Abstract
Objective
Potential clinical utility of galanin or peptidic analogs has been hindered by poor metabolic stability, lack of brain penetration, and hyperglycemia due to GalR1 receptor activation. NAX 810-2, a GalR2-preferring galanin analog, possesses 15-fold greater affinity for GalR2 over GalR1 and protects against seizures in the mouse 6 Hz, corneal kindling, and Frings audiogenic seizure models. The purpose of these studies was to further evaluate the pre-clinical efficacy and pharmacokinetics of NAX 810-2 in mice.
Methods
NAX 810-2 was administered by intravenous (IV; tail vein, bolus) injection to fully kindled (corneal kindling assay) or naïve CF-1 mice (6 Hz assay and pharmacokinetic studies). Plasma NAX 810-2 levels were determined from trunk blood samples. NAX 810-2 was also added to human plasma at various concentrations for determination of plasma protein binding.
Results
In the mouse corneal kindling model, NAX 810-2 dose-dependently blocked seizures following IV administration (ED50 0.5 mg/kg). In the mouse 6 Hz (32mA) seizure model demonstrated that NAX 810-2 dose-dependently blocked seizures following IV bolus administration (0.375 – 1.5 mg/kg, IV; ED50 0.7 mg/kg), with a time-to-peak effect of 0.5 h post-treatment. Motor impairment was observed at 1.5 mg/kg IV whereas one-half of this dose, 0.75 mg/kg IV, was maximally effective in the 6 Hz test. Plasma levels of NAX 810-2 show linear pharmacokinetics following IV administration and a half-life of 1.2 h. Functional agonist activity studies demonstrate that NAX 810-2 effectively activates GalR2 receptors at therapeutic concentrations.
Significance
These studies further suggest the potential utility of NAX 810-2 as a novel therapy for epilepsy.
Keywords: galanin, epilepsy, seizure, blood-brain barrier, neuropeptide, pharmacokinetic, antiseizure drug, drug discovery
Introducton
Galanin is one of several neuropeptides with demonstrated anticonvulsant activity1; 2. Both galanin receptor subtype 1 (GalR1) and type 2 (GalR2) receptor subtypes have been shown to play a role in seizures3; 4. However, the development of galanin as an anticonvulsant has been hindered by poor metabolic stability, a lack of effective penetration across the blood-brain barrier, and GalR1-mediated insulin inhibition in several species5. Galanin and its receptors are localized in several regions of the CNS, including the hippocampus, where a high density of galanin-immunoreactive fibers are found in the dentate granule cell layer6. Galanin has also been shown to inhibit glutamate release via presynaptic action7; 8 and galanin knockout mice produce a larger release of glutamate in response to depolarization9. Anticonvulsant effects of galanin have been demonstrated in several animal models. Central administration of galanin decreases the severity of evoked seizures in a rat kindling model10. Furthermore, both GalR1 and GalR2 agonists have been used in a performant path stimulation-induced model of status epilepticus (SE) to demonstrate that GalR1 and GalR2 play differential roles in initiation and maintenance of SE, respectively11. Similarly, infusion of with GalR2 complementary peptide nucleic antisense oligonucleotides increased the time spent in seizures in a hippocampal stimulation seizure model3.
We previously described high-affinity and systemically-active galanin analogs, with preference for either GalR1 or GalR2 receptors, that were potently active in the 6 Hz model of psychomotor seizures12. We further characterized a lead GalR1-preferring analog, NAX 505-5, in the 6 Hz (including 22, 32 and 44 mA stimulus intensities), corneal kindling, Frings audiogenic seizure, maximal electroshock, and pentylenetetrazol seizure models13. We also demonstrated that galanin analogs could be engineered to discriminate between GalR1 and GalR2 subtypes and retain anticonvulsant efficacy14. The lead GalR2-preferring analog, NAX 810-2, was designed and previously shown to prevent seizures in a variety of animal models15; 16. Initially, NAX 810-2 was evaluated for antiseizure efficacy following intraperitoneal (IP) administration and demonstrated efficacy in the 6 Hz model (32, 44 mA), audiogenic seizures, and corneal kindling15. In addition, this compound was further evaluated in the 6 Hz and corneal kindling models following IV administration16. These studies extend previous antiseizure efficacy observations by 1) using an optimized formulation of NAX 810-2 for evaluation in mouse seizure models (e.g., corneal kindling and 6 Hz seizure models) following IV administration and 2) comparing IV NAX 810-2 efficacy in the 6 Hz model with corresponding plasma levels in a pharmacokinetic-pharmacodynamic interaction study. Additional analysis was conducted to determine several pharmacokinetic parameters and evaluate whether the compound demonstrates linear pharmacokinetics.
Methods
Animals
Male albino CF-1 mice (18-38g; Charles River, Kingston, NY) were used as experimental animals. Mice evaluated in the 6 Hz model were 22-32 g whereas mice evaluated in the corneal kindling model were generally 30-38 g at the time of testing. All animals were allowed free access to food and water, except during testing procedures. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Utah and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
NAX 810-2 Preparation and Formulation
NAX 810-2 (mol. wt. 2124 g/mol) was synthesized by PolyPeptide Laboratories (San Diego, CA), and delivered as a lyophilized material (acetate salt, purity > 95%). The compound was supplied as a diastereoisomeric mixture (40/60) arising from a racemic mixture of N-methyl-trypophan, the N-terminal amino acid of the peptide (structure described previously15). The compound was dissolved in a vehicle (VEH) solvent consisting of 2% hydroxyl-propyl-beta cyclodextrin (HPβCD, Sigma, St. Louis, MO) in acetate buffer (0.1 M acetic acid, 0.1M sodium acetate; pH 4.5; 0.1M acetic acid prepared from glacial acetic acid stock, Sigma, St. Louis MO; sodium acetate, Sigma, St. Louis, MO).
Mouse 6 Hz Psychomotor Seizure Model
Seizures were induced via corneal stimulation (6 Hz, 0.2 msec rectangular pulse, 3 sec duration; 32 mA stimulus intensity) using a Grass S48 stimulator17. Prior to stimulation, 1-2 drops of tetracaine were applied to each eye. The limbic seizures that arise from corneal stimulation in this assay are characterized by automatistic behaviors including stun, forelimb clonus, twitching of the vibrissae, and Straub-tail. Animals that did not demonstrate these behaviors following corneal stimulation (up to 1-min observation post-stimulation) were considered “protected”.
Mouse Corneal Kindling Model
The corneal kindled mouse displays phenotypic secondarily generalized seizures (Racine scale18) and a pharmacologic profile consistent with other models of kindling (e.g., amygdala and hippocampal kindled rats). Mice received twice daily sub-threshold corneal stimulations (60 Hz, 3 mA, 3 sec; 5 days/week, ∼3 weeks19) until reaching the criterion of 5 consecutive secondarily generalized seizures (Racine scale 5), at which point they were considered “fully kindled.” One day prior to treatment, the fully kindled state was verified (presence of a stage 5 seizure) by a single stimulation. NAX 810-2 was administered by IV bolus (0.1 ml per 10 g) into a lateral tail vein of each kindled mouse. Animals were considered “protected” if they displayed a Racine seizure score of 0-3.
Rotarod Test for Motor Impairment
Testing in the rotarod assay was conducted immediately prior to corneal stimulation in 6 Hz and corneal kindling studies. When mice are placed on a 1-inch knurled rod rotating at a speed of 6 rpm, the animals can maintain equilibrium for long periods of time. Motor impairment was assessed by determining whether mice remained on the rotarod during a 1-min observation period; i.e., three falls during a 1-min period was considered a rotarod failure.
Sample Collection for Determination of Plasma NAX 810-2 Levels
Following testing in the mouse 6 Hz model, trunk blood was collected (time points: 0.08, 0.25, 0.5, 0.75, 1, 2, 3, 4, and 6 h) into K2EDTA-coated microcentrifuge tubes (500 ml capacity; Beckton Dickinson Company, Franklin Lakes, New Jersey, USA). Following centrifugation (10,000 × g) at 4°C for 10 min, plasma samples were transferred to uncoated microcentrifuge tube and stored (-80 °C) until processing for NAX 810-2 concentrations.
Plasma Protein Binding
NAX 810-2 was added to human plasma at various concentrations (0.5, 5, and 50 μM) and in a centrifuge tube and subjected to centrifugation for 16-24 hours at 200,000 × gmax. The concentration of NAX 810-2 in the supernatant was determined by a liquid chromatography/tandem mass spectrometry (LC/MS/MS) method using LC-20A Shimadzu HPLC and API4000 AB SCIEX mass spectrometer. For HPLC separations, a reverse-phase C18 column (3 mm, 2.1 × 50 mm, Shiseido) and a linear gradient acetonitrile in 0.2% formic acid were used. A positive control, warfarin (10 μM) was included as a positive control.
GalR Functional Activity
NAX 810-2 was evaluated using EMD Millipore's GPCRProfiler® Assay. NAX 810-2 was dissolved in dimethyl sulfoxide and prepared in various assay buffer concentrations (up to 3x× higher than highest assay concentration: 2.0/0.5/0.1 μM) using a modified Hanks balanced salt solution (HBSS) with an addition of 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 2.5 mM probenecid, pH 7.4. Reference controls for GalR1 and GalR2 were galanin (amino acids 1-30) (GalR1, Emax 0.0375 μM; GalR2, Emax 0.125 μM). Each concentration was evaluated in duplicate. The agonist assay was conducted on a FLIPRTETRA instrument wherein NAX 810-2, vehicle controls, and galanin (amino acids 1-30) were added to the assay plate after a baseline fluorescence/luminescence was determined. Maximum fluorescence/luminescence values were corrected for baseline values and a percentage of activation was determined: [(maximum activity, relative luminescence units) – (baseline average activity)] / [positive average – baseline average].
LC-MS Method for Determination of Plasma NAX 810-2 Levels
Plasma levels of NAX 810-2 were determined using liquid-liquid extraction followed by LC/MS. Plasma samples (25 μL) were added to 96-well plates followed by addition of an internal standard solution (200 μL) containing NAX 810-1 (internal standard) in acetonitrile with 1% formic acid. The final solutions were mixed and subjected to centrifugation (3000 rpm) for 5 min after which 100 μL of the supernatant was removed and added to a solution of water (100 μL) containing 1% formic acid. NAX 810-2 levels were evaluated by an LC method using an HPLC system (Shimadzu SCL-10A), reverse-phase column (Varian Metasil AQ C18 50x×2.1 mm), and a linear gradient of mobile phases consisting of water and acetonitrile with 1% formic acid. Following liquid chromatography, samples were added to an API-5500 mass spectrometer using electrospray ionization (5000 V, 700 °C). NAX 810-2 levels were evaluated based on a mass transition of 709.0 → 693.8 m/z.
Median Effective Dose (ED50) Determination and Statistical Analysis
ED50 and 95% CI values in the corneal kindling and 6 Hz assays were calculated using Probit analysis. Data are presented as means ± standard error. Individual means were compared using a t-test and multiple means were compared using a one-way ANOVA followed by a Dunnett's post-hoc evaluation for comparison to VEH.
Pharmacokinetic Parameter Analysis
A non-compartmental analysis was employed in order to obtain pharmacokinetic parameters. The half-life (t1/2) was calculated as 0.693 / λz, where λz is the terminal slope of the drug concentration vs. time curve. Area under-the-curve (AUC), corresponding to the area under the drug concentration vs. time curve was calculated using the trapezoidal rule and extrapolated to infinity. Clearance (CL) was calculated from the quotient dose / AUC. The apparent volume of distribution (Vd/F) or estimated volume of distribution was calculated as CL/F divided by the linear terminal slope, where F (bioavailability) is 1 following IV administration.
Results
NAX 810-2: Mouse IV Efficacy in the Corneal Kindling Model
A time-of-peak effect in corneal kindling for NAX 810-2 following IV administration was previously determined to be 1h in a time-course study (data not shown). Using this time point, several doses of NAX 810-2 (dose range 0.2 – 3.0 mg/kg) were administered to fully kindled mice. NAX 810-2 showed dose-dependent activity in the corneal kindling assay, with an ED50 (95% CI) of 0.5 mg/kg (0.1-0.9 mg/kg) (see Table 1).
Table 1.
NAX 810-2 Efficacy in the Mouse Corneal Kindling Model Following IV Administration.
| NAX 810-2 Dose (IV) (mg/kg) |
Efficacy (# protected / # tested) |
|---|---|
| 0.2 | 1/7 |
| 0.5 | 3/7 |
| 0.75 | 10/12 |
| 3 | 6/7 |
| ED50 (95% CI) 0.5 mg/kg (0.14 – 0.88) | |
NAX 810-2: Mouse IV Efficacy and in the 6 Hz (32 mA) Model
NAX 810-2 was administered to separate groups of naïve mice prior to testing (0.08 – 6 h post-treatment) in the 6 Hz (32 mA) assay (see Table 2). Immediately prior to 6 Hz testing, mice were evaluated for motor impairment using a rotarod (see Table 2). NAX 810-2 was effective in blocking seizures in a dose-dependent manner at several time points (0.08 – 2h), with peak activity observed 0.25 – 0.75 h following treatment. Using activity observed 0.5h following treatment, the calculated ED50 (95% CI) for NAX 810-2 in this assay was 0.7 mg/kg (0.5 – 0.9). Mild motor impairment was observed at doses of 0.75 mg/kg IV (0.75h post-treatment) and 1.5 mg/kg (0.75 – 1h post-treatment). Moderate motor impairment occurred 0.25h following treatment in the 1.5 mg/kg group.
Table 2.
Dose-Response Evaluation of NAX 810-2 Following IV Bolus Administration to Male CF-1 Mice in the 6 Hz (32 mA) Model of Psychomotor Seizures.
| NAX 810-2 Dose (mg/kg) |
6 Hz (32 mA) Efficacy (# protected / # tested) Test Time (h) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.08 | 0.25 | 0.5 | 0.75 | 1 | 2 | 3 | 4 | 6 | |
| 0.375 | 2/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 1/8 |
| 0.5625 | 1/8 | 2/8 | 3/8 | 4/8 | 3/8 | 1/8 | 0/8 | 0/8 | 0/8 |
| 0.75 | 1/8 | 5/8 | 7/8 | 8/8 | 3/8 | 2/8 | 2/8 | 1/8 | 1/8 |
| 1.5 | 4/8 | 7/8 | 7/8 | 8/8 | 8/8 | 6/8 | 0/8 | 1/8 | 0/8 |
| ED50 (95% CI): 0.7 mg/kg (0.5 – 0.9) | |||||||||
|
| |||||||||
|
Motor Impairment (# impaired / # tested) |
|||||||||
| 0.375 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| 0.5625 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| 0.75 | 0/8 | 1/8 | 0/8 | 2/8 | 1/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| 1.5 | 0/8 | 4/8 | 1/8 | 2/8 | 2/8 | 1/8 | 0/8 | 0/8 | 0/8 |
Pharmacokinetic-Pharmacodynamic Interaction Study of NAX 810-2 Following IVAdministration in CF-1 Mice
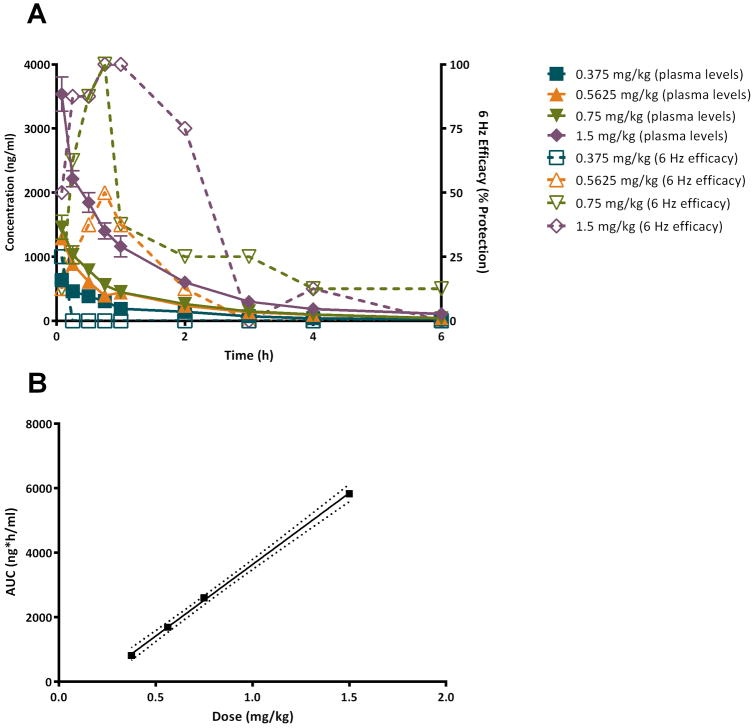
Figure 1 shows plasma levels of NAX 810-2 following IV bolus administration compared with 6 Hz efficacy (compare with Table 2). Plasma concentrations were greatest at the initial time point following treatment (0.08 h post dose). 6 Hz efficacy was maximal at 0.08 h (0.375 mg/kg; 25% protection), 0.75 h (0.5625 mg/kg, 50% protection; 0.75 mg/kg, 100% protection), and 0.75 – 1 h (1.5 mg/kg) (see Fig. 1A). AUC values were derived from the NAX 810-2 concentration vs. time curve are shown in Fig. 1B and show a linear increase with increasing doses.
Figure 1.
Pharmacokinetic-pharmacodynamic analysis of NAX 810-2 following intravenous (IV) administration of NAX 810-2 and evaluation in the 6 Hz (32 mA) psychomotor seizure model. (A) Following administration with NAX 810-2 (IV bolus), male CF-1 mice (N=8 per group) received corneal stimulation in the 6 Hz test, and the number of mice “protected” (no seizures) / N was determined for each treatment group (expressed as a % protection). Trunk blood samples were used for determination of NAX 810-2 plasma concentrations using an LC-MS assay. Treatment groups included 0.375 mg/kg (blue; plasma levels - closed squares, solid line, left Y-axis; 6 Hz efficacy – open squares, dotted line, right Y-axis), 0.5625 mg/kg (orange; plasma levels – closed triangle, solid line, left Y-axis; 6 Hz efficacy – open triangle, dotted line, right Y-axis), 0.75 mg/kg (green; plasma levels – closed inverted triangle, solid line, left Y-axis; 6 Hz efficacy – open inverted triangle, dotted line, right Y-axis). (B) The plasma concentration vs. time curve was used for determination of area under-the-curve (AUC) values for each treatment group. The solid line indicates the linear regression (R2=0.999), with dotted lines representing 95% confidence intervals.
Pharmacokinetic parameters derived from analysis of the NAX 810-2 plasma-concentration vs. time relationship and are shown in Table 3. CL, Vd, and terminal half-life (t1/2) were as follows: CL 0.32-0.48 L/h, Vd 0.53-0.82 L and T1/2 1.14-1.19h. CL were similar at the highest (1.5 mg/kg, 0.475 L/h) and lowest dose (0.375 mg/kg, 0.452 L/h) whereas doses of 0.5625 mg/kg and 0.75 mg/kg yielded lower CL values (0.340 L/h and 0.319 L/h, respectively), representing 71.6% and 67.2% of maximal CL values for 0.375 mg/kg. Similarly, Vd values were greater for the largest (1.5 mg/kg, 0.76 L) and smalles doses (0.375 mg/kg, 0.82 L) whereas 0.5625 mg/kg and 0.75 mg/kg yielded Vd values of 0.58 and 0.53 L, respectively. AUC and extrapolated initial concentrations (C0) showed dose-dependent increases that were approximately proportional to increases in dose (see also Figure 1B for AUC changes with dose).
Table 3.
Pharmacokinetic Parameters Derived Following IV (Bolus) Administration of NAX 810-2 to Male CF-1 Mice.
| Pharmacokinetic Parameter Dose (mg/kg, IV) |
||||
|---|---|---|---|---|
| 0.375 | 0.5625 | 0.75 | 1.5 | |
| CL (L/h) | 0.46 | 0.33 | 0.29 | 0.43 |
| Vd (L) | 0.80 | 0.57 | 0.48 | 0.72 |
| T1/2 (h) | 1.19 | 1.19 | 1.14 | 1.17 |
| AUC (ng*h/ml) | 809 | 1687 | 2596 | 5825 |
| C0 (ng/ml) | 475 | 834 | 1007 | 2365 |
To further evaluate the relationship between behavioral observations (6 Hz and rotarod assays) and plasma NAX 810-2 levels, plasma levels were compared at the times of peak 6 Hz protection (0.5h and 0.75h; see Figure S1-A) and peak motor impairment in the rotarod assay (0.25h and 0.75h; see Figure S1-B). Plasma NAX 810-2 increase in a dose-dependent manner to efficacy and motor impariment.
Plasma Protein Binding
NAX 810-2 was evaluated at several concentrations (0.5, 5, 50 μM) for plasma protein binding in human plasma in comparison to Warfarin (10 μM). At all concentrations tested, NAX 810-2 was observed to be hightly protein bound: 0.5 μM, 99.5 ± 0.1% bound; 5 μM, 99.7 ± 0.1% bound,; 50 μM, 100.0 ± 0.0% bound (see Table S1).
GalR Functional Activity
Activity of NAX 810-2 in functional agonist activity assays for GalR1 and GalR2 are shown in Table 4. The compound was evaluated at concentrations of 0.1, 0.5, and 2.0 μM at each receptor. NAX 810-2 shows preferential activity for GalR2 over GalR1 at concentrations of 0.1 and 0.5 μM whereas agonist activity was similar at both receptors at the highest concentration tested (2.0 μM).
Table 4.
Functional GalR Agonist Activity for NAX 810-2.
| Concentration (μM) | GalR1 | GalR2 |
|---|---|---|
| % Activationa | ||
| 0.1 | -0.8 | 64.5 |
| 0.5 | 54.7 | 95.9 |
| 2.0 | 93.3 | 104.8 |
Percent activation normalized to maximal activity for positive control samples, galanin (1-30).
Discussion
This work confirms and expands our initial findings that GalR2-preferring analogs have favorable preclinical properties as antiseizure drug leads. The pre-clinical pharmacology of NAX 810-2 following IP and IV administration has been previously described15; 16. NAX 810-2 dose-dependently blocks seizures in the mouse 6 Hz model at both 32 mA and 44 mA stimulus intensities and in the mouse corneal kindling model following IP administration and is effective following IV administration in the mouse 6 Hz (32 mA) and corneal kindling models. Following these initial studies, an optimized formulation of NAX 810-2 was prepared to improve solubility for IV administration studies. NAX 810-2 dose-dependently reduced seizures in the 6 Hz (32 mA) and corneal kindling models. Doses up to 0.75 mg/kg IV were generally well-tolerated, producing minimal motor impairment in the rotarod assay. The experiments reported in this mansucript therefore extend previous studies by demonstrating dose-dependent efficacy in mouse corneal kindling and mouse 6 Hz seizure models using an optimized formulation of NAX 810-2. Further, using pharmacokinetic-pharmacodynamic analysis, we observed that the compound has linear pharmacokinetics, and peak plasma concentrations coincide with peak antiseizure activity.
NAX 810-2 demonstrates linear pharmacokinetics, with dose-dependent increases in AUC and plasma levels. Several of the currently available antiseizure drugs exhibit pharmacokinetic properties that create challenges for use in clinical settings. For example, phenytoin, carbamazepine, and valproic acid can exhibit non-linear pharmacokinetics, wherein increases in dose do not result in proportional increases in plasma concentrations, thereby furthering the risk of untoward effects28. Therefore, demonstration of linear pharmacokinetics at therapeutically relevant concentrations for NAX 810-2 is a distinct advantage for this novel antiseizure drug. However, it is noteworthy that NAX 810-2 also demonstrated a high degree of plasma protein binding. At a concentration of 0.5 μM, which corresponds to concentrations of approximately 1000 ng/ml (within the range of plasma levels associated with antiseizure efficacy in the 6 Hz model) NAX 810-2 was 99.5% protein bound. This suggests that small changes in the ratio of bound/unbound NAX 810-2 may have dramatic effects on efficacy and toxicity. Further development of this compound as a novel therapeutic will therefore require careful consideration of co-administered drugs.
NAX 810-2 is effective in the corneal kindling model but not the maximal electroshock seizure model16 and therefore demonstrates a pre-clinical seizure profile similar to levetiracetam20; 21. Despite this similar profile, the mechanism of action of NAX 810-2 is distinct from that of levetriacetam. By binding to SV2A22, levetiracetam has a unique mode of action via pre-synaptic inhibition of neurotransmitter release23. Though the mechanism of action of GalR2 receptors in epileptic neural circuits hasn't been fully described, galanin stores can be mobilized during acute seizure insults24 and GalR2 receptors are highly expressed in the granule cell layer of the dentate gyrus25. These and other observations have led to the hypothesis that galanin acts as an endogenous anticonvulsant. Furthermore, the anticonvulsant actions of galanin analogs may occur via both pre- and post-synaptic modes of action26; 27. In addition, future studies could include co-administration of NAX 810-2 with levetiracetam to determine whether there are additive or synergistic effects arising from combined administration of compounds with pre-synaptic mechanisms of action.
Functional agonist studies for GalR1 and GalR2 demonstrated that that NAX 810-2 is GalR2-preferring at concentrations of 0.1 and 0.5 μM but was equally active for GalR1 and GalR2 at 2.0 μM. In addition, NAX 810-2 0.5 μM corresponds to 1062 ng/ml, which is similar to the plasma concentration observed following a dose of 0.75 mg/kg, which was maximally effective in the 6 Hz assay. Therefore, these data suggest that NAX 810-2 is GalR2-preferring at therapeutically relevant doses and this GalR2 preference diminishes at supratherapeutic doses.
Previous efforts to develop compounds that act on galanin receptors have included the small molecules, galmic6; 29; 30 and galnon6; 31, galanin peptide fragments6, and galanin allosteric modulators32. Galmic and galnon were hindered as novel therapeutics due to low affinity (i.e. compared to peptide ligands) and a lack of selectivity for GalR1 and/or GalR2 receptors6; 33. Galanin and galanin peptide fragments have proven effective against seizures when administered centrally6; 24, although systemic administration has been challenged by metabolic stability and lack of blood-brain barrier penetration. By contrast, NAX 810-2 is systemically active in acute seizure models, following both IV and IP administration, and this efficacy was observed at doses that did not produce motor impairment. Efficacy in the mouse 6 Hz and corneal kindling seizure models suggests that this compound crosses the blood-brain barrier and affects neuronal excitability. Previously we have observed that the galanin fragment Gal(1-16) and synthesized galanin analogs are effective in the mouse 6 Hz model following intracerebroventricular injection12; 34 even when these analogs are restricted to the periphery34, suggesting that central activation of galanin receptors can block seizures. Future studies will will include determination of brain NAX 810-2 levels for confirmation of brain penetration and comparison to antiseizure activity.
Supplementary Material
Acknowledgments
Financial Support: These studies were supported by a U01 Translational Research grant from the National Institutes of Neurological Diseases and Stroke of the National Institutes of Health (1U01 NS066911-01A1).
Footnotes
Author's Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest: CSM and BDK are previous full-time employees of NeuroAdjuvants, Inc. HSW and GB are scientific co-founders of NeuroAdjuvants, Inc.
References
- 1.Clynen E, Swijsen A, Raijmakers M, et al. Neuropeptides as targets for the development of anticonvulsant drugs. Mol Neurobiol. 2014;50:626–646. doi: 10.1007/s12035-014-8669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson CR, Flynn SP, White HS, et al. Anticonvulsant neuropeptides as drug leads for neurological diseases. Nat Prod Rep. 2011;28:741–762. doi: 10.1039/c0np00048e. [DOI] [PubMed] [Google Scholar]
- 3.Mazarati A, Lu X, Kilk K, et al. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur J Neurosci. 2004;19:3235–3244. doi: 10.1111/j.0953-816X.2004.03449.x. [DOI] [PubMed] [Google Scholar]
- 4.Mazarati A, Lu X, Shinmei S, et al. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience. 2004;128:431–441. doi: 10.1016/j.neuroscience.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindskog S, Ahren B. Galanin: effects on basal and stimulated insulin and glucagon secretion in the mouse. Acta Physiol Scand. 1987;129:305–309. doi: 10.1111/j.1748-1716.1987.tb08073.x. [DOI] [PubMed] [Google Scholar]
- 6.Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Zini S, Roisin MP, Armengaud C, et al. Effect of potassium channel modulators on the release of glutamate induced by ischaemic-like conditions in rat hippocampal slices. Neurosci Lett. 1993;153:202–205. doi: 10.1016/0304-3940(93)90322-c. [DOI] [PubMed] [Google Scholar]
- 8.Zini S, Roisin MP, Langel U, et al. Galanin reduces release of endogenous excitatory amino acids in the rat hippocampus. Eur J Pharmacol. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- 9.Mazarati AM, Hohmann JG, Bacon A, et al. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazarati AM, Halaszi E, Telegdy G. Anticonvulsive effects of galanin administered into the central nervous system upon the picrotoxin-kindled seizure syndrome in rats. Brain Res. 1992;589:164–166. doi: 10.1016/0006-8993(92)91179-i. [DOI] [PubMed] [Google Scholar]
- 11.Mazarati A, Lu X. Regulation of limbic status epilepticus by hippocampal galanin type 1 and type 2 receptors. Neuropeptides. 2005;39:277–280. doi: 10.1016/j.npep.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Bulaj G, Green BR, Lee HK, et al. Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem. 2008;51:8038–8047. doi: 10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]
- 13.White HS, Scholl EA, Klein BD, et al. Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics. 2009;6:372–380. doi: 10.1016/j.nurt.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson CR, Scholl EA, Pruess TH, et al. Engineering galanin analogues that discriminate between GalR1 and GalR2 receptor subtypes and exhibit anticonvulsant activity following systemic delivery. J Med Chem. 2010;53:1871–1875. doi: 10.1021/jm9018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Res. 2013;103:2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: A summary of the Twelfth Eilat Conference (EILAT XII) Epilepsy Res. 2015;111:85–141. doi: 10.1016/j.eplepsyres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Barton ME, Klein BD, Wolf HH, et al. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 18.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure Electroencephalogr. Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 19.Rowley NM, White HS. Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: Correlation with other seizure and epilepsy models. Epilepsy Res. 2010;92:163–169. doi: 10.1016/j.eplepsyres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Klitgaard H, Matagne A, Gobert J, et al. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 21.Loscher W, Honack D. Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats. Eur J Pharmacol. 1993;232:147–158. doi: 10.1016/0014-2999(93)90768-d. [DOI] [PubMed] [Google Scholar]
- 22.Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XF, Weisenfeld A, Rothman SM. Prolonged exposure to levetiracetam reveals a presynaptic effect on neurotransmission. Epilepsia. 2007;48:1861–1869. doi: 10.1111/j.1528-1167.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 24.Mazarati AM, Liu H, Soomets U, et al. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Donnell D, Ahmad S, Wahlestedt C, et al. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- 26.Casillas-Espinosa PM, Powell KL, O'Brien TJ. Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epilepsia. 2012;53(Suppl 9):41–58. doi: 10.1111/epi.12034. [DOI] [PubMed] [Google Scholar]
- 27.Mazarati A, Lundstrom L, Sollenberg U, et al. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 28.Perucca E, Johannessen SI. The ideal pharmacokinetic properties of an antiepileptic drug: how close does levetiracetam come? Epileptic Disord. 2003;5(Suppl 1):S17–26. [PubMed] [Google Scholar]
- 29.Bartfai T, Lu X, Badie-Mahdavi H, et al. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci U S A. 2004;101:10470–10475. doi: 10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceide SC, Trembleau L, Haberhauer G, et al. Synthesis of galmic: a nonpeptide galanin receptor agonist. Proc Natl Acad Sci U S A. 2004;101:16727–16732. doi: 10.1073/pnas.0407543101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saar K, Mazarati AM, Mahlapuu R, et al. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci U S A. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartfai T, Wang MW. Positive allosteric modulators to peptide GPCRs: a promising class of drugs. Acta Pharmacol Sin. 2013;34:880–885. doi: 10.1038/aps.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floren A, Sollenberg U, Lundstrom L, et al. Multiple interaction sites of galnon trigger its biological effects. Neuropeptides. 2005;39:547–558. doi: 10.1016/j.npep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Klein BD, Metcalf CS, et al. Incorporation of monodisperse oligoethyleneglycol amino acids into anticonvulsant analogues of galanin and neuropeptide y provides peripherally acting analgesics. Mol Pharm. 2013;10:574–585. doi: 10.1021/mp300236v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.