Sex differences in asthma phenotypes and prevalence have been well described. In experimental animal models of asthma, female mice have increased airway hyperresponsiveness, eosinophil influx, and increased type 2 cytokine production (ie, interleukin [IL] 4, IL-5, and IL-13) in the lungs after allergen challenge when compared with males. Although CD4+ TH2 cells are known to produce type 2 cytokines, the type 2 innate lymphoid cells (ILC2s) are better described for producing much larger quantities of IL-5 and IL-13 compared with TH2 cells.1, 2 ILC2s are a rare yet potent lung immune cell population implicated in allergic asthma. Shortly after ILC2s were discovered, the release of the airway-localized cytokines IL-25 and IL-33 were found to activate IL-5 and IL-13 production in ILC2s.3, 4 IL-5 recruits and drives proliferation of eosinophils in allergic asthma. Moreover, a supporting role for ILC2s in the expansion and survival of eosinophils after viral infection has been described.5 IL-13 is recognized to generate mucous hypersecretion, leading to airway obstruction in severe asthma.6 What is currently unknown is whether ILC2s respond differently in the male vs female host in their response to allergen challenge and asthma.
In this study, we evaluated sex differences in ILC2s isolated from male and female saline- and ovalbumin-treated, age-matched BALB/c mice after ex vivo activation with IL-33. Mice were randomly assigned to either a saline group or an ovalbumin treatment group to induce experimental asthma. The ovalbumin-treated mice were sensitized by intraperitoneal injection of chicken ovalbumin (500 µg/mL) adsorbed with aluminum hydroxide (20 mg/mL) then received daily airway challenges for 5 days with 1.5% ovalbumin diluted in sterile saline in a whole-body nebulization chamber. All mice were handled and treated according to approved Institutional Animal Care and Use Committee guidelines.
The day after the final ovalbumin challenge, lung lymphocytes were separated from lung parenchymal cells using a Ficoll gradient. Lymphocytes were labeled with antimouse antibodies, and ILC2s were acquired using the FACS Aria cell sorter (BD Biosciences, San Jose, California). After exclusion of the LIN populations, the remaining cells were gated as ST2+ and Sca-1+. Subsequent gating analysis confirmed that these cells were positive for inducible T-cell costimulator, IL-7 receptor α, IL-2 receptor γ, and IL-25 receptor. ILC2s were maintained in culture with IL-2 (40 ng/mL) with or without IL-33 (80 ng/mL) in RPMI media supplemented with 10% fetal bovine serum for up to 72 hours in 5% carbon dioxide in a 37°C incubator. Supernates and cell pellets were separated by centrifugation (300g for 10 minutes), and supernates were tested for IL-5 and IL-13 proteins by enzyme-linked immunosorbent assay.
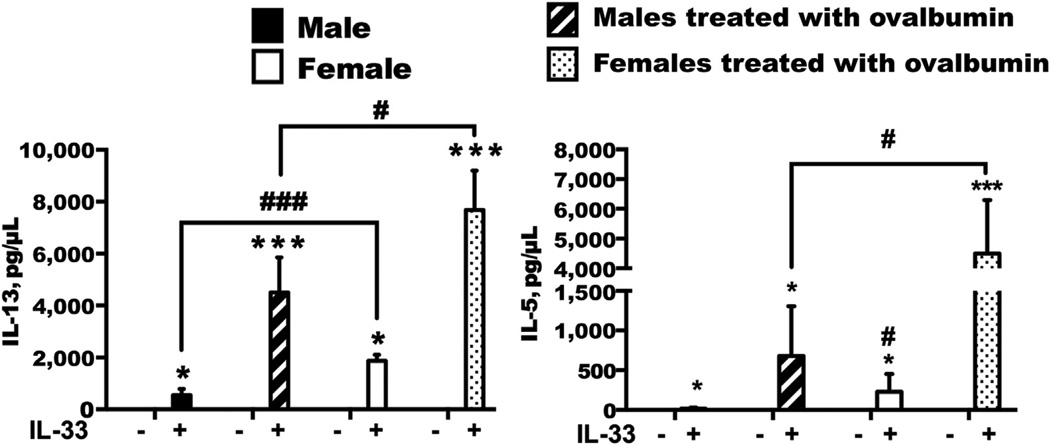
IL-33 stimulation of lung ILC2s induced a significant increase in IL-5 (up to 7 ng/mL) and IL-13 (up to 8 ng/mL) (P < .05) compared with media control wells (Fig 1). Regardless of whether the female mice were in vivo treated with ovalbumin or saline, ex vivo IL-33 stimulation of ILC2s resulted strikingly in increased protein levels of IL-13 and IL-5 (P < .05), which were not observed in the male animals. In addition, there were significant increases in IL-4 messenger RNA (2.5-fold) and IL-13 messenger RNA (4-fold) in IL-33–stimulated female ILC2s compared with male ILC2s (data not shown). This finding suggests an intrinsic sex-specific responsiveness of ILC2s, leading to increased type 2 cytokine gene expression and protein levels in females.
Figure 1.
Type 2 innate lymphoid cells (ILC2s) were isolated from whole-lung cells of male and female, saline- and ovalbumin-treated mice and maintained in culture with interleukin (IL) 2. ILC2s were stimulated with IL-33 (80 ng/mL) (n = 3 repeats) for 72 hours. IL-13 and IL-5 protein levels were quantified by enzyme-linked immunosorbent assay. Data are expressed as means; error bars indicate SE. Results are representative of 3 independent experiments. * P <.05, ** P <.01, and *** P <.001 for ovalbumin vs saline. # P < .05 and ## P < .01 for male vs female. Plus sign indicates positive; minus sign, negative.
IL-33 is present in the lungs shortly (<24 hours) after lung injury (induced by cigarette smoke, allergen, or viral infection) and has been described as a type 2 immune-promoting cytokine.4, 7 We chose to evaluate the stimulation of isolated ILC2s from male and female mice with IL-33 to assess potential sex differences in activation with this potent cytokine. We found a significant increase in IL-5 and IL-13 after IL-33–induced activation of ILC2s isolated from female mice, which has not been previously reported. In clinical observations, sputum ILC2s are proposed to promote eosinophil influx in patients with mild asthma compared with those with severe asthma through production of IL-5 and IL-13.8 This study, however, did not evaluated IL-33 protein levels in the blood, sputum, or lungs, respectively, and although one of few reports of ILC2s in patients with asthma, it did not account for differences in males vs females.
To our knowledge, no studies exist to assess sex-based differences in the IL-33 signaling pathway when this signaling pathway may be key to explaining differences between sexes in ILC2-driven airway disease. Future studies will focus on sex-based differences in IL-33 signaling, which is dependent on MyD88, as well as other signaling molecules, including IL-1 receptor–associated kinase 4 and tumor necrosis factor receptor–associated factor 6 in both males and females comparatively. Furthermore, the effect of specific sex hormones, including estrogen, progesterone, and testosterone, in ILC2 responses could also be investigated. Finally, these animal studies would support human study investigations to determine whether blood and/or airway ILC2s differ in male vs female patients with asthma. This information might be important in designing future, personalized, sex-based therapeutic strategies in patients with asthma. In summary, we found a profound effect of sex-specific differences in the activation status of the ILC2 population isolated from mice exposed to the standard ovalbumin model of allergic asthma.
Acknowledgments
Funding Sources: This study was supported by grants R01 ES019325 from the National Institute of Environmental Health Sciences (Dr Poole) and R01 AA008769 from the National Institute on Alcohol Abuse and Alcoholism (Mr Sisson), grant I01 BX003635 from the US Department of Veterans Affairs (Dr Wyatt), and the Dr. Eugene Kenney Memorial Fund for Asthma Research (Drs Wyatt and Warren).
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty TA, Broide DH. Group 2 innate lymphoid cells: new players in human allergic diseases. J Invest Allergol Clin Immunol. 2015;25:1–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Eberl G, Di Santo JP, Vivier E. The brave new world of innate lymphoid cells. Nature Immunol. 2015;16:1–5. doi: 10.1038/ni.3059. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DJ, Makrinioti H, Rana BM, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson JD, Alevy Y, Malvin NP, et al. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy. 2016;12:397–409. doi: 10.1080/15548627.2015.1056967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comeau M, Jessup H, Martin T, Vissinga C. Characterization of type 2 innate lymphoid cell (ILC2) responses to thymic stromal lymphopoietin (TSLP), IL-25, and IL-33. Am J Respir Crit Care Med. 2014;189:A6625. [Google Scholar]
- 8.Liu T, Wu J, Zhao J, et al. Type 2 innate lymphoid cells: A novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med. 2015;109:1391–1396. doi: 10.1016/j.rmed.2015.09.016. [DOI] [PubMed] [Google Scholar]