Abstract
This study was conducted to evaluate risk factors for mortality and treatment outcome of bloodstream infections due to extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae (ESBL-EK). ESBL production in stored K. pneumoniae and E. coli blood isolates from Jan 1998 to Dec 2002 was phenotypically determined according to NCCLS guidelines and/or the double-disk synergy test. A total of 133 patients with ESBL-EK bacteremia, including 66 patients with ESBL-producing K. pneumoniae and 67 with ESBL-producing E. coli, were enrolled. The overall 30-day mortality rate was 25.6% (34 of 133). Independent risk factors for mortality were severe sepsis, peritonitis, neutropenia, increasing Acute Physiology and Chronic Health Evaluation II score, and administration of broad-spectrum cephalosporin as definitive antimicrobial therapy (P < 0.05 for each of these risk factors). In 117 of the 133 patients, excluding 16 patients who died within 3 days after blood culture sample acquisition, the 30-day mortality rates according to definitive antibiotics were as follows: carbapenem, 12.9% (8 of 62); ciprofloxacin, 10.3% (3 of 29); and others, such as cephalosporin or an aminoglycoside, 26.9% (7 of 26). When patients who received appropriate definitive antibiotics, such as carbapenem or ciprofloxacin, were evaluated, mortality in patients receiving inappropriate empirical antimicrobial therapy was found not to be significantly higher than mortality in those receiving appropriate empirical antimicrobial therapy (18.9 versus 15.5%; P = 0.666). Carbapenem and ciprofloxacin were the most effective antibiotics in antimicrobial therapy for ESBL-EK bacteremia. A delay in appropriate definitive antimicrobial therapy was not associated with higher mortality if antimicrobial therapy was adjusted appropriately according to the susceptibility results. Our data suggest that more prudent use of carbapenem as empirical antibiotic may be reasonable.
Escherichia coli and Klebsiella pneumoniae are major nosocomial pathogens causing intra-abdominal infection, urinary tract infection, and primary bacteremia (5). These organisms had been uniformly susceptible to oxymino-β-lactam antibiotics. During the past 2 decades, broad-spectrum cephalosporins, including oxymino-β-lactam antibiotics, have been used worldwide, and antibiotic-resistant strains that produce extended-spectrum β-lactamases (ESBL) have emerged among the Enterobacteriaceae, predominantly in E. coli and K. pneumoniae (3, 8). Since the initial description of ESBL production by K. pneumoniae isolates in 1983 (14), strains that are resistant to broad-spectrum cephalosporins are being increasingly recognized (2, 7). The marked increase in the incidence of infections due to ESBL-producing organisms in recent years is of great concern (2, 7, 21, 23). Because ESBL-producing organisms are frequently resistant to multiple antimicrobial agents, therapeutic options for these infections are severely limited.
There have been many reports of outbreaks caused by these organisms (10, 16, 19, 23, 24), and it has been demonstrated that ESBL production by infecting organisms adversely affects the clinical outcome (11, 12, 15, 26). At present, carbapenems are recommended for the treatment of infections caused by ESBL-producing organisms. This recommendation is based primarily on the in vitro effect, the results of animal experiments (25, 28), and limited clinical data (12, 22, 26, 32). However, there are few data on treatment outcomes of bloodstream infections due to ESBL-producing organisms. We aimed to evaluate treatment outcomes of bloodstream infections due to ESBL-producing E. coli and K. pneumoniae (ESBL-EK) and the factors associated with mortality. Special emphasis was placed on determining the correlation between the antimicrobial therapy and the outcome.
(This study was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., September 2003 [C.I. Kang, S. H. Kim, D. M. Kim, et al., Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. K-720, 2003].)
MATERIALS AND METHODS
Patients.
The database at our clinical microbiology laboratory (Seoul National University Hospital, Seoul, Korea) was reviewed in order to identify patients with E. coli and K. pneumoniae bacteremia. In the period from January 1998 to December 2002, a total of 1,154 episodes of E. coli bacteremia and 578 episodes of K. pneumoniae bacteremia, in 1,045 and 499 patients, respectively, were identified. Only the first bacteremic episode of each patient was included in the analysis.
Bacterial strains.
All of the blood isolates were collected by the clinical microbiology laboratory in our hospital. Of the stored blood isolates from patients, 982 strains of E. coli and 471 strains of K. pneumoniae were successfully recovered for inclusion in the study. Species identification was carried out with VITEK-GNI cards (bioMérieux, Hazelwood, Mo.) by standard methods (9). Only one isolate from each bacteremic episode was included in the analysis. If there was more than one isolate, we selected the more resistant isolate.
Microbiologic methods.
The antibiotic susceptibility of each isolate was determined by the disk diffusion method, employing the criteria of the National Committee for Clinical Laboratory Standards (NCCLS) (17). Mueller-Hinton agar (Becton Dickinson, Sparks, Md.) was inoculated, and antibiotic disks (Becton Dickinson) were placed. The antibiotics included in the susceptibility test were cefotaxime, ceftriaxone, ceftazidime, aztreonam, cefpodoxime, cefoxitin, cefepime, amoxicillin-clavulanic acid, ciprofloxacin, amikacin, gentamicin, tobramycin, and imipenem. For isolates from patients who had received extended-spectrum cephalosporin, susceptibility testing for the actual agent used for the treatment was conducted. MICs were determined by the broth microdilution method, as described by NCCLS (18). ESBL production was screened and determined by the disk diffusion method according to NCCLS performance standards (17). In brief, we determined the diameters of the inhibition zones on cefotaxime and ceftazidime disks (30 μg each), alone and in combination with clavulanic acid (10 μg). An increase of ≥5 mm in zone diameter when either of the antimicrobial agents was combined with clavulanic acid was considered evidence of ESBL production. Isolates that either were resistant to cefotaxime, ceftazidime, or cefpodoxime; did not synergize with clavulanic acid; or gave a ≤5-mm increase in zone diameter were subjected to the double-disk diffusion test with cefotaxime, ceftazidime, and cefepime disks (31), as described by Thomas and Sanders (29), except that the ceftazidime and amoxicillin-clavulanic acid disks were placed 15 mm apart (15). After incubation, an enhanced zone of inhibition between any one of the β-lactam disks and the clavulanic acid disk was interpreted as presumptive evidence for the presence of an ESBL. The production of AmpC type β-lactamase was suspected for isolates that were resistant to either cefotaxime or ceftazidime, did not synergize with clavulanic acid, and were resistant to both amoxicillin-clavulanic acid and cefoxitin (27). Organisms that demonstrated AmpC type β-lactamase production were classified as ESBL-producing organisms. Two control organisms, E. coli ATCC 25922 and K. pneumoniae ATCC 700603, were inoculated in each set of tests for quality control.
Clinical analysis.
We reviewed the medical records of the patients, and a retrospective cohort study was conducted. The data collected included age, sex, underlying disease, primary site of infection, severity of illness as calculated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score (13), duration of hospital stay before onset of bacteremia, antimicrobial regimen, and any antimicrobial therapy in the 30 days prior to onset of bacteremia. The presence of the following comorbid conditions was also documented: neutropenia, presentation with septic shock, care in intensive care unit, use of immunosuppressive agents within 30 days prior to onset of bacteremia, corticosteroid use, postoperative state, and invasive procedures in the 72 h prior to onset of bacteremia. In addition, the presence of a central venous catheter, indwelling urinary catheter, or mechanical ventilation was assessed. As this study was retrospective, the patients' physicians, not the researchers, had chosen the antimicrobial therapy regimens.
The main outcome measures used were initial response to treatment and 30-day mortality rate. The initial response to treatment was assessed at 72 h after starting antimicrobial therapy and was classified as follows: complete response for patients who had resolution of fever, leukocytosis, and all signs of infection; partial response for patients who had abatement but not complete resolution of these parameters; and treatment failure for patients who had either no abatement or a deterioration in any of their clinical parameters or who died (15, 32). The 30-day mortality rate was calculated as total number of deaths/total number of cases.
Definitions.
Bacteremia was defined as the finding of an organism in a blood culture specimen. Clinically significant bacteremia was defined as at least one positive blood culture together with clinical features compatible with systemic inflammatory response syndrome (1), and the patients with significant bacteremia were included in this study.
Nosocomial infection was defined as an infection that occurred >48 h after admission to the hospital, an infection that occurred <48 h after admission to the hospital in patients who had been hospitalized in the 2 weeks prior to admission, or an infection that occurred <48 h after admission to the hospital in patients that had been transferred from another hospital or nursing home. Nosocomial bloodstream infections, as well as other nosocomial infections, were defined according to the criteria proposed by the Centers for Disease Control and Prevention (6). Neutropenia was defined as an absolute neutrophil count of below 500/mm3.
The antimicrobial therapies were classified as empirical or definitive, with the former being defined as the initial therapy before the results of blood culture were available and the latter being defined as therapy after the results of antibiotic susceptibility tests had been received. Empirical antimicrobial therapy was defined as treatment that included at least one antibiotic and that was started no later than 24 h after the index positive blood sample for culture had been drawn. Also, definitive antimicrobial therapy was defined as antimicrobial therapy that was continued or commenced on the day that the antibiogram results were reported to the clinicians and that was started no later than 120 h after the index positive blood sample for culture had been drawn. The antimicrobial therapy was considered appropriate if the treatment regimen included antibiotics active in vitro and the dosage and route of administration were in conformity with current medical standards. Cephalosporin monotherapy as definitive antimicrobial therapy was considered inappropriate irrespective of the MIC. Cefotaxime, ceftriaxone, ceftizoxime, and ceftazidime were defined as broad-spectrum cephalosporins.
Statistical analysis.
The Student t test was used to compare continuous variables, and the χ2 or Fisher exact test was used to compare categorical variables. In identifying the independent risk factors for mortality, a backward stepwise logistic regression analysis was used to control for the effects of confounding variables. Variables with a P value of <0.05 in the univariate analysis were candidates for multivariate analysis, as was the main variable of interest (i.e., cephalosporin therapy). The Kaplan-Meier method was used for survival analysis. All P values were two-tailed, with a P value of <0.05 considered statistically significant. The SPSS (version 10.0) software package was used for these analyses.
RESULTS
Demographic characteristics.
A total of 1,154 episodes of E. coli bacteremia and 578 episodes of K. pneumoniae bacteremia, in 1,045 and 499 patients, respectively, were identified. Of the stored blood isolates from these patients, 982 strains of E. coli and 471 strains of K. pneumoniae were successfully recovered for inclusion in the study. Of these, 7.7% (76 of 982) of the E. coli strains and 15.9% (75 of 471) of the K. pneumoniae strains were ESBL-producing organisms. A total of 133 patients, including 67 patients with E. coli bacteremia and 66 patients with K. pneumoniae bacteremia, were analyzed, since the remaining had unavailable medical records or had no significant bacteremia. Among the 67 patients with ESBL-producing E. coli bacteremia and 66 patients with ESBL-producing K. pneumoniae bacteremia, three cases and four cases, respectively, were considered to be infections caused by AmpC type β-lactamase hyperproducers.
A total of 63.9% of the patients were males, and their median age was 54 (range, 16 to 87) years. Demographic data and risk factors for infection are shown in Table 1. A total of 78.9% of the episodes were nosocomial infections, and 71.4% of the patients had received any antibiotics within previous 30 days (Table 1). The most common underlying disease of the study population was solid tumor (n = 45; 33.8%), and the most common primary site of infection was the pancreaticobiliary tract (n = 58; 43.6%).
TABLE 1.
Demographic characteristics of study population
| Characteristic | Value |
|---|---|
| Age, mean yr ± SD (median, range) | 54 ± 15.18 (56, 16-87) |
| No. male:female | 85:48 (63.9%:36.1%) |
| No. with K. pneumoniae:E. coli | 66:67 (49.6%:59.4%) |
| APACHE II score, mean ± SD (range) | 10.26 ± 4.60 (0-24) |
| Hospital stay before bacteremia, mean days ± SD | 22 ± 33.87 (10, 0-230) |
| (median, range) | |
| No. with hospital stay of ≥2 weeks | 61 (45.9%) |
| No. with nosocomial infection | 105 (78.9%) |
| No. with neutropenia | 24 (18%) |
| No. with presentation with septic shock | 20 (22.6%) |
| No. with care in intensive care unit | 11 (8.3%) |
| No. postsurgery | 10 (7.5%) |
| No. with indwelling urinary catheter | 30 (22.6%) |
| No. with central venous catheterization | 36 (27.1%) |
| No. with invasive procedure within previous 72 h | 34 (25.6%) |
| No. with prior use of any antibiotics within | 95 (71.4%) |
| previous 30 days | |
| No. of kinds of antibiotics administered | 2.03 ± 1.60 (2, 0-6) |
| within previous 30 days, mean ± SD (median, range) |
Thirty-day mortality and risk factors for mortality.
The 30-day mortality rate for all patients was 25.6% (34 of 133); it was 31.8% (21 of 66) for patients with K. pneumoniae bacteremia and 19.4% (13 of 67) for patients with E. coli bacteremia. The mortality rates according to underlying diseases and sites of infection are shown in Table 2. The mortality rate for patients with peritonitis was 68.4% (13 of 19), and that for patients with unknown primary site of infection was 33.3% (11 of 33) (Table 2). There were four patients with pneumonia and five patients with liver abscess caused by K. pneumoniae, whereas there were no patients with such infections caused by E. coli. Of four patients with pneumonia due to ESBL-producing K. pneumoniae, two patients died. However, all patients with liver abscess survived.
TABLE 2.
Thirty-day mortality rates for bloodstream infections caused by ESBL-producing K. pneumoniae and E. coli
| Disease or infection site | No. of deaths/total (% mortality)
|
||
|---|---|---|---|
| Overall | K. pneumoniae infection | E. coli infection | |
| Overall | 34/133 (25.6) | 21/66 (31.8) | 13/67 (19.4) |
| Underlying disease | |||
| Leukemia | 7/18 (38.9) | 3/8 (37.5) | 4/10 (40) |
| Lymphoma | 2/3 (66.7) | 1/2 (50) | 1/1 (100) |
| Solid organ transplantation | 2/5 (40) | 2/5 (40) | 0/0 |
| Bone marrow transplantation | 1/2 (50) | 1/2 (50) | 0/0 |
| Liver cirrhosis | 9/22 (40.9) | 6/15 (40) | 3/7 (42.9) |
| End stage renal disease | 2/3 (66.7) | 2/2 (100) | 0/1 (0) |
| Benign pancreaticobiliary tract disease | 2/20 (10) | 1/7 (14.3) | 1/13 (7.7) |
| Solid tumor | 6/45 (13.3) | 3/15 (20) | 3/30 (10) |
| Autoimmune disease | 2/5 (40) | 2/4 (50) | 0/1 (0) |
| Human immunodeficiency virus infection | 0/1 (0) | 0/0 | 0/1 (0) |
| Other | 1/6 (16.7) | 0/4 (0) | 1/2 (50) |
| None | 0/3 (0) | 0/2 (0) | 0/1 (0) |
| Primary site of infection | |||
| Pancreaticobiliary tract | 6/58 (10.3) | 3/22 (13.6) | 3/36 (8.3) |
| Liver abscess | 0/5 (0) | 0/5 (0) | 0/0 |
| Pneumonia | 2/4 (50) | 2/4 (50) | 0/0 |
| Urinary tract | 2/14 (14.3) | 1/4 (25) | 1/10 (10) |
| Peritonitis (including spontaneous bacterial peritonitis) | 13/19 (68.4) | 8/13 (61.5) | 5/6 (83.3) |
| Unknown | 11/33 (33.3) | 7/18 (38.9) | 4/15 (26.7) |
From univariate analysis, variables significantly associated with mortality included the following: administration of a broad-spectrum cephalosporin as definitive antimicrobial therapy, neutropenia, presentation with septic shock, care in an intensive care unit, peritonitis, immunosuppressive treatment, prior corticosteroid use, and increasing APACHE II score (Table 3). However, administration of a broad-spectrum cephalosporin as empirical antimicrobial therapy was not associated with a higher mortality (odds ration [OR] = 1.10; 95% confidence interval [95% CI] = 0.42 to 2.87; P = 0.842) (Table 3).
TABLE 3.
Factors influencing mortality in bloodstream infections due to ESBL-producing K. pneumoniae and E. coli
| Parameter | No. of deaths/no. of episodes (% mortality) | OR (95% CI) | P value |
|---|---|---|---|
| Age, yr | |||
| <65 | 27/104 (26) | 0.91 (0.35-2.36) | 0.842 |
| ≥65 | 7/29 (24.1) | ||
| Gender | |||
| Male | 22/85 (25.9) | 0.96 (0.42-2.15) | 0.911 |
| Female | 12/48 (25) | ||
| Organism | |||
| E. coli | 13/67 (19.4) | 1.94 (0.87-4.30) | 0.101 |
| K. pneumoniae | 21/66 (31.8) | ||
| Definitive antimicrobial therapy | |||
| Noncephalosporin | 16/101 (15.8) | 6.83 (2.84-16.46) | <0.001 |
| Broad-spectrum cephalosporin | 18/32 (56.3) | ||
| Empirical antimicrobial therapy | |||
| Noncephalosporin | 7/29 (24.1) | 1.10 (0.42-2.87) | 0.842 |
| Broad-spectrum cephalosporin | 27/104 (26) | ||
| Origin of infection | |||
| Community | 5/28 (17.9) | 1.76 (0.61-5.05) | 0.293 |
| Hospital | 29/105 (27.6) | ||
| Neutropenia | |||
| No | 23/109 (21.1) | 3.16 (1.25-7.98) | 0.012 |
| Yes | 11/24 (45.8) | ||
| Presentation with septic shock | |||
| No | 10/103 (9.7) | 37.20 (12.30-112.56) | <0.001 |
| Yes | 24/30 (80) | ||
| Care in intensive care unit | |||
| No | 27/112 (22.1) | 6.16 (1.68-22.61) | 0.006 |
| Yes | 7/11 (63.6) | ||
| Peritonitis | |||
| No | 21/114 (18.4) | 9.60 (3.27-28.17) | <0.001 |
| Yes | 13/19 (68.4) | ||
| Immunosuppressive treatment | |||
| No | 27/120 (22.5) | 4.02 (1.25-12.97) | 0.021 |
| Yes | 7/13 (53.8) | ||
| Corticosteroids use | |||
| No | 23/108 (21.3) | 2.90 (1.16-7.24) | 0.019 |
| Yes | 11/25 (44) | ||
| Hospital stay, days | |||
| ≤14 | 15/72 (20.8) | 1.72 (0.78-3.77) | 0.174 |
| >14 | 19/61 (31.1) | ||
| APACHE II score | |||
| ≤7 | 4/35 (11.4) | <0.001 | |
| 8-15 | 15/81 (18.5) | ||
| ≥16 | 15/17 (88.2) |
Multivariate analysis using a logistic regression model, which included the variables associated with mortality by univariate analysis (P < 0.05), demonstrated that the independent risk factors for 30-day mortality were as follows: administration of a broad-spectrum cephalosporin as definitive antimicrobial therapy, neutropenia, peritonitis, presentation with septic shock, and increasing APACHE II score (Table 4).
TABLE 4.
Independent risk factors for mortality in bloodstream infections due to ESBL-producing K. pneumoniae and E. colia
| Risk factor | Adjusted OR (95% CI) | P value |
|---|---|---|
| Administration of broad-spectrum cephalosporin as definitive antimicrobial therapy | 9.18 (1.55-54.51) | 0.015 |
| Neutropenia | 9.03 (1.24-65.97) | 0.030 |
| Peritonitis | 10.25 (1.26-83.25) | 0.029 |
| Presentation with septic shock | 45.25 (6.55-312.84) | <0.001 |
| Increasing APACHE II score (per one-point increments) | 1.44 (1.11-1.87) | 0.006 |
Multivariate analysis with a logistic regression model was used.
Treatment outcome.
To assess the initial clinical response at 72 h after empirical antimicrobial therapy, 127 patients who had received broad-spectrum cephalosporins, fluoroquinolones, or carbapenem as empirical antimicrobial therapy were included in the analysis. The remaining six patients were excluded because they had received piperacillin with or without an aminoglycoside, and of these, five patients had experienced treatment failure. When the clinical response at 72 h was assessed, patients with bloodstream infections caused by ESBL-producing K. pneumoniae had higher a treatment failure rate than those with infections caused by E. coli (32 of 64 [50%] versus 21 of 63 [33.3%]; P = 0.057). A trend toward a higher treatment failure rate was observed for cephalosporin regimens compared with noncephalosporin regimens (47 of 104 [45%] versus 6 of 23 [26%]; P = 0.093). This trend was more prominent in patients with infection caused by E. coli than in those with infection caused by K. pneumoniae (39 versus 14% [P = 0.086] and 51 versus 44% [P = 0.719], respectively).
A further breakdown of treatment failure according to the MICs of cephalosporins administered as empirical antimicrobial therapy is shown in Table 5. There was a trend toward a significant increase in treatment failure rate as MICs rose (P = 0.088). Eight patients were treated with an in vitro active broad-spectrum cephalosporin, that is, one for which the MIC was ≤8 μg/ml, and of these, two died. Six patients received a broad-spectrum cephalosporin with an MIC of ≤2 μg/ml, and of these, only one experienced treatment failure. The clinical details for these patients are summarized in Table 6.
TABLE 5.
Outcome of empirical treatment with a cephalosporin in bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae according to the MIC of the cephalosporin administered
| MIC (μg/ml) | Treatment failurea,b | 30-day mortalitya |
|---|---|---|
| ≤1 | 0/2 (0) | 0/2 (0) |
| 2 | 1/4 (25) | 1/4 (25) |
| 4 | NCc | NC |
| 8 | 2/2 (100) | 1/2 (50) |
| 16 | 4/6 (66.7) | 3/6 (50) |
| 32 | 8/11 (72.7) | 3/11 (27.3) |
Data are number of cases/number of episodes (percent).
Treatment failure at 72 h after empirical antimicrobial therapy.
NC, no case identified.
TABLE 6.
Clinical outcomes for 8 patients with ESBL-producing K. pneumoniae and E. coli bacteremia treated empirically with broad-spectrum cephalosporins to which the causative organisms were susceptible in vitro
| Age (yr)/sex | Underlying condition(s)a | Primary site of infection | Organism | Antibiotic regimenb | MIC (μg/ml) | Treatment outcome |
|---|---|---|---|---|---|---|
| 17/male | BMT, neutropenic | Unknown | K. pneumoniae | ZOX, 2 g q8h | 8 | Failure; continued fever after 7 days, changed to imipenem and ciprofloxacin but patient died on 22nd day of treatment |
| 26/male | Acute leukemia, neutropenic | Unknown | E. coli | ZOX, 2 g q8h; AMK, 7.5 mg/kg q12h | 8 | Failure; continued fever after 3 days, changed to imipenem and amikacin with cure |
| 39/female | ESRD, DM, neurogenic bladder | Urinary tract | K. pneumoniae | CAZ, 1 g q8h; AMK, 7.5 mg/kg q12h | 2 | Failure; progression to renal abscess, changed to imipenem but patient died on 28th day of treatment |
| 79/male | Renal tumor | Unknown | E. coli | CTX, 1 g q6h; | 2 | Cure; partial response to initial |
| AMK, 7.5 mg/kg q12h | antimicrobial therapy, changed to ciprofloxacin with cure | |||||
| 75/male | Cholangiocarcinoma | Cholangitis | E. coli | CTX, 1 g q6h | 2 | Cure; partial response to initial antimicrobial therapy, changed to ciprofloxacin with cure |
| 69/male | CBD stone | Cholangitis | E. coli | CTX, 1 g q6h | 2 | Cure; complete response to initial antimicrobial therapy |
| 65/female | CBD stone | Cholangitis | E. coli | CTX, 1 g q6h; AMK, 7.5 mg/kg q12h | ≤1 | Cure; complete response to initial antimicrobial therapy |
| 48/male | DM | Liver abscess | K. pneumoniae | CTX, 1 g q6h; AMK, 7.5 mg/kg q12h | ≤1 | Cure; antimicrobial therapy with percutaneous drainage |
BMT, bone-marrow transplantation; ESRD, end stage renal disease; DM, diabetes mellitus; CBD, common bile duct.
ZOX, ceftizoxime; CTX, cefotaxime; CAZ, ceftazidime; AMK, amikacin.
We also assessed outcomes for patients who had received a cephalosporin to which the organism was apparently susceptible as definitive antimicrobial therapy. There were three cases due to ESBL producers for which the MIC of the antibiotic which was used to treat the infection was ≤2 μg/ml, and all of these patients were cured. The MICs of cephalosporin were ≤1 μg/ml in two cases and 2 μg/ml in one case. However, in three cases due to ESBL producers for which the MIC of the cephalosporin which was used to treat the infection was 16 μg/ml, all of the patients experienced treatment failure and died.
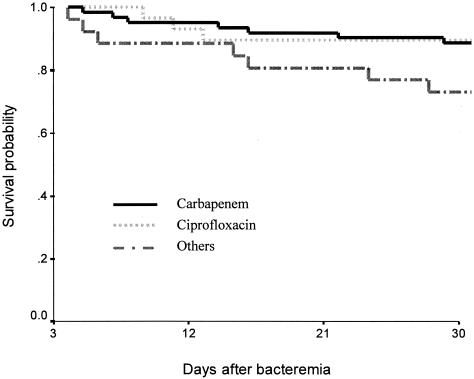
To assess the 30-day mortality rates according to the definitive antimicrobial therapy regimens, 117 patients who had survived during more than 3 days after onset of bacteremia were analyzed. The remaining 16 patients, who died within 3 days after blood culture sample acquisition, were excluded from the analysis. The 30-day mortality rates were as follows: carbapenem, 12.9% (8 of 62), ciprofloxacin, 10.3% (3 of 29); and others, such as cephalosporin or an aminoglycoside, 26.9% (7 of 26). Compared with others, the carbapenem and ciprofloxacin group had lower mortality, although statistical significance was not reached (P = 0.128 and 0.164, respectively). The survival curve is shown in Fig. 1.
FIG. 1.
Survival curve obtained by the Kaplan-Meier method for bloodstream infections due to ESBL-producing E. coli and K. pneumoniae according to definitive antimicrobial therapy regimens. The 16 patients who died within 3 days after blood culture sample acquisition were excluded from the analysis. The 30-day mortality rates were as follows: carbapenem, 12.9% (8 of 62), ciprofloxacin, 10.3% (3 of 29); others, 26.9% (7 of 26).
Among 91 patients who received carbapenem or ciprofloxacin as definitive antimicrobial therapy, 24 (26.4%) received combination therapy and the remaining 67 (73.6%) received monotherapy. There was no significant difference in mortality between the combination therapy group and monotherapy group (6 of 24 [25%] versus 5 of 67 [7.5%]; P = 0.061). None of the patients had received cefepime or cephamycin.
Influence of delay in appropriate antimicrobial therapy on mortality.
The patients who had received a broad-spectrum cephalosporin as definitive antimicrobial therapy had significantly higher mortality than those received a noncephalosporin (56.3 versus 15.8%; P < 0.001), whereas administration of a broad-spectrum cephalosporin as empirical antimicrobial therapy was not associated with higher mortality (26 versus 24.1%; P = 0.842). To evaluate the influence of a delay in appropriate antimicrobial therapy on the mortality, only the 95 patients who had received appropriate definitive antibiotics, such as carbapenem or ciprofloxacin, which are active in vitro against the causative microorganisms, were analyzed. The remaining 38 were excluded because they had received inappropriate definitive antibiotics, i.e., cephalosporins or aminoglycosides. The mean duration (± standard deviation) of the delay in appropriate antimicrobial therapy was 3.3 ± 1.8 days. Of the 95 patients that had received appropriate definitive antibiotics, the 30-day mortality rates were 15.5% (9 of 58) for those treated with an appropriate empirical antibiotic regimen and 18.9% (7 of 37) for those treated with an inappropriate regimen (P = 0.666) (Table 7).
TABLE 7.
Thirty-day mortality rates in patients with ESBL-producing K. pneumoniae and E. coli bacteremia according to the appropriateness of empirical antimicrobial therapya
| Organism | Mortalityb with:
|
P value | |
|---|---|---|---|
| Appropriate empirical antimicrobial therapyc | Inappropriate empirical antimicrobial therapyd | ||
| Overall | 9/58 (15.5) | 7/37 (18.9) | 0.666 |
| K. pneumoniae | 7/33 (21.2) | 3/14 (21.4) | 1.000 |
| E. coli | 2/25 (8) | 4/23 (17.4) | 0.407 |
Of 133 patients with bloodstream infection due to ESBL-EK, 95 patients who had received appropriate antimicrobial agents, such as a carbapenem or ciprofloxacin, as definitive therapy were examined. The remaining 38 patients were excluded because they had received inappropriate definitive antimicrobial therapy.
Data are number of deaths/number of episodes (percent).
This group included patients who had received antimicrobial agents to which the ESBL-EK isolate was susceptible in vitro as initial empirical antibiotics.
This group included patients who had not received any empirical antimicrobial agents or who had received antimicrobial agents to which the ESBL-EK isolate was not susceptible in vitro as initial empirical antibiotics.
DISCUSSION
In the present study we found that the independent risk factors for mortality in bloodstream infections due to ESBL-EK were neutropenia, peritonitis, septic shock, higher APACHE II score, and administration of a cephalosporin as definitive antimicrobial therapy. In addition, we demonstrated greater initial treatment failure in the cephalosporin treatment group.
Suboptimal clinical outcomes have been documented when cephalosporins are used to treat serious infections due to ESBL-producing organisms (10, 12, 22, 26, 32). Furthermore, several investigators have concluded that initial treatment of bloodstream infections caused by ESBL-producing strains with noncarbapenem agents may be associated with higher mortality than treatment with a carbapenem agent (22, 26). However, details of the treatment of most of the patients in those studies were not provided (22, 26). On the basis of in vitro susceptibility tests, coupled with a limited number of reports of clinical outcome, carbapenem has emerged as the agent of choice for the treatment of serious infections associated with ESBL-producing organisms. In the present study, the outcome of cephalosporin treatment of ESBL-EK bacteremia was also poor. However, initial treatment with a cephalosporin as empirical antimicrobial therapy was not associated with higher mortality. In addition, a delay in appropriate treatment of ESBL-EK did not have an adverse outcome, even in bloodstream infections. Similarly, in a report by Lautenbach et al., a delay in effective treatment for infection due to ESBL-producing organisms did not result in poorer clinical outcomes (15). Thus, more prudent use of a carbapenem as an initial empirical antibiotic may be justified. However, the empirical use of antibiotics should be based on the resistance background of the microorganisms and the underlying diseases of the patients.
There was low mortality in patients with ESBL-EK bacteremia associated with pancreaticobiliary tract infection. This could result from early intervention for biliary decompression in the majority of the patients and might indicate that nonmedical interventions, such as decompression, are also important in the treatment of biliary infection. However, patients with peritonitis had significantly higher mortality, and peritonitis was one of the independent risk factors for death. Most of our patients with peritonitis had advanced liver cirrhosis, with spontaneous bacterial peritonitis, and might not have tolerated ineffective initial antimicrobial therapy, due to their impaired liver function.
Schiappa et al. suggested that treatment with noncarbapenemagents is associated with higher mortality than treatment with a carbapenem agent (26). Wong-Beringer et al. also demonstrated that ceftazidime treatment was associated with treatment failure in all patients with bloodstream infections due to ESBL-producing organisms (32). Currently, the NCCLS recommends that ESBL-producing strains should be considered by microbiology laboratories to be resistant to all penicillins, cephalosporins, and aztreonam (17). These criteria and other expert recommendations may promote the use of carbapenems for the treatment of serious infections caused by ESBL-producing organisms, limiting clinical experience with the use of other agents. Furthermore, increased empirical use of carbapenems in response to outbreaks of ESBL-EK infections has been accompanied by the rapid emergence of carbapenem resistance in other nosocomial pathogens (4, 30). Therefore, therapeutic options other than carbapenems would be attractive. When ESBL-EK isolates are susceptible to the fluoroquinolones, these agents have been effective and may constitute an alternative antimicrobial therapy (10, 20). However, data on treatment outcomes of infection due to ESBL-EK, especially on ciprofloxacin treatment, are limited to case reports or descriptions of nosocomial outbreaks. In the present study, the outcome of ciprofloxacin treatment was similar to that of carbapenem treatment and was more favorable than that of treatment with broad-spectrum cephalosporins.
In this study, outcomes for patients receiving cephalosporin therapy were more favorable when the MICs for the infecting organisms were ≤2 μg/ml than when they were 8 μg/ml or greater. In this study, there were six cases of bloodstream infections due to ESBL producers for which the MIC of the antibiotic which was used to treat the infection was ≤2 μg/ml. Death occurred in only one of these patients, and two patients required a change in therapy to effect a cure. The remaining three patients experienced a complete response and cure. However, the number of patients in this category is too small to accurately state whether cephalosporins can be reliably used in this setting.
It is unlikely that randomized controlled trials of therapy for infections due to ESBL-EK will be performed in the near future. Therefore, further clinical experience with antibiotics other than carbapenem is warranted to fully evaluate the potential usefulness of these antibiotics against serious infections due to ESBL-producing organisms.
Although retrospective studies may be limited by the availability of medical records, we found that 90% of the charts were complete and available for review. Another potential limitation is that molecular epidemiologic analysis and characterization of ESBL types was not carried out in our study. However, there was no evidence of clonal spread, based on the antimicrobial susceptibility patterns of the isolates (data not shown). Also, we did not evaluate the relationship between the type of ESBLs and treatment outcomes. This study included three cases of infection by E. coli isolates and four cases of infection by K. pneumoniae isolates with different mechanisms of cephalosporin resistance, probable AmpC producers. AmpC-producing E. coli and K. pneumoniae isolates are not considered to be classic ESBL-producing organisms. However, this study consisted mainly of clinical analysis regarding treatment outcome, not regarding the epidemiology, laboratory detection methods, or molecular characterization of ESBLs. When data for patients infected by isolates that did not meet the classic definition were removed, the conclusions were not altered (data not shown). Thus, the results of this study may not be confounded by the inclusion those isolates.
Previous studies have focused on the epidemiology, laboratory detection methods, and molecular characterization of ESBL-producing organisms. However, data on treatment outcomes are limited. In this study, we evaluated the clinical outcome in 133 patients with bloodstream infections due to ESBL-EK. To our knowledge, this is the largest clinical analysis so far of treatment outcome for bloodstream infections caused by ESBL-EK.
In conclusion, independent risk factors for mortality were severe sepsis, peritonitis, neutropenia, increasing APACHE II score, and administration of a broad-spectrum cephalosporin as definitive antimicrobial therapy. The outcome of cephalosporin treatment for ESBL-EK bacteremia was poor. Ciprofloxacin and carbapenems were the most effective antibiotics in antimicrobial therapy for ESBL-EK bacteremia. However, a delay in appropriate definitive antimicrobial therapy was not associated with higher mortality if antimicrobial therapy was adjusted appropriately according to the susceptibility results. Our data suggest that ciprofloxacin may be an alternative antimicrobial therapy for ESBL-EK infections, even for bloodstream infections, and that more prudent use of carbapenems as initial empirical antibiotics may be reasonable.
REFERENCES
- 1.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20:864-874. [PubMed] [Google Scholar]
- 2.Burwen, D. R., S. N. Banerjee, and R. P. Gaynes. 1994. The National Nosocomial Infections Surveillance System. Ceftazidime resistance among selected nosocomial gram-negative bacilli in the United States. J. Infect. Dis. 170:1622-1625. [DOI] [PubMed] [Google Scholar]
- 3.Bush, K. 2001. New β-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 4.Corbella, X., A. Montero, M. Pujol, M. A. Dominguez, J. Ayats, M. J. Argerich, F. Garrigosa, J. Ariza, and F. Gudiol. 2000. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J. Clin. Microbiol. 38:4086-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenstein, B. I., and D. F. Zaleznik. 2000. Enterobacteriaceae, p. 2294-2310. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 6.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 7.Itokazu, G. S., J. P. Quinn, C. Bell-Dixon, F. M. Kahan, and R. A. Weinstein. 1996. Antimicrobial resistance rates among aerobic gram-negative bacilli recovered from patients in intensive care units: evaluation of a national postmarketing surveillance program. Clin. Infect. Dis. 23:779-784. [DOI] [PubMed] [Google Scholar]
- 8.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen, J. H., J. D. Turnidge, and J. A. Washington. 1999. Antibacterial susceptibility tests: dilution and disk diffusion methods, p. 1526-1543. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology. 7th ed. American Society for Microbiology, Washington, D.C.
- 10.Karas, J. A., D. G. Pillay, D. Muckart, and A. W. Sturm. 1996. Treatment failure due to extended-spectrum beta-lactamase. J. Antimicrob. Chemother. 37:203-204. [DOI] [PubMed] [Google Scholar]
- 11.Kim, B. N., J. H. Woo, M. N. Kim, J. Ryu, and Y. S. Kim. 2002. Clinical implications of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteremia. J. Hosp. Infect. 52:99-106. [DOI] [PubMed] [Google Scholar]
- 12.Kim, Y. K., H. Pai, H. J. Lee, S. E. Park, E. H. Choi, J. Kim, J. H. Kim, and E. C. Kim. 2002. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knaus, W. A., E. A. Drapier, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 14.Knothe, H., P. Shah, V. Kremery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 15.Lautenbach, E., J. B. Patel, W. B. Bilker, P. H. Edelstein, and N. O. Fishman. 2001. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 32:1162-1171. [DOI] [PubMed] [Google Scholar]
- 16.Meyer, K. S., C. Urban, J. A. Eagen, B. J. Berger, and J. J. Rahal. 1993. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann. Intern. Med. 119:353-358. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A7, 7th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard. NCCLS document M100-S13. National Committee for Clinical Laboratory Standards. Wayne, Pa.
- 19.Naumovski, L., J. P. Quinn, D. Miyashiro, M. Patel, K. Bush, S. B. Singer, D. Graves, T. Palzkill, and A. M. Arvin. 1992. Outbreak of ceftazidime resistance due to a novel extended-spectrum beta-lactamase in isolates from cancer patients. Antimicrob. Agents Chemother. 36:1991-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paterson, D. L. 2000. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs). Clin. Microbiol. Infect. 6:460-463. [DOI] [PubMed] [Google Scholar]
- 21.Paterson, D. L., W-C. Ko, A. V. Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann. Intern. Med. 140:26-32. [DOI] [PubMed] [Google Scholar]
- 22.Paterson, D. L., W.-C. Ko, A. V. Gottberg, J. M. Casellas, L. Mulazimoglu, K. P. Klugman, R. A. Bonomo, L. B. Rice, J. G. McCormack, and V. L. Yu. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum β-lactamases: implications for the clinical microbiology laboratory. J. Clin. Microbiol. 39:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pena, C., M. Pujol, C. Ardanuy, A. Ricart, R. Pallares, J. Linares, J. Ariza, and F. Gudiol. 2001. An outbreak of hospital-acquired Klebsiella pneumoniae bacteraemia, including strains producing extended-spectrum beta-lactamase. J. Hosp. Infect. 47:53-59. [DOI] [PubMed] [Google Scholar]
- 24.Pena, C., M. Pujol, C. Ardanuy, A. Ricart, R. Pallares, J. Linares, J. Ariza, and F. Gudiol. 1998. Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 42:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice, L. B., L. L. Carias, R. A. Bonomo, D. M. Shlaes. 1996. Molecular genetics of resistance to both ceftazidime and beta-lactam-beta-lactamase inhibitor combinations in Klebsiella pneumoniae and in vivo response to beta-lactam therapy. J. Infect. Dis. 173:151-158. [DOI] [PubMed] [Google Scholar]
- 26.Schiappa, D. A., M. K. Hayden, M., G. Matushek, F. N. Hashemi, J. Sullivan, K. Y. Smith, D. Miyashiro, J. P. Quinn, R. A. Weinstein, and G. M. Trenholme. 1996. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J. Infect. Dis. 174:529-536. [DOI] [PubMed] [Google Scholar]
- 27.Steward, C. D., J. K. Rasheed, S. K. Hubert, J. W. Biddle, P. M. Raney, G. J. Anderson, P. P. Williams, K. L. Brittain, A. Oliver, J. E. McGowan, and F. C. Tenover. 2001. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum β-lactamase detection methods. J. Clin. Microbiol. 39:2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thauvin-Eliopoulos, C., M. F. Tripodi, R. C. Moellering, and G. M. Eliopoulos. 1997. Efficacies of piperacillin-tazobactam and cefepime in rats with experimental intra-abdominal abscesses due to an extended-spectrum beta-lactamase-producing strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 41:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomason, K. S., and C. C. Sanders. 1992. Detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae: comparison of the double-disk and three-dimensional tests. Antimicrob. Agents Chemother. 36:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troillet, N., M. H. Samore, and Y. Carmeli. 1997. Imipenem-resistant Pseudomonas aeruginosa: risk factors and antibiotic susceptibility patterns. Clin. Infect. Dis. 25:1094-1098. [DOI] [PubMed] [Google Scholar]
- 31.Tzelepi, E., P. Giakkoupi, D. Sofianou, V. Loukova, A. Kemeroglou, and A. Tsakris. 2000. Detection of extended-spectrum β-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 38:542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong-Beringer, A., J. Hindler, M. Loeloff, A. M. Queenan, N. Lee, D. A. Pegues, J. P. Quinn, and K. Bush. 2002. Molecular correlation for the treatment outcomes in bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae with reduced susceptibility to ceftazidime. Clin. Infect. Dis. 34:135-146. [DOI] [PubMed] [Google Scholar]