Abstract
Pain catastrophizing is one of the most powerful predictors of poor outcomes in youth and adults with pain; however, little is known about differential impacts of pain catastrophizing on outcomes as a function of age. The current study examined the predictive value of pain catastrophizing on pain interference and pain intensity across children, adolescents, and two age groups of young adults with chronic pain. Cross-sectional data are presented from the adult and pediatric Collaborative Health Outcomes Information Registry (CHOIR), including measures of pain catastrophizing, pain intensity, pain interference and emotional distress from 1028 individuals with chronic pain. Results revealed that age moderated the relation between pain catastrophizing and pain interference, with the strength of these effects declining with age. The effect of pain catastrophizing on pain interference was strongest in adolescents and relatively weak in all three other groups. Emotional distress was the strongest predictor of pain interference for children, whereas pain intensity was the strongest predictor for both adult groups. Pain catastrophizing was found to predict pain intensity and, although age was a significant moderator, statistical findings were weak. Developmental considerations and clinical implications regarding the utility of the construct of pain catastrophizing across age groups are discussed.
Keywords: Developmental, Catastrophizing, Chronic pain, Pain interference, Adolescents, Collaborative Health Outcomes Information Registry (CHOIR)
Introduction
Chronic pain is a common problem for children, adolescents, and young adults [30,34,40], interfering with psychosocial functioning [14,31,50] and generating high economic costs [20]. Pain catastrophizing (PC) - a negative cognitive and emotional pattern characterized by rumination, magnification and helplessness toward actual or anticipated pain [10,55] - is among the most powerful predictors of poor outcomes in chronic pain samples. In adults, strong associations have been found between PC and increased pain intensity, disability, psychological distress and lower quality of life [27,50,69]. In youth, PC has been associated with similar poor outcomes [10,21,32,61]. Although PC is known to predict heightened pain intensity and interference with daily activities across adults and youth, only a few studies have explored whether PC impacts outcomes differentially for individuals of different ages [10,28,37,57].
In youth with chronic pain, PC has been found to decline with age [10]. Relatedly, Tran and colleagues (2015) have demonstrated that PC is a stronger predictor of functional disability and pain intensity in children compared to adolescents [15]. It is reasonable to expect that, as the cognitive abilities of children change in accordance with normal developmental trajectories, their responses to pain may also change. Adaptive responses to stress and pain have been found to increase as children age. Brown and colleagues (1986) theorized that as more opportunities to apply coping strategies arise, a greater repertoire of coping responses is available [4]. Thus, across life stages and as a function of normative developmental processes, cognitive response styles such as catastrophizing, may vary in salience and differentially impact outcomes related to pain and function.
In children, the process of PC may not be due to maladaptive cognitive coping attempts as is speculated in adults [49,53] but rather, a developmentally normative process due to lack of coping resources [13]. It would follow that as children mature into adolescents and early adults, a wider array of cognitively complex resources may help minimize the impact of PC on functional outcomes, as alternative coping methods and styles are learned and more readily accessible. Literature suggests that adolescents may use more active and accommodative coping methods compared to children, which may be due to the cognitive resources and executive functions needed to employ these types of strategies [9]. For adults on the other hand, coping styles and response sets (adaptive and maladaptive) have been found to be more stable [28]. Particularly in the instance of chronic pain, maladaptive cognitive patterns such as pain catastrophizing, may become entrenched and shape the trajectory of functional outcomes [18]. With age, the development of greater emotion-focused coping may also lead to increased capacities for maladaptive rumination, a characteristic subsumed within the construct of pain catastrophizing [70].
To date, no published study has compared PC from childhood into adulthood, nor examined the predictive value of PC on pain interference and pain intensity specifically across cohorts of children, adolescents, and young adults with chronic pain. The primary aim of this study was to determine whether the relationships of PC with pain interference and pain intensity vary as a function of age in a sample comprised of children, adolescents, and young adults. We predicted that the effect of PC on pain interference, while controlling for emotional distress and pain intensity, would be strongest in our youngest and oldest age groups due to the narrow array of coping strategies and solidified cognitive processes around pain, respectively. Our second aim was to investigate if PC predicted pain intensity while controlling for emotional distress, and examine the moderating effect of age. Again, we hypothesized that there would be higher levels of PC in the youngest and oldest age groups, with strongest relations to pain intensity in these two groups. As we expected PC to show differential effects on pain interference across age groups, we also conducted an exploratory analysis to determine whether age moderated the effects of pain intensity and emotional distress, which are both salient predictors of pain interference.
Methods
The current study accessed data from both the adult and pediatric Collaborative Health Outcomes Information Registry (CHOIR and Peds-CHOIR, respectively, http://choir.stanford.edu), utilizing an open-source learning healthcare system to assess patient-reported outcomes across children, adolescents and young adults with chronic pain [2]. All methods and procedures were approved by the University's institutional review board as a retrospective chart review.
Participants
Although research is unclear regarding socio-cognitive and neuro-developmental transition from adolescence to adulthood, literature often cites mid-20's as the time period for which brain development completes [17,43,51]. Thus adult age groupings were generated to contain an equal distribution of ages within each bin (i.e., 18-23 years of age and 24-29 years of age). When referring to children and adolescents as a group the term “youth” will be utilized; whereas the term “children” will demarcate 8-12 year-olds and “adolescents” will refer to 13-17 year-olds. Children under the age of 8 and over the age of 17 were excluded from youth analyses due to the valid age range of pediatric PROMIS measures. Although empirical literature recommends alternate child groupings (e.g., 6-11; 12-18) [67], theoretical perspectives on child development suggest soft boundaries between children 7 to 8 years olds as well as those 11 to 12 years old comprising different developmental stages [41]. The current groupings were made to accommodate validity of the measures used in the study and sample size constraints, while considering developmental literature.
Data were collected from 703 adults, ages 18-29 who presented for initial medical evaluation between December 2012 and July 2015 at a large, tertiary care pain clinic. The mean age for the adults 18-23 was 21.02 (SD = 1.77) and for the adults age 24-29 was 27.08 (SD = 1.73). Youth data were collected at initial interdisciplinary evaluations from 325 pediatric patients ages 8-17 in a moderate-sized, tertiary care pediatric pain clinic. The mean age for the children in the sample was 10.94 (SD = 1.33) and for the adolescents, 15.50 (SD = 1.36). Both clinics were located on the west coast and affiliated with the same university housed within the department of anesthesiology, perioperative and pain medicine. Demographics, including diagnostic categories, for the adult and youth sample are listed in Table 1 and Table 2, respectively. Diagnostic bins were created according to billing codes entered by the pain physician at initial clinic visits, reflecting broad characteristics of presenting pain complaints. Differences between diagnostic categories existed between the adult and pediatric clinics as can be gleaned in Tables 1 and 2. Notably, in the adult sample, 156 patients carried diagnoses in a single category, 160 patients had diagnoses in 2 categories, 108 patients had diagnoses in 3 categories, 78 patients had diagnoses in 4 categories, and 149 patients had diagnoses in 5 or more categories. No diagnostic information was available for 39 patients in the adult sample.
Table 1. Demographic and Diagnostic Characteristics of Adult Sample.
| % | N | |
|---|---|---|
| Gender | ||
| Female | 65.6% | 461 |
| Male | 33.9% | 238 |
| Missing | 0.5% | 4 |
| Race | ||
| Caucasian | 62.3% | 438 |
| Asian | 4.8% | 34 |
| African American | 3.0% | 21 |
| American Indian or Alaskan | 0.5% | 4 |
| Native Hawaiian or Pacific Islander | 0.9% | 6 |
| Other | 22.6% | 159 |
| Declines to state | 1.8% | 13 |
| Unknown | 2.7% | 19 |
| Missing | 1.3% | 9 |
| Primary Pain Diagnoses | ||
| Thoracolumbar pain | 37.1% | 256 |
| Musculoskeletal pain | 37.0% | 255 |
| Headache | 33.0% | 228 |
| Nerve pain | 28.1% | 194 |
| Abdominal pain | 27.7% | 191 |
| Fibromyalgia/myofascial pain | 26.2% | 181 |
| Cardiac conditions | 17.0% | 117 |
| Neck pain | 14.6% | 101 |
| CNS-based pain | 11.4% | 79 |
| Pelvic pain | 10.1% | 70 |
| Orofacial pain | 9.3% | 64 |
| Substance abuse disorders | 9.1% | 63 |
| Connective tissue diseases | 8.7% | 60 |
| Dermatological conditions | 6.4% | 44 |
| Neurologic diseases | 5.5% | 38 |
| Vascular diseases | 4.6% | 32 |
| Chest pain | 4.3% | 30 |
| Cancer | 3.6% | 25 |
| Rheumatologic diseases | 3.2% | 22 |
| Endocrine diseases | 2.6% | 18 |
| Complex regional pain syndrome | 2.6% | 18 |
| Pulmonary diseases | 0.7% | 5 |
| Urologic diseases | 0.4% | 3 |
Table 2. Demographic and Diagnostic Characteristics of Youth Sample.
| % | N | |
|---|---|---|
| Gender | ||
| Female | 73.8% | 240 |
| Male | 25.5% | 83 |
| Missing | 0.6% | 2 |
| Race | ||
| Caucasian | 68.6% | 223 |
| Asian | 6.8% | 22 |
| African American | 3.1% | 10 |
| American Indian or Alaskan | 0.6% | 2 |
| Native Hawaiian or Pacific Islander | 1.2% | 4 |
| Other | 11.4% | 37 |
| Declines to state | 2.2% | 7 |
| Missing | 6.1% | 20 |
| Primary Pain Diagnoses | ||
| Musculoskeletal pain | 37.2% | 121 |
| Abdominal pain | 15.4% | 50 |
| Headache | 17.8% | 58 |
| Complex regional pain syndrome | 9.8% | 32 |
| Fibromyalgia | 5.5% | 18 |
| Primary psychological diagnoses | 0.6% | 2 |
| Rheumatologic conditions | 0.9% | 3 |
| Ehlers-Danlos syndrome | 0.9% | 3 |
| Other | 9.5% | 31 |
| Missing | 2.1% | 7 |
Procedures
Prior to initial evaluation and as a part of the assessment process, patients completed a demographic questionnaire followed by a series of patient-reported outcome measures through the CHOIR and Peds-CHOIR systems. Questionnaires were completed at home by most patients through a secure URL link emailed to them upon registering for their clinic appointment. The link is hosted on a HIPAA-compliant and university-approved Oracle database. Patients who arrived without completed questionnaires, were provided encrypted computer tablets before their appointment to finish the survey.
Measures
Pain Catastrophizing Scale (PCS and PCS-C)
The adult Pain Catastrophizing Scale (PCS) measures catastrophic thoughts and feelings about pain [55], yielding a total catastrophizing score and three subscale scores assessing rumination, magnification, and helplessness. The PCS is a 13-item self-report measure utilizing a 5-point Likert response scale (0 = “Not at all,” 1 = “To a slight degree,” 2 = “to a moderate degree,” 3 = “To a great degree,”to 4 = “All the Time”), with a scoring range of 0-52, where higher scores indicate greater levels of catastrophic thoughts and feelings about pain. The PCS shows excellent internal consistency (PCS α = .66-.93) [39,55]. The clinical reference point is 30 or above for the PCS [54]. The PCS used in our study is a modified electronic version based on the validated PCS.
The Pain Catastrophizing Scale for children (PCS-C) is an adaptation of the adult PCS for ages 8-16, which assesses catastrophic thoughts and feelings about pain on similar domains as the PCS (i.e., helplessness, rumination, and magnification) [10]. Like the PCS, the PCS-C is also a self-report measure comprised of 13-items employing a 5-point Likert response scale and the same range of scores as the adult measure. The PCS-C, however, exhibits differences in the Likert options (0 = “Not at all,” 1 = “Mildly” 2 = “Moderately,” 3 = “Severely”, to 4 = “Extremely”). The PCS-C was developed to include adaptations (e.g., rewording of items, repeating the beginning sentence stem for each item) to ensure comprehension for youth [10] yet the item content of all items of the PCS-C remains conceptually consistent with the PCS. The PCS-C employed in the current study was a modified electronic version with the same Likert options, yet retained the item wording from the PCS. Additionally, due to electronic administration practices, the word stems were separated out at the top of the screen with each item delivered on a separate page. The PCS-C is reported to have adequate internal consistency of the full measure and subscales (PCS-C α = 0.68-0.87) [10]. The clinical reference point is a score of 26 or above [42]. Instructions for both PCS and PCS-C were modified to reflect electronic administration.
Pain Intensity
Pain intensity was assessed using a unidimensional 11-point numeric rating scale (NRS-11) ranging from 0 (No pain) to 10 (Worst pain possible) [24,52]. At the time of initial assessment, pediatric patients reported their current pain intensity in addition to average, highest and lowest pain intensity in the last month (i.e., 30 days), while adult patients reported these scores over the previous 7 days. Only the average pain intensity score was used for the current analyses [35,62]. The NRS-11 has demonstrated evidence for validity in assessing pain intensity in clinical and non-clinical samples of children as young as six and eight, respectively [36]. In adult populations, the NRS is a commonly used measure for pain intensity and has shown excellent psychometric properties [12].
PROMIS Instruments
The Patient Reported Outcome Measurement Information System (PROMIS) was developed by an NIH Roadmap for Medical Research Initiative with the aim to provide clinicians and researchers access to efficient, validated, and patient-reported measures of health and well-being [8]. PROMIS instruments are normed against the United States general population as well as multiple disease populations [45]. Scores are based on T-score distribution with a mean of 50 points and a standard deviation of 10 [8]. PROMIS instruments utilize item response theory (IRT) in order to improve patient-reported outcomes measurement quality and precision compared to static composite scale responses[15]. PROMIS instruments were administered via computer adaptive testing (CAT), an approach where items are selected based on patients' responses to previously administered items [6,16,65] thereby reducing patient burden [15,16,25,45,63]. Psychometric research suggests that CAT is a valid method to measure constructs that contain multi-item banks of questions [15,48]. Even though different questions are answered within each domain, the final score (i.e., T- score) enables comparisons across populations or studies, within and between reporters, as scores yielded from IRT are assumed to reflect the same underlying construct. For this study, PROMIS item banks for Pain Interference, Anxiety, and Depression were included. The adult version of the PROMIS measures were administered to study participants 18 years old and above, whereas the pediatric version of the PROMIS was administered to the youth in the study. Comparable item banks exist for adults and youth with only minor differences in content areas. A Likert scale (1= “Never/Not able to do” to 5 = “Almost always/With no trouble”) is used to measure symptoms or functioning over the past 7 days. Higher scores signify greater severity of symptoms.
Pain Interference
The PROMIS Pain Interference item bank (adult and pediatric) assesses the impact of pain on physical, psychosocial, recreational activities, sleep, and emotional functioning [1,60]. For the adult version, item examples include: “How much did pain interfere with your household chores,” and “How often did pain prevent you from standing for more than 30 minutes;” for youth items include “I had trouble doing schoolwork when I had pain” and “It was hard for me to walk one block when I had pain.”
Anxiety
The PROMIS Anxiety item bank for both adults and youth assesses fears, worry or dread, and hyperarousal [7,23]. The adult measure additionally assesses somatic symptoms related to autonomic arousal (e.g., “I had a racing or pounding heart” and “I felt fidgety”) [7]. Examples of youth items include: “I was afraid of going to school” and “I worried about what could happen to me.”
Depression
The PROMIS Depression item bank for adults and pediatric patients assesses negative mood, negative self-perceptions, decreased positive affect and negative social cognition [7,23]. Both the adult and pediatric item banks exclude items assessing somatic symptoms of depression due to poor fit of the IRT model and possible overlap between somatic symptoms of depression and markers of disease/illness [7,8]. This measure is best characterized as an assessment of depressive symptoms rather than a diagnostic tool [7,23]. Examples from the adult item bank include: “I felt that nothing could cheer me up” and “I withdrew from other people.” From the pediatric item bank examples items include: “Being sad made it hard for me to do things with my friends” and “I could not stop feeling sad.”
Emotional Distress
Due to the high correlation between anxiety and depression in the current sample (r = .78) and the known relation between these variables with pain catastrophizing [5,32,42], a composite “emotional distress” variable (i.e., mean score of anxiety and depression) was created and used as a covariate in order to determine the unique contribution of pain catastrophizing on outcomes, above and beyond symptoms of anxiety and depression.
Analytic Plan
All analyses were conducted using Mplus version 6.12 [38]. The two dependent variables utilized for analyses were pain interference and pain intensity. Pain intensity additionally served as an independent variable (i.e., covariate) in models examining pain interference as an outcome. Age, as a categorical variable, served as a moderator and emotional distress was included as a covariate in all models. Our primary clinical predictor (pain catastrophizing; PC) was modeled in two different sets of multiple regression analyses. The first model examined PC concurrently with covariates (pain intensity and emotional distress) predicting pain interference, along with a categorical age variable (representing children, adolescents, adults 18-23, and adults 24-29). Age was tested as a moderator of the effects of PC, pain intensity, and emotional distress on pain interference. Although the interaction of age with PC was of primary substantive interest, we opted to include interactions between age and both pain intensity and emotional distress as a secondary analysis, based on the assumption that the age-varying salience of PC may have implications for both pain intensity and emotional distress as predictors of pain interference. Moderating effects were tested by constructing an interaction term representing the interaction of age with each moderator, and including this interaction term as a separate predictor in an equation with all lower-order effects present. Regression equations representing these multiple regression model testing the interaction of age and PC, as well as models testing the interaction of age with key covariates in predicting pain interference, are listed below:
If an interaction was found to be significant, differences in the size of this effect between each age group were tested using a Wald chi-square test. All coefficients in the current model are standardized, to represent the relative size of each effect in the model. A second model explored PC as a predictor of pain intensity, with emotional distress and age serving as covariate and moderator, respectively, using the same analytic formulation described above. All continuous variables were centered on the grand mean, as were interaction terms in the moderation models.
Results
Descriptive Statistics
A one-way analysis of variance (ANOVA) was run to determine mean differences in study variables as a function of age. Descriptive values, including means, standard deviations, and results of ANOVA can be found in Table 3. PC and pain intensity scores did not significantly differ between either of the youth-report groups (i.e., children or adolescents) and the adult groups. Post-hoc Tukey's HSD was examined for distress given omnibus differences (F (3, 1014) = 21.86, p < 0.001) which indicated that mean scores were significantly higher for emotional distress in the adult age groups compared to both the children and adolescents; whereas no significant differences in emotional distress were found between the two adult groups nor between the child and adolescent group. There were also significant differences between the groups on pain interference (F (3, 1024) = 67.34, p < .001; however because Levene's test was violated, the Games-Howell post-hoc test was used to explore differences. Differences in pain interference were found between both child and adolescent groups compared to both adult groups, with higher scores found for the eldest age groups. Significant post-hoc differences were also demonstrated between adults 18-23 and adults 24-29 with older adults endorsing higher scores; whereas scores only approached significance between children and adolescents. Correlational analyses depicted in Table 4 reflected significant associations between all study variables across the full sample and within age groups, except for the relation between PC and pain intensity in children, which was non-significant.
Table 3. Descriptive Statistics and Comparison of Group Means.
| Children (N = 73) | Adolescents (N = 252) | Adults 18-23 (N = 294) | Adults 24-29 (N = 409) | ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Measures | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | P |
|
|
||||||||||
| PC | 22.45 | 12.92 | 23.13 | 12.30 | 22.93 | 12.72 | 23.80 | 13.01 | 0.28 | 0.84 |
| Pain Intensity | 5.66 | 2.40 | 5.51 | 2.04 | 5.33 | 2.11 | 5.66 | 2.23 | 1.34 | 0.26 |
| Pain Interference | 56.99 | 9.51 | 59.97 | 7.94 | 65.15 | 6.85 | 66.55 | 6.64 | * 67.34 | 0.00 |
| Distress | 51.49 | 10.63 | 53.60 | 9.82 | 57.70 | 9.40 | 58.46 | 9.07 | **21.86 | 0.00 |
= Significant difference between both adult age groups compared to both the child and adolescent groups, as well as between adults 18-23 and adults 24-29.
= Significant differences between both adult age groups compared to both the child and adolescent groups
Table 4. Correlations between Study Variables from the Total Sample and within Discrete Age Groups.
| Variables | 1 | 2 | 3 | |
|---|---|---|---|---|
| Total Sample | 1. PC | - | - | - |
| 2. Pain Intensity | .438** | - | - | |
| 3. Pain Interference | .442** | .585** | - | |
| 4. Distress | .611** | .298** | .466** | |
|
| ||||
| Children | 1. PC | - | - | - |
| 2. Pain Intensity | .184 | - | - | |
| 3. Pain Interference | .476** | .448** | - | |
| 4. Distress | .720** | .248* | .558** | |
|
| ||||
| Adolescents | 1. PC | - | - | - |
| 2. Pain Intensity | .296** | - | - | |
| 3. Pain Interference | .483** | .421** | - | |
| 4. Distress | .645** | .216** | .460** | |
|
| ||||
| Adults 18-23 | 1. PC | - | - | - |
| 2. Pain Intensity | .237** | - | - | |
| 3. Pain Interference | .473** | .565** | - | |
| 4. Distress | .642** | .224** | .475** | |
|
| ||||
| Adults 24-29 | 1. PC | - | - | - |
| 2. Pain Intensity | .438** | - | - | |
| 3. Pain Interference | .442** | .585** | - | |
| 4. Distress | .611** | .298** | .466** | |
= p < 0.01
= p < .05
Pain Interference
When the full model was estimated for the entire sample, PC (β = .137, p < .001), pain intensity (β = .355, p < .001), and emotional distress (β = .276, p < .001) were all found to be significantly and positively related to pain interference. Of note, pain intensity showed the strongest relative effect on pain interference, followed by emotional distress, and PC scores showed the smallest relative effect. There was also a main effect of age, such that pain interference scores were significantly higher in older patients than younger patients, above and beyond the effects of PC, pain intensity, and emotional distress (β = .313, p < .001).
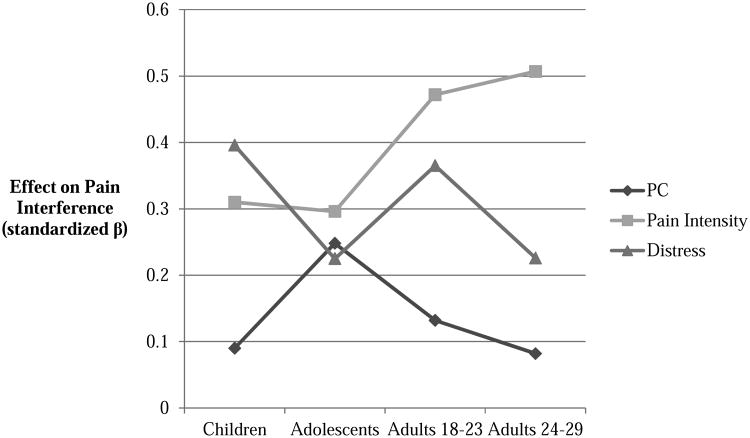
Age was tested as a moderator of the effects of each predictor (PC, pain intensity, and emotional distress) on pain interference. Results from the moderation analysis suggested that age was a significant moderator of the effects of both PC (interaction β = -.180, p = .016) and emotional distress (interaction β = -.400, p = .017), suggesting that the effects of PC and emotional distress on pain interference declined as a function of age. Age did not moderate the effects of pain intensity on pain-related interference (interaction β = .039, p = .65). When the estimates of each relative effect were plotted as a function of patient age (see Figure 1), visual inspection of the results suggested that the effect of PC appeared to be non-linear across age groups. More specifically, we noted a significant increase in the relative size of this effect in teens, whereas PC scores were the weakest predictors in other age groups. Consequently, a term was constructed representing the quadratic effect of PC scores in interaction with age; however, this term was not statistically significant (quadratic interaction β = -.191, p = .41). When Wald chi square tests were used to determine which age groups significantly varied from one another on the effects of PC on pain interference, a significant difference was noted between the teenage group and adults, ages 24-29 (Wald statistic = 4.410, p = .036). This effect suggested that PC was a significantly stronger predictor of pain interference scores in teenagers than in the adults, age 24-29. No other comparisons suggested significant age-related differences in the effects of pain catastrophizing or emotional distress on pain interference scores.
Figure 1. Predictive value of Pain Catastrophizing, Pain Intensity and Distress on Pain Interference as a Function of Age.
Pain Intensity
When PC, emotional distress, and age were modeled as concurrent predictors of pain intensity, only PC was found to significantly predict pain intensity (β = .284, p < .001). When age was tested as a moderator of the effect of PC on pain intensity, this interaction was found to be significant (interaction β = .234, p = .017). When Wald chi-square tests were used to examine pairwise differences between different age groups in this effect, there was a significantly stronger effect of PC on pain intensity for adults 24-29 than for children (Wald statistic = 5.442, p = .020). As emotional distress did not significantly predict pain intensity, no interaction between distress and age was tested.
Conclusions
Relatively few studies have examined children and adolescents with chronic pain separately and comparatively on the relation between PC and functional outcomes [10,57]; furthermore, no studies have examined these relations in a developmental context in patients ranging from childhood to early adulthood. The current study investigated the moderating effect of age on the relation between PC and pain interference in a cross-sectional analysis, while accounting for emotional distress (i.e., depression and anxiety) and pain intensity. Our results suggested that age did, in fact, moderate the relation between PC and pain interference, with the strength of these effects declining with age. Specifically, the decline was seen from adolescents through adults 18-23 and adults 24-29; yet the strength of association for children was comparable to that of the adults. The effect of PC on pain interference was strongest in the adolescent group, yet the relation between PC and pain interference for children was weak.
Previous research examined the unique contributions of anxiety and catastrophizing on functional disability (i.e., Child Activities Limitations Questionnaire; CALQ) [22] and health-related quality of life (i.e., PedsQL) [59], constructs conceptually similar to pain interference [26], in children and adolescents indicating contradictory findings to the current study [57]. In that study, PC was found to be a stronger predictor of disability in children than in adolescents, though pain catastrophizing was noted to be strongly associated with functional disability in both groups [57]. In that same study, anxiety was a stronger predictor of disability and quality of life in adolescents compared to children [57], whereas the current study revealed that emotional distress was a stronger predictor of pain interference in children compared to adolescents. Furthermore, our findings suggest that emotional distress was a better predictor of pain interference in children than specific catastrophic thinking about pain. Different analytic approaches utilized between the two studies (2015) may account for the discrepancies [57]. Additionally, although studies have begun to report on the construct validity between pediatric PROMIS measures and legacy measures (e.g., FDI and PedsQL) [26], replication comparing pain interference with other commonly used measures in the pediatric pain literature (e.g., CALQ) appears warranted given these incompatible findings [22,59,64]. Given the results found for the child subset in our study it is possible that, PC, as measured by the version of the PCS-C used in our study, may not be a good prognosticator for pain interference in children.
Our results may align with the conceptualization put forth by Eccelston and colleagues (2012), such that catastrophic thinking in children may be normative or even re-conceptualized as worry [13] and that such reappraisal may focus intervention efforts on better understanding children's fears in the context of worry or anxiety, rather than catastrophic thinking per se. Prior research with a school-based sample (with at least one pain complaint) suggests that adolescents engage in more emotion-focused avoidance (including catastrophizing) than children [47] which is speculated to be related to physiological and behavioral changes that occur during adolescence. Yet other research documents a decline in PC as a function of age, from childhood to adolescence [10]. Results of this study are partially consistent with our hypotheses suggesting that within different age groups, normative cognitive/emotional resources to cope with pain may be present, reducing the impact of catastrophic thinking on activity interference, yet does not explain the results for the child subset of the sample. Our findings suggest that for adolescents, emotional distress, PC, and pain intensity may all be important constructs predictive of pain interference and furthermore appear to share variance in accounting for the cognitive and affective determinants of pain interference.
Consistent with the broad literature documenting PC as a predictor of a host of poor outcomes, PC was found to predict pain interference in adults. However, our models revealed that pain intensity held greater predictive value than PC. Of note, adults overall reported significantly higher levels of pain interference (as well as emotional distress) than either of the youth-report groups indicating that adults in the study tended to have poorer emotional functioning and greater interference with daily activities due to pain, despite comparable PC and pain intensity scores. Children and adolescents receiving treatment for chronic pain not only have medical providers encouraging function, but additionally have caregivers who are responsible for promoting engagement in daily activities. Thus adults may not be held accountable in the same way as children and adolescents, or receive similar support from providers or caregivers. Alternatively, this difference may reflect measurement differences in the PROMIS pain interference construct between pediatric- and adults-report instruments.
With regard to the findings for pain intensity, our study replicated prior work indicating that PC is an important predictor of pain intensity across our sample [19,50,58,66]. Although age was found to moderate the relation between PC and pain intensity, statistical findings were weak and only suggested a difference between adults 24-29 and children, with the former reflecting the strongest relation between PC and pain intensity. Previous work has demonstrated PC as a predictor of pain intensity in adults and trends reflect that PC manifests similarly across age groups [28]. Future research exploring this same relation by adult age group (e.g., younger adult, middle adult, older adult) may be important to further assess developmental differences among adults and determine if young adult samples are comparable to or representative of adult samples of other ages with chronic pain.
Limitations
Given this study's primary interest in developmental differences, a major limitation is the use of cross-sectional data. Using separate cohorts clustered by age allows for examination of group differences of important constructs in chronic pain, but precludes causal inferences or the ability to draw conclusions over the lifespan. The version of the PCS-C used in our study combined elements of the adult PCS and child version rendering comparisons between youth and adults more viable; however, we acknowledge that our version is not identical to the child adapted PCS-C validated in the literature [10], thereby limiting generalizability of the findings. An additional limitation concerns the unequal sample sizes between age cohorts, as there were comparatively fewer children included in our analysis than adolescents or adults. We note similar concerns about the differing time frames for assessment of pain intensity in the youth (previous 30 days) and in the adult groups (previous 7 days). It is conceivable that these methodological differences may have contributed to the differences noted between these groups, though prior research suggests high degree of correspondence between pain intensity ratings of 7-day and 4-week periods [3]. Lastly, samples in this study were drawn from tertiary pain clinics and thus, caution should be taken when generalizing results to the broader population.
Future Directions
Despite its limitations, this study sheds light on the way PC is conceptualized across developmental stages [13] to guide treatment interventions based on the most salient factors that predict pain interference. Future investigations may include longitudinal tracking of patients over time to determine if relations to outcomes change as a function of age. Additionally, data collection using comparable measures in pediatric and adult clinics offers an effective platform for longitudinal research to better understand predictors of persistent pain into adulthood and the ways in which cognitive factors such as PC impact functional outcomes. In fact, recent research has documented a reasonable correspondence between adult and pediatric PROMIS domains of emotional functioning, yet more research is needed to further elucidate such overlaps and determine compatibility among other measures and domains [46]. Literature has also explored the importance of parent-reported PC compared to youth self-report [68]. Given that differences between children and adolescents were not as pronounced as expected, next steps may include an investigation of the same relations explored above, utilizing parent-report of PC as a predictor. For younger children in particular, parents' catastrophic thoughts about pain may play a more salient role in predicting children's function (i.e. pain interference). Sex differences in the pain experiences of adults and youth have also been found in the literature; therefore future investigation exploring the interaction between sex and age in our sample may be useful [29,33,44].
Eccleston and colleagues (2012) have suggested that aspects of catastrophic thinking in children may be normative developmental responses to pain (e.g., worry) and have cautioned against over-pathologizing this response style in children [13]. In that regard, worries about pain might not be implicated as such potent predictors of poor outcomes in children, as the current study suggests [13]. Consistent with Eccleston's work (2012), future item analysis to determine which PCS items were endorsed most frequently may help to elucidate which facets of catastrophizing are most salient within youth and young adult age groups [13], and further identify responses consistent with catastrophic thinking compared to expected worry or normative pain-related fears.
Given that PC has become an important target of treatment [11,56], greater understanding of age-related differences in PC will be informative for successfully intervening with patients across developmental levels, and may lend support for differential application of cognitive behavioral interventions. However, the differential salience of pain and psychological predictors across age groups suggests that, from a clinical standpoint, interventions that target factors such as catastrophic thinking about pain or emotional distress may be more relevant for certain developmental stages compared to others. In alignment with other research, adolescents with chronic pain may need interventions that target emotional distress and PC; whereas children mayneed treatments more specific to managing symptoms of depression and anxiety when presenting for pain management. Future research is needed, not only to further elucidate differences between children and adolescents with chronic pain, but also to better understand how young adults differ from their middle-age and older adult counterparts, and how psychosocial interventions may vary within age groups.
Highlights.
Developmental differences in catastrophizing were explored in chronic pain patients
Age moderated the relation between pain catastrophizing and pain interference
The effect of catastrophizing on pain interference was strongest in adolescents
Psychosocial interventions should be optimized based on age and developmental level
Perspective.
This article explores differences in pain catastrophizing as predictors of pain interference and pain intensity across cohorts of children, adolescents, and two age groups of young adults. This work may stimulate further research on chronic pain from a developmental perceptive and inform developmentally tailored treatment interventions that target catastrophizing, emotional distress and pain intensity.
Acknowledgments
Funding: The authors wish to acknowledge funding from the National Institutes of Health (NIH HHSN 271201200728P K24 DA029262; NIH/NIDA 3T32DA035165-02S1), as well as the Redlich Pain Endowment and The William and Gretchen Kimball Endowment for Pediatric Pain Management.
Other acknowledgement: We wish to thank Kim Nguyen, B.S. for her assistance with preparation of aspects of the manuscript.
Footnotes
Disclosures: The authors have no financial conflicts to disclose. Amanda Feinstein is a trainee member of Pain in Child Health, a Strategic Training Initiative in Health Research of the Canadian Institutes of Health Research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhandari RP, Feinstein AB, Huestis SE, Krane EJ, Dunn AL, Cohen LL, Kao MC, Darnall BD, Mackey SC. Pediatric-Collaborative Health Outcomes Information Registry (Peds-CHOIR): A Learning Health System to Guide Pediatric Pain Research and Treatment. Pain. 2016;157:2033–44. doi: 10.1097/j.pain.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139:146–157. doi: 10.1016/j.pain.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JM, O'Keeffe J, Sanders SH, Baker B. Developmental changes in children's cognition to stressful and painful situations. Journal of pediatric psychology. 1986;11:343–357. doi: 10.1093/jpepsy/11.3.343. [DOI] [PubMed] [Google Scholar]
- 5.Buenaver LF, Edwards RR, Smith MT, Gramling SE, Haythornthwaite JA. Catastrophizing and pain-coping in young adults: associations with depressive symptoms and headache pain. The journal of pain : official journal of the American Pain Society. 2008;9:311–319. doi: 10.1016/j.jpain.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2007;16(1):133–141. doi: 10.1007/s11136-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 7.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of clinical epidemiology. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Medical care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compas BE, Jaser SS, Dunn MJ, Rodriguez EM. Coping with chronic illness in childhood and adolescence. Annual review of clinical psychology. 2012;8:455–480. doi: 10.1146/annurev-clinpsy-032511-143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 11.Darnall BD, Sturgeon JA, Kao MC, Hah JM, Mackey SC. From Catastrophizing to Recovery: a pilot study of a single-session treatment for pain catastrophizing. Journal of pain research. 2014;7:219–226. doi: 10.2147/JPR.S62329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Immpact Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Eccleston C, Fisher EA, Vervoort T, Crombez G. Worry and catastrophizing about pain in youth: a reappraisal. Pain. 2012;153:1560–1562. doi: 10.1016/j.pain.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Forgeron PA, King S, Stinson JN, McGrath PJ, MacDonald AJ, Chambers CT. Social functioning and peer relationships in children and adolescents with chronic pain: A systematic review. Pain research & management : the journal of the Canadian Pain Society. 2010;15:27–41. doi: 10.1155/2010/820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fries JF, Bruce B, Cella D. The promise of PROMIS: using item response theory to improve assessment of patient-reported outcomes. Clinical and experimental rheumatology. 2005;200523(5 Suppl 39):S53–57. [PubMed] [Google Scholar]
- 16.Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W. The development of a clinical outcomes survey research application: Assessment Center. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2010;19:677–685. doi: 10.1007/s11136-010-9634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 18.Gil KM, Wilson JJ, Edens JL. The stability of pain coping strategies in young children adolescents, and adults with sickle cell disease over an 18-month period. The Clinical journal of pain. 1997;13:110–115. doi: 10.1097/00002508-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. The Clinical journal of pain. 2005;21:439–445. doi: 10.1097/01.ajp.0000135236.12705.2d. [DOI] [PubMed] [Google Scholar]
- 20.Groenewald CB, Essner BS, Wright D, Fesinmeyer MD, Palermo TM. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. The journal of pain : official journal of the American Pain Society. 2014;15:925–933. doi: 10.1016/j.jpain.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guite JW, McCue RL, Sherker JL, Sherry DD, Rose JB. Relationships among pain, protective parental responses, and disability for adolescents with chronic musculoskeletal pain: the mediating role of pain catastrophizing. The Clinical journal of pain. 2011;27:775–781. doi: 10.1097/AJP.0b013e31821d8fb4. [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth KR, Davies WH, Khan KA, Weisman SJ. Development and preliminary validation of the child activity limitations questionnaire: flexible and efficient assessment of pain-related functional disability. The journal of pain : official journal of the American Pain Society. 2007;8:746–752. doi: 10.1016/j.jpain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2010;19:595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55:195–203. doi: 10.1016/0304-3959(93)90148-I. [DOI] [PubMed] [Google Scholar]
- 25.Kao MC, Cook K, Olson G, Pacht T, Darnall BD, Weber SC, Mackey SC. American Medical Informatics Associations (AMIA) 2014 Joint Summits on Translational Science. San Francisco, CA: 2014. SNAPL-CAT: Catalyzing the Rate-Limiting Step of Big Data Psychometrics with Item-Response Theory and Advanced Computerized Adaptive Testing (Poster Presentation) [Google Scholar]
- 26.Kashikar-Zuck S, Carle A, Barnett K, Goldschneider KR, Sherry DD, Mara CA, Cunningham N, Farrell J, Tress J, DeWitt EM. Longitudinal evaluation of patient-reported outcomes measurement information systems measures in pediatric chronic pain. Pain. 2016;157:339–347. doi: 10.1097/j.pain.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37:51–56. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 28.Keefe FJ, Williams DA. A comparison of coping strategies in chronic pain patients in different age groups. Journal of gerontology. 1990;45:P161–165. doi: 10.1093/geronj/45.4.p161. [DOI] [PubMed] [Google Scholar]
- 29.Keogh E, Eccleston C. Sex differences in adolescent chronic pain and pain-related coping. Pain. 2006;123:275–284. doi: 10.1016/j.pain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 30.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Logan DE, Simons LE, Stein MJ, Chastain L. School impairment in adolescents with chronic pain. The journal of pain : official journal of the American Pain Society. 2008;9:407–416. doi: 10.1016/j.jpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, Goldschneider KR. The interplay of parent and adolescent catastrophizing and its impact on adolescents' pain, functioning, and pain behavior. The Clinical journal of pain. 2013;29:681–688. doi: 10.1097/AJP.0b013e3182757720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Sex and age differences in coping styles among children with chronic pain. Journal of pain and symptom management. 2007;33:208–216. doi: 10.1016/j.jpainsymman.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Mallen C, Peat G, Thomas E, Croft P. Severely disabling chronic pain in young adults: prevalence from a population-based postal survey in North Staffordshire. BMC musculoskeletal disorders. 2005;6:42. doi: 10.1186/1471-2474-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L, PedImmpact Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. The journal of pain : official journal of the American Pain Society. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Miro J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. European journal of pain. 2009;13:1089–1095. doi: 10.1016/j.ejpain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Molton I, Jensen MP, Ehde DM, Carter GT, Kraft G, Cardemas DD. Coping with chronic pain among younger, middle-aged, and older adults living with neurological injury and disease. Journal of aging and health. 2008;20:972–996. doi: 10.1177/0898264308324680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthén LK, Muthén BO. Mplus User's Guide. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- 39.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. Journal of behavioral medicine. 1997;20:589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 40.Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J, van der Wouden JC. Pain in children and adolescents: a common experience. Pain. 2000;87:51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 41.Piaget J. Intellectual Evolution from Adolescence to Adulthood. Human Development. 1971;15:1–12. [Google Scholar]
- 42.Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. Pain. 2014;155:2360–2367. doi: 10.1016/j.pain.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of neurology. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- 44.Ramirez-Maestre C, Esteve R. The role of sex/gender in the experience of pain: resilience, fear, and acceptance as central variables in the adjustment of men and women with chronic pain. The journal of pain : official journal of the American Pain Society. 2014;15:608–618 e601. doi: 10.1016/j.jpain.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D, Group PC. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Medical care. 2007;45(5 Suppl 1):S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 46.Reeve BB, Thissen D, DeWalt DA, Huang IC, Liu Y, Magnus B, Quinn H, Gross HE, Kisala PA, Ni P, Haley S, Mulcahey MJ, Charlifue S, R AH, Slavin M, Jette A, Tulsky DS. Linkage between the PROMIS((R)) pediatric and adult emotional distress measures. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2016;25:823–833. doi: 10.1007/s11136-015-1143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reid GJ, Gilbert CA, McGrath PJ. The Pain Coping Questionnaire: preliminary validation. Pain. 1998;76:83–96. doi: 10.1016/s0304-3959(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 48.Revicki DA, Cella DF. Health status assessment for the twenty-first century: item response theory, item banking and computer adaptive testing. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1997;6:595–600. doi: 10.1023/a:1018420418455. [DOI] [PubMed] [Google Scholar]
- 49.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 50.Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. The Clinical journal of pain. 2001;17:165–172. doi: 10.1097/00002508-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 52.Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125:143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Sturgeon JA, Zautra AJ. State and trait pain catastrophizing and emotional health in rheumatoid arthritis. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2013;45:69–77. doi: 10.1007/s12160-012-9408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sullivan MJ. The Pain Catastrophizing Scale: User Manual. In: Montreal, editor. DoP McGill University, Medicine, and Neurology School of Physical and Occupational Therapy. McGill University; [Google Scholar]
- 55.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 56.Thorn BE, Pence LB, Ward LC, Kilgo G, Clements KL, Cross TH, Davis AM, Tsui PW. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. The journal of pain : official journal of the American Pain Society. 2007;8:938–949. doi: 10.1016/j.jpain.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Tran ST, Jastrowski Mano KE, Hainsworth KR, Medrano GR, Anderson Khan K, Weisman SJ, Davies WH. Distinct Influences of Anxiety and Pain Catastrophizing on Functional Outcomes in Children and Adolescents With Chronic Pain. Journal of pediatric psychology. 2015;40:744–755. doi: 10.1093/jpepsy/jsv029. [DOI] [PubMed] [Google Scholar]
- 58.Turner JA, Jensen MP, Warms CA, Cardenas DD. Catastrophizing is associated with pain intensity, psychological distress, and pain-related disability among individuals with chronic pain after spinal cord injury. Pain. 2002;98:127–134. doi: 10.1016/s0304-3959(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 59.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Medical care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, Yeatts K, Dewalt DA. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. The journal of pain : official journal of the American Pain Society. 2010;11:1109–1119. doi: 10.1016/j.jpain.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vervoort T, Goubert L, Eccleston C, Bijttebier P, Crombez G. Catastrophic thinking about pain is independently associated with pain severity, disability, and somatic complaints in school children and children with chronic pain. Journal of pediatric psychology. 2006;31:674–683. doi: 10.1093/jpepsy/jsj059. [DOI] [PubMed] [Google Scholar]
- 62.von Baeyer CL. Children's self-reports of pain intensity: scale selection, limitations and interpretation. Pain research & management : the journal of the Canadian Pain Society. 2006;11:157–162. doi: 10.1155/2006/197616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wainer H, Dorans NJ, Flaugher R, Green BF, Mislevy RJ, Steinberg L, Thissen D. Computerized adaptive testing: a primer. Hillsdale, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 64.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of pediatric psychology. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 65.Ware JE, Jr, Kosinski M, Bjorner JB, Bayliss MS, Batenhorst A, Dahlof CG, Tepper S, Dowson A. Applications of computerized adaptive testing (CAT) to the assessment of headache impact. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2003;12:935–952. doi: 10.1023/a:1026115230284. [DOI] [PubMed] [Google Scholar]
- 66.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Experimental brain research. 2008;186:79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 67.Williams K, Thomson D, Seto I, Contopoulos-Ioannidis DG, Ioannidis JP, Curtis S, Constantin E, Batmanabane G, Hartling L, Klassen T, Sta RCHG. Standard 6: age groups for pediatric trials. Pediatrics. 2012;129(3):S153–160. doi: 10.1542/peds.2012-0055I. [DOI] [PubMed] [Google Scholar]
- 68.Wilson AC, Moss A, Palermo TM, Fales JL. Parent pain and catastrophizing are associated with pain, somatic symptoms, and pain-related disability among early adolescents. Journal of pediatric psychology. 2014;39:418–426. doi: 10.1093/jpepsy/jst094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wollaars MM, Post MW, van Asbeck FW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. The Clinical journal of pain. 2007;23:383–391. doi: 10.1097/AJP.0b013e31804463e5. [DOI] [PubMed] [Google Scholar]
- 70.Zimmer-Gembeck MJ, Skinner EA. Review: The development of coping across childhood and adolescence: An integrative review and critique of research. International Journal of Behavioral Development. 2011;35:1–17. [Google Scholar]