Abstract
Nosocomial infections caused by Pseudomonas aeruginosa in critically ill patients are often difficult to treat due to resistance to multiple antimicrobials. The purpose of this study was to evaluate antimicrobial resistance among P. aeruginosa isolates from intensive care unit patients in the United States from 1993 to 2002 by using the Intensive Care Unit Surveillance Study database. Over the 10-year period, susceptibility of 13,999 nonduplicate isolates of P. aeruginosa was analyzed. From 1993 to 2002, nationwide increases in antimicrobial resistance were greatest for ciprofloxacin, imipenem, tobramycin, and aztreonam. Rates of multidrug resistance (resistance to ≥3 of the following drugs: ceftazidime, ciprofloxacin, tobramycin, and imipenem) increased from 4% in 1993 to 14% in 2002. The lowest dual resistance rates were observed between aminoglycosides or fluoroquinolones with piperacillin-tazobactam while the highest were for those that included β-lactams and ciprofloxacin. Ongoing surveillance studies are crucial in monitoring antimicrobial susceptibility patterns and selecting empirical treatment regimens.
Pseudomonas aeruginosa is one of the most common gram-negative pathogens associated with nosocomial infections (11). Unfortunately, resistance to available antipseudomonal agents is on the rise, jeopardizing selection of appropriate treatment and subsequently increasing morbidity and mortality in patients infected with this pathogen (1, 9). In fact, inadequate empirical therapy has been associated with mortality exceeding 30% (6), and delays in the initiation of appropriate therapy contribute to increased length of hospital stay and persistence of infection (8).
The selection of appropriate antimicrobial therapy requires active surveillance of emerging resistance trends and continuing education among the health care providers and institution(s) involved. The objectives of this study were to analyze data from the Intensive Care Unit Surveillance Study (ISS) to assess the rates of resistance and multidrug resistance among P. aeruginosa isolates in intensive care units (ICUs) in the United States from 1993 to 2002 and to use these data to evaluate current recommendations for empirical antibiotic regimens.
(These data were presented as a poster presentation at the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy on 17 September 2003.)
MATERIALS AND METHODS
The ISS is a national postmarketing surveillance program sponsored by Merck and Company Inc. (Rahway, N.J.) since 1989. Participating institutions submitted susceptibility data for approximately 100 to 200 consecutive gram-negative aerobic isolates from ICU patients annually. All organisms were identified to the species level and susceptibility testing was conducted at each institution using a standardized custom microdilution MIC panel (Microscan MKD MIC; Dade International MicroScan, Sacramento, Calif.) (10). The P. aeruginosa quality control strain ATCC 27853 was used weekly in each laboratory.
In this study, independent of the research sponsor, we evaluated the susceptibility of nonduplicate isolates of P. aeruginosa from 1993 to 2002. Nonduplicate isolates consisted of initial or unique isolates from an individual patient. A total of 17 antimicrobials were included in the standardized custom microdilution MIC panel. With respect to this study, only the susceptibilities to commonly recognized antipseudomonal agents were evaluated, including the aminoglycosides (amikacin, tobramycin, and gentamicin); antipseudomonal cephalosporins (ceftazidime and cefepime); extended-spectrum penicillins (piperacillin); β-lactam-β-lactamase inhibitor combinations (ticarcillin-clavulanate and piperacillin-tazobactam); fluoroquinolones (ciprofloxacin and levofloxacin); aztreonam; and imipenem. In 2001, gentamicin was replaced with levofloxacin in the standardized MIC panel. For purposes of study analysis, any antimicrobial displaying intermediate susceptibility according to the NCCLS guidelines was considered resistant.
P. aeruginosa isolates were considered to be multidrug resistant if the isolate was resistant to at least three of the following four drugs: imipenem, ceftazidime, ciprofloxacin, and tobramycin. These agents were selected as representatives of the primary antibiotic classes used to treat P. aeruginosa infections in addition to availability of MIC data for the entire ISS study duration from 1993 through 2002. This study also evaluated dual resistance rates among β-lactam antibiotics and aminoglycosides or fluoroquinolones. Dual resistance was defined as resistance to both agents within a two-drug combination regimen.
Statistical analysis.
All statistical analyses were performed using SAS software, version 8 (SAS Institute, Cary, N.C.). Data are presented as percentages unless otherwise stated. The variables analyzed included resistance rates of individual antipseudomonal agents as well as multidrug resistance and dual resistance rates among commonly used antipseudomonal combinations over the 10-year period. Comparisons utilized a chi-square test for an R × C contingency table with nine degrees of freedom. A P value of ≤0.05 was considered significant.
RESULTS
Over the 10-year period from 1993 to 2002, 45 to 117 ICUs participated in the study annually, representing 43 states plus the District of Columbia. These institutions consisted primarily (>80%) of teaching hospitals with bed sizes ranging from 250 to 1,500, and a majority of the hospitals (>80%) repeatedly took part in this program during the study period. Susceptibility of antipseudomonal agents for 13,999 nonduplicate isolates of P. aeruginosa was evaluated during the 10-year period. Although P. aeruginosa isolates represented a significant portion of the 100 to 200 consecutive gram-negative isolates submitted from each institution, omission of repeat isolates yielded approximately 20 to 25% of all isolates from each ICU being evaluated for this study. The patient locations and culture sites from which P. aeruginosa strains were isolated are described in Table 1. During the study period, the majority of strains were isolated from patients in the medical ICU (44 to 53%) or surgical ICU (28 to 34%). P. aeruginosa was most commonly isolated from respiratory sources (bronchial washings, pleural fluid, sputum, tracheal aspirates, and lung tissue samples) for all years.
TABLE 1.
Individual P. aeruginosa isolates by patient location and culture site, 1993 to 2002
| Isolate parameter | Result for:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | |
| No. of isolates | 960 | 1,041 | 1,122 | 1,075 | 1,338 | 2,312 | 1,716 | 1,623 | 1,861 | 951 |
| Patient location (%) | ||||||||||
| Burn ICU | 1 | 3 | 0 | 2 | 2 | 1 | 3 | 4 | 5 | 4 |
| Medical ICU | 46 | 52 | 55 | 48 | 46 | 50 | 52 | 46 | 44 | 44 |
| Neurology ICU | 3 | 4 | 4 | 2 | 4 | 5 | 4 | 6 | 5 | 6 |
| Surgical ICU | 31 | 28 | 33 | 34 | 34 | 34 | 29 | 29 | 32 | 31 |
| Pediatric/Newborn ICU | 4 | 7 | 3 | 3 | 3 | 3 | 3 | 8 | 5 | 8 |
| Others | 15 | 6 | 5 | 11 | 11 | 7 | 9 | 7 | 9 | 7 |
| Culture (%) | ||||||||||
| Blood | 3 | 7 | 7 | 8 | 9 | 7 | 7 | 8 | 8 | 7 |
| Pulmonary sourcesa | 63 | 65 | 64 | 67 | 64 | 64 | 60 | 62 | 62 | 66 |
| Urine | 13 | 13 | 10 | 9 | 10 | 12 | 15 | 13 | 10 | 10 |
| Wound | 4 | 4 | 6 | 5 | 6 | 7 | 6 | 6 | 5 | 6 |
| Othersb | 17 | 11 | 13 | 11 | 11 | 10 | 12 | 11 | 15 | 11 |
Pulmonary sources include sputum, bronchial washings, tracheal aspirates, pleural fluid, and lung tissue.
Other sources included abdomen or peritoneal cavity, bone, cerebrospinal fluid, liver, pancreas, spleen, and catheter tips.
Resistance rates to common antipseudomonal agents among the isolated P. aeruginosa strains are presented in Table 2. Over the 10-year period, susceptibilities against P. aeruginosa isolates declined significantly for all drug classes. From 1993 to 2002, dramatic increases in antimicrobial resistance occurred with ciprofloxacin (15 to 32%, P < 0.0001), imipenem (15 to 23%, P < 0.0001), tobramycin (9 to 16%, P < 0.0001), and aztreonam (26 to 32%, P < 0.0001). Although susceptibility data were not available for cefepime during the entire study period, there was a significant increase in resistance rates (16% in 1998 to 25% in 2002, P < 0.0001). For most antimicrobial agents, steady increases in resistance occurred over the study period with rates peaking in 2001 followed by a slight decrease in 2002; exceptions to these trends were imipenem and levofloxacin, which peaked in 2002.
TABLE 2.
In vitro antimicrobial resistance rates for P. aeruginosa, 1993 to 2002a
| Yr | No. of isolates | IMP | CAZ | CEF | P/T | PI | TIM | AZT | AK | TOB | GEN | CIP | LEV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | 960 | 15 | 15 | NA | NA | 11 | 14 | 26 | 7 | 9 | 29 | 15 | NA |
| 1994 | 1,041 | 15 | 16 | NA | NA | 13 | 18 | 29 | 12 | 11 | 29 | 17 | NA |
| 1995 | 1,122 | 15 | 16 | NA | NA | 11 | 17 | 27 | 8 | 9 | 26 | 19 | NA |
| 1996 | 1,075 | 15 | 18 | NA | NA | 12 | 17 | 27 | 8 | 10 | 25 | 19 | NA |
| 1997 | 1,338 | 16 | 18 | NA | 9 | 14 | 20 | 29 | 8 | 9 | 26 | 22 | NA |
| 1998 | 2,312 | 13 | 17 | 16 | 10 | 13 | 18 | 29 | 8 | 12 | 32 | 25 | NA |
| 1999 | 1,716 | 17 | 18 | 24 | 12 | 16 | 20 | 33 | 9 | 14 | 36 | 28 | NA |
| 2000 | 1,623 | 16 | 19 | 24 | 12 | 15 | 19 | 33 | 9 | 12 | 32 | 28 | NA |
| 2001 | 1,861 | 19 | 21 | 28 | 13 | 19 | 21 | 35 | 13 | 17 | NA | 34 | 30 |
| 2002 | 951 | 23 | 19 | 25 | 10 | 15 | 17 | 32 | 10 | 16 | NA | 32 | 34 |
| P valueb | <0.0001 | 0.0002 | <0.0001 | 0.0005 | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.024 |
IMP, imipenem; CAZ, ceftazidime; CEF, cefepime; P/T, piperacillin-tazobactam; PI, piperacillin; TIM, ticarcillin-clavulanate; AZT, aztreonam; AK, amikacin; TOB, tobramycin; GEN, gentamicin; CIP, ciprofloxacin; LEV, levofloxacin; NA, not available. Twelve of 17 antibiotics tested against P. aeruginosa are shown. Data presented as percentages.
P values representative of available resistance data.
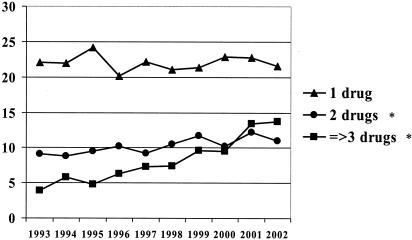
The incidence of multidrug resistance is reported in Fig. 1. While resistance to one agent remained relatively stable over the 10-year period (P > 0.05), a significant increase in resistance rates to two drugs occurred (P = 0.04). Overall, multidrug resistance increased from 4% in 1993 to 14% in 2002 (P < 0.0001).
FIG. 1.
Number of antipseudomonal drugs (imipenem, ceftazidime, ciprofloxacin, and tobramycin) to which P. aeruginosa isolates were resistant, 1993 to 2002. Resistance to at least three of these drugs was considered multidrug resistance. *, indicates P value of <0.05 for the 10-year study period.
Resistance to both agents in commonly used antibiotic combinations was also assessed and is reported in Table 3. The dual resistance rates increased significantly among all examined regimens. Of note, the dual resistance was highest for ciprofloxacin combinations in comparison to aminoglycosides. Although the dual resistance rates with amikacin and β-lactam antipseudomonal agents remained relatively low (approximately 5% in 2002), dual resistance with combinations of tobramycin and β-lactam agents increased (approximately 3% in 1993 to 10% in 2002, P ≤ 0.0002). Among β-lactam antibiotics, aztreonam, cefepime, and imipenem combinations showed dual resistance rates that were higher with ciprofloxacin or aminoglycosides than with piperacillin-tazobactam.
TABLE 3.
Resistance of P. aeruginosa isolates to both agents in potential combination regimensa
| Yr | Cefepime
|
Imipenem
|
Piperacillin-tazobactam
|
Aztreonam
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | TOB | AK | CIP | TOB | AK | CIP | TOB | AK | CIP | TOB | AK | |
| 1993 | NA | NA | NA | 5 | 3 | 2 | NA | NA | NA | 7 | 4 | 4 |
| 1994 | NA | NA | NA | 6 | 5 | 6 | NA | NA | NA | 11 | 8 | 8 |
| 1995 | NA | NA | NA | 6 | 3 | 3 | NA | NA | NA | 10 | 5 | 5 |
| 1996 | NA | NA | NA | 6 | 4 | 3 | NA | NA | NA | 10 | 6 | 6 |
| 1997 | NA | NA | NA | 9 | 4 | 3 | 5 | 2 | 2 | 11 | 5 | 5 |
| 1998 | 10 | 5 | 4 | 8 | 5 | 2 | 7 | 4 | 3 | 14 | 6 | 5 |
| 1999 | 15 | 10 | 6 | 10 | 6 | 4 | 8 | 6 | 3 | 16 | 9 | 6 |
| 2000 | 16 | 8 | 6 | 10 | 6 | 3 | 8 | 4 | 3 | 17 | 7 | 6 |
| 2001 | 19 | 11 | 9 | 13 | 8 | 5 | 9 | 5 | 4 | 20 | 10 | 8 |
| 2002 | 17 | 11 | 6 | 16 | 9 | 5 | 7 | 4 | 4 | 17 | 11 | 7 |
| P valueb | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.013 | <0.0001 | <0.0001 | <0.0001 |
AK, amikacin; CIP, ciprofloxacin; TOB, tobramycin; NA, not available. Data presented as percentages.
P values representative of available resistance data over the 10-year period.
DISCUSSION
The present study demonstrates significant increases in resistance to single antipseudomonal agents, multidrug resistance, and dual resistance to commonly prescribed combination therapies among P. aeruginosa isolates during the 10-year period from 1993 to 2002. Consistent with previous surveillance studies, the present study found that P. aeruginosa was most commonly isolated from the respiratory tract (3, 5, 7, 12). In addition, the present study found that a majority of strains were isolated from patients in the medical (44 to 53%) or surgical (28 to 34%) ICUs.
Decreasing susceptibility to all antipseudomonal agents was observed with the greatest declines involving ciprofloxacin (85 to 68%), imipenem (85 to 77%), tobramycin (91 to 84%), aztreonam (74 to 68%), piperacillin (89 to 85%), amikacin (93 to 90%), and ceftazidime (85 to 81%). Other surveillance studies have also shown decreasing susceptibility of P. aeruginosa isolates, particularly with fluoroquinolones and β-lactam antibiotics. When comparing P. aeruginosa isolates from 1994-1998 and 1999, the National Nosocomial Infections Surveillance System reported resistance increases of 49 and 20% with quinolones and imipenem, respectively (11). The SENTRY study reported a decline in aztreonam susceptibility from 67 to 62.3%, 88 to 80.9% for imipenem, and 79.8 to 75.4% for ciprofloxacin in the United States from 1997 to 1999 (3). Data from The Surveillance Network (TSN) from 1999 to 2002 indicated the greatest decline in susceptibilities against P. aeruginosa isolates in ICU patients occurred with ciprofloxacin (74.4 to 57.9%), ticarcillin-clavulanate (72.1 to 56.9%), and ceftazidime (83.2 to 65%) (2). In the present study, piperacillin-tazobactam represents the β-lactam with the lowest rate of resistance, with 90% of isolates remaining susceptible in 2002. Higher susceptibility rates with piperacillin-tazobactam were also reported in the Meropenem Yearly Susceptibility Test Information Collection (MYSTIC) study and the TSN program when compared to other β-lactam antimicrobials (2, 13). Another interesting finding is the lower susceptibility rate with cefepime than with ceftazidime. This trend has also been reported in the MYSTIC and TSN programs (2, 13).
Susceptibility to aminoglycosides decreased significantly over the 10-year period in the present study. In 2002, amikacin and tobramycin susceptibilities fell to 90 and 84%, respectively. Gentamicin susceptibility testing was discontinued in 2001, with the last reported rate of 68% in 2000. However, not all studies have demonstrated a decrease in aminoglycoside susceptibilities. While the TSN study reported a decline in gentamicin susceptibility from 73% in 1999 to 69.4% in 2002, the susceptibility to amikacin remained similar over the 4-year period with rates of 91 and 90% in 1999 and 2002, respectively (2). In the SENTRY study, amikacin susceptibilities were 95% in 1997 and 97% in 1999 while tobramycin susceptibilities were 91% in 1997 and 92% in 1999 (3). The MYSTIC study data for 2000 also reported higher aminoglycoside susceptibility rates than the present study with tobramycin and gentamicin susceptibilities at 92 and 82%, respectively (13).
In the present study, multidrug resistance increased from 4% in 1993 to 14% in 2002. Flamm et al. reported multidrug resistance (defined as resistance to ≥3 of the following agents: amikacin, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, piperacillin, piperacillin-tazobactam, ticarcillin-clavulanate, or tobramycin) to occur in 29.5% of isolates from ICU patients with ticarcillin-clavulanate, ciprofloxacin, and gentamicin being most commonly involved in multidrug-resistant P. aeruginosa (2). These large surveillance studies indicate multidrug resistance has risen significantly and provide cause for concern. In addition, these studies further stress the need for ongoing surveillance from multiple databases to measure the true magnitude of antimicrobial resistance.
Increasing multidrug resistance in P. aeruginosa isolates complicates the selection of empirical therapy in critically ill patients and highlights the importance of reporting dual resistance rates to commonly used combination therapy to furnish clinicians with comprehensive information. In the present study, combinations which included piperacillin-tazobactam resulted in the lowest rates of dual resistance, with >90% of P. aeruginosa isolates being susceptible to at least one agent in 2002. In comparison, regimens which included aztreonam, cefepime, and imipenem resulted >80% susceptibility to at least one agent in the combination. The highest rates of dual resistance occurred with ciprofloxacin-containing regimens, especially when combined with cefepime or imipenem. Trends in high cumulative rates of dual resistance between ciprofloxacin and imipenem or ceftazidime were also reported for P. aeruginosa from the 1994 to 2000 ISS data (12). However, increasing dual resistance in tobramycin and β-lactam antibiotic combinations is noteworthy in the present study.
Several limitations of this study need to be mentioned. Molecular typing was not required for this study; therefore, contribution of clonal outbreaks within an institution to increased resistance rates cannot be evaluated. However, the susceptibility data from numerous institutions across the United States limit any significant impact of outbreaks within one institution or region. Secondly, documentation of infection was not required for this study, and thus, it is difficult to assess whether the organisms were true pathogens or mere colonizers. Finally, due to the unavailability of antimicrobial usage data for each institution, correlation of antimicrobial use with rising resistance cannot be evaluated during the study period.
As multidrug resistance rates increase among nosocomial pathogens such as P. aeruginosa, appropriate surveillance programs may assist in empirical antibiotic selection and reduce the number of nosocomial infections. This was illustrated by the Study on the Efficacy of Nosocomial Infection Control, which reported a 32% decrease in nosocomial infection rates after implementation of a surveillance program, compared to an 18% increase in other institutions over a 5-year period (4). Such programs facilitate early detection of outbreaks as well as resistance patterns and allow for institution or alteration of infection control policies, especially within individual institutions where rates may be higher than those stated in national surveillance reports.
In conclusion, susceptibility of antipseudomonal agents against ICU isolates decreased while multidrug resistance and dual resistance rates increased from 1993 to 2002. Significant reduction in susceptibilities of P. aeruginosa isolates may compromise the ability to choose efficacious empirical regimens for treatment of this formidable pathogen in critically ill patients. The present nationwide surveillance study provides valuable information related to emerging trends in resistance, and dual resistance rates as provided in the present study are vital to clinicians in the selection of reliable empirical therapy for P. aeruginosa infections in ICU patients.
Acknowledgments
Data kindly provided by Merck and Company Inc. (Rahway, N.J.). We thank Richard Reinert and Gail Gallagher of Merck and Company for their time and effort in obtaining information related to study protocol and database.
REFERENCES
- 1.Alverez-Lerma, F., et al. 1996. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med. 22:387-394. [DOI] [PubMed] [Google Scholar]
- 2.Flamm, R. K., M. K. Weaver, C. Thornsberry, M. E. Jones, J. A. Karlowsky, and D. F. Sahm. 2004. Factors associated with relative rates of antibiotic resistance in Pseudomonas aeruginosa isolates tested in clinical laboratories in the United States from 1999 to 2002. Antimicrob. Agents Chemother. 48:2431-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gales, A. C., R. N. Jones, J. Turnidge, R. Rennie, and R. Ramphal. 2001. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32:S146-S155. [DOI] [PubMed] [Google Scholar]
- 4.Haley, R. W., D. H. Culver, J. W. White, W. M. Morgan, T. G. Emori, V. P. Munn, and T. M. Hooton. 1985. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am. J. Epidemiol. 121:182-205. [DOI] [PubMed] [Google Scholar]
- 5.Hanberger, H., J. A. Garcia-Rodriguez, M. Gobernado, et al. 1999. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. JAMA 281:67-71. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 7.Itokazu, G. S., J. P. Quinn, C. Bell-Dixon, F. M. Kahan, and R. A. Weinstein. 1996. Antimicrobial resistance rates among aerobic gram-negative bacilli recovered from patients in intensive care units: evaluation of a national postmarketing surveillance program. Clin. Infect. Dis. 23:779-784. [DOI] [PubMed] [Google Scholar]
- 8.Kollef, M. H. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 9.Leibovici, L., I. Shraga, M. Drucker, H. Konigsberger, Z. Samra, and S. D. Pitlik. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379-386. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Nosocomial Infections Surveillance System. 1999. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990-May 1999, issued June 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 12.Neuhauser, M. M., R. A. Weinstien, R. Rdyman, L. H. Danziger, G. Karam, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., R. N. Jones, and D. J. Biedenbach. 2001. Antimicrobial resistance trends in medical centers using carbapenems: report of 1999 and 2000 results from the MYSTIC program (USA). Diagn. Microbiol. Infect. Dis. 41:177-182. [DOI] [PubMed] [Google Scholar]