Abstract
Obesity induced low-grade inflammation (metaflammation) impairs insulin receptor signaling (IRS). This has been implicated in the development of insulin resistance. Insulin signaling in the target tissues is mediated by stress kinases such as p38 mitogen-activated protein kinase (MAPK), c-Jun NH2-terminal kinase (JNK), inhibitor of NF-kB kinase complex beta (IKKβ), AMP activated protein kinase (AMPK), protein kinase C (PKC), Rho associated coiled-coil containing protein kinase (ROCK) and RNA-activated protein kinase (PKR), etc. Most of these kinases phosphorylate several key regulators in glucose homeostasis. The phosphorylation of serine residues in the insulin receptor (IR) and IRS-1 molecule results in diminished enzymatic activity in the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. This has been one of the key mechanisms observed in the tissues that are implicated in insulin resistance especially in Type II Diabetes Mellitus (T2-DM). Identifying the specific protein kinases involved in obesity induced chronic inflammation may help in developing the targeted drug therapies to minimize the insulin resistance. This review is focused on the protein kinases involved in the inflammatory cascade and molecular mechanisms and their downstream targets with special reference to obesity induced T2-DM.
Keywords: Obesity, Insulin resistance, Inflammation, Protein kinases
Introduction
Obesity has been implicated as a major risk factor for the development of metabolic syndrome. The incidence of obesity and metabolic syndrome are increasing rapidly in the developed as well as in developing countries [1]. It has been estimated that more than one third of adults and 17% of young population in the United States are obese. Obesity has been recognized as a major health problem in US [2]. Obesity-induced chronic inflammation is critical in the pathogenesis of insulin resistance, diabetes, and metabolic syndrome.
Inflammatory cell infiltration and activation of the pro-inflammatory cytokine network in hypertrophied and hyperplasic adipocytes in obesity along with hypoxia, oxidant stress, and endoplasmic reticulum (ER) stress leads to chronic inflammation [3]. Diseases such as rheumatoid arthritis, multiple sclerosis and type-2 diabetes (T2-DM) are known to be induced by low grade chronic inflammation [4]. Low grade chronic inflammation leads to impaired insulin receptor signaling and metabolic instability termed as “metaflammation” [5]. The intracellular signal transduction pathways are usually activated in response to the inflammation resulting in increased secretion of pro-inflammatory cytokines. Pro-inflammatory cytokines further mediate the cascade of events leading to insulin resistance [6].
Insulin signaling is essential for maintaining glucose homeostasis and the regulation of its metabolism in the liver, muscle and adipose tissues. Inflammation and pro-inflammatory cytokines affect the insulin signaling, thereby modulating glucose absorption [7]. Additionally, the inflammatory signal transduction cascade is known to function at both cellular and molecular level. Hence understanding the regulation of the inflammatory response and its effects on insulin signaling is essential towards developing novel therapeutic approaches to treat the inflammatory mediated metabolic conditions such as T2-DM. This review highlights the major signaling pathways associated with insulin resistance.
Immune cells and cytokines in obesity
The key immune cell population that regulates chronic inflammation and insulin resistance includes macrophages, T cells, dendritic cells (DCs), natural killer T cells (NKTs), B cells, neutrophils, and eosinophils. Apart from their role in mediating the immune response, cytokines secreted by these cells play a major role in obesity induced T2-DM [8]. Studies have shown that the number of tissue macrophages directly correlated with the adipose size in obesity [9]. The hypertrophied adipocyte induced inflammation increases circulating cytokines (TNF-α, IL-6 and IL-1β) and chemokines (MCP-1) [10] levels, ultimately leading macrophage recruitment. Further, macrophage recruitment can also be mediated by neutrophil elastase, a protease secreted by neutrophils plays a potential role in insulin resistance [11]. This contributes to the increased number of tissue macrophages and pro-inflammatory environment thereby playing a key and important role in insulin resistance [12].
The T-cell profile in adipose tissue also plays an important role in obesity and insulin resistance. Pathogenic CD4+ and CD8+ T cells have been implicated in obesity-associated inflammation. CD4+ T cells in adipose tissue secretes IFN-γ and induces the polarization of macrophages towards the M1 classically activated phenotype [13]. Fabbrini et al., [14] demonstrated that there was an increase in Th17 and Th22 cells in adipose tissue of metabolically abnormal insulin-resistant obese subjects. Additionally, the obese state is also characterized by an increased accumulation of Th1 cells and a reduction of regulatory T cells. This changes may influence the cytokine level and their effect on inflammation and insulin resistance [15]. Increased IL-6 and STAT3 levels from T cells subsets have been identified as key mediators in insulin resistance associated with obesity [16]. The pro-inflammatory cytokines from T cells can be synergistically augmented by B cells. DeFuria et al., [17] reported that B cells increase the pro-inflammatory T-cell function in obesity/T2-DM through contact-dependent mechanisms; thereby mediating the obesity induced insulin resistance. NKT cells in adipose tissue were recently identified to be involved in the development of inflammation mediated by obesity, but the molecular mechanism behind the principle remains uncertain [18]. Adipose tissue DCs play a prominent role in obesity mediated chronic inflammation by increasing the production of pro-inflammatory Th17 cells [19].
Thus T cells contribute to obesity induced insulin resistance by increasing the pro-inflammatory M1 macrophages through pro-inflammatory cytokines. This increased pro-inflammatory cytokines and M1 macrophages further enhancing the inflammation. Thus inflammatory cells amplify the axes of inflammation and also contribute in the development of obesity induced insulin resistance.
Protein kinases and inflammatory signaling
The inflammatory signals perceived at cellular level are mediated by the corresponding cytokine and chemokine receptors and intracellular specific kinases. The pro-inflammatory cytokine induced activation of receptors such as IL-1, toll like receptor-4, TNF-α and IL-6 leads to stimulation of downstream protein kinases. These protein kinases further increase the release of additional mediators which increases inflammation [8] and potentially results in insulin resistance. Thus the status of insulin resistance is determined by the type of activated kinases and their downstream regulation [20, 21].
Apart from inflammatory cells, the abnormalities in the lipid metabolism and lipid metabolites do play a role in the pathophysiology of insulin resistance [22]. Inflammatory cells and higher levels of saturated fatty acids (SFA) and lipids, and ceramide (composed of sphingosine and a fatty acid) interlinks the inflammatory and protein kinase pathways involved in obesity induced insulin resistance. Stimulation with SFA, ceramides and liposaccharide (LPS) in pro-inflammatory M1 macrophages induces pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) through inflammatory protein kinase cascades [23]. Therefore, SFA and its derivative ceramide converge with inflammatory pathway IKKβ/NFκB, JNK1/AP1 and PKC signaling pathways to stimulate inflammation and thus can potentially induce insulin resistance [24].
Insulin sensitivity and resistance depends on the specific kinases such as AMP-activated protein kinase (AMPK), IκB kinase (IKK), protein kinase C (PKC) and mitogen-activated protein kinases (MAPKs) that acts on the insulin receptor substrate [8]. Similarly the role of Rho associated coiled-coil containing protein kinase (ROCK), [25, 26] and RNA-activated protein kinase (PKR) [27] in pathogenesis of insulin resistance has been documented. Insulin mediated activation of intrinsic tyrosine kinase in the insulin receptor (IR) leads to tyrosine phosphorylation of IRS1. This further activates its substrates phosphatidylinositol 3-kinase (PI3K) and Akt, leading to increased glycogen synthesis, glucose uptake and protein synthesis [28]. AMPK is considered a positive regulator of insulin sensitivity. It is reported to be associated with increased GLUT4 translocation and subsequent glucose uptake [29]. Studies reported that dysregulation of AMPK as the central mechanism behind the insulin resistance mediated diabetes [30].
The mitogen-activated protein kinases (MAPKs) are well known for their role in inflammatory responses through phosphorylation of serine/threonine residues of target proteins [31]. The deficiency of MAPKs has been associated with reduced insulin sensitivity [32]. In insulin resistant conditions, serine kinases such as IB kinase (IKK) and JNK become activated by pro-inflammatory stimuli [33] and impair insulin sensitivity. Thus, understanding the interplay between inflammation, obesity and protein kinases, and identifying the specific protein kinases involved in phosphorylation may help in developing the targeted drug therapies to minimize the insulin resistance. This might play a key role in the prevention of diabetes.
AMP activated protein kinase (AMPK) and its upstream kinases
AMPK is a serine/threonine kinase composed of α, β and γ subunits [34]. The AMPKs are activated in the cytoplasm by upstream kinase mediated phosphorylation of Thr172 on the serine/threonine protein kinase domain at its N-terminus [35]. The upstream kinases of AMPKs includes liver kinase B1 (LKB1), the calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ), and the transforming growth factor-beta-activated kinase 1 (TAK1) [36–38]. The role of AMPK and its upstream kinases using in-vitro and in-vivo models are highlighted in Table 1.
Table 1.
Functional validation of kinases in obesity associated complications
| Sl. No | Research Model | Observation | Reference |
|---|---|---|---|
| 1. | Over expression of AMPKα2 cDNA in mice | Decrease in blood glucose level with increase in hepatic lipid metabolism | [39] |
| 2. | Over expression of AMPKα1 hyperlipidemic type 2 diabetic rats | Decrease in blood glucose concentration, plasma and hepatic triglyceride content. | [40] |
| 3. | AMPK α1/2 siRNA transfected myotubes | Decreased Chlorogenic acid mediated glucose transport | [41] |
| 4. | CaMKKβ Over expressed in CCL13 cells | Increased phosphoryation of AMPK | [37] |
| 5. | Overexpression of AMPK in pancreatic islets of mice | Decreased β-cell function | [42] |
| 6. | Genetic inhibition of PKC-ζ | Inhibited metformin mediated phosphorylation of AMPK-Thr172 and its upstream kinase | [43] |
Many studies have demonstrated the role of activated AMPK in regulating the glucose metabolism. Activated AMPK controls the glucose uptake by increasing expression and translocating GLUT4 or by phosphorylation of the 160 kDa Akt substrate [44] (Fig. 1). AMPK also contributes to glucose control by reducing gluconeogenesis through suppression of the expression of glycolytic genes encoding phosphoenol pyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) [45]. AMPK enhances insulin sensitivity either by directly regulating PI3K or by suppressing the negative feedback loop of IRS1 regulation via inhibition of mTOR/S6K [46]. Along with regulating insulin signaling, AMPK is an upstream kinase for metabolic enzymes such as acetyl-CoA carboxylase (ACC) [47] and HMG-CoA reductase [48]. It also regulates the fatty acid synthesis and plays a potential role in hepatic steatosis [49]. Glucagon mediated inhibitory protein kinase (PKA) increases the inhibitory phosphorylation of AMPK Ser173 and reduces the activating phosphorylation of AMPK Thr172 in the regulation of insulin signaling [50].
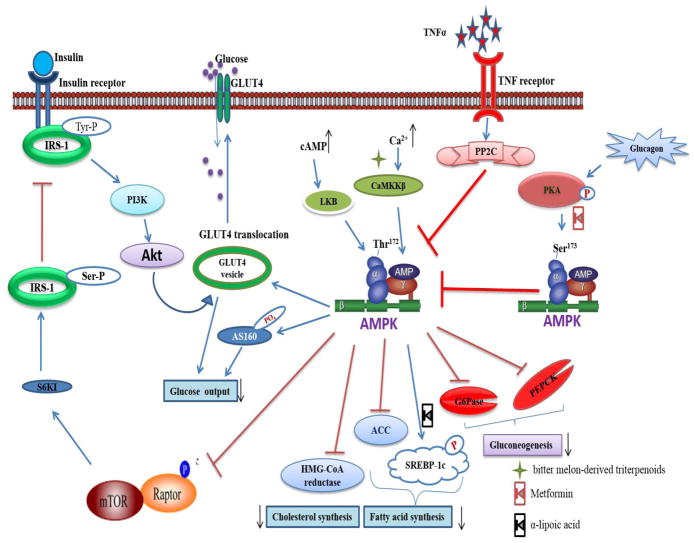
Fig. 1.
AMPK mediated regulation of glucose uptake. Under increase cAMP or Ca2+ content, AMPK gets activated by Thr172 phosphorylation via the upstream kinases LKB, CaMKKβ and TAK. The positive phosphorylation improves glucose uptake by either increasing the expression of GLUT-4 or by phosphorylation of Akt substrate of 160 kDa (AS160). AMPK promotes PI3K mediated GLUT-4 translocation indirectly by inhibiting the mTOR mediated inhibitory Ser phosphorylation of IRS-1. AMPK is the upstream kinase of factors important for gluconeogenesis, Fatty acid and cholesterol synthesis and down regulates their actions. The glucagon mediated PKA phosphorylation induces the inhibitory phosphorylation of AMPK Ser173 and reverses the whole positive effect of AMPK. Obesity induced low grade inflammatory signals such as TNFα are involved in PP2C mediated inhibition of AMPK Thr172 phosphorylation. The metformin has been recognized as PKA inhibitor and α-lipoic acid was noticed to inhibit SREBP activation. Whereas bitter melon-derived triterpenoids activates the CaMKKβ.
Expression of AMPK is essential for decreasing the pro-inflammatory stimuli (TNFα, IL-6 and IL-1) and increasing the production of the anti-inflammatory cytokine, IL-10 [51, 52]. Stimulation of inflammatory signals down regulates the AMPK activity [51]. The reduction in expression/activity of AMPK in inflammatory cells in obesity can potentially lead to development of inflammation induced diabetes [53]. The pro-inflammatory cytokine, TNFα, reduces the phosphorylation of AMPK Thr172 that was reported to suppress the activation of AMPK via protein phosphatase 2C (PP2C) [54]. Increased TNFα leads to PP2C mediated inactivation of AMPK, which increases fatty acid levels and potentially insulin resistance. However, such reduction in AMPK activity was not noticed in TNF receptor knockdown mice which indicates that AMPK reduction is multifactorial and several other pro and anti-inflammatory mediators may play important role. A recent study shows that AMPK in macrophage promotes FA oxidation to reduce macrophage inflammation and insulin resistance [55].
AMPK regulates insulin homeostasis by reducing the phosphorylation of mTOR in the cytoplasm. Increased mTOR signaling has been implicated in the pathogenesis of obesity and the development of insulin resistance in metabolic syndrome [56] and modulation of mTOR signaling may suppress insulin resistance and T2-DM [57] The disruption of mTORC1 signaling in macrophages reduce inflammation and insulin resistance by inhibiting JNK/NFκB pathway activation in obesity [58]. The interplay between NF-κB signaling pathway and liver AMPK/mTOR/autophagy axis in relation to hepatic steatosis and insulin resistance has also been discussed [59]. The role of suppressing mTOR/SREBP-1 mediated lipogenesis in the liver and restoring insulin signaling in skeletal muscle has also been shown [60]. These studies suggest the role of mTOR in insulin resistance pathogenesis. Understanding the mTOR signaling may provide future therapeutics and interventions against obesity, insulin resistance, and diabetes.
Most of the anti-diabetic drugs reported to date target and activate AMPK indirectly by inhibiting mitochondrial Complex I. Side effects of the existing drugs could be eliminated by identifying anti-diabetic agents which could activate AMPK directly without affecting mitochondrial respiration [61]. Though activation of muscle and liver AMPK is advantageous [62] in terms of glucose uptake, its activation in pancreas inhibits glucose stimulated insulin secretion and β-cell function in vivo [42]. Treatment with Astragalus polysaccharide induces phosphorylation of AMPK Thr172 along with the upstream kinases CaMKKβ and LKB1 and results in increased glucose uptake [63]. Since inhibition of satellite cell-specific AMPKα1 is involved in the obesity induced muscle degeneration, preventing its inhibition and enhancing the activation of AMPK may be useful [64].
In summary, AMPK is a positive regulator of glucose uptake and its activity depends on its phosphorylation state and inflammatory molecules are the major contributor of AMPK inhibitory phosphorylation. Other than the inflammatory molecules, kinases mediating the synthesis of inflammatory cytokines are also considered as a source of AMPK inhibitory phosphorylation. Thus decrease in AMPK activation leads to activation of pro-inflammatory downstream signaling pathways like IKK, PKCs, MAPK (ERK, JNK, p38) and results in obesity induced insulin resistance.
IκB kinase (IKK)
The transcription factor NF-κB is an important mediator of the inflammatory response. Under normal conditions NF-κB remains inactive in the cytoplasm by interacting with IκB proteins. The subtypes of IκB include IκBα, IκBβ and IκBε and their phosphorylation is essential for activating NF-κB. Phosphorylated IκB undergoes ubiquitination mediated proteasomal degradation which allows for NF-κB translocation into the nucleus [65, 66]. IκB kinase (IKK) has been identified as the upstream kinase of IκB. To date, four different IKK namely IKKα, IKKβ, IKKε and TANK-binding kinase 1(TBK1) have been reported [67].
In addition to NF-κB activation, IKKs are also involved in the phosphorylation of other substrates [68], one such target being IRS1 [69]. IKKβ-mediated serine phosphorylation of IRS1 inhibits tyrosine phosphorylation of IRS1 and subsequent insulin signaling (Fig. 2). The emerging studies from the past decade have revealed the role of IKK mediated activation of NF-kB in obesity induced metabolic disorders especially diabetes. Chiang et al. [70] reported the role IKKβ in high-fat diet induced NF-kB activation subsequently increased IKKβ in liver, adipocytes and adipose tissue macrophages suggesting it as an attractive therapeutic target for obesity induced diabetes.
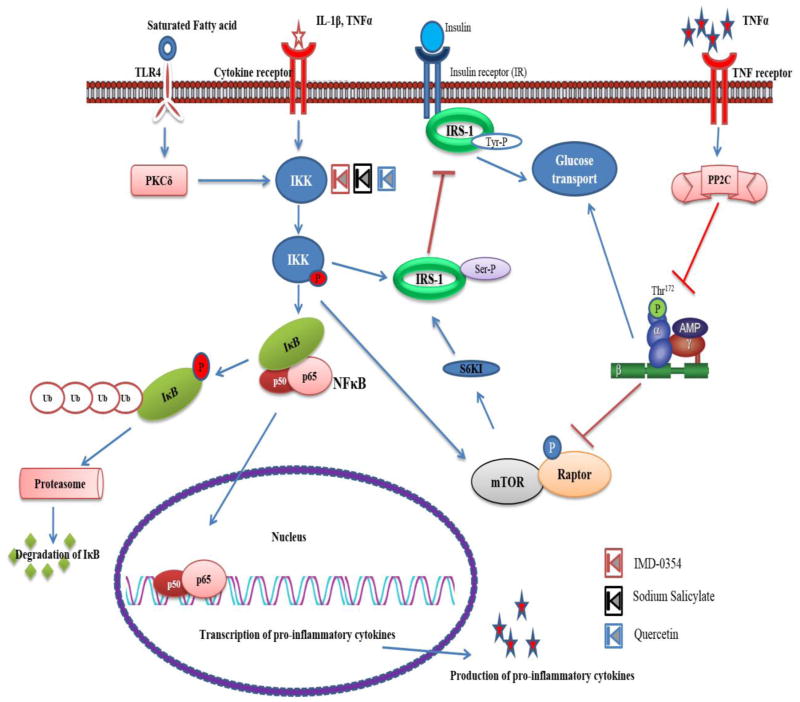
Fig. 2.
IKK mediated insulin resistance under the influence of inflammatory signal. The inflammatory signal perceived at the receptor activates IKK and leads to subsequent activation and nuclear translocation of NFκB. The NFκB mediated transcription of inflammatory cytokines mediates insulin resistance via inhibitory phosphorylation of AMPK. The compound IMD-0354, Sodium Salicylate and Quercetin inhibits the IKK.
Studies with IKKβ knockdown mice revealed an increase in the level of adiponectin and hepatic insulin sensitivity [71]. Absence of IKKβ in hepatocytes provides local insulin responsiveness, whereas absence of IKKβ in myeloid cells provides protection towards the global insulin resistance in a mouse model [72]. Knocking out the interferon regulatory factors 3 (IRF3) results in diet induced hepatic insulin resistance and steatosis. Conversely over-expressed IRF3 interacts with IKKβ in the cytoplasm and preserves glucose and lipid homeostasis in liver [73]. The cytoplasmic interaction of IRF3 and the kinase domain of IKKβ represses the inhibitor of nuclear factor kappa B kinase beta subunit/nuclear factor kappa B (IKKβ/NF-κB) signaling and plays a regulatory role in insulin resistance. Kamon et al. [74] documented that inhibition of IKKβ ameliorates TNFα-mediated down-regulation of adiponectin secretion and stimulates insulin-stimulated Akt activity in adipocytes. This suggests that inhibitors of IKKβ reduce insulin resistance by targeting the TNFα signaling pathway. In normal conditions the IKKβ/NF-kB complex remains inactive in the hypothalamus, whereas in obese condition IKKβ/NF-kB gets activated and disrupts insulin/leptin signaling [75]. Recent report have shown that using SC-514 [76] and peptide-based inhibition [77] of IKK attenuated NF-κB activation with suppressed inflammation and reduced development of long-term diabetes inflammatory complications.
Though there are reports on IKKβ as a potent target of obesity induced insulin resistance, Rohl et al. [78] noticed that expression of muscular IKKβ is not essential for obesity induced insulin resistance. In addition to the existing, firmly established IKKα and IKKβ, Reilly et al., [67] explained the role of the additional kinase IKKε. Regulation of IKKε in the hypothalamus of obese mice has been recently analyzed and reported as the main inflammatory mediator in the hypothalamus. In both liver and fat of IKKε knockdown mice, the modulated gene was noticed as the target gene for IRFs regulation but its mechanism remained unclear [79]. Studies by Chiang et al., [70] revealed higher levels of IKKε in liver and adipose tissue of high-fat diet induced obese animal models. IKKε knockout (KO) mice are protected from the diet induced obesity and insulin resistance. Inhibition of IKKε with either chemical means on small interfering RNA suggested a reduction in the inhibitory phosphorylation of IRS1Ser307 and insulin resistance via IR/IRS-1/Akt and JAK2/STAT3 pathways [80].
These results and literature clearly suggest that IKK plays a tissue specific role and identification of the correct IKK isoform and its specific inhibitor with global effect can prevent obesity induced insulin resistance and subsequent development of metabolic syndrome. Furthermore, TNFα produced via IKK signaling inhibits AMPK Thr172 phosphorylation and increases the level of saturated fatty acid (SFA) [23]. Thus suppression of IKK in target tissues associated with obesity can be a potential therapeutic target for the treatment of insulin resistance in obesity.
Role of Protein kinase C in obesity and insulin resistance
Fatty acid metabolites are known to impair insulin signaling by tissue-specific activation of specific isoforms of protein kinase C (PKC) in the cytoplasm. Lipid dependent PKC activation has been associated with insulin resistance in type 2 diabetics [81]. To date, 12 isoforms of three major groups: conventional/Ca2+ and phospholipid dependent (α, βI, βII and γ), novel/Ca2+ independent and phospholipid dependent (δ, ε, η, θ and μ), and atypical/Ca2+ and phospholipid independent (ζ and ι/λ) have been reported [82].
In skeletal muscle, PKCα is the most abundant among the various isoforms of PKC and its activation is associated with high FFA content. Activation of PKCα mediates the phosphorylation of IRS1 (IRS1 Ser307, IRS1Ser1101) [28]. Similar to PKCα, PKC-θ was noticed to be activated by cytosolic diacylglycerol and induced by direct phosphorylation of IRS1Ser1101 [83]. The isoform PKCβ is the upstream kinase for Jun amino-terminal kinase, IKK mitogen/extracellular-regulated kinase. These isoforms promote the respective Ser307 and Ser612 phosphorylation of IRS1 [84]. Increased expression of PKCβ is associated with the increase in white adipose tissue mass and its knock-out results in reduction in triglyceride level and WAT mass [85].
Among the various isoforms of PKC, the skeletal muscle isoform was found to be enriched with the novel/Ca2+ independent and phospholipid dependent PKC-θ [86], while the PKCε was shown to be expressed in liver, adipocytes and 3T3 fibroblasts [87]. Kumashiro et al., [88] reported that activation of PKCε increased proportionally with hepatic diacylglycerol (DAG) content, which is a predictor of insulin resistance. Using antisense oligonucleotides against liver PKCε, it has been demonstrated that targeting PKCε with a specific inhibitor could be a novel therapeutic target for fat-induced hepatic insulin resistance and type 2 diabetes [87]. Although other PKCs are reported to induce insulin resistance by mediating inhibitory phosphorylation of IRS Ser307/612/1101, PKCε mediates insulin resistance by directly targeting the expression of insulin receptor (IR) [28] (Fig. 3). However, some studies also reported the inverse correlation between IR expression and PKCε level in diabetic obese rats has also been reported [89].
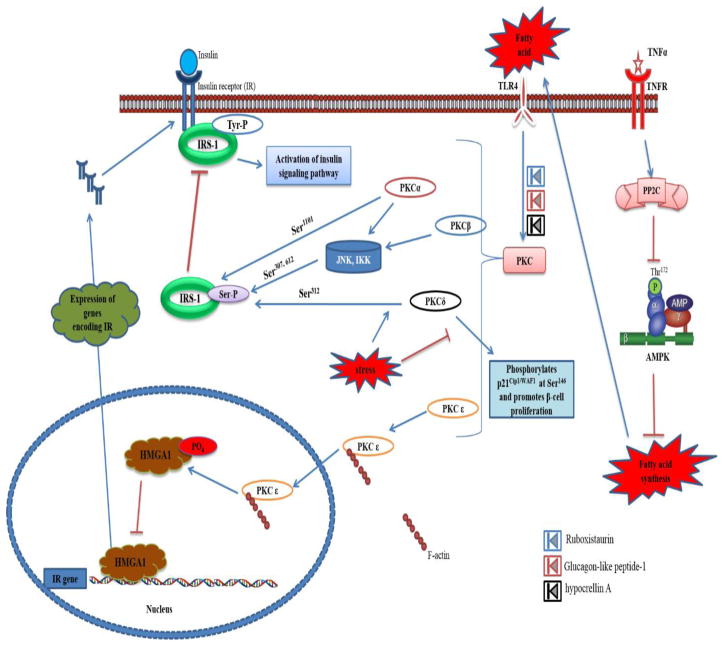
Fig. 3.
Role of PKC in insulin resistance. Upon activation by the fatty acids, PKC α,β,δ either directly involves in inhibitory phosphorylation of IRS or activates JNK or IKK for their subsequent Ser phosphorylation of IRS. Whereas the FA activated PKCε translocates to nucleus with the help of F-actin and suppresses the transcription of insulin receptor by phosphorylating the HMGA1. The activation of PKCs were inhibited by Ruboxistaurin, Glucagon-like peptide-1 and hypocrellin A.
The High-mobility group AT-hook 1 (HMGA1) protein has been recognized as a transcriptional regulator of IR gene expression and its defect decreases IR expression with an increase in type 2 diabetes mellitus susceptibility [90]. Under the phosphorylated state, the DNA binding ability of HMGA1 declines and thus the mechanism behind the phosphorylation/dephosphorylation of HMGA1 will determine the expression of IR [91]. Upon FA induced phosphorylation, PKCε translocates to the nuclear region and mediates phosphorylation of HMGA1. The absence of a nuclear localization signal (NLS) makes it unclear as to how PKCε is mobilized to the nuclear region for HMGA1 phosphorylation. This could be explained by recent studies conducted by Dasgupta et al., [92] which reported that F-actin recognizes the phosphorylated form of PKCε and mobilizes it to the nuclear compartment.
Increased proliferation of pancreatic endocrine cells is an essential event to fulfil insulin demand. In stress-free conditions PKCδ displays a specific dual role; it mediates the inhibitory phosphorylation of p21Cip1/WAF1 (cell cycle inhibitor) at Ser146 and promotes β-cell proliferation by nuclear extrusion and induced cytosolic accumulation of p21Cip1/WAF1 [93]. Under FFA induced stress however, PKCδ promotes nuclear accumulation of pro-apoptotic factors [94]. Mingo-Sion et al., [95] reported PKCδ mediated inhibition of insulin receptor signaling in diabetic models via IRS-1 Ser312 phosphorylation. Genetic inhibition of PKCδ with RNA interference and pharmacological inhibition with rottlerin revealed a decline in p21Cip1/WAF1 Ser146 phosphorylation increasing nuclear accumulation and subsequent apoptosis [96]. On the other hand, PKCδ WT transgenic mice overexpressing PKCδ increased cytosolic accumulation of the cell cycle inhibitory p21Cip1/WAF1. Different splice variants of PKCδ involving in apoptosis (PKCδI) and pro-survival pathway (PKCδII and PKCδVIII) have also been reported [97, 98]. Patel et al. [99] demonstrated that TRA2B regulated splice variants PKCδI is essential for clonal expansion of pre-adipocytes and its expression decreases with terminal differentiation. The molecular switch which operates the dual role of PKCδ is unclear and has to be elucidated in order to facilitate favorable tuning of PKCδ activation. Further studies to identify the molecular mechanisms behind the alternative splicing will be helpful in the development of a novel therapeutic target for management of obesity induced insulin resistance.
To summarize, PKCs activation by ongoing inflammation results in IRS-1 Ser phosphorylation. This leads to decrease in binding of insulin with its receptor. Activated PKCs also enhance the production of pro-inflammatory mediators, thus increasing the inflammation contributing to obesity induced insulin resistance.
Mitogen activated protein kinase (MAPK) and insulin signaling
MAPKs have now been identified in a wide range of inflammatory disease states ranging from cancer to obesity induced diabetes. Broadly, MAPKs consist of seven families and are classified into two groups [100]. The first group, referred to as classical MAPKs, includes extracellular regulated kinase 1/2 (ERK1/2), p38 kinase, c-Jun N-terminal kinase (JNK) and ERK5. The second group, referred as atypical MAPKs, consists of ERK3, ERK4, ERK7 and Nemo-like kinase (NLK). Activation of inflammatory responsive MAPKs is mediated by protein kinase cascades containing a series of three or more upstream protein kinase; MAP3Ks activate MAPK MAP2Ks by dual phosphorylation on serine/threonine residues, MAP2Ks then activate MAPKs by dual phosphorylation on tyrosine and threonine residues. The phosphorylation in target amino acid varies with specific MAPKs. Though most of the MAPKs require dual phosphorylation of threonine and a tyrosine at T-X-Y motif, ERK3 and 4 as well as NLK require a single tyrosine as the phosphorylation site [101]. Activated MAPKs can either phosphorylate the subsequent protein kinase or non-protein kinases such as transcriptional factors. Downstream of the activating stimuli, the kinase cascades may themselves be stimulated by combinations of small G proteins, MAP4Ks, scaffolds, or oligomerization of the MAP3K in a pathway [102]. Activation of upstream kinases of the MAPK cascade, MAPKs and the target proteins has been reviewed by Broom et al. [101]. The role of protein kinase Map4k4 has been further discussed in obesity-induced hyperinsulinemia and insulin resistance by promoting insulin secretion from β cells [103]. Involvement of the MAPK signaling pathways has been recently reported in mediating the beneficial effects of tartary buckwheat flavonoids on redox balance and insulin resistance in insulin-resistant HepG2 cells [104].
Role of ERKs in glucose metabolism and insulin resistance
The extracellular signal-regulated kinase (ERK) signaling pathway in the cytoplasm is activated by inflammatory signals. It activates downstream kinases and transcriptional factors, and mediates insulin resistance in target tissues (Fig. 4). Activation of ERK1/2 (p44/42 mitogen-activated protein kinases) has been characterized as a key event in insulin signaling and diabetes. ERK1/2 activation requires phosphorylation at Thr202 and Tyr204. The activation cascade comprises of two upstream kinases, MEK1/2 and a upstream Raf kinase (ERK1/2 kinases kinases) [105, 106]. The Raf kinase is the only MAP3K recruited by receptor tyrosine kinases for activation of the ERK pathway. It plays a major role in glucose uptake and metabolism in liver and skeletal muscles, regulates insulin secretion as well as insulin production at the transcriptional and translational levels. The hormone mediated insulin secretion also increases ERK1/2 activity [107], thus playing an important role in insulin signaling and metabolism.
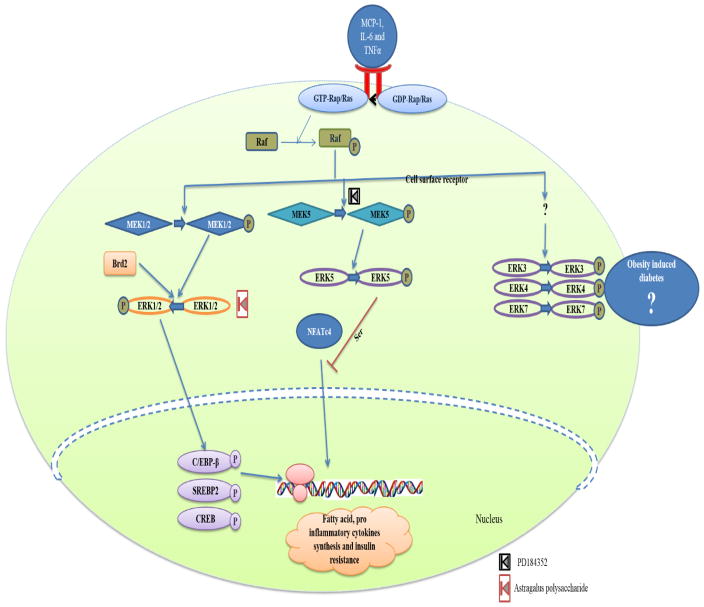
Fig. 4.
Role of ERK in obesity mediated diabetes. The inflammatory signals received as the result of obesity phosphorylates the Raf (MAP3K) via activation of G-protein coupled receptor. Activated MAP3K activates ERKs via activation of ERKs specific MAP2Ks (MEKs). The Brd involves in raf independent activation of ERKs. Activation of ERK1/2 phosphorylates its target transcriptional factors and mediates production of fatty acid, inflammatory cytokines and mediates development of Insulin resistance. ERK5 prevents the above insulin resistance by preventing the nuclear translocation of AP1 synergic partner NFATc4 via ser phosphorylation. The Astragalus polysaccharide was reported to inhibit the activity of ERK and the compound PD184352 inhibits the activity of MEK.
Elevated blood glucose levels lead to calcium entry and calcineurin induced ERK activation. The other nutrients which influence blood glucose concentration and hormones like glucagon-like peptide I (Glp1) are also involved in ERK activation [108]. The activation of ERK1/2 decreased the responsiveness of insulin gene promoter by elevated glucose levels and induce insulin resistance [109]. This inhibition of insulin gene promoter is induced by expression of CCAAT/enhancer binding protein beta (C/EBP-β). This protein binds to the insulin gene promoter in an ERK1/2-dependent manner and regulates insulin transcription in beta cells. In type II diabetes, ERK1/2 also inhibits insulin gene transcription by phosphorylating the factors that inhibit the insulin gene promoter. Insulin-activated ERK1/2 phosphorylates SREBP2 [110] and SREBP1a, thereby enhancing their transactivation potential and linking ERK1/2 signaling with key regulators of lipid metabolism. Phosphorylation of ERK1/2 is mediated by Raf, however it can be Raf independent [111]. Raf independent phosphorlylation is mediated by Bromodomain-containing protein 2 (Brd2). Brd2 is a nuclear serine/threonine kinase which also regulates the activation of ERK1/2 with special reference to adipogenesis. Although, the role of ERK and its activation in mediating the insulin resistance has been studied in detail, there is a need to further investigate the physiological significance of these modifications in-vivo, as well as the effect of inhibition of ERK on insulin resistance. Recently Liu et al., [112] reported, serum and glucocorticoid-regulated protein kinase 1 (SGK1) negatively regulate the activation of ERK1/2 and play a significant role in insulin signaling.
ERK-5 is also termed as big MAP kinase 1 (BMK1). The basic function of ERK5 relates to endothelial function and vascular integrity [113]. It has been documented that the expression of ERK5 in endothelial cells in heart, placenta, lung, kidney and skeletal muscle tissue remains dominant [114]. ERK5 could regulate the signaling either by phosphorylating further downstream kinases or by transcriptional activation of the target genes. Activation of ERK5 is similar to other MAPKs and is mediated by dual phosphorylation at Thr218/Tyr220 via a specific upstream kinase MEK5 [115]. ERK5 has an opposing action to calcineurin on NFATc4-mediated gene transcription which changes the levels of adipokines expression. The deletion of ERK5 increased adiposity with dysregulation in adipokines secretion, leptin resistance, and impaired glucose handling [116]. It has been identified that above effect of ERK5 in diabetic nephropathy is mediated by TGFβ1 induced phosphorylation of MEK5 without involvement of the MAP3K Ras [117]. There are no extensive studies on ERK3, ERK4 and ERK7, hence there is a need to study their role in insulin resistance.
Taken together, ERK has a bimodal activity. ERK1/2 activation by obesity induced inflammation induces the production of free fatty acids and pro-inflammatory cytokines. This leads to increased inflammation and insulin resistance. Whereas, ERK5 activation alternates the nuclear translocation of AP-1 and decrease the production of pro-inflammatory cytokines.
Obesity, insulin resistance and JNK
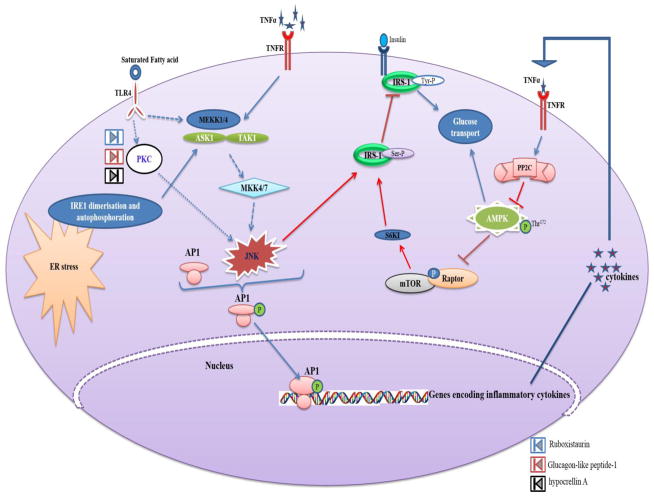
Studies have suggested the role of cJun NH2-terminal kinase (JNK) in the mechanism of obesity-induced insulin resistance [118]. A high fat diet causes activation of the JNK1 signaling pathway leading to insulin resistance and obesity, and manifests a chronic low-grade inflammatory response. This results in activation of stress protein kinase pathways (including the cJun NH2-terminal kinase JNK1) in the cytoplasm and plays a critical role in the pathogenesis of obesity-induced insulin resistance [119] (Fig. 5). Three isoforms of JNKs include JNK1, JNK2 and JNK3. JNK1 and JNK2 are expressed ubiquitously, whereas JNK3 is expressed in a limited number of tissues, including brain and heart [20].
Fig. 5.
Activation of JNK in response obesity induced inflammatory signals and its role in insulin resistance. JNK gets activated by four routes; in first route the TNFα mediated activation of MAP3Ks (MEKK1/4, ASK1 and TAK-1) activates JNK via MAP2K (MKK4/7); in second route JNK activation occurs by Endoplasmic Reticulum (ER) stress mediated activation of MAP3K (ASK1); in third route, MAP3K (MEKK1/4) activated by TLR4 with perception of fatty acid signals activated the JNK; in fourth route TLR4 perceives the fatty acid signal and activates JNK independent of MAP3K via PKC. The activated JNK promotes nuclear translocation of AP1 transcription factor and mediates synthesis of inflammatory cytokines responsible for development of insulin resistance. In addition to induction of inflammatory cytokines mediated impairment in insulin signaling, JNK induces insulin resistance by direct inhibitory ser phosphorylation of IRS-1. The Ruboxistaurin, Glucagon-like peptide-1 and hypocrellin A inhibits the activity of PKC, thereby further activation of JNK will be attenuated.
There are several potential mechanisms involved in JNK activation in obesity. High fat diet induces ER stress [120] and the unfolded protein response (UPR) pathway that leads to JNK1 activation [121]. Saturated fatty acids might act as ligands for toll-like receptors that activate the JNK pathway [122]. These fatty acids can also activate the JNK pathway by protein kinase C-mediated activation of the mixed-lineage protein kinase (MLK) group of MAP3Ks [123] and subsequent JNK activation is mediated by the JIP1 scaffold protein [124]. HFD-induced insulin resistance is associated with chronic low-grade inflammation and the expression of inflammatory cytokines such as tumor necrosis factor [125] and adipokines can cause JNK activation [20]. The relative contribution and mechanistic relationships between these above mentioned pathways remains to be determined.
In the experimental approach to confirm the role of JNK, it has been demonstrated that Jnk1−/− mice are protected against HFD-induced insulin resistance [126]. Specifically, feeding a HFD to mice with tissue-specific Jnk1-deficiency in insulin target tissues (e.g. fat and muscle) causes casual obesity, yet these mice exhibit elevated insulin sensitivity compared with non-ablated mice [127]. In Jnk2−/− mice, though JNK2 is involved in metabolic regulation but its function could be masked by complete expression of JNK1 [128]. Similarly, a recent study revealed that though JNK deficiency in macrophages does not alter the obesity in HFD mice, but improves insulin sensitivity [129]. In spite of its role in the development of type 2 diabetes development, disruption of the Mapk9 gene encoding JNK2 resulted in protection against autoimmune destruction of β-cells by maintaining the Th1/Th2 balance [130]. In contrast to the well documented negative roles of JNKs (JNK1 and JNK2) in an obesity associated signaling pathway, JNK3 has been reported to be a mediator of anti-apoptotic activity in insulin-secreting cells [131] and a potential role of JNK3 as a candidate marker to assess islet quality prior to transplantation has been reported [132]. These results suggest that JNK2 is an important contributor for both T1-DM and T2-DM. Knockout and inhibition of JNK1 reduces HFD adiposity, improves glucose tolerance and insulin sensitivity suggesting the crucial role of JNK1 in development of insulin resistance.
P38 kinases and insulin resistance
The p38 is among the stress activated serine/threonine protein kinase MAPKs. Its family is composed of four members, α, β, γ and δ. Expression of the isoforms of p38 varies between tissues; the α and β isoforms are expressed ubiquitously, the γ isoform is specific to skeletal muscle and the expression of δ isoform was found in the testes, pancreas and small intestines [133]. The p38 MAPKs are activated by the MAP3K and MAP2K cascade (Fig. 6). They are subject to activation by one of the several upstream MAP3Ks such as TAK1, ASK1, LK3, MEKK1-4 and TAO1-3 [134]. The activation of p38 MAPKs results in lipotoxicity in adipocytes and stellate cells. Significantly increased phosphorylation of p38 MAPK has been found in adipose and bowel tissue [135]. The p38 MAPK plays important roles in regulating glucose metabolism in skeletal muscle and adipose tissue. Studies with cultured skeletal muscle cells revealed that p38 MAPK enhances insulin and exercise or AMPK-induced glucose uptake by activating GLUT4 [136, 137].
Fig. 6.
MAPK p38 activation in response to obesity induced inflammation. As a result of inflammatory stress mediated by TNFα, MAP3Ks (MKK6, TAK1 and MKK4) gets activated. Further the MAP3Ks activates the respective MAP2Ks. The phosphorylation of MAP2Ks activates the specific p38 MAPKs, which consequently activates the transcription factors of genes involved in synthesis of cytokines and fatty acid. Inspite of the inflammation mediated activations, p38 MAPKs are even activated by oxidative stress released by NADP oxidase. The compound GS-444217 inhibits the activity of ASK1 and the 5Z-7-oxozeaenol inhibits the activity of TAK1
Insulin has been identified as a regulatory molecule for ERKs and p38 in adipogenesis, but the molecular mechanism has not been fully characterized. Recently, Liu et al. [138] reported the role of Steroid Receptor RNA Activator (SRA) in adipogenesis. Overexpression of SRA inhibits the phosphorylation of p38 MAPK and JNK and SRA knockdown results in reduced insulin receptor levels with decreased phosphorylation of IRS-1 and Akt resulting in the activation of p38 MAPK and JNK. Inhibition of p38MAPK has been discussed as a novel strategy to alleviate FFA induced hepatic insulin resistance in obesity-associated disorders [139]. Makeeva et al., [140] reported that nitric oxide-induced p38 activation promotes TAB1 independent activation of ERK in β-cell death resulting in decreased insulin secretion. MAPKs are important in the development of insulin resistance in skeletal muscle tissue, via a p38 MAPK-dependent mechanism.
These studies suggest the important roles of p38 MAPKs in obesity and insulin resistance. However, it has been reported that activation of p38 MAPK in the livers of obese mice reduces ER stress in obese and diabetic mice [141]. This positive effect of p38 MAPK is mediated by Thr48 and Ser61 phosphorylation of X-box binding protein 1 (XBP1s) transcriptional factor and is associated with improvement in its nuclear migration. XBP1s is an important regulator of adiponectin multimerization and its over expression improves glucose tolerance and insulin sensitivity in both lean and obese (ob/ob) mice [142]. Since there are mixed results in relation to the role of MAPKs in obesity and insulin resistance, there is a need to further investigate their effects on cellular metabolism in obesity mediated type 2 diabetes and insulin resistance.
Rho kinase (ROCK)
Rho-kinase (ROCK) is a Serine/Threonine protein kinase with two isoforms, ROCK1 (ROCKβ) and ROCK2 (ROCKα) [143]. ROCK1 has been suggested as a novel regulator of glucose homeostasis and insulin sensitivity [25]. The depletion of ROCK1 in mice results in decreased insulin-stimulated PI3K associated IRS-1 signaling, and decreased phosphorylation of Akt, AS160, S6K, and S6 in the skeletal muscle, thereby causing systemic insulin resistance by impairing insulin signaling [25]. In muscle of obese diabetic human subjects, insulin stimulated ROCK1 was noticed to be decreased with up-regulation of its kinase inhibitor RhoE [144]. These studies suggest that decreased ROCK1 in the skeletal muscle is associated with insulin resistance. However, the role of ROCK1 in adipose tissue is suggested to be inducing insulin resistance. These have been reported in diet induced or genetically obese mice where increased ROCK1 mediated negative regulation of insulin signaling [26] The role of ROCK2 also seems to induce obesity mediated insulin resistance and cardiac dysfunction [145]. The role of ROCK in insulin homeostasis appear to be dependent on tissue specificity and isoform function [145]. Further studies are needed to determine the role of ROCK and to develop further potential target strategies to improve insulin resistance.
RNA-activated protein kinase (PKR)
Inflammatory pathways involved in obesity induced insulin resistance are also regulated by the activation of double-stranded RNA-dependent protein kinase (PKR), a pattern recognition receptor (PRR) [146]. PKR is a key constituent of the metaflammasome, activated by nutrients such as fatty acids and by ER stress [147]. The interaction between the PKR, and inflammatory kinases, such as IKK and JNK, IRS1 has also been reported [148]. The absence of PKR leads to decreased activation of JNK and IKKβ which are key kinases in the development of insulin resistance. This suggests that PKR is an important modulator of insulin signaling in obesity [27]. Activation of PKR has been associated with the inhibition of pancreatic β-cell proliferation through sumoylation-dependent stabilization of P53 in the development of T2-DM [146]. PKR also directly interacts with insulin receptor signaling components and inhibits insulin action [149]. These studies suggest the role of PKR activation in the pathogenesis of the insulin resistance, and inhibition of the PKR activation may be a potential therapeutic approach to improve insulin resistance. This has been reported in mice model with small molecules inhibitors of PKR [147].
Recent therapies targeting protein kinases to wrestle against obesity induced type2 diabetes
A wide range of therapeutic molecules targeting the phosphorylation of obesity-associated kinases has been reported to act against insulin resistance and type 2 diabetes mellitus (Table 2).
Table 2.
Molecules reported with overcome obesity induces diabetes and their respective target protein kinases
| Sl. No | Therapeutic molecule | Therapeutic effect | Target Kinase | Model | Reference |
|---|---|---|---|---|---|
| 1. | Astragalus polysaccharide | Stimulates glucose uptake | AMPK | In-vitro | [63] |
| 2. | 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) | LKB1-independent AMPK phosphorylation | AMPK | In-vitro | [150] |
| 3. | Conjugated linoleic acid | Stimulates glucose uptake | AMPK | In-vitro | [151] |
| 4. | Metformin | Inhibits PKA dependent inhibitory phosphorylation of AMPK | AMPK | In-vitro | [50] |
| 5. | Glucagon-like peptide-1 | Decreases body weight, serum FFA, and triglyceride | AMPK | In-vivo | [152] |
| 6. | Thienopyridone (Compound A-769662) | Improves glucose homeostasis | AMPK | In-vivo | [153] |
| 7. | C24 | reduce blood glucose and lipid levels and improved glucose tolerance | AMPK | In-vivo | [154] |
| 8. | Resveratrol | Stimulates glucose uptake | AMPK | In-vivo | [155] |
| 9. | α-lipoic acid | Reduces hepatic lipogenesis | AMPK | In-vivo | [156] |
| 10. | Chlorogenic acid | Stimulates glucose transport in skeletal muscle | AMPK | In-vivo | [41] |
| 11. | Bitter melon-derived triterpenoids | Improve glucose disposal | CaMK Kβ | In-vivo | [61] |
| 12. | IMD-0354 | Stimulates adiponectin levels and overcomes glucose intolerance | IKKβ | In-vivo | [74] |
| 13. | Sodium Salicylate | Prevents FFA-induced hepatic insulin resistance | IKKβ | In-vivo | [157] |
| 14. | Amlexanox | Increases thermogenesis and improves weight loss and insulin sensitivity | IKKε | In-vivo | [67] |
| 15. | CAYMAN10576 | Decreased adiposity, hypophagia and increased in energy and glucose metabolism | IKKε | In-vivo | [80] |
| 16. | Ursolic acid | Restoration of insulin signaling | IKKβ | In-vivo | [158] |
| 17. | Hypocrellin A | Improves glucose and lipid metabolism under insulin resistant and diabetic condition | PKC | ex vivo | [86] |
| 18. | Ruboxistaurin | ameliorates insulin resistance | PKC | In-vivo | [158] |
| 19. | Aurothiomalate | Prevents complications such as hyperglycemia, hyperinsulinemia, abdominal obesity, hepatosteatosis, hypertriglyceridemia, and hypercholesterolemia | PKC-λ/ι | In-vivo | [159] |
| 20. | Quercetin | Prevents early diabetes tissue injury | IKK | In-vivo | [160] |
| 21 | PD184352 | Improves insulin sensitivity and glucose tolerance | MEK | In-vivo | [161] |
| 22 | GS-444217 | reduce renal inflammation under diabetic nephropathy condition | ASK1 | In-vivo | [162] |
| 23 | 5Z-7-oxozeaenol | Delays onset of autoimmune diabetes | TAK1 | In-vivo | [163] |
| 24 | Fish oil | Activates AMPK, inhibits MAPK and RAS pathways | AMPK | In-vivo | [164] |
Atorvastatin, a powerful drug to decrease cholesterol level, has been identified to have anti-inflammatory potency, decreased macrophage infiltration and expression levels of TNF-alpha, IL-6, and GLUT4 in adipose tissue [165]. It is unclear whether these anti-inflammatory properties will have any long term effect on insulin resistance. Similarly, Glucagon-like peptide-1 has been developed against Type 2 diabetes and is known to induce active phosphorylation of AMPK Thr172. This reduces body weight, serum FFA, and triglyceride levels [152, 166]. Unlike other compounds, Thienopyridone (Compound A-769662) is a direct activator of AMPK [167]. The main demerit of A-769662 is that it exhibits AMPK-independent effects and has poor oral absorption [168]. Recently C24 has been reported as a small molecule which was structurally optimized from PT1 to improve oral bioavailability [154]. Both PT1 and C24 caused an increase in AMPK activity and ACC phosphorylation [154, 169, 170]. Triterpenoids derived from bitter melon showed an anti-diabetic effect via CaMKKβ, a key upstream kinase responsible for the activation of AMPK [61]. In addition to IKK mediated NFκB activation, IKK is also reported to be involved in inhibitory phosphorylation of IRS1 [69], Furthermore, Kamon et al., [74] documented an increase in glucose tolerance by inhibiting IKKβ using IMD-0354. Salicylate was reported to impair FFA- induced insulin resistance by targeting IKK and preventing IKK induced serine phosphorylation of IRS-1 (Ser 307) and IRS-2 (Ser 233) [157]. Hypocrellin A, a fungal perylene quinonoid pigment from Hypocrella bambuase targets a specific PKC isoform other than PKC-θ and ameliorates insulin resistant induced abnormalities in glucose and lipid metabolism [86]. Two small molecules, which are inhibitors against PKC-λ/ι, have been developed by Sajan et al., [159] and have been shown to prevent abdominal obesity, hepatosteatosis, hyper triglyceridemia and hypercholesterolemia in obese rodent models. Komers et al., [171] reported that p38 activity was noticed to be increased in renal cortices of diabetic rats. Similarly, a p38 MAPK inhibitor compound (PHA666859) was documented as a novel therapeutic molecule against diabetic retinopathy [172]. A recent report by Ozaki et al., [161] revealed improvement in insulin sensitivity and glucose tolerance in the diabetic mice treated with MEK inhibitor PD184352. Specific inhibition of TAK1 with administration of 5Z-7-oxozeaenol delayed the onset of autoimmune diabetes in spontaneous and cyclophosphamide-induced experimental diabetic mice [163]. Thus targeting the p38 MAPK with its specific inhibitor could be a novel strategy to protect against diabetic associated tissue damage. Bargut et al., [164] have reported that fish-oil diet prevents adiposity and modulates white adipose tissue inflammation through activation of AMPK and inhibition of MAPK and RAS pathways. Metformin, a biguanide agent activates AMPK via inhibition of PKA dependent inhibitory phosphorylation of AMPK [50] and increase in LKB1 mediated AMPK activation [173]. These drugs and therapies render a hope to develop better therapies to counter the kinases involved in insulin resistance.
Conclusion
In conclusion, protein kinases play an important role in downstream signals perceived from the cell surface. The role of IKK, PKC, MAPK (ERK, JNK, p38) in inducing insulin resistance has been well established in several animal models. However, developing targeted therapies, either drugs or inhibitors, has not been very successful to date. Changes in the cell surface molecules that can further influence the cytoplasmic protein kinases can be reasonable as good therapy to prevent the development of resistance. Among the kinases reported, AMPK alone was noticed as positive regulator of insulin signaling. Hence, there is a need to study and research the role of other kinases in relation to insulin resistance, so that a potential novel therapy can be designed. It is now well established that kinase signaling is required for the metabolic events and kinase specific activation is associated with detrimental effects in obesity that contribute to Type 2 diabetes. Strategies to prevent and/or ameliorate obesity remain important in the overall treatment of insulin resistance and type 2 diabetes.
Acknowledgments
This study was supported by a research grant to Dr. Kalyana C Nandipati from the LB692 Nebraska Tobacco Settlement Funds to Creighton University, and by the research grants R01HL116042 and R01HL128063 from the National Institutes of Health, USA to DK Agrawal. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no other relevant affiliations or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Conflict of interest All authors have read the journal’s policy on disclosure of potential conflict of interest and declares no potential conflicts of interest relevant to this article.
References
- 1.Teng K-T, Chang C-Y, Chang LF, Nesaretnam K. Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutr J. 2014;13:12. doi: 10.1186/1475-2891-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawasaki N, Asada R, Saito A, et al. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Kim SC, Yu T, et al. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:352371. doi: 10.1155/2014/352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura T, Furuhashi M, Li P, et al. Double-Stranded RNA-Dependent Protein Kinase Links Pathogen Sensing with Stress and Metabolic Homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz) 2013;61:119–25. doi: 10.1007/s00005-012-0210-1. [DOI] [PubMed] [Google Scholar]
- 7.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McArdle MA, Finucane OM, Connaughton RM, et al. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 2013 doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amano SU, Cohen JL, Vangala P, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–71. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talukdar S, Oh DY, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eljaafari A, Robert M, Chehimi M, et al. Adipose Tissue-Derived Stem Cells From Obese Subjects Contribute to Inflammation and Reduced Insulin Response in Adipocytes Through Differential Regulation of the Th1/Th17 Balance and Monocyte Activation. Diabetes. 2015;64:2477–88. doi: 10.2337/db15-0162. [DOI] [PubMed] [Google Scholar]
- 13.Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol. 2014;5:379. doi: 10.3389/fimmu.2014.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabbrini E, Cella M, McCartney SA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–74. e1–3. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin T, Liu L-F, Lamendola C, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637–43. doi: 10.1161/ATVBAHA.114.304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priceman SJ, Kujawski M, Shen S, et al. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2013;110:13079–84. doi: 10.1073/pnas.1311557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–8. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37:365–71. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Tian J, Tian X, et al. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 2014;9:e92450. doi: 10.1371/journal.pone.0092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 21.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 22.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–11. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lyons CL, Kennedy EB, Roche HM. Metabolic Inflammation-Differential Modulation by Dietary Constituents. Nutrients. 2016 doi: 10.3390/nu8050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–45. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DH, Shi J, Jeoung NH, et al. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284:11776–80. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S-H, Huang H, Choi K, et al. ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2014;306:E332–43. doi: 10.1152/ajpendo.00619.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvalho-Filho MA, Carvalho BM, Oliveira AG, et al. Double-stranded RNA-activated protein kinase is a key modulator of insulin sensitivity in physiological conditions and in obesity in mice. Endocrinology. 2012;153:5261–74. doi: 10.1210/en.2012-1400. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Soos TJ, Li X, et al. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) J Biol Chem. 2004;279:45304–7. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 29.Fazakerley DJ, Holman GD, Marley A, et al. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J Biol Chem. 2010;285:1653–60. doi: 10.1074/jbc.M109.051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–72. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer JF, Dikkers A, Jurdzinski A, et al. Mitogen-activated protein kinase-activated protein kinase 2 deficiency reduces insulin sensitivity in high-fat diet-fed mice. PLoS One. 2014;9:e106300. doi: 10.1371/journal.pone.0106300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gual P, Le Marchand-Brustel Y, Tanti J-F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Woods A, Vertommen D, Neumann D, et al. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;278:28434–42. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- 36.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Momcilovic M, Hong S-P, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–43. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 39.Foretz M, Ancellin N, Andreelli F, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–9. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 40.Seo E, Park E-J, Joe Y, et al. Overexpression of AMPKalpha1 Ameliorates Fatty Liver in Hyperlipidemic Diabetic Rats. Korean J Physiol Pharmacol. 2009;13:449–54. doi: 10.4196/kjpp.2009.13.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong KW, Hsu A, Tan BKH. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7:e32718. doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards SK, Parton LE, Leclerc I, et al. Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo. J Endocrinol. 2005;187:225–35. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z, Dong Y, Scholz R, et al. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–62. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treebak JT, Glund S, Deshmukh A, et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–8. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 45.Lochhead PA, Salt IP, Walker KS, et al. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 46.Tao R, Gong J, Luo X, et al. AMPK exerts dual regulatory effects on the PI3K pathway. J Mol Signal. 2010;5:1. doi: 10.1186/1750-2187-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SH, Gammon SR, Knippers JD, et al. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol. 2002;92:2475–82. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- 48.Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–46. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aw DKL, Sinha RA, Xie SY, Yen PM. Differential AMPK phosphorylation by glucagon and metformin regulates insulin signaling in human hepatic cells. Biochem Biophys Res Commun. 2014;447:569–73. doi: 10.1016/j.bbrc.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 51.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–41. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong HW, Hsu KC, Lee J-W, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–64. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 53.Viollet B, Horman S, Leclerc J, et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–95. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinberg GR, Michell BJ, van Denderen BJW, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–74. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Galic S, Fullerton MD, Schertzer JD, et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–15. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–81. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Yang X, Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell Signal. 2016;28:1099–1104. doi: 10.1016/j.cellsig.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Jiang H, Westerterp M, Wang C, et al. Macrophage mTORC1 disruption reduces inflammation and insulin resistance in obese mice. Diabetologia. 2014;57:2393–404. doi: 10.1007/s00125-014-3350-5. [DOI] [PubMed] [Google Scholar]
- 59.Zeng T, Zhou J, He L, et al. Blocking Nuclear Factor-Kappa B Protects against Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. PLoS One. 2016;11:e0149677. doi: 10.1371/journal.pone.0149677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H-W, Wei C-C, Chen Y-J, et al. Flavanol-rich lychee fruit extract alleviates diet-induced insulin resistance via suppressing mTOR/SREBP-1 mediated lipogenesis in liver and restoring insulin signaling in skeletal muscle. Mol Nutr Food Res. 2016 doi: 10.1002/mnfr.201501064. [DOI] [PubMed] [Google Scholar]
- 61.Iseli TJ, Turner N, Zeng X-Y, et al. Activation of AMPK by bitter melon triterpenoids involves CaMKKβ. PLoS One. 2013;8:e62309. doi: 10.1371/journal.pone.0062309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, Zhang J, Lu J, et al. Astragalus polysaccharide stimulates glucose uptake in L6 myotubes through AMPK activation and AS160/TBC1D4 phosphorylation. Acta Pharmacol Sin. 2013;34:137–45. doi: 10.1038/aps.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu X, Zhu M, Zhang S, et al. Obesity Impairs Skeletal Muscle Regeneration Through Inhibition of AMPK. Diabetes. 2016;65:188–200. doi: 10.2337/db15-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hinz M, Arslan SÇ, Scheidereit C. It takes two to tango: IκBs, the multifunctional partners of NF-κB. Immunol Rev. 2012;246:59–76. doi: 10.1111/j.1600-065X.2012.01102.x. [DOI] [PubMed] [Google Scholar]
- 67.Reilly SM, Chiang S-H, Decker SJ, et al. An inhibitor of the protein kinases TBK1 and IKK-improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313–21. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu MC-T, Lee D-F, Xia W, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 69.Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 70.Chiang S-H, Bazuine M, Lumeng CN, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–75. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 73.Wang X-A, Zhang R, She Z-G, et al. Interferon regulatory factor 3 constrains IKKβ/NF-κB signaling to alleviate hepatic steatosis and insulin resistance. Hepatology. 2014;59:870–85. doi: 10.1002/hep.26751. [DOI] [PubMed] [Google Scholar]
- 74.Kamon J, Yamauchi T, Muto S, et al. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun. 2004;323:242–8. doi: 10.1016/j.bbrc.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Zhang G, Zhang H, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Negi G, Sharma SS. Inhibition of IκB kinase (IKK) protects against peripheral nerve dysfunction of experimental diabetes. Mol Neurobiol. 2015;51:591–8. doi: 10.1007/s12035-014-8784-8. [DOI] [PubMed] [Google Scholar]
- 77.Oguiza A, Recio C, Lazaro I, et al. Peptide-based inhibition of IκB kinase/nuclear factor-κB pathway protects against diabetes-associated nephropathy and atherosclerosis in a mouse model of type 1 diabetes. Diabetologia. 2015;58:1656–67. doi: 10.1007/s00125-015-3596-6. [DOI] [PubMed] [Google Scholar]
- 78.Röhl M, Pasparakis M, Baudler S, et al. Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest. 2004;113:474–81. doi: 10.1172/JCI18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eguchi J, Yan Q-W, Schones DE, et al. Interferon regulatory factors are transcriptional regulators of adipogenesis. Cell Metab. 2008;7:86–94. doi: 10.1016/j.cmet.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weissmann L, Quaresma PGF, Santos AC, et al. IKKε is key to induction of insulin resistance in the hypothalamus, and its inhibition reverses obesity. Diabetes. 2014;63:3334–45. doi: 10.2337/db13-1817. [DOI] [PubMed] [Google Scholar]
- 81.Huang W, Bansode R, Mehta M, Mehta KD. Loss of protein kinase Cbeta function protects mice against diet-induced obesity and development of hepatic steatosis and insulin resistance. Hepatology. 2009;49:1525–36. doi: 10.1002/hep.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Idris I, Donnelly R. Protein kinase C beta inhibition: A novel therapeutic strategy for diabetic microangiopathy. Diab Vasc Dis Res. 2006;3:172–8. doi: 10.3132/dvdr.2006.026. [DOI] [PubMed] [Google Scholar]
- 83.Szendroedi J, Yoshimura T, Phielix E, et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111:9597–602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo K, Liu Y, Zhou H, et al. Involvement of protein kinase C beta-extracellular signal-regulating kinase 1/2/p38 mitogen-activated protein kinase-heat shock protein 27 activation in hepatocellular carcinoma cell motility and invasion. Cancer Sci. 2008;99:486–96. doi: 10.1111/j.1349-7006.2007.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bansode RR, Huang W, Roy SK, et al. Protein kinase C deficiency increases fatty acid oxidation and reduces fat storage. J Biol Chem. 2008;283:231–6. doi: 10.1074/jbc.M707268200. [DOI] [PubMed] [Google Scholar]
- 86.Qu X, Dang L, Seale JP. Inhibitory effect of hypocrellin A on protein kinase C in liver and skeletal muscle of obese Zucker rats. Am J Chin Med. 2003;31:871–8. doi: 10.1142/S0192415X03001624. [DOI] [PubMed] [Google Scholar]
- 87.Samuel VT, Liu Z-X, Wang A, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–45. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–5. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikeda Y, Olsen GS, Ziv E, et al. Cellular mechanism of nutritionally induced insulin resistance in Psammomys obesus: overexpression of protein kinase Cepsilon in skeletal muscle precedes the onset of hyperinsulinemia and hyperglycemia. Diabetes. 2001;50:584–92. doi: 10.2337/diabetes.50.3.584. [DOI] [PubMed] [Google Scholar]
- 90.Brunetti A, Manfioletti G, Chiefari E, et al. Transcriptional regulation of human insulin receptor gene by the high-mobility group protein HMGI(Y) FASEB J. 2001;15:492–500. doi: 10.1096/fj.00-0190com. [DOI] [PubMed] [Google Scholar]
- 91.Sgarra R, Maurizio E, Zammitti S, et al. Macroscopic differences in HMGA oncoproteins post-translational modifications: C-terminal phosphorylation of HMGA2 affects its DNA binding properties. J Proteome Res. 2009;8:2978–89. doi: 10.1021/pr900087r. [DOI] [PubMed] [Google Scholar]
- 92.Dasgupta S, Bhattacharya S, Maitra S, et al. Mechanism of lipid induced insulin resistance: activated PKCε is a key regulator. Biochim Biophys Acta. 2011;1812:495–506. doi: 10.1016/j.bbadis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Oh Y-T, Chun KH, Park BD, et al. Regulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1 by protein kinase Cdelta-mediated phosphorylation. Apoptosis. 2007;12:1339–47. doi: 10.1007/s10495-007-0066-8. [DOI] [PubMed] [Google Scholar]
- 94.Cantley J, Boslem E, Laybutt DR, et al. Deletion of protein kinase Cδ in mice modulates stability of inflammatory genes and protects against cytokine-stimulated beta cell death in vitro and in vivo. Diabetologia. 2011;54:380–9. doi: 10.1007/s00125-010-1962-y. [DOI] [PubMed] [Google Scholar]
- 95.Mingo-Sion AM, Ferguson HA, Koller E, et al. PKCdelta and mTOR interact to regulate stress and IGF-I induced IRS-1 Ser312 phosphorylation in breast cancer cells. Breast Cancer Res Treat. 2005;91:259–69. doi: 10.1007/s10549-005-0669-0. [DOI] [PubMed] [Google Scholar]
- 96.Ranta F, Leveringhaus J, Theilig D, et al. Protein kinase C delta (PKCδ) affects proliferation of insulin-secreting cells by promoting nuclear extrusion of the cell cycle inhibitor p21Cip1/WAF1. PLoS One. 2011;6:e28828. doi: 10.1371/journal.pone.0028828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel NA, Song SS, Cooper DR. PKCdelta alternatively spliced isoforms modulate cellular apoptosis in retinoic acid-induced differentiation of human NT2 cells and mouse embryonic stem cells. Gene Expr. 2006;13:73–84. doi: 10.3727/000000006783991890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apostolatos H, Apostolatos A, Vickers T, et al. Vitamin A metabolite, all-trans-retinoic acid, mediates alternative splicing of protein kinase C deltaVIII (PKCdeltaVIII) isoform via splicing factor SC35. J Biol Chem. 2010;285:25987–95. doi: 10.1074/jbc.M110.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patel RS, Carter G, Cooper DR, et al. Transformer 2β homolog (Drosophila) (TRA2B) Regulates Protein Kinase C δI (PKCδI) Splice Variant Expression during 3T3L1 Preadipocyte Cell Cycle. J Biol Chem. 2014;289:31662–72. doi: 10.1074/jbc.M114.592337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochim Biophys Acta. 2007;1773:1376–87. doi: 10.1016/j.bbamcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Broom OJ, Widjaya B, Troelsen J, et al. Mitogen activated protein kinases: a role in inflammatory bowel disease? Clin Exp Immunol. 2009;158:272–80. doi: 10.1111/j.1365-2249.2009.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 103.Roth Flach RJ, Danai LV, DiStefano MT, et al. Protein Kinase Mitogen Activated Protein Kinase Kinase Kinase Kinase 4 (MAP4K4) Promotes Obesity-Induced Hyperinsulinemia. J Biol Chem. 2016 doi: 10.1074/jbc.M116.718932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu Y, Hou Z, Liu D, Yang X. Tartary buckwheat flavonoids protect hepatic cells against high glucose-induced oxidative stress and insulin resistance via MAPK signaling pathways. Food Funct. 2016;7:1523–36. doi: 10.1039/c5fo01467k. [DOI] [PubMed] [Google Scholar]
- 105.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 106.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 107.Arnette D, Gibson TB, Lawrence MC, et al. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278:32517–25. doi: 10.1074/jbc.M301174200. [DOI] [PubMed] [Google Scholar]
- 108.Gibson TB, Lawrence MC, Gibson CJ, et al. Inhibition of glucose-stimulated activation of extracellular signal-regulated protein kinases 1 and 2 by epinephrine in pancreatic beta-cells. Diabetes. 2006;55:1066–73. doi: 10.2337/diabetes.55.04.06.db05-1266. [DOI] [PubMed] [Google Scholar]
- 109.Lawrence MC, McGlynn K, Park B-H, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem. 2005;280:26751–9. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- 110.Kotzka J, Lehr S, Roth G, et al. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding Protein-2 at serine residues 432 and 455 in vivo. J Biol Chem. 2004;279:22404–11. doi: 10.1074/jbc.M401198200. [DOI] [PubMed] [Google Scholar]
- 111.Zang K, Wang J, Dong M, et al. Brd2 inhibits adipogenesis via the ERK1/2 signaling pathway in 3T3-L1 adipocytes. PLoS One. 2013;8:e78536. doi: 10.1371/journal.pone.0078536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu H, Yu J, Xia T, et al. Hepatic serum- and glucocorticoid-regulated protein kinase 1 (SGK1) regulates insulin sensitivity in mice via extracellular-signal-regulated kinase 1/2 (ERK1/2) Biochem J. 2014;464:281–9. doi: 10.1042/BJ20141005. [DOI] [PubMed] [Google Scholar]
- 113.Hayashi M, Tapping RI, Chao TH, et al. BMK1 mediates growth factor-induced cell proliferation through direct cellular activation of serum and glucocorticoid-inducible kinase. J Biol Chem. 2001;276:8631–4. doi: 10.1074/jbc.C000838200. [DOI] [PubMed] [Google Scholar]
- 114.Regan CP, Li W, Boucher DM, et al. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc Natl Acad Sci U S A. 2002;99:9248–53. doi: 10.1073/pnas.142293999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drew BA, Burow ME, Beckman BS. MEK5/ERK5 pathway: the first fifteen years. Biochim Biophys Acta. 2012;1825:37–48. doi: 10.1016/j.bbcan.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu H, Guariglia S, Li W, et al. Role of extracellular signal-regulated kinase 5 in adipocyte signaling. J Biol Chem. 2014;289:6311–22. doi: 10.1074/jbc.M113.506584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Badshah II, Baines DL, Dockrell ME. Erk5 is a mediator to TGFβ1-induced loss of phenotype and function in human podocytes. Front Pharmacol. 2014;5:71. doi: 10.3389/fphar.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Belgardt BF, Mauer J, Brüning JC. Novel roles for JNK1 in metabolism. Aging (Albany NY) 2010;2:621–6. doi: 10.18632/aging.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010;35:490–6. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl 7):S52–4. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsukumo DML, Carvalho-Filho MA, Carvalheira JBC, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 123.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27:498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jaeschke A, Czech MP, Davis RJ. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 2004;18:1976–80. doi: 10.1101/gad.1216504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim WH, Lee JW, Gao B, Jung MH. Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal. 2005;17:1516–32. doi: 10.1016/j.cellsig.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 126.Pal M, Wunderlich CM, Spohn G, et al. Alteration of JNK-1 signaling in skeletal muscle fails to affect glucose homeostasis and obesity-associated insulin resistance in mice. PLoS One. 2013;8:e54247. doi: 10.1371/journal.pone.0054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sabio G, Das M, Mora A, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–43. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tuncman G, Hirosumi J, Solinas G, et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–6. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han MS, Jung DY, Morel C, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–22. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jaeschke A, Rincón M, Doran B, et al. Disruption of the Jnk2 (Mapk9) gene reduces destructive insulitis and diabetes in a mouse model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:6931–5. doi: 10.1073/pnas.0502143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abdelli S, Bonny C. JNK3 maintains expression of the insulin receptor substrate 2 (IRS2) in insulin-secreting cells: functional consequences for insulin signaling. PLoS One. 2012;7:e35997. doi: 10.1371/journal.pone.0035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdelli S, Papas KK, Mueller KR, et al. Regulation of the JNK3 signaling pathway during islet isolation: JNK3 and c-fos as new markers of islet quality for transplantation. PLoS One. 2014;9:e99796. doi: 10.1371/journal.pone.0099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res. 2007;86:800–11. doi: 10.1177/154405910708600902. [DOI] [PubMed] [Google Scholar]
- 134.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:409–14. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 135.Dahan S, Roda G, Pinn D, et al. Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology. 2008;134:192–203. doi: 10.1053/j.gastro.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McGee SL, Hargreaves M. Exercise and skeletal muscle glucose transporter 4 expression: molecular mechanisms. Clin Exp Pharmacol Physiol. 2006;33:395–9. doi: 10.1111/j.1440-1681.2006.04362.x. [DOI] [PubMed] [Google Scholar]
- 137.Geiger PC, Wright DC, Han D-H, Holloszy JO. Activation of p38 MAP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2005;288:E782–8. doi: 10.1152/ajpendo.00477.2004. [DOI] [PubMed] [Google Scholar]
- 138.Liu S, Xu R, Gerin I, et al. SRA regulates adipogenesis by modulating p38/JNK phosphorylation and stimulating insulin receptor gene expression and downstream signaling. PLoS One. 2014;9:e95416. doi: 10.1371/journal.pone.0095416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pereira S, Yu WQ, Moore J, et al. Effect of a p38 MAPK inhibitor on FFA-induced hepatic insulin resistance in vivo. Nutr Diabetes. 2016;6:e210. doi: 10.1038/nutd.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Makeeva N, Roomans GM, Welsh N. Role of TAB1 in nitric oxide-induced p38 activation in insulin-producing cells. Int J Biol Sci. 2007;3:71–6. doi: 10.7150/ijbs.3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee J, Sun C, Zhou Y, et al. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med. 2011;17:1251–60. doi: 10.1038/nm.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sha H, Yang L, Liu M, et al. Adipocyte spliced form of X-box-binding protein 1 promotes adiponectin multimerization and systemic glucose homeostasis. Diabetes. 2014;63:867–79. doi: 10.2337/db13-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chun K-H, Choi K-D, Lee D-H, et al. In vivo activation of ROCK1 by insulin is impaired in skeletal muscle of humans with type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;300:E536–42. doi: 10.1152/ajpendo.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–82. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soliman H, Nyamandi V, Garcia-Patino M, et al. Partial deletion of ROCK2 protects mice from high-fat diet-induced cardiac insulin resistance and contractile dysfunction. Am J Physiol Heart Circ Physiol. 2015;309:H70–81. doi: 10.1152/ajpheart.00664.2014. [DOI] [PubMed] [Google Scholar]
- 146.Song Y, Wan X, Gao L, et al. Activated PKR inhibits pancreatic β-cell proliferation through sumoylation-dependent stabilization of P53. Mol Immunol. 2015;68:341–9. doi: 10.1016/j.molimm.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura T, Arduini A, Baccaro B, et al. Small-molecule inhibitors of PKR improve glucose homeostasis in obese diabetic mice. Diabetes. 2014;63:526–34. doi: 10.2337/db13-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sud N, Rutledge AC, Pan K, Su Q. Activation of the dsRNA-Activated Protein Kinase PKR in Mitochondrial Dysfunction and Inflammatory Stress in Metabolic Syndrome. Curr Pharm Des. 2016;22:2697–703. doi: 10.2174/1381612822666160202141845. [DOI] [PubMed] [Google Scholar]
- 149.Nakamura T, Furuhashi M, Li P, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–48. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun Y, Connors KE, Yang D-Q. AICAR induces phosphorylation of AMPK in an ATM-dependent, LKB1-independent manner. Mol Cell Biochem. 2007;306:239–45. doi: 10.1007/s11010-007-9575-6. [DOI] [PubMed] [Google Scholar]
- 151.Mohankumar SK, Taylor CG, Siemens L, Zahradka P. Activation of phosphatidylinositol-3 kinase, AMP-activated kinase and Akt substrate-160 kDa by trans-10, cis-12 conjugated linoleic acid mediates skeletal muscle glucose uptake. J Nutr Biochem. 2013;24:445–56. doi: 10.1016/j.jnutbio.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 152.Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–97. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 153.Cool B, Zinker B, Chiou W, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 154.Li Y-Y, Yu L-F, Zhang L-N, et al. Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice. Toxicol Appl Pharmacol. 2013;273:325–34. doi: 10.1016/j.taap.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 155.Price NL, Gomes AP, Ling AJY, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–90. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park K-G, Min A-K, Koh EH, et al. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 2008;48:1477–86. doi: 10.1002/hep.22496. [DOI] [PubMed] [Google Scholar]
- 157.Park E, Wong V, Guan X, et al. Salicylate prevents hepatic insulin resistance caused by short-term elevation of free fatty acids in vivo. J Endocrinol. 2007;195:323–31. doi: 10.1677/JOE-07-0005. [DOI] [PubMed] [Google Scholar]
- 158.Lu J, Wu D, Zheng Y, et al. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav Immun. 2011;25:1658–67. doi: 10.1016/j.bbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 159.Sajan MP, Nimal S, Mastorides S, et al. Correction of metabolic abnormalities in a rodent model of obesity, metabolic syndrome, and type 2 diabetes mellitus by inhibitors of hepatic protein kinase C-ι. Metabolism. 2012;61:459–69. doi: 10.1016/j.metabol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dias AS, Porawski M, Alonso M, et al. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005;135:2299–304. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- 161.Ozaki K-I, Awazu M, Tamiya M, et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab. 2016 doi: 10.1152/ajpendo.00445.2015. ajpendo.00445.2015. [DOI] [PubMed] [Google Scholar]
- 162.Tesch GH, Ma FY, Han Y, et al. ASK1 Inhibitor Halts Progression of Diabetic Nephropathy in Nos3-Deficient Mice. Diabetes. 2015;64:3903–13. doi: 10.2337/db15-0384. [DOI] [PubMed] [Google Scholar]
- 163.Cao H, Lu J, Du J, et al. TAK1 inhibition prevents the development of autoimmune diabetes in NOD mice. Sci Rep. 2015;5:14593. doi: 10.1038/srep14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bargut TCL, Mandarim-de-Lacerda CA, Aguila MB. A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice. J Nutr Biochem. 2015;26:960–9. doi: 10.1016/j.jnutbio.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 165.Furuya DT, Poletto AC, Favaro RR, et al. Anti-inflammatory effect of atorvastatin ameliorates insulin resistance in monosodium glutamate-treated obese mice. Metabolism. 2010;59:395–9. doi: 10.1016/j.metabol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 166.Lee J, Hong S-W, Chae SW, et al. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One. 2012;7:e31394. doi: 10.1371/journal.pone.0031394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 168.Moreno D, Knecht E, Viollet B, Sanz P. A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism. FEBS Lett. 2008;582:2650–4. doi: 10.1016/j.febslet.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 169.Pang T, Zhang Z-S, Gu M, et al. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–60. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yu L-F, Li Y-Y, Su M-B, et al. Development of Novel Alkene Oxindole Derivatives As Orally Efficacious AMP-Activated Protein Kinase Activators. ACS Med Chem Lett. 2013;4:475–80. doi: 10.1021/ml400028q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Komers R, Lindsley JN, Oyama TT, et al. Renal p38 MAP kinase activity in experimental diabetes. Lab Invest. 2007;87:548–58. doi: 10.1038/labinvest.3700549. [DOI] [PubMed] [Google Scholar]
- 172.Du Y, Tang J, Li G, et al. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci. 2010;51:2158–64. doi: 10.1167/iovs.09-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]