Abstract
Objectives
Uncovering heterogeneities in longitudinal patterns (trajectories) of opioid use among individuals with opioid use disorder can increase our understanding of disease progression and treatment responses to improve care. The present study aims to identify distinctive opioid use trajectories and factors associated with these patterns among participants randomized to treatment with methadone (MET) or buprenorphine + naloxone (BUP).
Methods
Growth mixture modeling was applied to identify distinctive opioid use trajectories among 795 opioid users after their enrollment in a multi-site trial during 2006–2009, with follow-up interviews conducted in 2011–2014.
Results
Four distinctive trajectories were identified based on opioid use over the follow-up period: Low Use (42.0%), High Use (22.3%), Increasing Use (17.1%), and Decreasing Use (18.6%). Greater odds of being in the High Use group (relative to Low Use) was associated with Hispanics (relative to African American, OR=3.21), injection drug use (OR=2.12), higher mental health functioning at baseline (OR=1.23), location on the West Coast (vs. East Coast, OR=2.15), and randomization to BUP (relative to MET, OR=1.53). High Use and Increasing Use groups had greater severity in problems related to drug, employment, legal, and social/family relationships, and worsened mental health functioning at follow-up. Participation in treatment significantly accounted for both within- and between-group differences in opioid use.
Conclusions
Continued treatment is necessary to reduce risk for opioid use and related adverse consequences, particularly among individuals (e.g., injecting drug) at risk for consistently high level of opioid use.
Keywords: opioid use trajectory, growth mixture model, methadone maintenance, buprenorphine
1. Introduction
Long-term observations of individuals with opioid addiction have revealed that it is a chronic, relapsing disorder with excess mortality, morbidities, and adverse psychosocial consequences (Hser et al., 2015). Recognition of this course has drawn attention to the critical need for longer-term care, or maintenance treatment programs. Both methadone (MET) and buprenorphine (BUP) are effective medications for opioid use disorders and often are used as maintenance treatment to stabilize abstinence from opioid use on a long-term basis (Connock et al., 2007; Mattick et al., 2008).
A recent study (“Starting Treatment with Agonist Replacement Therapy,” or START) randomized participants to MET versus BUP (Saxon et al., 2013). The follow-up of these participants provided an opportunity to examine opioid use 2 to 8 years subsequent to the randomization (Hser et al., 2016). Prior analyses showed that for both randomization conditions, opioid use dropped immediately after entering START and that treatment participation, either in MET or BUP, over the follow-up period was significantly related to the reduction in opioid use (Hser et al., 2016). That study provided valuable knowledge based on the mean change in opioid use over time for the START sample, but did not examine trends in individual variability of opioid use. The tremendous individual variation in opioid use over time suggests that identification of factors associated with different temporal course in opioid use may have important implications for improved care or intervention development.
The longitudinal pattern of opioid use with repeated measures over time depicts within-person patterns of change, often referred to as time trends, time paths, growth curves, or trajectories (Curran et al., 2010). These trajectories might take on a variety of different characteristics that vary from person to person: They might be flat (i.e., showing no change over time), they might be systematically increasing or decreasing over time, and they might be linear or curvilinear in form. Longitudinal models based on these trajectories allow the estimation of between-person differences in within-person change. Previous studies have shown that subpopulations may exist within a larger heterogeneous population, and using growth mixture modeling (GMM) may identify groups with distinctive trajectory patterns masked when group means as a whole are considered. Using this approach to assess treatment outcomes may facilitate the targeting of patients most likely to benefit when differential responders can be identified and categorized.
The purpose of the present study was to identify distinctive patterns of opioid use trajectories following randomization in the START study and to investigate factors and consequences associated with those trajectories. Based on previous experience with this population, we anticipate 4 distinctive groups or classes of trajectories of opioid use: some participants will maintain a high or low level of opioid use at all times, some may decrease their use over time after treatment entry, and others may decrease at the beginning due to treatment and relapse subsequently to increased use. We also hypothesize that randomization condition will have no effect in trajectory group membership, but treatment participation will be associated with reduced opioid use both within a trajectory and between the trajectory groups.
2. Methods
2.1. Study Design
Full details of the methods of the START follow-up study has been previously been described (Hser et al., 2016). Briefly, the original START trial randomized 1,269 individuals to BUP (n=740) or MET (n=529) at nine federally licensed opioid treatment programs during 2006–2009. Because of higher dropout in the BUP arm, midway through the trial the randomization scheme was changed from 1:1 to 2:1 to achieve targeted 300 evaluable BUP participants. This change accounts for the higher number randomized to BUP. Participants were provided study medication for 24 weeks, and were then tapered off medication over ≤8 weeks or referred for ongoing clinical treatment, with study completion by Week 32.
A follow-up study was conducted during 2011–2014, approximately 2 to 8 years (a mean of 4.5 years) post randomization. Two sites (189 participants) were dropped due to logistical difficulties (i.e., one site recruited only 2 participants and the other had difficulty conducting follow-ups). Of the 1,080 targeted participants, 89.4% were located with 797 interviewed, 49 deceased, 54 refused to be interviewed, 29 incarcerated, and 36 not interviewed. Among the 797 interviewed, two did not provide timeline follow-back data and were excluded from further analysis. There were no differences in the demographic characteristics of participants included (n=795) and omitted (n=285) from analysis.
2.2. Participants and Interview Procedures
Demographic information on 795 participants is as follows. Mean age at baseline was 37.4, 34.1% were female, 72.5% white, 11.3% Hispanic, 9.2% African American, and 7.0% other race/ethnicity. The two medication groups were all similar in baseline measures except that more participants in the MET group reported recent cocaine use (37.2%) than in the BUP group (30.2%).
Research staff at the clinics where participants were originally recruited conducted face-to-face follow-up interviews. The assessment interview lasted approximately one and a half to two hours. Staff also collected a urine sample for drug testing and a saliva swab for rapid HIV testing. Participants were compensated for their time in accord with local policies. All study procedures were approved by the IRB at UCLA and by the local IRB overseeing each study site. A federal Certificate of Confidentiality was obtained to protect against disclosure of sensitive information.
2.3. Main Measures
Opioid use
Opioid use over the follow-up period was measured by self-reported days of opioid use per month from enrollment to the follow-up interview using timeline follow back (TLFB) methodology (Sobell & Sobell, 1992) aided by a calendar and other memory prompts (Hser et al., 1992, Murphy et al., 2010). Current opioid use was established by positive urine specimen testing at follow-up.
Treatment participation
TLFB was also used to collect monthly treatment status over time from START enrollment to the follow-up interview, thus including periods during the original START trial. Types of treatment include (1) BUP, (2) MET, and (3) treatment with no opioid treatment medication.
Addiction Severity Index-Lite (ASI; McLellan et al., 1992)
The ASI is a structured interview that assesses problem severity in seven areas: alcohol use, drug use, employment, family and social relationships, legal, medical, and psychological. The ASI was administered at the follow-up visit.
Short Form 36-item Health Survey (SF-36)
The SF-36 is a self-report instrument to assess health status over a 4-week-period providing summary scores of physical and mental health components (Ware et al., 1992). It was administered at baseline and the follow-up interview.
2.4. Statistical Analyses
We conducted growth mixture modeling (GMM) (Muthen, 2004) using Mplus (Muthen & Muthen, 2007) in conjunction with multivariate comparisons of participants’ characteristics and related treatment outcomes with identified groups. The dependent variable was level of opioid use over time (with a maximum of 55 months), indicated by number of days of opioid use per month over the follow-up period after study enrollment. Lengths of the follow-up period varied among participants, with 58.5% of the participants (54% of BUP and 65% of MET) contributing complete data. Repeated measures of opioid use across months were modeled as curvilinear curves with intercept, slope, and quadratic parameters, which were estimated by a censored-normal model with j trajectory groups.
The GMM identifies subgroups in a sample based on shared growth parameters (e.g., intercept, slope). This approach assumes that multiple subpopulations exist in a sample; however, no assumptions are made about the number of subpopulations or their specific growth parameters. Hence, individuals are classified into possible unobserved subgroups based on common profile patterns and, subsequently, between-group difference can be examined. We handled missing data by applying the full information maximum likelihood method so that the analysis makes full use of all available data.
We estimated a series of models with the number of classes ranging from 1 to 6. The model fitting was assessed by goodness-of-fit index, including log-likelihood values, Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), Adjusted BIC, and Entropy, and was also visually inspected by plotting observed against model-predicted values. Lower values of AIC, BIC and adjusted BIC but higher values of log likelihood and Entropy were expected for a well fitted model. In addition, the Lo-Mendell-Rubin likelihood ratio test (LMR-LRT, Lo, Mendell & Rubin, 2001) and the Bootstrapped Likelihood Ratio Test (BLRT, McLachlan & Peel, 2000) were applied to test the significant improvement of a k class model over a k-1 class model. The optimal model was selected on the basis of a reasonably low AIC, BIC and adjusted BIC values, as well as parsimony of the model, clinical utility and interpretability of the distinguishable trajectories. The probability of group membership was estimated in a multinomial distribution. Based upon the estimated membership probability for each individual, individuals were placed into their most likely group.
The three-step procedure (Asparouhov & Muthen, 2014) with the “r3step” command in Mplus was applied to examine the association of study site and participant baseline characteristics with the membership of the identified trajectory groups. Post-hoc analyses were then conducted on outcomes associated with the most likely class membership.
3. Results
3.1. Identification of Distinctive Opioid Use Trajectory Groups
A series of GMM with increasing numbers of trajectories were fitted to identify the optimal unconditional model. The adjusted BIC value decreased from 152415.4 in the two-trajectory model to 148258.4 in the three-trajectory, 145862.9 in the four-trajectory, 144323.4 in the five-trajectory, and 143131.5 in the six-trajectory model. Similar patterns of decreasing values in AIC (152320.7, 148157.7, 145756.2, 144210.7, 143012.8) and BIC (152615.4, 148471.1, 146088.3, 144561.6, 143382.4) were observed. The BLRT on the improvement of a k trajectory model over a k-1 trajectory model was significant at p<0.01 with k = 2 and up to 6, while the LMR-LRT was significant at p<0.05 only with k= 2 and 3. In summary, the difference in the decreasing BIC values got smaller as the number of trajectory groups increased, indicating diminishing gains in estimating classes beyond a four-trajectory model. The corresponding Entropy (0.982, 0.976, 0.978, 0.976, 0.965) showed an increase from 3-trajectory to 4-trajectory models, but a decrease afterwards. Furthermore, the additional trajectories identified in the 5- and 6-trajectory models were parallel to and similar to other trajectories identified in the 4-trajectory models. Given the highest value of Entropy and decreased changes of AIC, BIC and adjusted BIC in models with higher classes, the 4-trajectory model was selected as the most parsimonious and informative description of the study data.
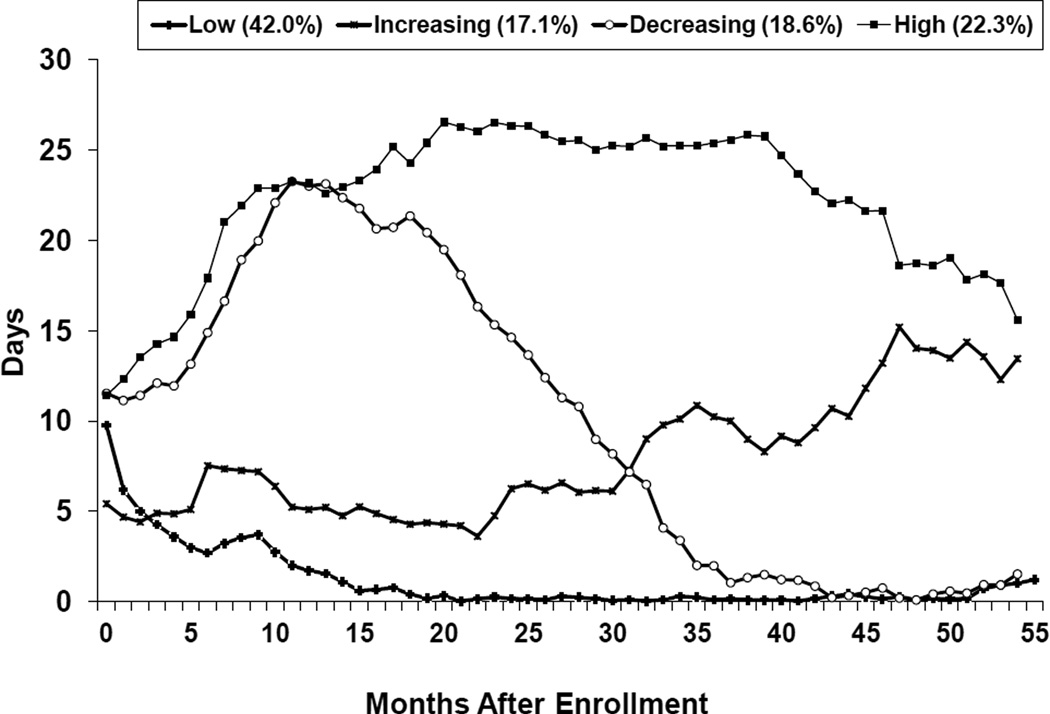
Figure 1 shows trajectories of estimated days of opioid use of the 4-trajectory model. About 22.3% of participants were classified in the High Use group; these individuals remained at a high level of opioid use (on average, using opioiod use more than 15 days per month) throughout the 55-month period. Another 18.6% were in the Decreasing Use group, exhibiting a linearly decreasing opioid use trajectory after an initial increase during the first year post randomization; opioid use increased from approximately 13 days per month to 23 days per month from months 1 to 12 and was consistently decreasing to almost no use from months 42 to 55. Another 17.1% were classified in the Increasing Use group, exhibiting a fairly linear increasing trajectory. These individuals had a low level of opioid use in the first 6 months after randomization, but exhibited a consistent increase in level of opioid use from months 7 to 55. The largest proportion of individuals (42.0%) belonged to the Low Use group, representing those who had a low level of opioid use throughout the whole observation period.
Figure 1.
Trajectories of Averaged Days of Opioid Use over 55 Months
Urine-positive rates at the follow-up interview corroborated the group classification with 16.4% testing positive for opioid use among the Low Use group, 36.3% among the Decreasing Use group, 45.3% among the Increasing Use group, and 69.8% among the High Use group.
3.2. Baseline Characteristics Associated with Distinctive Opioid Use Trajectories
Baseline clinical profiles associated with the four trajectories are provided in Table 1. The multinomial logistic regression results using the r3step command in Mplus (Table 2) showed that a greater odds of membership in the High Use group (relative to Low Use) was associated with living on the West Coast (vs. East Coast, OR=2.15), randomization to BUP (relative to MET, OR=1.53), Hispanic race/ethnicity (relative to African American, OR=3.21), injecting drugs (OR=2.12), and higher mental health functioning (OR=1.23). Randomization to BUP (relative to MET, OR=1.58), and injection drug use (OR=2.19) significantly increased the odds of being in the Decreasing Use group (relative to the Low Use group). No factors were associated with the likelihood of being in the Increasing Use group relative to the Low Use group.
Table 1.
Clinical profiles at baseline by 4 trajectory groups over the 55-month follow-up period
| High (n=177) |
Increasing (n=136) |
Decreasing (n=148) |
Low (n=334) |
Total (n=795) |
|
|---|---|---|---|---|---|
| Clinic site location, % ** | |||||
| East coast | 25.4 | 33.1 | 36.5 | 46.7 | 37.7 |
| West coast | 74.6 | 66.9 | 63.5 | 53.3 | 62.3 |
| Randomized conditions, % * | |||||
| Buprenorphine | 64.4 | 54.4 | 64.9 | 53.9 | 58.4 |
| Methadone | 35.6 | 45.6 | 35.1 | 46.1 | 41.6 |
| Age at baseline, Mean(SD) ** | 38.9 (10.6) | 38.2 (11.0) | 36.8 (11.5) | 36.6 (11.3) | 37.4(11.2) |
| Female, % | 32.2 | 35.3 | 31.1 | 35.9 | 34.1 |
| Race/Ethnicity, % ** | |||||
| White | 59.9 | 69.1 | 75.7 | 79.3 | 72.5 |
| African American | 10.2 | 13.2 | 7.4 | 7.8 | 9.2 |
| Hispanic | 20.9 | 11.0 | 10.1 | 6.6 | 11.3 |
| Other | 9.0 | 6.6 | 6.8 | 6.3 | 7.0 |
| # Cigarettes smoked per day, % | |||||
| 0 | 10.2 | 9.6 | 9.4 | 12.6 | 10.9 |
| <10 | 24.9 | 38.2 | 23.7 | 26.0 | 27.4 |
| 11–20 | 50.9 | 42.6 | 49.3 | 43.4 | 46.0 |
| 21–30 | 11.8 | 8.1 | 11.5 | 12.9 | 11.6 |
| 31+ | 2.2 | 1.5 | 6.0 | 5.1 | 4.0 |
| Alcohol use, % | 28.3 | 33.1 | 27.0 | 32.8 | 30.8 |
| Cocaine positive, % | 31.6 | 36.0 | 35.8 | 31.4 | 33.1 |
| Amphetamine positive, % | 10.2 | 10.3 | 6.1 | 5.1 | 7.3 |
| Cannabinoids positive, % | 20.9 | 25.0 | 19.6 | 20.7 | 21.3 |
| Drug injection in past 30 days, % ** | 77.4 | 66.2 | 75.0 | 55.4 | 65.8 |
| SF-36 Physical Component Summary, Mean (SD) |
49.5 (8.8) | 48.8 (9.2) | 49.5 (9.2) | 49.2 (9.4) | 49.3 (9.2) |
| SF-36 Mental Component Summary, Mean (SD) ** |
41.9 (12.0) | 39.0 (11.4) | 39.8 (13.0) | 37.9 (13.5) | 39.3 (12.8) |
p<0.05;
p<0.01
Table 2.
Characteristics associated with the four-trajectory membership in multinomial logistic regression model using the 3-step procedure in Mplus
| Opioid Use Trajectories (OR) |
|||
|---|---|---|---|
| High vs. Low |
Increasing vs. Low |
Decreasing vs. Low |
|
| Clinic Site Location | |||
| West Coast (vs. East Coast) | 2.15** | 1.46 | 1.37 |
| Randomization Conditions | |||
| Buprenorphine (vs. Methadone) | 1.53* | 1.02 | 1.58* |
| Age (by 1 year of change) | 1.00 | 1.00 | 0.99 |
| Male (vs. Female) | 0.94 | 0.96 | 1.11 |
| Race/Ethnicity | |||
| White (vs. African American/others) | 0.88 | 0.79 | 0.51 |
| Hispanic (vs. African American/others) | 3.21* | 1.45 | 0.80 |
| Age first regular heroin/opiate use | 0.99 | 1.00 | 1.00 |
| # cigarettes smoked per day (i.e., per 10 cigarettes of change) |
1.23 | 0.84 | 1.15 |
| Alcohol use (vs. no use) | 0.93 | 1.08 | 0.77 |
| Cocaine use positive (vs. negative) | 0.92 | 1.16 | 1.13 |
| Amphetamine use positive (vs. negative) | 1.47 | 1.96 | 1.03 |
| Cannabinoids use positive (vs. negative) | 1.07 | 1.35 | 0.95 |
| Drug injection in past 30 days (vs. no injection) | 2.12** | 1.44 | 2.19** |
| SF-36 Physical Component Summary (per 10 points of change) |
1.04 | 0.94 | 1.03 |
| SF-36 Mental Component Summary (per 10 points of change) |
1.23* | 1.04 | 1.10 |
p<0.05;
p<0.01
3.3. Treatment Participation by the Trajectory Groups
Descriptive statistics on treatment participation at the follow-up are provided in Table 3. Overall the sample spent a mean of 8.4 months in treatment over the follow-up period with a mean of 19.6 months in MET and 7.2 in BUP. The Low Use group had the highest number of months in treatment (M=36.6, SD=19.1), the High Use group had the least amount of time in treatment (M=16.2, SD=11.2), and the Decreasing and Increasing Groups were in-between (26.7 and 26.0, respectively). Treatment status at the follow-up follows a similar pattern, with highest treatment participation by the Low Use group (65.3%), followed by the Decreasing Use group (54.0%), Increasing Use Group (46.3%), and lowest among the High Use Group (35.8%).
Table 3.
Treatment retention and status at follow-up interview by the 4 trajectory groups
| High (n=177) |
Increasing (n=136) |
Decreasing (n=148) |
Low (n=334) |
Total (n=795) |
|
|---|---|---|---|---|---|
|
Mean (SD) Months in treatment over 60 months after START enrollment |
|||||
| Months in treatment ** | 16.2 (11.2) | 26.0 (15.2) | 26.7 (14.0) | 36.6 (19.1) | 28.4 (17.9) |
| Months in methadone treatment ** | 10.0 (11.9) | 17.8 (16.5) | 16.5 (15.5) | 26.8 (22.1) | 19.6 (19.3) |
| Months in buprenorphine treatment ** | 4.9 (5.5) | 6.2 (8.8) | 7.9 (10.1) | 8.5 (14.4) | 7.2 (11.3) |
| Months in other treatment | 0.0 (0.2) | 0.0 (0.0) | 0.1 (0.7) | 0.16 (2.7) | 0.1 (1.8) |
| Treatment status at follow-up | |||||
| Not in treatment (%) ** | 64.2 | 53.7 | 46.0 | 34.7 | 46.6 |
| In methadone treatment (%) ** | 30.0 | 32.4 | 42.6 | 51.8 | 41.8 |
| In buprenorphine treatment (%) | 6.3 | 14.0 | 10.8 | 13.2 | 11.3 |
p<0.05;
p<0.01
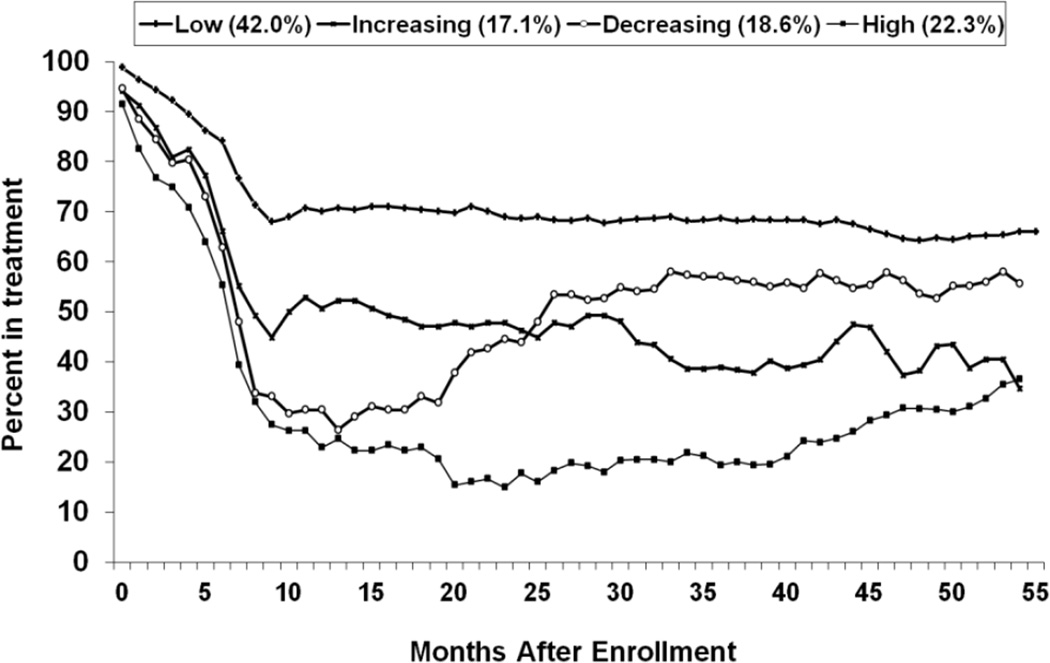
Figure 2 shows trajectories of the percent of people in treatment by the four opioid-trajectory groups. The trajectories of treatment statuses were significantly different among the four groups (i.e., F-tests of intercepts, slopes and quadratics are F(3,38975)=10.2, F(3,38975)=20.0, and F(3,38975)=33.3, respectively with p<0.01 for all three tests). Pairwise comparisons of the treatment trajectories between each pair of the four opioid-trajectory groups were significant, except the pair of the Decreasing and High Use groups. The treatment trajectories of the Decreasing and High Use groups paralleled each other, but participants in the Decreasing Use group exhibited a higher percent in treatment across all time points than those in the High Use group.
Figure 2.
Trajectories of Percent in Treatment over 55 Months
3.4. Addiction Severity Index and Quality Of Health Outcomes Associated With the Trajectory Groups
The ASI and SF-36 outcomes at the follow-up associated with the four trajectory groups are provided in Table 4. Not surprisingly, the ASI scores showed that the High Use and Increasing Use groups consistently showed worse outcomes in drug use, employment, legal, and social/family relationships.
Table 4.
Addiction Severity Index and SF-36 at follow-up by the four trajectory groups
| High (n=177) |
Increasing (n=136) |
Decreasing (n=148) |
Low (n=334) |
Total (n=795) |
|
|---|---|---|---|---|---|
|
Addiction Severity Index composite score, Mean (SD) |
|||||
| Alcohol * | 0.09(0.19) | 0.06(0.14) | 0.07(0.14) | 0.06(0.13) | 0.07 (0.15) |
| Drug ** | 0.26(0.14) | 0.21(0.13) | 0.16(0.14) | 0.14(0.11) | 0.18 (0.14) |
| Medical | 0.31(0.36) | 0.36(0.37) | 0.31(0.36) | 0.28(0.35) | 0.31 (0.36) |
| Employment ** | 0.76(0.28) | 0.68(0.30) | 0.63(0.32) | 0.58(0.33) | 0.64 (0.32) |
| Legal ** | 0.12(0.19) | 0.12(0.20) | 0.10(0.19) | 0.05(0.12) | 0.09 (0.17) |
| Social/Family ** | 0.17(0.19) | 0.17(0.18) | 0.12(0.17) | 0.12(0.18) | 0.14 (0.18) |
| Psychiatric | 0.24(0.23) | 0.25(0.22) | 0.21(0.23) | 0.22(0.22) | 0.23 (0.23) |
|
SF-36 Physical Component Summary, Mean (SD) |
46.6(11.1) | 47.2(10.3) | 47.2(11.0) | 48.3(10.5) | 47.5 (10.7) |
|
SF-36 Mental Component Summary, Mean (SD)** |
41.8(12.1) | 42.7(12.3) | 45.9(10.5) | 45.0(12.4) | 44.1 (12.0) |
p<0.05;
p<0.01
While simple comparisons of the SF-36 physical component summary did not show differences among the four groups, there was a significant difference by the four trajectory groups after controlling for the baseline SF-36 physical component summary. Relative to the score of the Low Use group, the High Use group exhibited the most severe (i.e., poorest functioning) SF-36 physical component summary at follow-up. The score of the High Use group was significantly lower than the score of the Low Use group (estimated difference=−1,80; t=−1.99; p=0.04). After controlling for the baseline SF-36 mental component summary, the SF-36 Mental component summary at follow-up was significantly different by the four trajectory groups. Relative to the score of the Low Use group, the High Use group and Increasing Use group exhibited the more severe (i.e., lower) SF-36 mental component summary at follow-up. The score of the High Use group was significantly lower than the score of the Low Use (estimated difference=−4.67; t=−4.42; p<0.01) and Decreasing Use groups (estimated difference=−4.45; t=−3.96; p<0.01), respectively. The score of the Increasing Use group was significantly lower than the score of the Low Use (estimated difference=−2.62; t=−2.29; p<0.01) and Decreasing Use groups (estimated difference=−2.91; t=−2.18; p=0.03), respectively.
4. Discussion
The study identified four distinctive trajectories of opioid use over the follow-up period after randomization to MET or BUP. A very promising finding is that the largest group (more than 40% of the sample) demonstrated a consistently low level of use after entering the medication trial. In contrast, approximately 17% of the participants gradually increased their opioid use after they initially reduced use, and another 19% did not respond well initially but gradually decreased use over time. The stable High Use group (22% of the sample) was of most concern, as their use not only increased from the initial level but continued at a high level over a long period of time.
Several baseline measures were associated with the membership in different trajectory groups. Relative to the Low Use group, the High Use group was characterized by being Hispanic, injecting drugs, better mental health at baseline, being on the West Coast, and being randomized to BUP. The Decreasing Drug Use group was characterized by being randomized to BUP and injecting drugs; characteristics of the Increasing Drug Use group were similar to those of the Low Use group. Injection drug use and randomization to BUP appeared to be the two most consistent factors associated with worse opioid use. It is well known that using drugs by injection indicates a more serious drug use problem. We have also reported previously that opioid use at the follow-up was higher among participants randomized to BUP relative to MET, mostly due to less treatment participation among participants randomized to BUP than MET (Hser et al., 2016). The trial lasted for only 6 months and across study sites or regions there was tremendous variability in post-trial treatment availability and/or availability of heroin and other opioids. These regional differences could influence variations in opioid use observed in the follow-up study, which should be further examined in future studies.
Because patients could drop out of the trial and switch to treatment to which they were not originally randomized, we further explored if the distinctive trajectory groups were associated with switching medication condition. Supplemental analysis of the treatment utilization characterizing each trajectory group indicated that a large majority of those randomized to BUP and who had a Low Use trajectory either remained on BUP (49%) or switched to MET (31%) during the initial six-month trial. Furthermore, 40% and 56% of individuals who remained on BUP as assigned or switched from BUP to MET, respectively, were belonged to the Low Use trajectory group. Conversely, those in the High Use group either remained on BUP (44%) or switched off of treatment entirely (43%). A larger proportion (34%) of individuals who transitioned out of treatment during the six-month trial were in the High Use group. Taken together, these results suggest that near-term treatment efficacy may predict better long-term substance use trajectories; however, more research is needed to explore this hypothesis. Small frequencies of individuals switching from MET to BUP or transitioning off of treatment entirely precluded a similar exploration of trajectory groups of those randomized to MET.
This study has several limitations. It was based on a randomized medication trial, and many participants dropped out, which influences the representativeness of the population and thereby the degree to which the study results can be generalized to the whole population of persons with opioid use disorder. Due to the long recruitment period, the follow-up periods varied across individuals, and some persons were lost to follow-up. Although we used growth modeling with full information maximum likelihood method, the assumption of missing at random could have influenced our outcomes. Another limitation is that most study measures were based on self-report. Nevertheless, underreporting was minimal as less than 3% of the sample that reported no opioid use in the past 30 days also tested positive for opioids. Retrospective reports on opioid use using TLFB could be subject to additional potential biases, but it is unlikely any such bias could be related to treatment status, and the trajectory patterns were consistent with urine testing results. Additionally, the growth mixture modeling is an analytic tool that empirically identifies distinctive trajectory patterns among the study sample. The reliability and generalizability of the identified trajectory models may need to be further verified using other samples along with a theoretical-based model (e.g., Sher, Jackson & Steinley, 2011). Finally, covariates are limited by the data collected at baseline, limiting the exploration of potential factors explaining or contributing to the distinctive opioid use trajectory patterns.
The study still revealed several important findings. Considerable heterogeneity in opioid use trajectories was identified among these opioid-dependent participants. Several baseline variables (region, ethnicity, drug use by injection, and mental health status) were associated with the High Use group suggesting risk factors needing attention for future targeted interventions. Further, treatment participation appears to be the main factor accounting for both within- and between-group variations in opioid use. Not surprisingly, the follow-up study also found that high levels of use were associated with worse outcomes in multiple key life domains measured by the ASI. Even though the High Use group started with better mental health functioning, their mental health worsened significantly compared to other groups. These observational data cannot determine whether the decrements in mental health were caused by opioid use, the opioid use was exacerbated by the mental health deterioration, or whether both pathways were operating. This topic needs further study; however, these findings are actionable now and suggest that the High Use group might benefit from psychiatric and/or behavioral interventions in addition to medication treatment.
The study findings underscore the importance of keeping opioid-dependent individuals in treatment and making treatment more widely available and accessible, which are consistent with recent initiatives by President Obama to expand treatment in order to address the recent national crisis in prescription opioid and heroin abuse epidemic. Identifying factors that may account for distinctive opioid use trajectory patterns can further inform policy decisions and clinic practice in targeting those at greatest need for opioid treatment.
Acknowledgments
The corresponding author, Yih-Ing Hser, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sincere appreciation to our participating networks: the Pacific Northwest Node and Evergreen Treatment Services; the Western States Node and CODA Inc. and Bi-Valley Medical Clinic; the New England Node and Connecticut Counseling Centers and Yale and Hartford Dispensary; the Delaware Valley Node and NET Steps; the Pacific Region Node and Matrix Institute; EMMES Corporation (CCC); the CCTN and NIDA.
Funding source:
Main study funding was provided by the National Institute on Drug Abuse (NIDA) through the Clinical Trials Network (CTN) through a series of grants provided to each participating node:
The Pacific Northwest Node (U10 DA01714)
The Western States Node (U10 DA015815)
The New England Node (U10 DA13038)
The Delaware Valley Node (U10 DA13043)
The Pacific Region Node (U10 DA13045)
The Greater New York Node (UG1 DA013035)
Funding was also provided by NIDA through grant number P30DA016383.
Footnotes
Declaration of Interest: Authors disclosing relevant financial interests, activities, relationships, and affiliations are:
Walter Ling: Consultant to Reckitt Benckiser Pharmaceuticals.
Andrew J. Saxon: Receives royalties as an editor for Up To Date.
George Woody: Consultant to Reckitt Benckiser Pharmaceuticals.
All other authors report no financial or other possible conflicts of interest.
Trial registration: The START Follow-up Study on ClinicalTrials.gov (NCT01592461).
References
- Asparouhov T, Muthen B. Mplus Web Notes: No. 15, Version 8, August 5, 2014. 2014. Auxiliary variables in mixture modelling: 3-Step approach using Mplus. [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Obeidat K, Losardo D. Twelve Frequently Asked Questions About Growth Curve Modeling. Journal of cognition and development: official journal of the Cognitive Development Society. 2010;11(2):121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y, Evans L, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harvard Review of Psychiatry. 2015;23(2):78–89. doi: 10.1097/HRP.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Hser Y, Evans L, Huang D, Weiss R, Saxon A, Carroll K, Woody G, et al. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site Trial. Addiction. 2016;111(4):695–705. doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109:79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y, Anglin MD, Chou CP. Reliability of retrospective self-report by heroin addicts. Psychol Assess. 1992;4:207–213. [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;16(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McLachlan G, Peel D. Finite mixture models. New York: Wiley; 2000. [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Murphy D, Hser Y, Huang D, Brecht L, Herbeck DM. Self-report of longitudinal substance use: A comparison of the UCLA Natural History Interview and the Addiction Severity Index. J Drug Issues. 2010;40:495–516. doi: 10.1177/002204261004000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Muthen L, Muthen B. Mplus User’s Guide. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128:71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Jackson KM, Steinley D. Alcohol use trajectories and the ubiquitous cat’s cradle: Cause for concern? Journal of Abnormal Psychology. 2011;120:322–335. doi: 10.1037/a0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36), I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]