Abstract
This protocol describes how to carry out theta-burst long-term potentiation (LTP) with extracellular field recordings in acute rodent hippocampal slices. This method is relatively simple and noninvasive and provides a way to sample many neurons simultaneously, making it suitable for applications requiring higher throughput than whole-cell recording.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous material used in this protocol.
RECIPE: Please see the end of this protocol for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Acute hippocampal rodent brain slices
Many different species can be used, e.g., rats, mice, guinea pigs, etc. The slices should be 200–500-μm thick. In thicker slices, gas and metabolite exchange become compromised, as diffusion time increases exponentially with distance. Gas exchange is better with an interface slice recording chamber (Reid et al. 1988), but high flow rate may alleviate this problem in a submerged slice recording chamber (Hajos et al. 2009). Brain tissue can be sliced in different orientations, e.g., coronally or horizontally, which may alter experiments because specific structures are preserved in some orientations but not in others (Skrede and Westgaard 1971; Marcaggi and Attwell 2007; Bartlett et al. 2011; Sherwood et al. 2012). It is typically easier to record from juvenile slices because young slices stay healthier for longer (see Troubleshooting). As a consequence, thicker slices or entire brain structures of neonatal rodent brain can be used for in vitro studies (Khalilov et al. 1997). Anecdotally, we find that irrespective of age, rat slices are easier to work with than mouse slices.
Artificial cerebrospinal fluid (ACSF) for synaptic plasticity studies <R>
Carbogen gas (95% O2/5% CO2)
This gas mixture keeps the slices oxygenated and the pH at ~7.4 during recording.
Equipment
Amplifier
An amplifier (e.g., Molecular Devices MultiClamp 700B, HEKA EPC 10, Dagan BVC-700A, or A-M Systems Model 1700 or 3000) is required to boost electrode signals. Patch-clamp amplifiers can act as field-recording amplifiers, thus providing a saving for those laboratories that already have them. However, field potential amplifiers are considerably cheaper if the sole intended purpose is field potential recordings.
Computer with acquisition and analysis software
There is a plethora of very good recording software packages available. Many of these can be downloaded for free (e.g., ePhus, Strathclyde Electrophysiology Software, or NeuroMatic), although they may require a software framework that is not free, such as Igor Pro (WaveMetrics) or MATLAB (MathWorks). Off-the-shelf electrophysiology software packages include AxoGraph, WinLTP, and pCLAMP. The choice of software is tremendously important, as it may determine what kinds of experiments can be performed and which acquisition boards must be used. A flexible alternative is to use custom-made programs written in, e.g., Igor Pro, MATLAB, or Labview. This simplifies data acquisition and analysis, but requires computer programming skills.
Data acquisition boards
The choice of data acquisition board depends critically on the choice of software (see above). Relatively inexpensive boards of a wide variety can be purchased from National Instruments (e.g., PCI-6229, PCI-6221-37, or USB-6003). Boards dedicated to electrophysiology can be purchased from Molecular Devices (e.g., Digidata 1550) or HEKA (e.g., InstruTech ITC-18). Dedicated boards come with advantages such as integration with specific amplifiers, but this may be of less importance for field potential recordings. Many types of boards can be used with general data analysis software frameworks such as Igor Pro (WaveMetrics) or MATLAB (Math-Works), although custom scripts may have to be written or downloaded.
Electrodes and electrode holders
Stimulation and recording electrodes can be made from borosilicate glass capillaries using a pipette puller. Capillaries of various thicknesses and diameters are available; we use those with an outer diameter of 1.5 mm and an inner diameter of 0.86 mm. We have used Sutter Instruments’ P-97 and P-1000 pullers, the Narishige Group’s PC-10 puller, and AutoMate Scientific’s Zeitz DMZ puller, and they all work very well—the chief differences are degree of automation, versatility, and price. Both recording and stimulation pipettes may be filled with ACSF, in which case the tip size should typically be 2–50 μm. It may be difficult to obtain a large enough tip diameter, in which case gently breaking the pipette tip against tissue paper may help. Capillaries with an internal filament are easier to fill. The recording pipette can also be filled with 1–4 m NaCl to increase the electrode conductance. In this case, however, the tip size has to be much smaller (maximally 1 μm) to avoid NaCl leakage, which may damage tissue and which may result in nonphysiologically high Cl− concentration locally. A chlorided silver wire attached to an electrode holder is placed inside the recording pipette. We know of two methods for chloriding electrode wires: either the wire is immersed in bleach for ~10 min (rinse carefully in distilled water afterwards), or a positive current is passed through the electrode dipped in ~1 m NaCl using, for example, a 4.5-V battery until a white layer appears. For stimulation, a variety of different types of electrodes can be used, for example, insulated tungsten electrodes (World Precision Instruments or FHC Inc.). These have lower resistance and capacitance, but larger tip diameter.
Faraday cage
A grounded metal cage used to reduce electric noise can be bought (e.g., from Harvard Apparatus or World Precision Instruments) or can be made by attaching a metal mesh to a frame. A Faraday cage is often not necessary.
Filters (see Step 6)
Ground electrodes
Two ground electrodes should be placed in the recording chamber: one connected to the amplifier and the other to the stimulus isolator. For the recording ground, use a chlorided silver wire. For stimulation, use a bare silver wire to avoid build-up of electrode polarization. If bipolar metal electrodes are used for stimulation, there is no need for a stimulation ground.
Micromanipulators
For field recordings, inexpensive mechanical micromanipulators (e.g., Narishige Group NMN-21, Scientifica LBM-7, or Sutter Instruments MP85) are normally adequate. Motorized micromanipulators cost more but simplify experiments—a nonexhaustive list includes PatchStar and MicroStar by Scientifica, Mini and Micro by Luigs & Neumann, and MP-285 and MP-225 by Sutter Instruments.
Microscope
There are several microscopes suitable for electrophysiology on the market, such as the Olympus BX-50, Scientifica SliceScope, Leica DM series, and Zeiss Axio Examiner. Important properties to consider include size, price, choice of contrast enhancement (differential interference, oblique illumination, Dodt contrast), and level of automation. For field-recording experiments, an inexpensive stereoscope may provide an excellent training environment.
Oscilloscope
We recommend having a dedicated oscilloscope to simplify debugging. Digital oscilloscopes of low sampling rate are not expensive (e.g., Tektronix TDS 200 series or PicoScope).
Perfusion system
To perfuse the slice with oxygenated ACSF, use a peristaltic pump (e.g., Gilson Minipulse 3 or World Precision Instruments Peri-Star) or a siphon combined with suction for removal. Peristaltic pumps can be used to recycle expensive drug-containing perfusion solutions to keep the overall ACSF volume low. Add a drip chamber to the inlet to visualize flow rate, to reduce electrical noise levels when using a recirculating pump, and to prevent perfusion line bubble formation.
Recording chamber
These are commercially available (e.g., from Harvard Apparatus or Scientifica), but can also be constructed in-house by making a hole in the bottom of a Petri dish and attaching a coverslip using silicon adhesive.
Slice holder (“harp”)
Use a flattened U-shaped platinum wire with individual nylon strings super-glued in place, forming the shape of a harp. Pantyhose is a good source of nylon strings. Human hair provides an alternative. Platinum is soft and easy to shape and is also inert, which reduces the risk of toxicity. Slice anchors are also commercially available (e.g., from Harvard Apparatus or Warner Instruments).
Stimulus isolator
To activate input fibers, a stimulus isolator (“stim box”) is needed. This unit takes a command signal from a computer or other external source, isolates it to minimize electrical noise, and injects current via the stimulation electrode into the preparation. There are many equivalent units on the market—we have had good experience with Dagan BSI-950, Digitimer DS2A, and AMPI ISO-Flex. Some isolators accept a command signal to control pulse amplitude, whereas others only accept a TTL-style gating pulse, with pulse amplitude controlled by a dial. Some have constant-current or constant-voltage modes, and some can be switched between mono- and biphasic pulses. We recommend biphasic pulses (two contiguous monophasic pulses of opposite sign), as they minimize electrochemical reactions at the electrode tip thus improving long-term stability.
Temperature controller
To obtain physiological ACSF temperature, use an inline heater (e.g., Harvard Apparatus CL-100 or Scientifica SM-4500). Measure temperature in the recording chamber, since it drops in the tubing system.
Transfer pipette
Carefully break off a few centimeters of the narrow part of a glass Pasteur pipette and attach a rubber bulb. The wide end is then used to transfer the slice. Alternatively, cutoff the tip of a plastic transfer pipette.
METHOD
Setup
-
1
Start the perfusion to circulate oxygenated ACSF into and out of the recording chamber.
Perfusion flow rate should be >1 drop per second (>2 mL/min). For recordings nominally at physiological temperatures, recording chamber temperature should be 31°C–34°C because at higher temperatures, slice quality suffers and evoked responses decay. Plasticity experiments are typically performed at nominally physiological temperature, although LTP may be observed at room temperature as well (Feldman 2000).
-
2
Attach the recording electrode to the electrode holder. Attach the stimulation electrode to one of the micromanipulators and connect it to the stimulus isolator.
Before starting recordings, check noise levels (see Troubleshooting).
Recording
-
3
Transfer a slice to the recording chamber using a transfer pipette. Secure the slice in place with a slice holder.
Reposition the slice so that the electrodes can be placed without touching the edges of the recording chamber. Slice holder strings should not run between stimulation and recording electrodes.
-
4
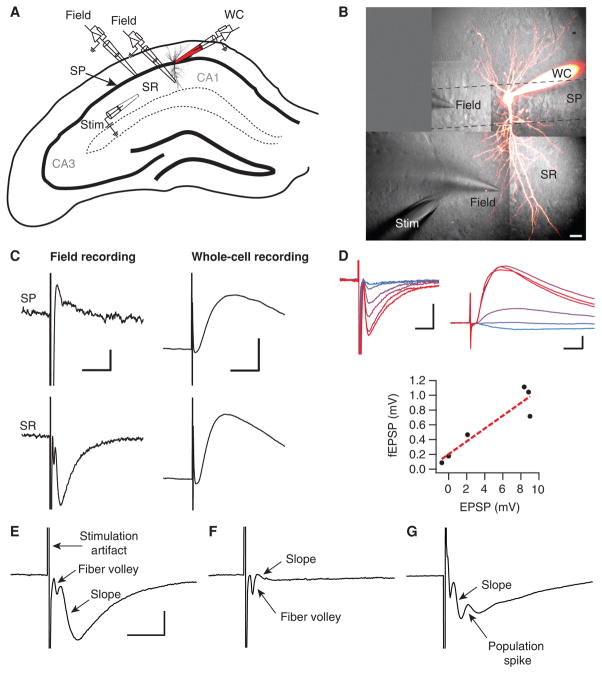
Position the stimulation and recording electrodes in the stratum radiatum (Fig. 1A).
Electrodes should penetrate the slice ~50 μm without damaging tissue appreciably. A white halo can typically be seen surrounding the pipette tip as it enters the tissue.
-
5
Set the stimulation strength for the baseline recording.
Adjust stimulation amplitude and duration to obtain the desired amplitude of the field excitatory postsyn-aptic potential (fEPSP). Typical stimulation values are 10–300 μA or 20–100 V, and 20–200 μs, but these vary enormously depending on, for example, the stimulation electrode and type of brain tissue. A monopolar glass electrode with 1- to 2-μm tip should reliably evoke responses in hippocampal P12 (postnatal day 12) slices with biphasic ±30–100 V pulses of 200-μs duration. Maximize fEPSP amplitude by adjusting electrode positions and stimulation strength (Fig. 1A–D). A fiber volley should ideally precede the fEPSP (Fig. 1E), although may not be evident if capacitive coupling between the stimulation and recording electrode generates a large stimulation artifact (Stangl and Fromherz 2008). The fiber volley represents presynaptic action potentials; variations in fiber volley amplitude are commonly used as a measure of stability, whereas a fEPSP that is smaller than the fiber volley indicates an unhealthy slice (Fig. 1F; see Troubleshooting). A larger distance between stimulation and recording electrodes separates the fiber volley from the fEPSP better, but at the expense of fEPSP amplitude. Increase stimulation strength until a population spike appears (Fig. 1G), then reduce it until fEPSP is halved.
-
6
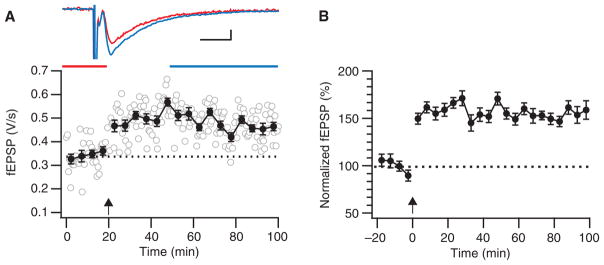
Record responses every 10–60 sec to obtain a ~30-min baseline period (Fig. 2).
Experiments without a stable baseline should be rejected, which may result in a 10%–50% rejection rate. To avoid bias, it is important to consistently apply the same stability criteria across experiments, e.g., baseline fEPSP amplitude should not significantly correlate with time (Pearson’s r at P < 0.05 level). Baseline length should also reflect experiment duration: recordings lasting several hours may require an hour-long baseline. To further ensure stability, an additional control stimulation electrode is often used (see Troubleshooting).
To reduce noise, recorded signals should be filtered with a 2-kHz low-pass filter. A 0.03-Hz high-pass filter removes any offsets, and a notch filter eliminates line frequency noise. Adaptive filters (e.g., The HumBug, Quest Scientific) work better than notch filters, but are more expensive. Proper systematic grounding of key equipment is the most efficient method to reduce noise, but can be quite time consuming.
-
7
Induce LTP (Fig. 2).
For theta-burst LTP, deliver trains of 4–5 pulses at 100 Hz every 200 ms, repeated 10–75 times (Larson and Lynch 1986; Larson et al. 1986; Staubli and Lynch 1987; Kirkwood et al. 1993; Kirkwood and Bear 1994). For tetanization, ~100 pulses are typically delivered at 100 Hz, repeated 2–4 times. (Bliss and Lømo 1973; Lynch et al. 1977; Dunwiddie and Lynch 1978). Theta-burst stimulation generally elicits LTP more reliably than tetanization (see Introduction: In Vitro Investigation of Synaptic Plasticity [Abrahamsson et al. 2016] and Larson and Lynch 1986).
-
8
Resume baseline recordings (Fig. 2).
Overall duration of the recording depends on which phases of LTP are studied. Early LTP may last up to 3 h after induction, whereas late LTP should be measured 3–4 h after induction (Frey and Morris 1998; Kandel 2001). Regardless, plasticity is typically measured 30–60 min after the induction.
-
9
Analyze the data.
The size of the responses is measured by determining the slope of the fEPSP, i.e., by calculating the linear regression of the rising phase of the fEPSP (see Fig. 1E). It is more appropriate to measure slope than amplitude, since field recordings represent a time derivative of extracellular current sources and sinks (Johnston and Wu 1994). In addition, spiking may contaminate peak amplitude measurements, leading to underestimation of the amplitude.
FIGURE 1.
Factors determining fEPSP recordings. (A) Schematic drawing of a hippocampal slice showing positioning of stimulation and recording electrodes. A stimulation electrode (“stim”) is placed in the stratum radiatum (SR) of the CA1 area, to activate axons originating from pyramidal cells in CA3. For comparison, a whole-cell recording electrode (WC) is placed in the cell body layer, stratum pyramidale (SP). The field-recording electrode (“field”) is moved between SP and SR positions. (B) 2-photon microscopy maximal intensity projection recorded pyramidal cell loaded with Alexa 594 is superimposed on infrared Dodt contrast images. Positions of stimulation and field-recording electrodes are shown, similar to the schematic in A (scale bar: 20 μm). (C) fEPSPs recorded in SP (top left) and SR (bottom left) have reversed polarities (scale bars: 200 μV, 10 ms). EPSP recorded in whole-cell configuration remains the same (right, scale bars: 2 mV, 10 ms). Note that the SR fEPSP (bottom left) has faster dynamics and the opposite sign compared with the intracellular EPSP (bottom right). (D) fEPSPs (top left) and whole-cell EPSPs (top right) recorded simultaneously at six different stimulation intensities (30, 40, 50, 70, 80, and 100 V, indicated by lines ranging from blue to red) are significantly correlated (bottom; Pearson’s r = 0.933, P < 0.05), indicating equivalency in terms of measuring synaptic strength (scale bars: 500 μV, 5 ms [top left]; 2 mV, 5 ms [top right]). (E) Example of a good fEPSP recording. The stimulus artifact, fiber volley, and slope are indicated. The fEPSP is large compared with the fiber volley, which indicates a healthy slice (scale bar: 500 μV, 5 msec). (F) A fEPSP that is much smaller than the fiber volley suggests that the slice is of poor quality; despite stimulating many axons, the response is very small. (G) A fEPSP that has a population spike (positive deflection) riding on the PSP interfering with the measurement of the peak. This occurs when the recorded neurons fire.
FIGURE 2.
Theta-burst LTP. (A) Sample theta-burst induction experiment showing robust LTP. During the induction (arrow), 12 trains of 4 pulses at 100 Hz were delivered at 5 Hz, and this was repeated three times at 0.1 Hz. Error bars indicate xxx. Inset top: fEPSPs averaged over indicated time periods before (red) and after (blue) induction (scale bars: 100 μV, 5 ms). (B) This ensemble average of five such theta-burst experiments indicates how robust this LTP paradigm is. Before averaging, experiments were normalized to their individual baseline periods, so error bars (S.E.M.) reflect the variability around the mean, not absolute amplitude variability across experiments.
TROUBLESHOOTING
Problem: The fEPSP is small despite using high stimulation strength.
Solution:
Electrodes may not be positioned correctly. Move the stimulation electrode vertically in the stratum radiatum to maximize fEPSP amplitude.
Slice may be unhealthy. fEPSP responses that are much smaller than the fiber volley indicate an unhealthy slice (Fig. 1E, F). Slice health is crucial for LTP and there are several ways to achieve and maintain healthier slices. Make sure the osmolality and pH of the ACSF are correct: the pH should be ~7.4, whereas the osmolality depends on the animal species being used (Bourque 2008). We recommend 320 mM for rat slices, adjusted with D-glucose. An essential step in the preparation of slices is the dissection of the brain, which should be relatively quick. Aim for <15 min from decapitation to the end of the slicing procedure. The decapitation and removal of the skin and skull should take <1 min. It may be easier to generate healthy slices from juvenile animals, but LTP mechanisms vary with developmental stage (Isaac et al. 1995; Liao et al. 1995; Yasuda et al. 2003; Massey and Bashir 2007; Abrahamsson et al. 2008; Larsen et al. 2010). With adult animals, slice quality can be improved by cardiac perfusion with ice-cold ACSF to cool the brain rapidly (Moyer and Brown 1998).
Problem: Response amplitudes are not stable.
Solution:
Electrodes may be drifting. If the slice or the electrodes are gradually moving, responses tend to run up or down. The harp should hold the slice firmly in place to minimize slice movement. Minimizing electrode drift can be more challenging. Drift can be monitored using a microscope, but can be very difficult to notice along the microscope z-axis. Causes of drift include: taut manipulator wires pulling on manipulator housing, ambient room temperature changes (e.g., air conditioning), and electrodes settling in their holder.
Gas exchange in the slice may be compromised. Dropping oxygen levels reduce fEPSPs amplitude but not the fiber volley. In addition, CO2 accumulation suppresses the fEPSP (Lee et al. 1996). Increase perfusion rate to enhance slice oxygenation. Use Teflon or Tygon tubing to prevent gas leakage (Hajos et al. 2009).
Perfusion temperature may be changing. Check heater settings. Bubbles in the perfusion line cause temperature transients; reduce bubble formation by filling the drip chamber a little at the beginning of the experiment. Pharmacological manipulations via perfusate changes also tend to cause bubble formation and temperature changes.
Slice may be unhealthy. Take a new slice from the incubation chamber, or dissect new slices.
An additional input may be needed to monitor fEPSP stability. One input will undergo LTP but the other will not. Slice deterioration, oxygen deprivation, and perfusion temperature fluctuations affect both inputs. The two stimulation electrodes are typically placed on opposite sides of the recording electrode, on different levels in the stratum radiatum. To ensure that the afferent pathways are independent, the two inputs are activated separately and simultaneously and then the algebraic sum of the separate fEPSPs is compared with the simultaneously evoked fEPSP.
Problem: Recording sweeps are disrupted by epileptiform activity.
Solution: Hyperexcitable acute slices display epileptiform activity. Epileptiform activity can be observed as multiple regenerative population spikes in the decay phase of the fEPSP. To prevent this, make a cut between CA3 and CA1. Increase the total concentration of Ca2+ and Mg2+ in the ACSF to 4 mM. Hyperexcitability can be a consequence of perfusing inhibitory blockers such as picro-toxin or bicuculline. If inhibitory blockade is essential, try applying drugs locally in the slice (Feldman 2000).
Problem: Recordings suffer from electrical noise.
Solution: Electrical noise is a major problem in electrophysiology, but noise debugging is an art form that we regrettably cannot cover exhaustively here. The Faraday cage should help reduce noise, but the key is proper grounding. The input to the amplifier should be floating, which means that the perfusion ground should not be shared with the equipment chassis ground—the latter is there to protect the user from electrical shock, whereas the former is a signal reference. There should be no ground loops, which means that equipment should only be grounded once. This is achieved by methodically using a star grounding pattern: the grounding leads should all connect to the same grounding point, thus forming a star shape. To debug for noise, measure root-mean-square noise level with the acquisition system and systematically turn equipment on and off until the identity of the poorly grounded culprit(s) is revealed. Noise frequency can give hints to the source: line frequency noise may be cause by transformers, cathode ray tubes, or lights, whereas double line frequency noise can be caused by fluorescent light tubes. High-frequency noise can result from equipment such as computers, monitors, or nearby lasers and can be filtered out with a low-pass filter. Nearby light sources often cause noise. Shielding using grounded aluminum foil may provide a temporary solution but is a last resort that we do not generally recommend.
DISCUSSION
Investigating LTP using extracellular field recordings is a technique that has been used for decades since LTP was first discovered (Bliss and Lømo 1973). Besides theta-burst stimulation, there are several other types of LTP induction protocols, e.g., tetanization (Bliss and Lømo 1973; Lynch et al. 1977; Dunwiddie and Lynch 1978), spike-timing-dependent plasticity (STDP; see Protocol: Using Multiple Whole-Cell Recordings to Study Spike-Timing-Dependent Plasticity in Acute Neocortical Slices [Lalanne et al. 2016] and Sjöström et al. 2001), and pairing of presynaptic spiking with postsynaptic depolarization (Artola et al. 1990). Note that different induction protocols may recruit different plasticity mechanisms that vary with synapse type, brain region, and age of the animal (Sjöström et al. 2008). These parameters have to be taken into account when comparing results from different studies. For example, whether LTP is expressed pre- or postsynaptically in hippocampus has been hotly debated (Malenka and Bear 2004; Kullmann 2012), but this disagreement can partly be caused by comparisons across different plasticity paradigms.
Extracellular field recordings are ideally used in laminated structures such as hippocampus, where pre- and postsynaptic cells are well separated. Here, this method enables rapid and relatively easy sampling of a large number of synaptic connections. Extracellular field recordings do have some drawbacks compared with patch-clamp recordings, however. Patch-clamp recordings offer cell specificity, the possibility of filling cells with drugs or dyes, etc. (see Protocol: Using Multiple Whole-Cell Recordings to Study Spike-Timing-Dependent Plasticity in Acute Neocortical Slices [Lalanne et al. 2016]; for additional background on both methods, also see Introduction: In Vitro Investigation of Synaptic Plasticity [Abrahamsson et al. 2016]). Ultimately, the choice of method depends on the experiment at hand. For example, it may make little sense to carry out high-throughput drug screening with technically demanding whole-cell recordings; field recordings would be more appropriate. The field recording technique will therefore remain an indispensable tool in the neuroscientist’s toolbox for the foreseeable future.
RECIPES
Artificial Cerebrospinal Fluid (ACSF) for Synaptic Plasticity Studies
Prepare the following 10× stock solution in double-distilled water (ddH2O). Store it at 4°C for a maximum of 1 wk.
| Compound | Concentration of 10× stock |
|---|---|
| NaCl | 1250 mM |
| KCl | 25 mM |
| NaH2PO4 | 12.5 mM |
| NaHCO3 | 260 mM |
On the day of the experiment, dilute the 10× solution 10-fold, bubble for 10 min with carbogen (95% O2/5% CO2), and supplement with the following. (artificial cerebrospinal fluid [ACSF]—in particular Ca2+ and Mg2+ concentrations—may be varied depending on what is to be studied. For example, higher Ca2+ concentration favors long-term potentiation [LTP] whereas lower Ca2+ concentration promotes long-term depression [LTD].) Adjust osmolality to ~320 mOsm with D-glucose for rat and ~338 mOsm for mouse.
| Compound | Final concentration |
|---|---|
| MgCl2 | 1 mM |
| CaCl2 | 2 mM |
| Glucose | ~26 mM |
Acknowledgments
We thank Ian Duguid, Karri Lamsa, Eric Hanse, David Stellwagen, Elvis Cela, and Jérôme Maheux for help and useful discussions, as well as Pauline Vitte and all the students in BIOL389 at McGill University in 2015, for helpful input and for testing this protocol. This work was funded by CFI LOF 28331 (P.J.S.), CIHR OG 126137 (P.J.S.), CIHR OG 130570 (A.J.W), CIHR NIA 288936 (P.J.S.), NSERC DG 418546-2 (P.J.S.), by an RI MUHC studentship award (T.L.), and by the Biology Department of McGill University.
References
- Abrahamsson T, Gustafsson B, Hanse E. AMPA silencing is a prerequisite for developmental long-term potentiation in the hippocampal CA1 region. J Neurophysiol. 2008;100:2605–2614. doi: 10.1152/jn.90476.2008. [DOI] [PubMed] [Google Scholar]
- Abrahamsson T, Lalanne T, Watt AJ, Sjöström PJ. In vitro investigation of synaptic plasticity. Cold Spring Harb Protoc. 2016 doi: 10.1101/pdb.top087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Lu J, Wang YT. Slice orientation and muscarinic acetylcholine receptor activation determine the involvement of N-methyl D-aspartate receptor subunit GluN2B in hippocampal area CA1 long-term depression. Mol Brain. 2011;4:41. doi: 10.1186/1756-6606-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T, Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol. 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/ III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Johnston D, Wu SMS. Foundations of cellular neurophysiology. The MIT Press; Cambridge, MA: 1994. [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Esclapez M, Medina I, Aggoun D, Lamsa K, Leinekugel X, Khazipov R, Ben-Ari Y. A novel in vitro preparation: the intact hippocampal formation. Neuron. 1997;19:743–749. doi: 10.1016/s0896-6273(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. The Mother of All Battles 20 years on: is LTP expressed pre- or postsynaptically? J Physiol. 2012;590:2213–2216. doi: 10.1113/jphysiol.2011.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne T, Abrahamsson T, Sjöström PJ. Using multiple whole-cell recordings to study spike-timing-dependent plasticity in acute neocortical slices. Cold Spring Harb Protoc. 2016 doi: 10.1101/pdb.prot091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Rao D, Manis PB, Philpot BD. STDP in the Developing Sensory Neocortex. Front Synaptic Neurosci. 2010;2:9. doi: 10.3389/fnsyn.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Lee J, Taira T, Pihlaja P, Ransom BR, Kaila K. Effects of CO2 on excitatory transmission apparently caused by changes in intracellular pH in the rat hippocampal slice. Brain Res. 1996;706:210–216. doi: 10.1016/0006-8993(95)01214-1. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Short- and long-term depression of rat cerebellar parallel fibre synaptic transmission mediated by synaptic crosstalk. J Physiol. 2007;578:545–550. doi: 10.1113/jphysiol.2006.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Brown TH. Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J Neurosci Methods. 1998;86:35–54. doi: 10.1016/s0165-0270(98)00143-5. [DOI] [PubMed] [Google Scholar]
- Reid KH, Edmonds HL, Jr, Schurr A, Tseng MT, West CA. Pitfalls in the use of brain slices. Prog Neurobiol. 1988;31:1–18. doi: 10.1016/0301-0082(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Sherwood JL, Amici M, Dargan SL, Culley GR, Fitzjohn SM, Jane DE, Collingridge GL, Lodge D, Bortolotto ZA. Differences in kainate receptor involvement in hippocampal mossy fibre long-term potentiation depending on slice orientation. Neurochem Int. 2012;61:482–489. doi: 10.1016/j.neuint.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Rancz EA, Roth A, Häusser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Skrede KK, Westgaard RH. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Res. 1971;35:589–593. doi: 10.1016/0006-8993(71)90508-7. [DOI] [PubMed] [Google Scholar]
- Stangl C, Fromherz P. Neuronal field potential in acute hippocampus slice recorded with transistor and micropipette electrode. Eur J Neurosci. 2008;27:958–964. doi: 10.1111/j.1460-9568.2008.06067.x. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by “theta” pattern stimulation. Brain Res. 1987;435:227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]