Abstract
CTX-M-25 is a novel extended-spectrum β-lactamase isolated from a single Canadian Escherichia coli isolate. Susceptibility testing demonstrated that this enzyme confers resistance to both cefotaxime and ceftazidime, but the level of resistance was reduced with the addition of β-lactamase inhibitors. The blaCTX-M-25 gene was detected on a 111-kb plasmid. It is a member of the CTX-M-8 group and has the closest amino acid identity (99%; three amino acid substitutions) with CTX-M-26. The blaCTX-M-26 gene was detected on a 100-kb plasmid isolated from a Klebsiella pneumoniae strain from the United Kingdom, and plasmid profiling revealed that it showed some homology to the blaCTX-M-25-harboring plasmid. Both CTX-M genes were located downstream of ISEcp1, although the copy upstream of blaCTX-M-25 was disrupted by IS50-A. Comparative kinetic studies of recombinant CTX-M-25 and CTX-M-26 enzymes showed that CTX-M-25 has a higher level of ceftazidime hydrolysis (kcat values, 33 and 0.005 s−1 for CTX-M-25 and CTX-M-26, respectively).
Recent years have seen the emergence and global spread from South America (7) and the Far East (11) of the CTX-M-type extended-spectrum β-lactamases (ESBLs), with reports of CTX-M for the first time in Britain (1), the United States (18), Italy (24), Turkey, and Bulgaria and Romania (30). All of these were reported between January 2002 and March 2004. A recent multicenter surveillance study has also revealed the presence of CTX-M type ESBLs in Canada (20). The CTX-M-type enzymes can be divided into four family groups on the basis of their amino acid identities: the CTX-M-1 group (CTX-M-1, CTX-M-3, CTX-M-15, etc.), the CTX-M-2 group (CTX-M-2, Toho-1, etc.), the CTX-M-8 group, and the CTX-CTX-M-9 group (CTX-M-9, CTX-M-14, Toho-2, etc.) (31). Recent evidence suggests that each of these family groups is derived from a progenitor β-lactamase gene from the genus Kluyvera. So far progenitor genes have been suggested for the CTX-M-1 group (KLUC-1 of Kluyvera cryocrescens), the CTX-M-2 group (KLUA-1 of K. ascorbata), and the CTX-M-8 group (KLUG-1 of K. georgiana) (12, 14, 26); however, they have not been suggested for the CTX-M-9 group.
Hitherto, only two members of the CTX-M-8 group have been described. The first description of CTX-M-8-producing isolates of the family Enterobacteriaceae was for Brazilian isolates recovered in 1997 (7). A second member of this family, CTX-M-26, was produced by isolates of cefotaxime-resistant Klebsiella pneumoniae recovered as part of a clonal outbreak in the United Kingdom (10).
Amino acid substitutions in TEM- and SHV-type β-lactamases are known to result in the development of their extended spectra of activity. In the case of SHV-2, the G238S substitution (coordinates according to Ambler et al. [2]) results in enlargement of the omega loop, giving larger molecule substrates, such as the oxyimino-cephalosporins, access to the active site (5). Similar events have occurred for the CTX-M enzymes, most notably, amino acid substitution D240G in CTX-M-15, CTX-M-16, and CTX-M-27, which results in the greater hydrolysis of ceftazidime (8, 9, 15).
Here we report on an Escherichia coli strain isolated from a hospitalized patient that harbored a plasmid containing blaCTX-M-25. The CTX-M-25 enzyme shares 99% amino acid identity with CTX-M-26. Furthermore, we compared the immediate genetic environments of blaCTX-M-25 and blaCTX-M-26, compared the plasmids harboring the two enzymes, and undertook a kinetic analysis of the two enzymes.
MATERIALS AND METHODS
Bacterial strains.
E. coli ESBL530 was isolated in 2000 from a hospitalized patient as a screening-positive, possible ESBL producer and was received by the National Microbiology Laboratory, Winnipeg, Manitoba, Canada, for routine confirmation of an ESBL-producing phenotype. K. pneumoniae isolate H610, identified in 2002, was part of an outbreak of predominantly urinary tract infection-causing Enterobacteriaceae isolates from Birmingham, United Kingdom (10). This strain was used in this study as an example of a blaCTX-M-26-carrying strain (GenBank accession number AF518567). E. coli DH10B (Invitrogen Ltd., Paisley, United Kingdom) was used for cloning and expression experiments.
PCR, plasmid isolation, and Southern analysis.
The blaCTX gene was identified with in-house-designed universal primers CTX-U1 and CTX-U2 in a PCR with the following cycling conditions: 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min, preceded by denaturation at 95°C for 10 min and followed by a 5-min extension at 72°C. Amplicons were purified by commercially available methods (Millipore, Nepean, Ontario, Canada). Plasmid DNA was isolated by using commercial isolation kits (Qiagen, Mississauga, Ontario, Canada), and was transformed into electrocompetent E. coli DH10B (Invitrogen Ltd.) with a Gene Pulser apparatus (Bio-Rad, Mississauga, Ontario, Canada). Transformants were selected on Luria-Bertani agar containing cefotaxime (5 mg/liter). Specifically, pCTX25 from E. coli ESBL530 and pCTX26 from K. pneumoniae H610 were isolated by the electrotransformation of plasmid DNA from the clinical strain into E. coli DH10B with selection on cefotaxime. Plasmid DNA digested with restriction enzymes was separated on a 0.7% agarose gel for 16 h at 2.8 V/cm with circulating 0.5× Tris-borate-EDTA buffer. Southern hybridization analysis was performed by standard methods (29) with probes labeled and detected with an enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, N.J.).
Cloning experiments and sequencing.
In order to clone blaCTX-M-25, two plasmid libraries were constructed, one containing E. coli ESBL530 EcoRI fragments cloned into the EcoRI site of pACYC184 and one containing partially digested Sau3A fragments cloned into the BamHI site of pACYC184. The libraries were electrotransformed into E. coli DH10B, and clones were selected on 5 mg of cefotaxime per liter and either 10 mg of tetracycline per liter (EcoRI library) or 30 mg of chloramphenicol per liter (Sau3A library). A plasmid isolated from the EcoRI plasmid library and used in this study was labeled pOZ5200. The primers used in this study are listed in Table 1. PCR analysis of blaCTX-M-26 and flanking regions was carried out with primer pairs ISEcp1-DN2 and ISEcp1-UP2, ISEcp1-DN1 and CTX-U2, and CTX-U1 and CTX25-UP2. The same primers were used for sequence analysis. A PCR product generated from K. pneumoniae H610 DNA with primers ISEcp1 (28) and C11, a primer designed specifically for this study, was cloned into the SrfI site of the pPCRScript-Cam (SK+) vector (Stratagene Inc., La Jolla, Calif.) to create plasmid pOZ2501. Selection was on Mueller-Hinton agar plates containing 100 mg of ampicillin per liter and 30 mg of chloramphenicol per liter. Sequencing of the recombinant plasmid inserts was carried out by standard methods.
TABLE 1.
Primers used in this study
| Primer name | Primer sequence (5′-3′)a |
|---|---|
| C11 | TAATCATACAGAAGTCGCAG |
| ISEcp1-DN1 | TCCGTACAAGGGAGTGTATG |
| ISEcp1-DN2 | TATGCATTCCTTCGAAATTC |
| ISEcp1-UP2 | TCACGAAGAATTTAGACTGC |
| CTX-U1 | ATGTGCAGYACCAGTAARGTKATGGC |
| CTX-U2 | TGGGTRAARTARGTSACCAGAAYCAGCGG |
| CTX25-UP2 | TCCACCAGCAGCATCAAGC |
R, purine; Y, pyrimidine; S, G or C.
Susceptibility testing.
MICs of selected β-lactams were determined by the agar dilution technique on Mueller-Hinton agar plates, as described previously (22), and were interpreted according to the NCCLS guidelines (23).
Biochemical analysis of CTX-M-25 and CTX-M-26.
Cultures of E. coli DH10B carrying plasmids pOZ5200 (blaCTX-M-25) and pOZ5201 (blaCTX-M-26) were grown for 18 h at 30°C in 4 liters of Luria-Bertani broth enriched with 6% glucose containing ampicillin (100 mg/liter) and induced with 1 mM isopropyl-β-d-thiogalactopyranoside. β-Lactamase extracts were obtained by the following method. Harvested cells were washed twice in 50 mM phosphate buffer (pH 7) before cell lysis by sonication (four times for 30 s each time at 20 W). Following centrifugation (10,000 × g for 20 min at 4°C), the resulting supernatant was concentrated by using Centrisart-C30 microcentrifuge filters (Sartorius, Goettingen, Germany) and dialyzed against 20 mM phosphate buffer (pH 7). Purification was by high-pressure liquid chromatography with a Shodex ion-exchange column (Phenomenex, Macclesfield, United Kingdom), 20 mM NaPO4 buffer (pH 7), and an increasing gradient of 0 to 50 mM NaCl as the eluent. β-Lactamase-positive fractions were pooled and concentrated with Centrisart-C30 microcentrifuge filters before purity was checked, and relative molecular masses were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein concentrations were measured with a DC protein assay kit (Bio-Rad, Hemel Hempstead, United Kingdom).
Purified β-lactamases were then used for kinetic measurements, which were performed at 37°C in 100 mM sodium phosphate buffer (pH 7.0). The initial rates of hydrolysis were determined as described previously (25) with a Perkin-Elmer Lambda 2UV/VIS spectrophotometer. The 50% inhibitory concentrations were determined as reported previously (25), but with cefotaxime as the substrate.
IEF analysis.
Analytical isoelectric focusing (IEF) of β-lactamase extracts from cultures of E. coli DH10B cells harboring recombinant plasmid pOZ5200 was performed with broad-range ampholine PAGplates (pH 3.5 to 9.5; Amersham Biosciences, Little Chalfont, United Kingdom) on a Multiphore II platform (Amersham Biosciences), as described previously (11). Focused β-lactamase was detected by overlaying of the gel with filter paper strips saturated with 1 mM nitrocefin diluted in 100 mM phosphate buffer (pH 7.0). β-Lactamases with a range of known pIs were used for comparison for determination of the pI values.
Nucleotide sequence accession numbers.
The nucleotide sequence of blaCTX-M-25 and flanking regions has been assigned GenBank accession number AF518567, and that of blaCTX-M-26 and flanking regions has been assigned GenBank accession number AY455830.
RESULTS
Molecular characterization of blaCTX-M-25 and comparison to blaCTX-M-26.
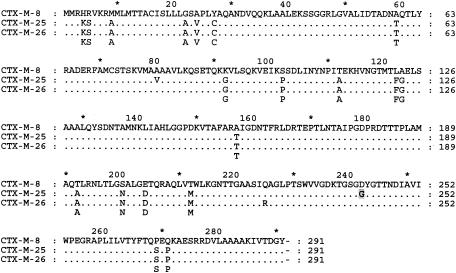
E. coli ESBL530 was initially identified as an ESBL-positive strain by disk diffusion by NCCLS methods (22). IEF showed that the strain harbored β-lactamases with pIs of 5.4 and 7.5, and PCR analysis showed that the strain harbored blaTEM and blaCTX-M β-lactamase genes. Cefotaxime resistance could be transferred to E. coli DH10B by electrotransformation of a plasmid isolated from E. coli ESBL530. IEF and PCR analyses of the transformant showed that it harbored blaTEM and blaCTX-M β-lactamase genes (data not shown). HpaI digests of the transformed plasmid, labeled pCTX25, revealed that it was 111 kb (Fig. 1). Sequence analysis of the 593-bp amplicon produced with the CTX-M-specific universal primers showed that the plasmid harbored a CTX-M-type gene with 90% identity to blaCTX-M-8. In order to isolate the entire gene, two plasmid libraries were screened for cefotaxime-resistant clones. Analysis of four clones from the EcoRI library revealed that all the clones contained a 3,481-bp EcoRI fragment insert. One plasmid, labeled pOZ5100, was selected for further analysis. Analysis of four clones from the partial Sau3A library revealed clones with inserts ranging from 5.5 to 8 kb. However, since no attempt was made to prevent a scrambled Sau3A library, only the partial sequence from one Sau3A clone which originated inside the cloned EcoRI fragment to the first Sau3A site outside of the one of the EcoRI sites was considered. In this way a colinear sequence of 3,854 bp was obtained (Fig. 2). Analysis revealed a novel 876-bp open reading frame encoding a CTX-M β-lactamase designated CTX-M-25. The blaCTX-M-25 gene exhibited identities of 99% to blaCTX-M-26 (10), 88.5% to blaCTX-M-8 (7), and 88.1% to blaKLUG-1 (26), the proposed progenitor of the CTX-M-8 group. An alignment of the CTX-M-8 group enzymes is shown in Fig. 3. There were three amino acid differences between CTX-M-26 and CTX-M-25, A80V, R225Q, and D242G (the coordinates are from the CTX-M enzyme). The last change corresponds to the D240G change (numbering of Ambler et al. [2]) that has been found in CTX-M-15, CTX-M-16, and CTX-M-27 and that is responsible for the increased hydrolysis of ceftazidime (8, 9, 15). A sequence identical to that of ISEcp1 was detected upstream of blaCTX-M-25. This insertion sequence element has been found upstream of many blaCTX-M genes and has been implicated in the mobilization of blaCTX-M-19 (27). Interestingly, the ISEcp1 transposase gene was disrupted by IS50-A, an IS50-IS50-R variant (6). Located 368 bp downstream of blaCTX-M-25 was the start of a partial open reading frame of 289 bp, orfX, whose putative product exhibited 33% identity in a 70-amino-acid overlap with an acid shock protein (GenBank accession number NP 460445) from Salmonella enterica serovar Typhimurium LT2. The precise end of orfX is unknown, as the sequence analyzed ends at a Sau3A site before a stop codon is encountered.
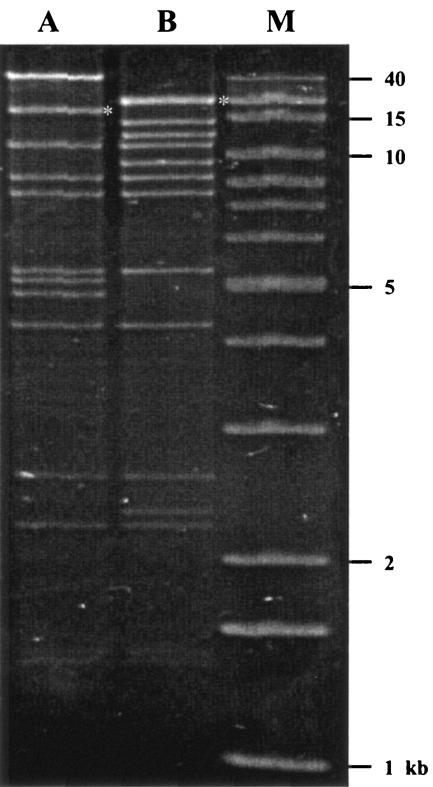
FIG. 1.
HpaI plasmid profiles of pCTX25 (lane A) and pCTX26 (lane B). The molecular weight marker (lane M) was a 1-kb extension ladder (Invitrogen).
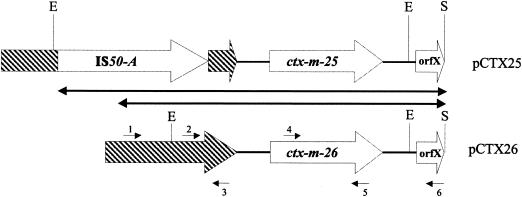
FIG. 2.
Schematic diagram of blaCTX-M-25 and blaCTX-M-26 and surrounding regions. The regions sequenced are indicated by doubled-headed arrows. ISEcp1 sequences are denoted by the arrows with cross-hatching. E, EcoRI; S, Sau3A. Only the Sau3A sites defining the one end of the sequenced regions are shown. Small numbered arrows indicate primers, as follows: 1, ISEcp1-DN2; 2, ISEcp1-DN1; 3, ISEcp1-UP2; 4, CTX-U1; 5, CTX-U2; 6, CTX25-UP2.
FIG. 3.
Comparison of amino acid sequence of CTX-M-25 with those of other members of the CTX-M-8 family group. Coordinates are from the CTX-M sequence except for the key amino acid change D240G (numbering of Ambler et al.[2]), which is highlighted in gray.
Due to their close amino acid identity, we wished to compare blaCTX-M-25 to blaCTX-M-26 more closely. blaCTX-M-26-containing plasmid pCTX26 was transformed into E. coli DH10B for isolation and analysis. An analysis of the HpaI profiles showed that pCTX26 was ∼100 kb, while pCTX25 was about ∼111 kb (Fig. 1). The two plasmids had nine fragments in common, but they also had nine band differences. Southern analysis showed that the blaCTX-M-25 gene is located on an ∼18-kb fragment and that the blaCTX-M-26 gene is located on an ∼20-kb fragment. Most of the size difference between the hybridizing bands can be accounted for by the presence of IS50-A (1.7 kb) in pCTX25.
We used PCR to amplify blaCTX-M-26 and its flanking regions from pCTX26 and compared the sequence to the corresponding region containing blaCTX-M-25 from strain ESBL530 (Fig. 2). We found that blaCTX-M-26 was located downstream of ISEcp1 in exactly the same position as blaCTX-M-25 and that the sequence downstream of the β-lactamase genes was identical. However, ISEcp1 was not disrupted by IS50-A in pCTX26. Thus, not including IS50-A and a direct repeat formed by its transposition, the only sequence differences between the two regions are the three nucleotide differences between the β-lactamase genes.
Antimicrobial susceptibility of cloned CTX-M.
The MICs of a range of β-lactam compounds for E. coli DH10B cells alone or E. coli DH10B cells harboring recombinant plasmid pOZ5200 or pOZ5201 were determined (Table 2). Both clones exhibited profiles typical for CTX-M-producing bacteria (17), with a reduction of the MICs with the addition of either β-lactamase inhibitor, clavulanic acid or tazobactam. However, the ceftazidime MIC was 16-fold higher for the CTX-M-25-producing clone than for CTX-M-26-producing strain E. coli DH10B, and the MICs of penicillins and other cephalosporins were also increased for the CTX-M-25-producing clone (Table 2).
TABLE 2.
MICs for E. coli DH10B alone or harboring recombinant plasmid pOZ5200 and pOZ5201 expressing CTX-M-25 and CTX-M-26, respectively
| β-Lactam(s)a | MIC (mg/liter)
|
||
|---|---|---|---|
| pOZ5200 | pOZ5201 | DH10B | |
| Ampicillin | >512 | >512 | 2 |
| Amoxicillin | >512 | >512 | 2 |
| Amoxicillin + CLA | 16 | 16 | 2 |
| Ticaracillin | >512 | 16 | 1 |
| Ticaracillin + CLA | 64 | 8 | 1 |
| Piperacillin | >512 | 16 | 1 |
| Piperacillin + TZB | 8 | 2 | 1 |
| Cephalothin | >512 | 512 | 4 |
| Cefuroxime | >512 | 512 | 4 |
| Cefotaxime | 256 | 256 | <0.06 |
| Cefotaxime + CLA | <0.06 | <0.06 | <0.06 |
| Cefotaxime + TZB | <0.06 | <0.06 | <0.06 |
| Ceftazidime | 64 | 4 | <0.06 |
| Ceftazidime + CLA | 0.25 | 0.25 | <0.06 |
| Ceftazidime + TZB | 1 | 1 | <0.06 |
| Cefriaxone | >512 | 16 | <0.06 |
| Cefpirome | >512 | 1 | <0.06 |
| Cefoxitin | 4 | 2 | 2 |
| Benzylpenicillin | >512 | >512 | 32 |
| Aztreonam | 64 | 64 | <0.06 |
| Imipenem | 0.25 | 0.25 | 0.12 |
CLA, clavulanic acid at a fixed concentration of 2 mg/liter; TZB, tazobactam at a fixed concentration of 4 mg/liter.
Biochemical properties of CTX-M.
Recombinant CTX-M-25 and CTX-M-26 were purified to ∼90% purity and appeared as bands of ∼29 kDa on sodium dodecyl sulfate-polyacrylamide gels. They were shown to have pIs of 7.5 and 8.0, respectively, by IEF. The 50% inhibitory concentrations of clavulanic acid for CTX-M-25 and CTX-M-26 were 0.45 and 0.16 μM, respectively. The kinetic parameters of CTX-M-25 and CTX-M-26 are shown in Table 3. Cephalothin was the best substrate tested for both enzymes (values two- to fourfold higher for cephalothin than for cefotaxime), with no activity seen with imipenem or cefoxitin. Almost no activity was seen for CTX-M-26 with ceftazidime, but CTX-M-25 showed a good enzymatic affinity (Km) to this substrate. Conversely, the catalytic activity was less in CTX-M-25 with all other substrates except cefuroxime.
TABLE 3.
Steady-state kinetics of purified CTX-M-25 and CTX-M-26 enzymesa
| Substrate | CTX-M-25
|
CTX-M-26
|
||||
|---|---|---|---|---|---|---|
| kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) | kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) | |
| Benzylpenicillin | 33 | 74 | 0.4 | 420 | 60 | 70 |
| Ampicillin | 5.9 | 7.7 | 0.8 | 12 | 24 | 0.5 |
| Ticaracillin | 8.2 | 2.3 | 3.5 | 84 | 27 | 3 |
| Cephalothin | 230 | 190 | 1.2 | 530 | 110 | 4.7 |
| Cefuroxime | 190 | 44 | 4.3 | 21 | 300 | 0.07 |
| Ceftazidime | 33 | 13 | 2.6 | 0.015 | 3,300 | ND |
| Cefotaxime | 101 | 28 | 3.6 | 120 | 150 | 0.77 |
| Imipenem | <0.01 | ND | ND | <0.01 | ND | ND |
| Aztreonam | 84 | 120 | 0.7 | 100 | 130 | 0.78 |
| Cefoxitin | <0.01 | ND | ND | <0.01 | ND | ND |
Values are the means of three independent measurements (standard deviations of the values were within 15%). ND, not determinable.
DISCUSSION
We have reported here details on CTX-M-25, only the third member of the CTX-M-8 group, which also includes CTX-M-26. Both blaCTX-M-25 and blaCTX-M-26, which are 99% identical, appear to have originated from the same genetic environment. Both genes are associated with ISEcp1 elements found upstream and share an identical 658-bp downstream sequence (Fig. 2). The one major difference is the presence of IS50-A, which interrupts the tnpA gene of ISEcp1 found upstream of blaCTX-M-25. As well, both genes are found on large plasmids that are similar in size and that appear to have, at the least, a distant relationship (Fig. 1).
CTX-M-25 is one of a growing number of CTX-M enzymes with significant ceftazidime hydrolytic activity. The first CTX-M mutant to be described with this increased antibiotic spectrum was a CTX-M-15 enzyme from India (15) that contained the amino acid substitution D240G. Subsequently, CTX-M-15, CTX-M-16, CTX-M-19, and CTX-M-27 have all been shown to have greater enzymatic efficiencies against ceftazidime but have reduced affinities (Km values) for penicillins (4, 8, 15, 25), with CTX-M-25 belonging to this growing group of enzymes. We found that an irresolvable discrepancy existed when the ratio of the difference in MICs of ceftazidime for E. coli DH10B cells producing CTX-M-25 and CTX-M-26 were compared to the kinetic parameters obtained, with differences similar to those found previously for other CTX-M enzymes (24).
Comparison of the amino acid structures of other CTX-M- type enzymes available in the GenBank database reveals a glycine molecule at position 240 for both CTX-M-28 and CTX-M-29 (GenBank accession numbers AY267213 and AJ549244, respectively), suggesting that these enzymes may also have ceftazidimase activities. These ceftazidime-hydrolyzing CTX-M enzymes are usually very closely related to previously described CTX-M enzymes and may vary by as little as one amino acid (i.e., CTX-M-15 and CTX-M-27 differ only by the substitution D240G from their respective derivatives, CTX-M-3 and CTX-M-14) and may represent the next evolutionary step for these enzymes. However, site-directed mutagenesis studies of CTX-M-9 by Aumeran and colleagues (3) demonstrated that a substitution at position 240 of D240K (instead of D240G) was similar to the mutations that promote ceftazidime activity found in the TEM and the SHV ESBLs but did not result in increased hydrolysis of ceftazidime for this enzyme. With the increasing numbers of CTX-M enzymes with this ceftazidime resistance phenotype, it may prove useful to subcategorize these enzymes into those that confer resistance to both ceftazidime and cefotaxime and those that confer resistance to cefotaxime alone.
In terms of epidemiology, there have been a number of recent reports of ceftazidime-resistant isolates of the family Enterobacteriaceae producing CTX-M enzymes (CTX-M-15, CTX-M-27, and in particular, CTX-M-15) in the Mediterranean basin (Greece, Egypt, Turkey, and Italy) (16, 24, 13, 32).
A multidrug-resistant E. coli clone from Canada expressing CTX-M-15 has been involved in an outbreak in a long-term-care facility in Toronto, Ontario, Canada (19). A number of CTX-M-15-producing isolates have also been recovered from sites throughout the United Kingdom (21). The increased frequency of isolation and reporting of these ESBLs is alarming and may represent the beginnings of a long-term global resistance problem resulting in the loss of oxyimino-cephalosporins as treatments for serious infections.
Acknowledgments
Part of this work was funded by Robotham Bequest grant NZZR EGF 1524 (to C. J. Munday) and BBSRC grant 6/JIF13209 to the Functional Genomics Laboratory, University of Birmingham.
REFERENCES
- 1.Alobwede, I., F. H. M'Zali, D. M. Livermore, J. Heritage, N. Todd, and P. M. Hawkey. 2003. CTX-M extended-spectrum β-lactamase arrives in the UK. J. Antimicrob Chemother. 51:470-471. [DOI] [PubMed] [Google Scholar]
- 2.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aumeran, C., C. Chanal, R. Labia, D. Sirot, J. Sirot, and R. Bonnet. 2003. Effects of Ser130Gly and Asp240Lys substitutions in extended-spectrum β-lactamase CTX-M-9. Antimicrob. Agents Chemother. 47:2958-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baraniak, A., J. Fiett, W. Hryniewicz, P. Nordmann, and M. Gniadkowski. 2002. Ceftazidime-hydrolysing CTX-M-15 extended-spectrum β-lactamase (ESBL) in Poland. J. Antimicrob. Chemother. 50:393-396. [DOI] [PubMed] [Google Scholar]
- 5.Barthelemy, M., J. Peduzzi, B. H. Yaghlane, and R. Labia. 1988. Single amino acid substitution between SHV-1 β-lactamase and cefotaxime-hydrolysing SHV-2 enzymes. FEBS Lett. 231:217-220. [DOI] [PubMed] [Google Scholar]
- 6.Berg, D. E., L. Johnsrud, L. McDivitt, R. Ramabhadran, and B. J. Hirschel. 1982. Inverted repeats of Tn5 are transposable elements. Proc. Natl. Acad. Sci. USA 79:2632-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet, R., J. L. Sampaio, R. Labia, C. De Champs, D. Sirot, C. Chanal, and J. Sirot. 2000. A novel CTX-M β-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob. Agents Chemother. 44:1936-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet, R., C. Dutour, J. L. Sampaio, C. Chanal, D. Sirot, R. Labia, C. De Champs, and J. Sirot. 2001. Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240→Gly. Antimicrob. Agents Chemother. 45:2269-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet, R., C. Recule, R. Baradue, C. Chanal, D. Sirot, C. De Champs, and J. Sirot. 2003. Effect of D240G substitution in a novel ESBL CTX-M-27. J. Antimicrob. Chemother. 52:29-35. [DOI] [PubMed] [Google Scholar]
- 10.Brenwald, N. P., G. Jevons, J. M. Andrews, J. H. Xiong, P. M. Hawkey, and R. Wise. 2002. An outbreak of a CTX-M-type β-lactamase-producing Klebsiella pneumoniae: the importance of using cefpodoxime to detect extended-spectrum β-lactamases. J. Antimicrob. Chemother. 51:195-196. [DOI] [PubMed] [Google Scholar]
- 11.Chanawong, A., F. H. M'Zali, J. Heritage, J. H. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decousser, J. W., L. Poirel, and P. Nordmann. 2001. Characterization of a chromosomally encoded extended-spectrum class A β-lactamase from Kluyvera cryocrescens. Antimicrob. Agents Chemother. 45:3595-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galani, I., M. Souli, G. Koratzanis, Z. Chryssouli, and H. Giamerellou. 2003. Alarming emergence of E. coli clinical isolates harboring a variety of newly acquired beta-lactamases in Athens, Greece, abstr. C1-695, p. 82. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.12499173
- 14.Humeniuk, C., G. Arlet, V. Gautier, P. Grimont, R. Labia, and A. Philippon. 2002. β-Lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob. Agents Chemother. 46:3045-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karim, A., L. Poirel, S. Nagarajan, and P. Nordmann. 2001. Plasmid-mediated extended-spectrum β-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 201:237-241. [DOI] [PubMed] [Google Scholar]
- 16.Lartigue, M. F., L. Poirel, C. Heritier, V. Tolun, and P. Nordmann. 2003. First description of CTX-M-15-producing Klebsiella pneumoniae in Turkey. J. Antimicrob. Chemother. 52:315-316. [DOI] [PubMed] [Google Scholar]
- 17.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moland, E. S., J. A. Black, A. Hossain, N. D. Hanson, K. S. Thomson, and S. Pottumarthy. 2003. Discovery of CTX-M-like extended-spectrum β-lactamases in Escherichia coli isolates from five U.S. states. Antimicrob. Agents Chemother. 47:2382-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller, M., A. McGeer, B. M. Willey, D. Reynolds, R. Malanczyj, M. Silverman, M. A. Green, and M. Culf. 2002. Outbreaks of multi-drug resistant Escherichia coli in long-term care facilities in the Durham, York, and Toronto regions of Ontario, 2000-2002. Can. Commun. Dis. Rep. 28:113-118. [PubMed] [Google Scholar]
- 20.Mulvey, M. R., E. Bryce, D. Boyd, M. Ofner-Agostini, S. Christianson, A. E. Simor, S. Paton, and Canadian Hospital Epidemiology Committee, Canadian Nosocomial Infection Surveillance Program, Health Canada. 2004. Ambler class A extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella spp. in Canadian hospitals. Antimicrob. Agents Chemother. 48:1204-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mushtaq, S., N. Woodford, N. Potz, and D. M. Livermore. 2003. Detection of CTX-M-15 extended-spectrum β-lactamase in the United Kingdom. J. Antimicrob. Chemother. 52:528-529. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standards, 6th ed. Document M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa
- 23.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing: 13th informational supplement. Document M100-S13 (M7). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Pagani, L., E. Dell'Amico, R. Migliavacca, M. M. D'Andrea, E. Giacobone, G. Amicosante, E. Romero, and G. M. Rossolini. 2003. Multiple CTX-M-type extended-spectrum β-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J. Clin. Microbiol. 41:4264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel, L., M. Gniadkowski, and P. Nordmann. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum beta-lactamase CTX-M-15 and of its structurally related β-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031-1034. [DOI] [PubMed] [Google Scholar]
- 26.Poirel, L., P. Kampfer, and P. Nordmann. 2002. Chromosome-encoded Ambler class A β-lactamase of Kluyvera georgiana, a probable progenitor of a subgroup of CTX-M extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 46:4038-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poirel, L., J.-W. Decousser, and P. Nordmann. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 47:2938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saladin, M., V. T. Cao, T. Lambert, J. L. Donay, J. L. Herrmann, Z. Ould-Hocine, C. Verdet, F. Delisle, A. Philippon, and G. Arlet. 2002. Diversity of CTX-M β-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161-168. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Schneider, I., E. Keuleyan, R. Markovska, E. Dragijeva, N. Gonullu, Z. Aktas, C. Bal, D. Buiuc, and A. Bauernfeind. 2003. Emergence of CTX-M-15 extended spectrum beta-lactamase producing Enterobacteriaceae in Bulgaria, Romania and Turkey. Clin. Microbiol. Infect. 9(Suppl. 1):94. [Google Scholar]
- 31.Tzouvelekis, L. S., E. Tzelepi, P. T. Tassios, and N. J. Legakis. 2000. CTX-M-type β-lactamases: an emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 14:137-142. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand, I., and M. H. M. Al-Agamy. 2003. First description of CTX-M enzymes in clinical E. coli strains from Egypt, abstr. C2-48, p. 108. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.