgenic mice failed to induce any sign of autoimmune reaction or disease during a 16-week period of observation (healthy status was defined as a glucose level in blood of <200 mg/dl and stable body weight). These results suggest that MALP-2 does not promote a break of peripheral anergy, thereby showing a good safety profile as adjuvant.
DISCUSSION
Mucosal epithelia constitute the primary sites for pathogen and antigen entry. Our knowledge on the mucosal barriers and the mucosal immune system has significantly expanded in recent years (5, 27). However, there is still limited information on the molecular events leading to the elicitation of mucosal immune responses after infection or vaccination. Furthermore, most of the experimental work on mucosal immunity was obtained using molecules administered by the oral route, while the upper respiratory tract has specific features that make it a distinct inductive site. Thus, it is essential to have a clear understanding on how immune responses are elicited after nasal challenge or vaccination.
The first line of defense against invading microorganisms relies on the innate immune system, which also represents a critical link for the stimulation of efficient adaptive immune responses. The stimulation of pattern recognition receptors present on the surface of the cells from the innate immune system, such as the TLR, is a fundamental initial event in the activation process. The data reported in the literature concerning the surface expression of TLR2 are controversial but suggest that there is poor expression in all cell types (17). This is in contrast to what has been observed by RT-PCR analysis and intracellular staining (13). However, two studies have clearly shown that TLR2 is expressed in mucosa-associated lymphoid tissues of human tonsils (8, 29). These results suggest that TLR2-mediated signaling may play an important role in the induction of immune responses at this mucosal inductive site.
In this study, we described the effects resulting from the activation of innate immune system receptor TLR2/6 through the binding of the adjuvant lipopeptide MALP-2 (4, 30). We analyzed TLR2 expression in cells from NALT by both RT-PCR and intracellular staining. The high constitutive expression of TLR2 by B cells from NALT is in accordance with their capacity to be activated in vitro by MALP-2 treatment, as demonstrated by the up-regulation of different activation markers (Fig. 2). The obtained results also showed that MALP-2 exerts a mitogenic effect on B cells in vitro. However, the in situ activation of NALT B cells by MALP-2 was rather modest (Fig. 3). In contrast, T cells from NALT, which do not constitutively express high levels of TLR2, exhibited a strong up-regulation of this receptor after MALP-2 application. This should result in an enhanced responsiveness to danger signals. In fact, it has been demonstrated that up-regulation of TLR2 (via a nonspecific signal, such as anti-CD3 stimulation) could lead to sensitization of T cells to TLR2 agonists (21). In accordance with the above-mentioned regulatory loop, NALT-derived T cells also showed an up-regulated expression of activation markers and intercellular adhesion molecules. This suggests an increased responsiveness to specific antigenic signals, as well as an enhanced capacity for naïve T cells priming in situ.
Activation and expansion of T cells is the central event in the development of adaptive immune responses against specific protein antigens. An optimal T-cell response (i.e., T-cell expansion, cytokine secretion, and development of helper and effector functions) requires two distinct stimuli. The first is an antigen-specific signal provided by the interaction through the TCR, whereas the second is a TCR-independent signal mediated by the engagement of T-cell surface molecules with costimulatory molecules expressed on antigen-presenting cells. The best-defined costimulatory molecules are B7-1 (CD80) and B7-2 (CD86) (22). The up-regulated expression of the costimulatory molecule CD80 on NALT-derived B cells suggests an increased potential for cross talk between T and B cells. This may in turn promote T-cell-dependent antibody production. The improved humoral responses observed after vaccination with MALP-2 as adjuvant are in agreement with these results (4, 30). The up-regulated expression of the adhesion receptor LFA-1 (CD11a/CD18) observed after treatment suggests that MALP-2 could favor T-cell binding to antigen-presenting cells, as well as trans-endothelial migration. The recruitment and activation of professional antigen-presenting cells observed after MALP-2 administration provides the ideal framework for enhanced immune responses. As demonstrated for other molecules exhibiting adjuvant activity, such as LPS, heat-labile enterotoxin of Escherichia coli, and CpG motifs (4, 9, 20, 36), MALP-2 also activates dendritic cells (18, 39). Accordingly, we demonstrated with in vitro-loaded macrophages and in vivo-loaded dendritic cells that antigen presentation is enhanced in the presence of MALP-2.
The global NALT microenvironment also appears to be geared toward improved immune responses after MALP-2 administration. We demonstrated that MALP-2 treatment acts as a proinflammatory stimulus for the nasal mucosa. We observed an increment in the expression of MCP-1 and CCR2, which are involved in monocyte recruitment (3, 23). The expression levels of IP-10 also matched those obtained by classical inflammatory stimuli, such as LPS and tumor necrosis factor alpha. This is in agreement with the reported recruitment and activation of macrophages after intraperitoneal or intranasal administration of MALP-2 (7, 30). The initiation of T-cell-independent effector mechanisms could be sustained and amplified by activated T cells at inflammation sites (37).
The expression of inflammatory chemokines and chemokine receptors (CCR1, CCR2, CCR5, and CCR6) regulates the migration of dendritic cells from peripheral tissues into T-cell areas of draining lymph nodes, where they initiate primary T-cell responses (23). The differential expression of chemokine receptors also dictates, to a large extent, the migration and tissue homing of Th1 or Th2 cells (3). It has also been proposed that the acquisition of a chemokine and chemokine receptor profile is an integral part of T-helper-cell differentiation (31, 40). Our results showed that MALP-2 induces the expression of IP-10 and MIP-1α, which preferentially correlate with a Th1 polarization, in nasal tissues (31). However, MCP-1 and CCR2, which correlate with a Th2 polarization, were also up-regulated by MALP-2 activation (32). Thus, the induction of a microenvironment characterized by the presence of molecules driving both Th1 and Th2 polarization matches the Th1/Th2 mixed responses observed after vaccination using MALP-2 as mucosal adjuvant (4, 30).
In vitro tests showed that MALP-2 represents a strong activation signal, as measured by the up-regulated expression of surface markers on B cells and dendritic cells (18). The presence of MALP-2 also resulted in the generation of a local immune-responsive environment in NALT. Discrepancies in the intensity of activation between in vitro and in vivo tests may reflect the fact that NALT are a highly tolerogenic milieu (1, 38). Alternatively, they may be related to the efficacy of MALP-2 transfer across the mucosal barrier.
Immune stimulation may lead to autoimmune reactions by breaking tolerance to self antigens. However, cell division studies proved that MALP-2 does not induce nonspecific T-cell proliferation, which would represent a major drawback for an adjuvant. This was further confirmed by the results obtained using the murine model of autoimmune diabetes. This model is highly suited for the study of immune modulation in vivo, as demonstrated by the fact that administration of a dimeric peptide-MHC class II chimera results in a down-regulation of the T-cell-mediated autoimmune response (6). In this study, we demonstrated that, although the NALT of double transgenic mice contained a majority of HA-specific T cells, repeated intranasal administration of MALP-2 did not break the peripheral tolerance.
In conclusion, the results obtained in the present work allow dissection of critical parameters concerning the cellular interactions and the antigen presentation processes after application of the novel mucosal adjuvant MALP-2. An efficient initiation of acquired immune responses is promoted by a TLR2/6-mediated activation via MALP-2. The adjuvant leads to improved antigen sampling and recognition through an increment in (i) the number of antigen-presenting cells, (ii) the expression of costimulatory molecules, (iii) the intercellular adhesion capacity of immune cells, and (iv) T-cell stimulation. MALP-2 also promotes the generation of a transient local microenvironment which favors the ability of immune cells to interact in a coordinated fashion, as well as to traffic and localize within mucosal tissues. MALP-2 also supports a direct B- and T-cell activation within nasal tissues, thereby lowering the threshold for antigen-specific activation. This explains the improved immune responses observed after intranasal vaccination with MALP-2 as adjuvant (4, 30). The emerging knowledge allows a better understanding of the mechanisms by which MALP-2 modulates innate immunity first, promoting strong acquired immune responses thereafter. This is expected to provide the rational basis for its optimal exploitation as adjuvant, as well as the design of new adjuvant molecules.
Acknowledgments
We are particularly grateful to Peter F. Mühlradt for providing MALP-2 and to Urte Jäger for her commitment and excellent technical help.
Editor: D. L. Burns
REFERENCES
- 1.Akbari, O., P. Stock, R. H. DeKruyff, and D. T. Umetsu. 2003. Mucosal tolerance and immunity: regulating the development of allergic disease and asthma. Int. Arch. Allergy Immunol. 130:108-118. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, T. A., T. F. Gajewski, D. Danielpour, E. B. Chang, K. W. Beagley, and J. A. Bluestone. 1992. Differential function of intestinal intraepithelial lymphocyte subsets. J. Immunol. 149:1124-1130. [PubMed] [Google Scholar]
- 3.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borsutzky, S., V. Fiorelli, T. Ebensen, A. Tripiciano, F. Rharbaoui, A. Scoglio, C. Link, F. Nappi, M. Morr, S. Butto, A. Cafaro, P. F. Muhlradt, B. Ensoli, and C. A. Guzman. 2003. Efficient mucosal delivery of the HIV-1 Tat protein using the synthetic lipopeptide MALP-2 as adjuvant. Eur. J. Immunol. 33:1548-1556. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., I. N. Farstad, and G. Haraldsen. 1999. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol. Today 20:267-277. [DOI] [PubMed] [Google Scholar]
- 6.Casares, S., A. Hurtado, R. C. McEvoy, A. Sarukhan, H. von Boehmer, and T. D. Brumeanu. 2002. Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide-MHC class II chimera. Nat. Immunol. 3:383-391. [DOI] [PubMed] [Google Scholar]
- 7.Deiters, U., and P. F. Muhlradt. 1999. Mycoplasmal lipopeptide MALP-2 induces the chemoattractant proteins macrophage inflammatory protein 1α (MIP-1α), monocyte chemoattractant protein 1, and MIP-2 and promotes leukocyte infiltration in mice. Infect. Immun. 67:3390-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flo, T. H., O. Halaas, S. Torp, L. Ryan, E. Lien, B. Dybdahl, A. Sundan, and T. Espevik. 2001. Differential expression of Toll-like receptor 2 in human cells. J. Leukoc Biol. 69:474-481. [PubMed] [Google Scholar]
- 9.Gierynska, M., U. Kumaraguru, S. K. Eo, S. Lee, A. Krieg, and B. T. Rouse. 2002. Induction of CD8 T-cell-specific systemic and mucosal immunity against herpes simplex virus with CpG-peptide complexes. J. Virol. 76:6568-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunzer, M., C. Weishaupt, L. Planelles, and S. Grabbe. 2001. Two-step negative enrichment of CD4+ and CD8+ T cells from murine spleen via nylon wool adherence and an optimized antibody cocktail. J. Immunol. Methods 258:55-63. [DOI] [PubMed] [Google Scholar]
- 11.Harmsen, A., K. Kusser, L. Hartson, M. Tighe, M. J. Sunshine, J. D. Sedgwick, Y. Choi, D. R. Littman, and T. D. Randall. 2002. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently of lymphotoxin-alpha (LT alpha) and retinoic acid receptor-related orphan receptor-gamma, but the organization of NALT is LT alpha dependent. J. Immunol. 168:986-990. [DOI] [PubMed] [Google Scholar]
- 12.Heritage, P. L., B. J. Underdown, A. L. Arsenault, D. P. Snider, and M. R. McDermott. 1997. Comparison of murine nasal-associated lymphoid tissue and Peyer's patches. Am. J. Respir. Crit. Care Med. 156:1256-1262. [DOI] [PubMed] [Google Scholar]
- 13.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 14.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol 20:197-216. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann, A., P. F. Muhlradt, D. Gemsa, and H. Sprenger. 1999. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans-derived lipoprotein MALP-2. Infect. Immun. 67:6303-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirberg, J., A. Baron, S. Jakob, A. Rolink, K. Karjalainen, and H. von Boehmer. 1994. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 180:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschning, C. J., and R. R. Schumann. 2002. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 270:121-144. [DOI] [PubMed] [Google Scholar]
- 18.Link, C., R. Gavioli, T. Ebensen, A. Canella, E. Reinhard, and C. A. Guzman. 2004. The Toll-like receptor ligand MALP-2 stimulates dendritic cell maturation and modulates proteasome composition and activity. Eur. J. Immunol. 34:899-907. [DOI] [PubMed] [Google Scholar]
- 19.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 20.Martin, M., A. Sharpe, J. D. Clements, and S. M. Michalek. 2002. Role of B7 costimulatory molecules in the adjuvant activity of the heat-labile enterotoxin of Escherichia coli. J. Immunol. 169:1744-1752. [DOI] [PubMed] [Google Scholar]
- 21.Matsuguchi, T., K. Takagi, T. Musikacharoen, and Y. Yoshikai. 2000. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood 95:1378-1385. [PubMed] [Google Scholar]
- 22.McAdam, A. J., A. N. Schweitzer, and A. H. Sharpe. 1998. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 165:231-247. [DOI] [PubMed] [Google Scholar]
- 23.Moll, H. 2003. Dendritic cells and host resistance to infection. Cell Microbiol. 5:493-500. [DOI] [PubMed] [Google Scholar]
- 24.Morr, M., O. Takeuchi, S. Akira, M. M. Simon, and P. F. Muhlradt. 2002. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur. J. Immunol. 32:3337-3347. [DOI] [PubMed] [Google Scholar]
- 25.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 27.Nagler-Anderson, C. 2001. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 1:59-67. [DOI] [PubMed] [Google Scholar]
- 28.Nishiguchi, M., M. Matsumoto, T. Takao, M. Hoshino, Y. Shimonishi, S. Tsuji, N. A. Begum, O. Takeuchi, S. Akira, K. Toyoshima, and T. Seya. 2001. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 166:2610-2616. [DOI] [PubMed] [Google Scholar]
- 29.Ochoa, M. T., A. J. Legaspi, Z. Hatziris, P. J. Godowski, R. L. Modlin, and P. A. Sieling. 2003. Distribution of Toll-like receptor 1 and Toll-like receptor 2 in human lymphoid tissue. Immunology 108:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rharbaoui, F., B. Drabner, S. Borsutzky, U. Winckler, M. Morr, B. Ensoli, P. F. Muhlradt, and C. A. Guzman. 2002. The mycoplasma-derived lipopeptide MALP-2 is a potent mucosal adjuvant. Eur. J. Immunol. 32:2857-2865. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto, F., A. Lanzavecchia, and C. R. Mackay. 1998. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today 19:568-574. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 33.Sansom, D. M., C. N. Manzotti, and Y. Zheng. 2003. What's the difference between CD80 and CD86? Trends Immunol. 24:314-319. [DOI] [PubMed] [Google Scholar]
- 34.Sarukhan, A., A. Lanoue, A. Franzke, N. Brousse, J. Buer, and H. von Boehmer. 1998. Changes in function of antigen-specific lymphocytes correlating with progression towards diabetes in a transgenic model. EMBO J. 17:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh, M., and D. O'Hagan. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075-1081. [DOI] [PubMed] [Google Scholar]
- 36.Sun, H., K. G. Pollock, and J. M. Brewer. 2003. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine 21:849-855. [DOI] [PubMed] [Google Scholar]
- 37.Taub, D. D., D. L. Longo, and W. J. Murphy. 1996. Human interferon-inducible protein-10 induces mononuclear cell infiltration in mice and promotes the migration of human T lymphocytes into the peripheral tissues and human peripheral blood lymphocytes-SCID mice. Blood 87:1423-1431. [PubMed] [Google Scholar]
- 38.Tlaskalova-Hogenova, H., L. Tuckova, R. Lodinova-Zadnikova, R. Stepankova, B. Cukrowska, D. P. Funda, I. Striz, H. Kozakova, I. Trebichavsky, D. Sokol, Z. Rehakova, J. Sinkora, P. Fundova, D. Horakova, L. Jelinkova, and D. Sanchez. 2002. Mucosal immunity: its role in defense and allergy. Int. Arch. Allergy Immunol. 128:77-89. [DOI] [PubMed] [Google Scholar]
- 39.Weigt, H., P. F. Muhlradt, A. Emmendorffer, N. Krug, and A. Braun. 2003. Synthetic mycoplasma-derived lipopeptide MALP-2 induces maturation and function of dendritic cells. Immunobiology 207:223-233. [DOI] [PubMed] [Google Scholar]
- 40.Wiley, J. A., R. J. Hogan, D. L. Woodland, and A. G. Harmsen. 2001. Antigen-specific CD8+ T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol. 167:3293-3299. [DOI] [PubMed] [Google Scholar]
- 41.Wu, H. Y., H. H. Nguyen, and M. W. Russell. 1997. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand. J. Immunol. 46:506-513. [DOI] [PubMed] [Google Scholar]