Abstract
Every year, nearly 300,000 people are diagnosed with pancreatic cancer worldwide, and an equivalent number succumb to this disease. One of the major challenges of pancreatic cancer that contributes to its poor survival rates is the development of resistance to the standard chemotherapy. Heterogeneity of the tumor, the dense fibroblastic stroma, and the aggressive biology of the tumor all contribute to the chemoresistant phenotype. In addition, the acellular components of the tumor microenvironment like hypoxia, stress pathways in the stromal cells, and the cytokines that are secreted by the immune cells, have a definitive role in orchestrating the chemoresistant property of the tumor. In this review, we systematically focus on the role played by the different microenvironmental components in determining chemoresistance of pancreatic tumors.
Introduction
Worldwide, almost 300,000 people are detected yearly with pancreatic cancer and an equivalent number succumb to this disease. In the US alone, the predicted number of pancreatic cancer cases in 2016 is estimated to be more than 53,000, and it is predicted that almost 41,000 of these will succumb to this disease (www.cancer.org). The 5-year survival is about 6 % in patients with pancreatic cancer and this figure has remained relatively unchanged over the past 25 years 1. The majority of patients present with locally advanced or metastatic disease, and such individuals have a grim median survival of 6–10 months, and 3–6 months, respectively 2. One of the major challenges that are responsible for this poor prognosis is the extreme chemoresistant phenotype of the tumor.
One of the main problems associated with chemotherapy has been that patient tumors with the same histology do not necessarily respond identically to the same therapeutic regimen. Identifying the presence of resistance mechanisms and other determinants for drug sensitivity, in order to classify tumors into response categories, has been an ongoing research effort. Recent studies have shown that the heterogeneity within the tumor contributes significantly to the tumor response to any kind of chemotherapy 3, 4. In addition to the heterogeneity within the tumor sub-types, current literature on pancreatic tumor biology has established beyond doubt that the tumor is a milieu of a large number of independent components that comprise its “microenvironment”. These include the fibro-inflammatory stroma, the secreted extracellular matrix, the infiltrating immune population, as well as the tumor initiating cell population5–9. Each of these components has a distinct role in conferring chemoresistance to the pancreatic tumor. The current review is focused on understanding how the tumor microenvironment may be instrumental in mediating the chemo-resistant property of pancreatic tumor (Figure 1).
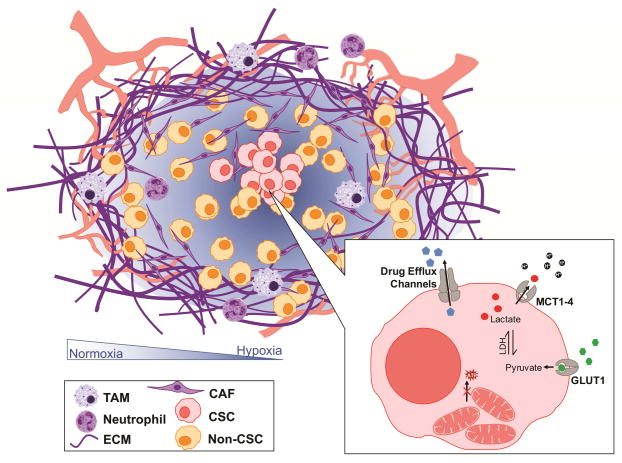
Figure 1.
A schematic displaying the role of the microenvironment in pancreatic adenocarcinomas (TME). The TME includes the immune cells (tumor associated macrophages (TAM) and neutrophils); stroma (extra cellular matrix (ECM) and cancer associated fibroblasts (CAF)); cancer stem cells (CSC) and non-CSC; in a hypoxic environment. Further, CSCs contain inactive mitochondria, higher levels of drug efflux channels, increased glucose uptake and lactate export.
Stroma as a physical barrier to drug delivery
The stroma has remained a controversial element in the pancreatic tumor microenvironment. To date, it has not been elucidated whether it plays a significant role in conferring chemoresistance. The fibro-inflammatory stroma develops along with the tumor. The primary components of the stroma are the fibroblasts, the immune cells, and the extracellular matrix (ECM) secreted by the cellular components. As the stroma develops, the interstitial pressure of the stromal cells and the ECM constrict the blood vessels, thereby not only reducing flow of oxygen and nutrients but also chemotherapeutic compounds 10, 11.
However, targeting the stroma has not yielded significant benefit it terms of the survival in pancreatic cancer patients. A study using the spontaneous pancreatic cancer mouse model (the KrasG12D; TP53 mutated mice or KPC mice) 12 proposed targeting the hyaluronan, a primary component of the ECM, in the pancreatic cancer stroma can relieve the pressure on the blood vessels thereby improving survival. This resulted in use of PEGylated hyaluronidase in combination with gemcitabine in the KPC mouse model. Though this study yielded promising results in mouse models, the Phase I clinical trial using this compound was terminated in 2014, as it did not offer any substantial benefit to patients compared to the current standard of care.
Intercepting stromal signaling pathways have been developed as another strategy to overcome the physical barrier posed by this tumor component. Of these, Sonic Hedgehog (SHH) signaling has been shown to be restricted to the stromal compartment 13. Thus, pharmacologic inhibition of the SHH pathway was hypothesized to have a positive impact on gemcitabine delivery, by reducing the desmoplastic stroma. However, though use of a smoothened inhibitor (IPI-926) with gemcitabine caused depletion of tumor stroma and resulted in increased micro-vessel density in animals, these inhibitors failed to improve survival in the recruited patients in clinical trials in 201214.
Later, a study by Rhim et. al in 2014 demonstrated that the role of stroma in pancreatic cancer may be restraining, and thus, inhibiting it particularly at an early stage of tumor development would likely be detrimental for survival and this would result in extensive metastasis of the cancer 15. In a parallel study by Ozdemir et. al. further showed that myofibroblast depletion in the tumor microenvironment led to extensive remodeling of the tumor ECM, with a significant decrease in tumor tissue stiffness and total collagen content. Interestingly, in this study, treatment with gemcitabine following myofibroblast depletion did not lead to increased survival 16. The study further showed that the most dramatic impact of myofibroblast depletion was on the composition of the immune infiltrate in the tumor microenvironment. The interplay between cancer-associated fibroblasts and immune cells has long been recognized as a major contributor of cancer development 17, 18. This study also demonstrated that the immune response (innate and adaptive) associated with pancreatic adenocarcinoma (PDAC) was significantly impaired when fibrosis was reduced starting at the PanIN stage or at the PDAC stage.
The debate of whether the stroma is beneficial for pancreatic tumor progression and resistance to therapy continues. In keeping with this concept, it can be argued that while depleting stroma in a tumor will result in relieving the interstitial pressure on the blood vessels and promote drug delivery to the tumor, the tumor cells need to be obliterated as well in order to prevent their extravasation and spread to a distant location. This hypothesis is supported by a recent publication on two experimental drugs: one of the studies published in 2015, showed that ormeloxifene, a non-hormonal, non-steroidal oral contraceptive to be effective in reducing desmoplasia as well as induce tumor cell death in pancreatic cancer19–21. The other study, also from 2015, evaluated a water-soluble diterpene triepoxide, Minnelide against both patient tumor derived xenografts as well as the spontaneous KPC model for pancreatic cancer. Minnelide was also able to decrease desmoplasia, increase drug delivery and induce tumor cell death at the same time 22. Early studies using Minnelide in multiple animal models have also shown that it prevents metastasis, which supports the hypothesis that the depletion of stromal component in pancreatic cancer will only be effective in tumor regression if the anti stromal compound is used in combination with anti-tumor and anti-metastatic drugs.
Pancreatic Stellate Cells conferring chemoresistance
Pancreatic Stellate Cells (PSCs) are a quiescent population of cells within the pancreas, which are an integral part of the tumor microenvironment. In a normal, disease-free state, these cells express desmin, glial fibrillary acidic protein (GFAP), and store cytoplasmic vitamin A-containing lipid droplets 23–25. The vitamin A droplets make them a distinct cell type that is different from the normal pancreatic fibroblast. Upon onset of disease, accumulation of reactive oxygen species, cytokines, and growth factors secreted by injured cells, activate PSCs stimulating them to differentiate into a myofibroblast like phenotype26, 27.
hPSC secretions have been shown to confer a chemoresistant cancer cell phenotype by (i) suppressing H2O2-induced apoptosis and increased survival of pancreatic cancer cells 28; and (ii) decreased pancreatic cancer cell sensitivity to gemcitabine and radiation therapy 29. In addition, pancreatic cancer cells cultured with ECM proteins, produced by PSCs, promoted resistance to 5-fluorouracil (5-FU), cisplatin, and doxorubicin 30. However, a limitation in the field is that no studies have examined the influence of PSCs on proteins, which protect tumor cells against chemotherapy agents (for example multi-drug resistant drug transporters). Further, recent work by Liu, et al have shown that periostin, exclusively overexpressed by the pancreatic stellate cells, confer resistance to gemcitabine in pancreatic cells and tumors31. In an independent study, it was also reported that PSCs promoted the expression of Hes1 in pancreatic cancer cells, which in turn contributed to their resistance to chemotherapy32. Additionally, the SDF1/CXCR4 axis has recently emerged as an important regulator of tumor-stroma interaction. SDF1 is secreted by the PSCs, while its receptor CXCR4, is secreted by the pancreatic cancer cells. A study by Zhang, et al revealed that PSCs promote the chemoresistance of pancreatic cancer cells to gemcitabine by paracrine SDF-1 α/CXCR4 signaling-induced activation of the intracellular FAK-AKT and ERK1/2 signaling pathways and a subsequent IL-6 autocrine loop in the cancer cells33.
It is important to note that in addition to the impact which stellate cells have on pancreatic cancer chemoresistance, it is now well established that the immune cells also impact tumor progression and chemoresistance 34. Immune cells within the tumor microenvironment activate PSCs, which may further potentiate the effect of stellate cells on chemoresistance.
Pancreatic Stellate Cells and radio-resistance
Almost ~40% of pancreatic cancer patients undergo external beam radiation therapy (EBRT) 35. Radiation therapy plays a significant role in locally advanced pancreatic cancer, particularly when patients do not have distant metastasis following a chemotherapy regimen. However, clinical trials testing the ability of radiation therapy to extend progression-free survival or increase overall survival either alone or in combination with chemotherapy, have not shown very conclusive results 35, 36. In addition, only 20% of pancreatic primary tumors show any significant response to radiation35.
The molecular pathways contributing to apparent resistance to ionising radiation (IR) in PC remain poorly understood37, 38. However, recent research has shown that pancreatic stellate cells may play a significant role in contributing to the radio-resistance of the tumor. Radioresistance conferred by the stellate cells was shown to be mediated by β-Intergrin-FAK signaling in the tumor cells39. Since this initial report, several studies have highlighted the radio-protective role played by the PSCs on the tumor. Recent studies by Al-Assar et al in 201440 as well as in 201641 showed that the presence of PSCs in a co-culture with pancreatic cancer cells protect the cancer cells from radiation induced cell death42. The contribution of stellate cells to radioprotection of pancreatic tumor is an upcoming and developing field and much remains to be done in this respect in order to gain a complete insight into the molecular mechanisms that may be involved in the process.
Hypoxic microenvironment as a factor for chemoresistance
As the tumor progresses, the developing stroma exerts pressure on blood vessels, resulting in constriction and increasing hypoxic niches in the tumor. However, this hypoxia does not lead to increased angiogenesis in pancreatic adenocarcinoma like pancreatic neuroendocrine tumors 14, 43. Instead, this hypoxia activates multiple signaling pathways that may contribute to chemoresistance.
It is known that a hypoxic environment exists both in pancreatic cancer cells as well as in surrounding PSCs. A study by Masamune showed that hypoxia induced migration, type I collagen expression, and vascular endothelial growth factor (VEGF) production in PSCs, suggesting its pro-fibrogenic and pro-angiogenic responses in these cells 44. Similarly, a study by Erkan, et al showed that in the peritumoral stroma, PSCs contribute to the fibrotic-hypoxic milieu through abnormal extracellular matrix deposition, and by amplifying endostatin production of cancer cells45.
Low oxygen content in the tumor results in stabilization of the Hypoxia Inducible Factor 1 (HIF1A). HIF1A is the central node that mediates activation of a number of different signaling pathways that alter metabolic pathways, induce invasiveness, promote chemoresistance, and lead to a poor prognosis of the patient 46. HIF has been reported to participate in drug and radiotherapy resistance in cancer treatment. Radiotherapy (ionizing radiation) causes DNA damage directly and indirectly. Ionizing radiation and some chemotherapeutic drugs cause intracellular/tissue water ionizing to produce free radical (to impair DNA). Lack of oxygen reduces the cytotoxic effects of drugs and radiotherapy directly. Therefore, areas of hypoxic tumor tissue are more resistant to treatment and are associated with a poor clinical prognosis 47.
HIF1A is a tightly regulated protein in the tumor cell that is degraded under normal oxygen content. However, upon hypoxic stress, HIF1A accumulates and compensates for low oxygen by increasing glycolysis and glucose uptake in the cells. This in turn results in the switch of cellular metabolism from oxidative phosphorylation to aerobic glycolysis, or the Warburg effect. The Warburg effect is not only a critical cellular metabolic adaptation to cyclic hypoxia, it is also an obviously beneficial trade-off for cancer cells to increase chemoresistance, mutation rate, and invasion/metastatic ability 48. The increase in glycolytic metabolism also results in the production of lactate, which results in the acidification of the extracellular environment. The resulting extracellular acidification coupled with HIF-1a-induced expression of carbonic anhydrases causes a significant change in the pH ratio between the intracellular and extracellular environment. This pH shift decreases the passive absorption of many drugs that would otherwise accumulate at a greater concentration within the cell49, 50.
Despite their roles in energy biogenesis, mitochondria also play an important role in the control of cell death. Mitochondria regulate cell death pathways not only through control of intrinsic apoptotic pathways but also the generation of reactive oxygen species 51. It has been shown that chemotherapy induced tumor cell death is mediated through the generation of reactive oxygen species. The generation of reactive oxygen species is suppressed in tumor cells under hypoxia by HIF1A thus conferring the drug resistance of tumor cells 52, 53.
Microenvironment niches promote stemness and chemoresistance within a tumor
Recent research in the field has shown that the enrichment of cancer stem cells depends on the microenvironment niches54 In pancreatic cancer, in addition to regulating altered metabolism in order to combat hypoxic stress in the tumor, HIF1A also regulates stemness in the tumor microenvironment by upregulating self-renewal genes in the tumor 6, 55–57. Thus, the hypoxic niche in the tumor microenvironment is one of the major driving forces for the tumor initiating population within it. In addition to hypoxia, the stromal cells can also enrich for tumor initiating cells. Recent work by Lonardo et al have also shown that pancreatic stellate cells are able to provide a niche for the cancer stem cells as well58.
Tumor initiating cells (TICs) are a quiescent population within a tumor that escape chemotherapeutic drug induced cell death by either having an increased drug efflux property or altered proliferative index. While standard chemotherapy targets rapidly diving cell population, the TICs, being quiescent, remain unaffected by these drugs. A number of recent researches have been focused on understanding the mechanism by which this rare population within a tumor escapes chemotherapeutics drugs56, 59–64.
One of the characteristics of TICs is an increased drug efflux. These cells have an abnormally high activity of ATP driven xenobiotic transporters like the ABC transporter family, which help in keeping the intracellular drug concentration low and thereby help the cell to evade the cytotoxic effects of the drugs. Increased activity of ABC transporters is typically fueled by increased metabolic needs of the tumor cells. This further stresses the role of hypoxia in the tumor microenvironment mediating altered metabolic needs of the tumor cells resulting in increased efflux activity of ABC transporters in the TICs 65–67.
Inflammation, Immune cells and chemoresistance
Role of inflammation and inflammatory molecules in progression of pancreatic cancer is well known8, 17, 68. The involvement of these in regulating the chemoresistant phenotype of the pancreatic tumor are becoming apparent by recent studies 69–71. As the pancreatic tumor progresses, the cross talk between the different cell types in the microenvironment get actively involved in recruiting the fibro-inflammatory stroma. Paracrine signaling, involving the cytokines and chemokines from the infiltrating cells, facilitate a tumor stroma interaction, resulting in formation of a reactive stroma that contributes to the chemoresistant nature of the tumor.
Inflammation within the pancreatic tumor microenvironment has been mechanistically linked to tumor progression and chemoresistance through NF-κB, IL-6, toll-like receptor and TGF-β signaling pathways 72, 73. Unlike ovarian and colorectal cancers, survival gains from immune cell infiltration into the tumor microenvironment have not been conclusively demonstrated in pancreatic cancer 74, 75. In a recent study, Delitto et al examined the inflammatory milieu present in the pancreatic cancer microenvironment from 36 freshly resected tumor specimens using a forty-one-item panel of cytokines, chemokines and growth factors. This study showed that among others, increased intratumoral IL-8 concentrations were associated with larger tumors and poor differentiation; the administration of neoadjuvant chemotherapy was associated with reduced IL-8 concentrations8. Similarly, elevated levels of pro-inflammatory cytokines IL-1β and TNFα were associated with a poor histopathologic response to neoadjuvant therapy 8. However, the molecular mechanisms of how this is instrumental in conferring a chemoresistant phenotype to the tumor needs to be determined by future studies.
Anti-tumor necrosis factor-alpha (TNF-α) antibodies have promising effects in a number of PDAC pre-clinical models. Inhibition of TNF-α has also been shown to synergize with chemotherapy in PDAC and result in a better pre-clinical response by killing tumor cells as well as diminishing desmoplasia and inflammation in PDAC tumor stroma76.
Another predominant component of the microenvironment constituting a “niche” are the immune cells. Together with the stroma, these cells play a significant role in determining the chemoresistant phenotype of the pancreatic tumor. The immune system monitors and eliminates pathogens as well as developing tumors. Early studies of hematological malignancies suggested that tumor-associated macrophages (TAMs) can promote chemoresistance by directly interacting with malignant cells within the tumor microenvironment 77. It is now clear that TAMs can influence the response of cancer cells to chemotherapy in the context of a process known as environment-mediated drug resistance 78, 79.
A study by Amit et al showed that TAMs stimulate chemoresistance by promoting the expression of CDA, the enzyme responsible for the inactivation of gemcitabine, by cancer cells. Using TAM-conditioned medium, the study showed a decrease in apoptosis of malignant cells exposed to gemcitabine. The co-culture of TAMs and cancer cells had similar effect on gemcitabine-induced apoptosis compared to exposure of the latter to TAM-conditioned medium, suggesting that one or more soluble factors secreted by TAMs mediate(s) this effect. In line with this finding, macrophage-depleted mice were more sensitive to the antineoplastic effects of gemcitabine than their wild-type counterparts. This study thus demonstrated for the first time that macrophages could increase the resistance of cancer cells to chemotherapy through the upregulation of CDA, an intracellular enzyme which catabolizes the active form of gemcitabine 80.
Conclusion
Pancreatic cancer poses a major therapeutic challenge. So far, only small progress has been in the diagnosis and management of patients with this disease. The recent discoveries in the field of genetics, biology, metabolism and immunology of pancreatic cancer have created new opportunities to develop novel approaches for earlier diagnosis and more effective treatment. More research is clearly needed to improve our biological understanding. It remains to be demonstrated whether therapeutic targeting of the different components of the tumor microenvironment, either alone or in combination with conventional treatments, will improve the outcome of patients with pancreatic cancer in the near future. Thus, understanding the role of microenvironment in tumor progression as well as its response to intervention is of extreme importance for development of novel therapy against this devastating disease.
Acknowledgments
This study was funded by NIH grants R01-CA170946 and CA124723 (to AKS); NIH grant R01-CA184274 (to SB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Binkley CE, Simeone DM. Pancreatic Cancer. Elsevier; USA: 2004. [Google Scholar]
- 3.Roberts NJ, Norris AL, Petersen GM, et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer Discov. 2016;6:166–175. doi: 10.1158/2159-8290.CD-15-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waghray M, Yalamanchili M, di Magliano MP, Simeone DM. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol. 2013;29:537–543. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan HX, Xu JW, Wu D, Zhang TP, Hu SY. Pancreatic cancer stem cells: new insight into a stubborn disease. Cancer Lett. 2015;357:429–437. doi: 10.1016/j.canlet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Ilmer M, Horst D. Pancreatic CSCs and microenvironment. Genes Cancer. 2015;6:365–366. doi: 10.18632/genesandcancer.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delitto D, Black BS, Sorenson HL, et al. The inflammatory milieu within the pancreatic cancer microenvironment correlates with clinicopathologic parameters, chemoresistance and survival. BMC cancer. 2015;15:783. doi: 10.1186/s12885-015-1820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, Wilson JS. Pancreatic cancer: The microenvironment needs attention too! Pancreatology. 2015;15:S32–38. doi: 10.1016/j.pan.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher FC. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32:445–451. doi: 10.1093/carcin/bgq280. [DOI] [PubMed] [Google Scholar]
- 14.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY) 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 19.Khan S, Chauhan N, Yallapu MM, et al. Nanoparticle formulation of ormeloxifene for pancreatic cancer. Biomaterials. 2015;53:731–743. doi: 10.1016/j.biomaterials.2015.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan S, Ebeling MC, Chauhan N, et al. Ormeloxifene suppresses desmoplasia and enhances sensitivity of gemcitabine in pancreatic cancer. Cancer Res. 2015;75:2292–2304. doi: 10.1158/0008-5472.CAN-14-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher DM, Khan S, Nordquist JL, et al. Ormeloxifene efficiently inhibits ovarian cancer growth. Cancer Lett. 2015;356:606–612. doi: 10.1016/j.canlet.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee S, Modi S, McGinn O, et al. Impaired synthesis of stromal components in response to Minnelide improves vascular function, drug delivery and survival in pancreatic cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apte MV, Pirola RC, Wilson JS. Battle-scarred pancreas: role of alcohol and pancreatic stellate cells in pancreatic fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S97–S101. doi: 10.1111/j.1440-1746.2006.04587.x. [DOI] [PubMed] [Google Scholar]
- 25.McCarroll JA, Phillips PA, Santucci N, Pirola RC, Wilson JS, Apte MV. Vitamin A inhibits pancreatic stellate cell activation: implications for treatment of pancreatic fibrosis. Gut. 2006;55:79–89. doi: 10.1136/gut.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. The American journal of pathology. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vonlaufen A, Phillips PA, Xu Z, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:7707–7710. doi: 10.1158/0008-5472.CAN-08-1132. [DOI] [PubMed] [Google Scholar]
- 29.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Li F, Gao F, et al. Periostin promotes the chemotherapy resistance to gemcitabine in pancreatic cancer. Tumour Biol. 2016;37:15283–15291. doi: 10.1007/s13277-016-5321-6. [DOI] [PubMed] [Google Scholar]
- 32.Cao F, Li J, Sun H, Liu S, Cui Y, Li F. HES 1 is essential for chemoresistance induced by stellate cells and is associated with poor prognosis in pancreatic cancer. Oncol Rep. 2015;33:1883–1889. doi: 10.3892/or.2015.3789. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Wu H, Guan J, et al. Paracrine SDF-1alpha signaling mediates the effects of PSCs on GEM chemoresistance through an IL-6 autocrine loop in pancreatic cancer cells. Oncotarget. 2015;6:3085–3097. doi: 10.18632/oncotarget.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol. 2012;3:270. doi: 10.3389/fphys.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazard L. The role of radiation therapy in pancreas cancer. Gastrointest Cancer Res. 2009;3:20–28. [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman KA, Hajj C. Role of radiation therapy in the management of pancreatic cancer. J Surg Oncol. 2013;107:86–96. doi: 10.1002/jso.23137. [DOI] [PubMed] [Google Scholar]
- 37.Brunner TB, Cengel KA, Hahn SM, et al. Pancreatic cancer cell radiation survival and prenyltransferase inhibition: the role of K-Ras. Cancer Res. 2005;65:8433–8441. doi: 10.1158/0008-5472.CAN-05-0158. [DOI] [PubMed] [Google Scholar]
- 38.Kimple RJ, Vaseva AV, Cox AD, et al. Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res. 2010;16:912–923. doi: 10.1158/1078-0432.CCR-09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Assar O, Demiciorglu F, Lunardi S, et al. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother Oncol. 2014;111:243–251. doi: 10.1016/j.radonc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Al-Assar O, Bittner MI, Lunardi S, Stratford MR, McKenna WG, Brunner TB. The radiosensitizing effects of Nelfinavir on pancreatic cancer with and without pancreatic stellate cells. Radiother Oncol. 2016;119:300–305. doi: 10.1016/j.radonc.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Cabrera MC, Tilahun E, Nakles R, et al. Human Pancreatic Cancer-Associated Stellate Cells Remain Activated after in vivo Chemoradiation. Front Oncol. 2014;4:102. doi: 10.3389/fonc.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson P, Chu GC, Perry SR, Nolan-Stevaux O, Hanahan D. Imaging guided trials of the angiogenesis inhibitor sunitinib in mouse models predict efficacy in pancreatic neuroendocrine but not ductal carcinoma. Proc Natl Acad Sci U S A. 2011;108:E1275–1284. doi: 10.1073/pnas.1111079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709–717. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- 45.Erkan M, Reiser-Erkan C, Michalski CW, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497–508. doi: 10.1593/neo.81618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doktorova H, Hrabeta J, Khalil MA, Eckschlager T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:166–177. doi: 10.5507/bp.2015.025. [DOI] [PubMed] [Google Scholar]
- 47.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 48.Kondoh H. Cellular life span and the Warburg effect. Exp Cell Res. 2008;314:1923–1928. doi: 10.1016/j.yexcr.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 50.Maftouh M, Avan A, Sciarrillo R, et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172–182. doi: 10.1038/bjc.2013.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Gao P, Fukuda R, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 54.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell stem cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nomura A, Dauer P, Gupta V, et al. Microenvironment mediated alterations to metabolic pathways confer increased chemo-resistance in CD133+ tumor initiating cells. Oncotarget. 2016 doi: 10.18632/oncotarget.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamada S, Shimosegawa T. Pancreatic cancer stem cell and mesenchymal stem cell. In: Grippo PJ, Munshi HG, editors. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India): 2012. [PubMed] [Google Scholar]
- 57.Meng F, Li C, Li W, Gao Z, Guo K, Song S. Interaction between pancreatic cancer cells and tumor-associated macrophages promotes the invasion of pancreatic cancer cells and the differentiation and migration of macrophages. IUBMB Life. 2014;66:835–846. doi: 10.1002/iub.1336. [DOI] [PubMed] [Google Scholar]
- 58.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–1290. doi: 10.4161/cc.19679. [DOI] [PubMed] [Google Scholar]
- 59.Bhagwandin VJ, Bishop JM, Wright WE, Shay JW. The Metastatic Potential and Chemoresistance of Human Pancreatic Cancer Stem Cells. PloS one. 2016;11:e0148807. doi: 10.1371/journal.pone.0148807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santisteban M. ABC transporters as molecular effectors of pancreatic oncogenic pathways: the Hedgehog-GLI model. J Gastrointest Cancer. 2010;41:153–158. doi: 10.1007/s12029-010-9144-1. [DOI] [PubMed] [Google Scholar]
- 61.Van den Broeck A, Gremeaux L, Topal B, Vankelecom H. Human pancreatic adenocarcinoma contains a side population resistant to gemcitabine. BMC cancer. 2012;12:354. doi: 10.1186/1471-2407-12-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weng CC, Kuo KK, Su HT, et al. Pancreatic Tumor Progression Associated With CD133 Overexpression: Involvement of Increased TERT Expression and Epidermal Growth Factor Receptor-Dependent Akt Activation. Pancreas. 2016;45:443–457. doi: 10.1097/MPA.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 63.Yao J, Cai HH, Wei JS, et al. Side population in the pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with cancer stem-like cells. Oncol Rep. 2010;23:1375–1382. doi: 10.3892/or_00000774. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z, Duan Q, Zhao H, et al. Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-kappaB/STAT3 signaling cascade. Cancer Lett. 2016;382:53–63. doi: 10.1016/j.canlet.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 65.Cojoc M, Mabert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol. 2015;31:16–27. doi: 10.1016/j.semcancer.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Krishnamurthy P, Ross DD, Nakanishi T, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 67.Sarkadi B, Ozvegy-Laczka C, Nemet K, Varadi A. ABCG2 -- a transporter for all seasons. FEBS Lett. 2004;567:116–120. doi: 10.1016/j.febslet.2004.03.123. [DOI] [PubMed] [Google Scholar]
- 68.Ali N, Chandrakesan P, Nguyen CB, et al. Inflammatory and oncogenic roles of a tumor stem cell marker doublecortin-like kinase (DCLK1) in virus-induced chronic liver diseases. Oncotarget. 2015;6:20327–20344. doi: 10.18632/oncotarget.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fokas E, O’Neill E, Gordon-Weeks A, Mukherjee S, McKenna WG, Muschel RJ. Pancreatic ductal adenocarcinoma: From genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochim Biophys Acta. 2015;1855:61–82. doi: 10.1016/j.bbcan.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Grimmig T, Matthes N, Hoeland K, et al. TLR7 and TLR8 expression increases tumor cell proliferation and promotes chemoresistance in human pancreatic cancer. Int J Oncol. 2015;47:857–866. doi: 10.3892/ijo.2015.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muerkoster S, Wegehenkel K, Arlt A, et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004;64:1331–1337. doi: 10.1158/0008-5472.can-03-1860. [DOI] [PubMed] [Google Scholar]
- 73.Hausmann S, Kong B, Michalski C, Erkan M, Friess H. The role of inflammation in pancreatic cancer. Adv Exp Med Biol. 2014;816:129–151. doi: 10.1007/978-3-0348-0837-8_6. [DOI] [PubMed] [Google Scholar]
- 74.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 76.Zhao X, Fan W, Xu Z, et al. Inhibiting tumor necrosis factor-alpha diminishes desmoplasia and inflammation to overcome chemoresistance in pancreatic ductal adenocarcinoma. Oncotarget. 2016 doi: 10.18632/oncotarget.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng Y, Cai Z, Wang S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Palma M, Lewis CE. Cancer: Macrophages limit chemotherapy. Nature. 2011;472:303–304. doi: 10.1038/472303a. [DOI] [PubMed] [Google Scholar]
- 79.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amit M, Gil Z. Macrophages increase the resistance of pancreatic adenocarcinoma cells to gemcitabine by upregulating cytidine deaminase. Oncoimmunology. 2013;2:e27231. doi: 10.4161/onci.27231. [DOI] [PMC free article] [PubMed] [Google Scholar]