Abstract
Background
Type 1, Type 17 and other pro-inflammatory cytokines are known to play an important role in resistance to pulmonary tuberculosis. The role of these cytokines in tuberculous lymphadenitis (TBL) is not well characterized.
Methods
To estimate the systemic and mycobacterial antigen – stimulated cytokine concentrations of Type 1, Type 17, other pro-inflammatory and regulatory cytokines in TBL, we examined both the systemic and the antigen–specific concentrations of these cytokines in TBL (n = 31) before and after chemotherapy and compared them with those with latent tuberculosis infection (LTB, n = 31).
Results
We observed significantly reduced systemic concentrations of the pro-inflammatory cytokines – IL-1β and IL-18 but not other Type 1 or Type 17 cytokines in TBL compared to LTB. Following standard anti-tuberculosis (TB) treatment, we observed a significant increase in the concentrations of both IL-1β and IL-18. In addition, we also observed significantly reduced baseline or mycobacterial – antigen or mitogen stimulated concentrations of IL-1β and IL-18 in TBL individuals. Similar to systemic cytokine concentrations, anti-TB treatment resulted in significantly increased concentrations of these cytokines following antigen stimulation.
Conclusions
TBL is therefore, characterized by reduced systemic and antigen – specific concentrations of IL-1β and IL-18, which are reversible following anti-TB treatment, indicating that these cytokines are potential correlates of protective immunity in TBL.
Keywords: Tuberculosis, Lymphadenitis, Cytokines
Introduction
Tuberculous lymphadenitis (TBL) is the most common presentation of extrapulmonary TB, accounting for 30–40% of cases [1]. The incidence of TBL is increasing worldwide every year. The cervical lymph nodes are the most commonly affected followed by inguinal, axillary, mesenteric, mediastinal and intra mammary lymph nodes [2]. TBL is thought to be the result of hematogenous or lymphatic dissemination of mycobacteria from the lungs, although its exact mechanism of pathogenesis is still not clear [2]. TBL differs from pulmonary tuberculosis in being more predominant in women and in occurring in an younger age group [3].
While the host immune response against pulmonary TB is well characterized [4], the immune response induced in TBL is poorly understood as are the correlates of protective immunity against TBL. Thus, both Type 1, especially IFNγ and TNFα, and Type 17 cytokines, especially IL-17 are considered to be important in host protective immunity against pulmonary tuberculosis (TB) [4]. In addition, the induction of innate cytokines, especially of the IL-1 family (including IL-1α, IL-1β and IL-18) and IL-12, which drives Type 1 cytokines responses, are also known to be an important pathway of protective immunity to pulmonary TB [5]. In contrast, regulatory cytokines such as IL-10 and TGFβ play a detrimental role in protective immunity to pulmonary TB [6]. The role of most of these cytokines has not been explored in detail in TBL.
In order to characterize the association between systemic and antigen – stimulated concentrations of Type 1, Type 17, other pro-inflammatory and regulatory cytokines with TBL pathogenesis, we examined the concentrations of these cytokines in TBL individuals before and after standard anti-TB therapy. Moreover, we compared these cytokines responses to those present in latent tuberculosis (LTB) infection. We show that TBL is predominantly characterized by reduced systemic and antigen or mitogen induced concentrations of IL-1β and IL-18, which are then reversed following treatment.
METHODS
Study Population
We studied a group of 31 individuals with TBL and 31 with LTB (Table 1). Individuals with TBL were diagnosed on the basis of excision biopsy showing either culture positivity or histology confirming tuberculosis. LTB diagnosis was based on Tuberculin skin test (TST) and Quantiferon TB-Gold in Tube ELISA positivity and absence of chest radiograph abnormalities and absence of pulmonary symptoms. A positive TST result was defined as an induration at the site of tuberculin inoculation of at least 12mm in diameter to minimize false positivity due to exposure to environmental mycobacteria. All individuals were HIV negative. The individuals were not on any steroid treatment and consecutive samples were collected. Baseline blood samples were collected before any anti-TB medication was administered. All TBL individuals were administered standard anti-TB treatment for 6 months and at the end of treatment, blood was collected again from these individual. This study was specifically approved by the Institutional Ethics Committee of the National Institute of Research in Tuberculosis and informed written consent was obtained from all participants.
Table 1.
Demographics of the study population
| Study Demographics | TBL | LTB |
|---|---|---|
| No. of subjects recruited | 31 | 31 |
| Gender (M/F) | 09/22 | 13/18 |
| Median Age (Range) | 28 (18–53) | 37 (21–65) |
| Smear Grade (0/1+/2+/3+) | 7/17/6/1 | Not done |
ELISA
Plasma cytokines and chemokines were measured using Duoset or Quantikine ELISA kits from R&D systems. The parameters analyzed were IFNγ, TNFα, IL-2, IL-17A, IL-17F, IL-22, IL-1α, IL-1β, IL-18 IL-12p70, IL-10 and TGFβ.
Quantiferon supernatant ELISA
Whole blood was incubated with either no antigen or TB antigen (ESAT-6, CFP-10, TB 7.7) or mitogen according to the manufacturers instructions using the Quantiferon in Tube Gold kit. The unstimulated or TB antigen or mitogen stimulated whole blood supernatants were then used to analyze the concentrations of IFNγ, TNFα, IL-2, IL-1β, IL-18 and IL-10 using the Duo-set ELISA kits from R& D systems. 50 ul of sample in duplicate was used for each assay according to the manufacturer’s instruction.
Statistical Analysis
Data analyses were performed using GraphPad PRISM (GraphPad Software, Inc., San Diego, CA). Geometric means (GM) were used for measurements of central tendency. Statistically significant differences between two groups were analyzed using the nonparametric Mann-Whitney U test and between pre- and post – treatment concentrations were analyzed using Wilcoxon signed rank test. Multiple comparisons were corrected using the Holm’s correction.
RESULTS
Study population characteristics
The baseline characteristics including demographic and hematogical features of the study population are shown in Table I and II. As shown in Table I, the two groups did not diffr significantly in age or gender distribution. Also, as can be seen in Table II, compared to LTB, TBL individuals exhibit significantly lower percentages of lymphocytes but significantly higher percentages of neutrophils, eosinophils and basophils.
Table 2.
Hematological parameters
| Haematological profile | TBL | LTB | p value |
|---|---|---|---|
| Whole blood cells (μl) | 6.7 (3.8–12) | 8.5 (3.8–12.1) | NS |
| Red blood cells (μl) | 4.5 (3.90–5.70) | 4.63 (3.87–6.27) | NS |
| Lymphocytes (%) | 29. 1 (17.6– 55.7) | 33.9 (19.1–53.7) | 0.0226 |
| Neutrophils (%) | 60.8 (27.9–73.4) | 51.5 (34.4–72.6) | 0.0323 |
| Monocytes (%) | 6.6 (2–14.5) | 6.4 (2–11.5) | NS |
| Eosinophils (%) | 2.5 (0.9–12.0) | 0.33 (0.11–1.24) | <0.0001 |
| Basophils (%) | 0.8 (0.3–1.5) | 0.08 (0.02–0.19) | <0.0001 |
| Platelets | 317 (138–406) | 293 (185–507) | NS |
TBL is associated with decreased systemic concentrations of IL-1β and IL-18
To determine the cytokine profile in TBL, we measured the systemic concentrations of Type 1 (IFNγ, TNFα and IL-2), Type 17 (IL-17A, IL-17F and IL-22), other pro-inflammatory (IL-1α, IL-β, IL-12 and IL-18) and regulatory cytokines (IL-10 and TGFβ) in TBL and LTB individuals (Figure 1). As shown in Figure 1A, there were no significant differences in the systemic concentrations of IFNγ, TNFα, IL-2, IL-17A, IL-17F and IL-22 in TBL compared to LTB individuals. In contrast, as shown in Figure 1B, the systemic concentrations of IL-β (Geometric mean of 16.7 pg/ml in TBL vs. 23.8 pg/ml in LTB) and IL-18 (GM of 124.3 pg/ml vs. 174 pg/ml) were significantly lower in TBL compared to LTB individuals. However, no significant differences in the concentrations of IL-1α, IL-12, IL-10 and TGFβ were found between the two groups. Thus, TBL is associated with reduced systemic concentrations of IL-1β and IL-18.
Figure 1. TBL is associated with reduced systemic concentrations of IL-1β and IL-18.
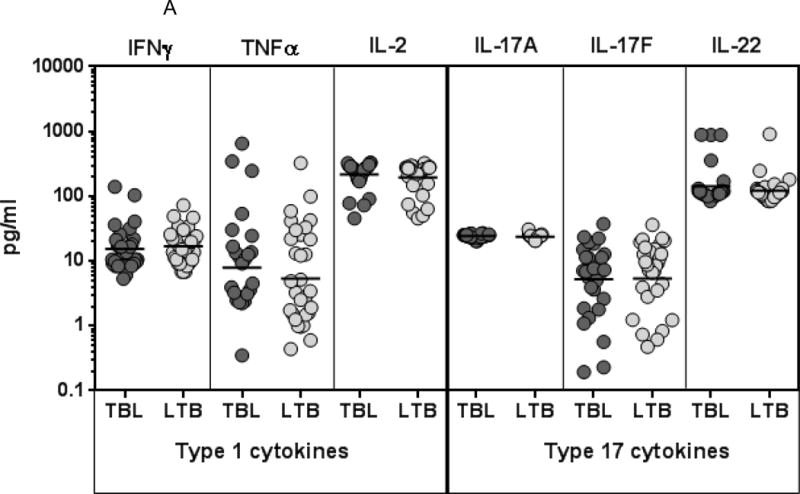

(A) The systemic concentrations of Type 1 (IFNγ, TNFα, IL-2) and Type 17 (IL-17A, IL-17F, IL-22) cytokines were measured in TBL (n=31) and LTB (n=31) individuals. (B) The systemic concentrations of pro-inflammatory (IL-1α, IL-1β, IL-12 and IL-18) and regulatory (IL-10, TGFβ) cytokines were measured in TBL (n=31) and LTB (n=31) individuals. The results are shown as scatter plots with each circle representing a single individual and the bar representing the GM. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons.
Treatment of TBL is associated with increased systemic concentrations of IL-1β and IL-18
To determine the effect of treatment on the cytokine profile in TBL, we measured the systemic concentrations of Type 1 (IFNγ, TNFα and IL-2), Type 17 (IL-17A, IL-17F and IL-22), other pro-inflammatory (IL-1α, IL-β, IL-12 and IL-18) and regulatory cytokines (IL-10 and TGFβ) in TBL individuals before (pre-T) and after (post-T) treatment (Figure 2). As shown in Figure 2A, there were no significant differences in the systemic concentrations of IFNγ, TNFα, IL-2, IL-17A, IL-17F and IL-22 in TBL individuals before and after treatment. In contrast, as shown in Figure 2B, the systemic concentrations of IL-β (GM of 16.7 pg/ml in pre-T vs. 22.2 pg/ml in post-T) and IL-18 (GM of 124.3 pg/ml vs. 172.1 pg/ml) were significantly higher post-treatment in TBL individuals compared to pre-treatment concentrations. However, no significant differences in the concentrations of IL-1α, IL-12, IL-10 and TGFβ were found before and after treatment. Thus, treatment of TBL is associated with enhanced systemic concentrations of IL-1β and IL-18.
Figure 2. Treatment modifies the systemic cytokine profile in TBL.
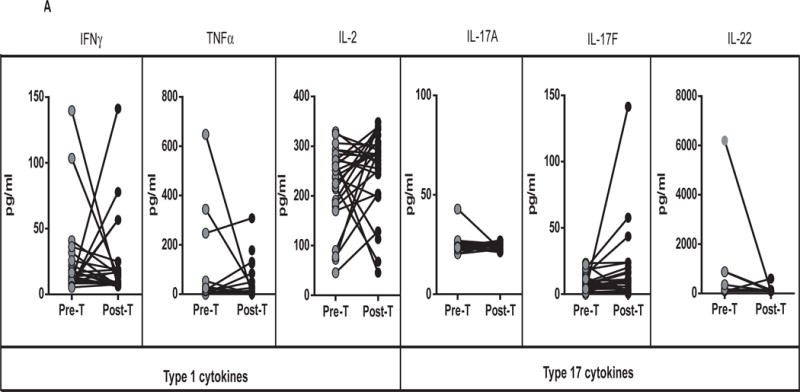

(A) The systemic concentrations of Type 1 (IFNγ, TNFα, IL-2) and Type 17 (IL-17A, IL-17F, IL-22) cytokines were measured in TBL (n=31) individuals before (pre-T) and 6 months after (post-T) anti-TB treatment. (B) The systemic concentrations of pro-inflammatory (IL-1α, IL-1β, IL-12 and IL-18) and regulatory (IL-10, TGFβ) cytokines were measured in TBL (n=31) individuals before (pre-T) and 6 months after (post-T) anti-TB treatment. The results are shown as line diagrams with each line representing a single individual. P values were calculated using the Wilcoxon signed rank test with Holm’s correction for multiple comparisons.
TBL is associated with decreased baseline, TB antigen and mitogen stimulated concentrations of IL-1β and IL-18
To determine the mycobacterial antigen stimulated cytokine profile in TBL, we measured the concentrations of these cytokines following stimulation of whole blood with no antigen or a cocktail of TB antigens (ESAT-6, CFP-10, TB 7.7) or mitogen in TBL and LTB individuals (Figure 3). As shown in Figure 3A, the spontaneously produced concentrations of IL-1β (GM of 135.5 pg/ml vs. 321.8 pg/ml) and IL-18 (GM of 183 pg/ml vs. 287.7 pg/ml) but not IFNγ, TNFα, IL-2 or IL-10 were significantly lower in TBL compared to LTB individuals. Similarly, as shown in Figure 3B, the TB antigen stimulated concentrations of IL-1β (GM of 326.6 pg/ml vs. 1003 pg/ml) and IL-18 (GM of 188.9 pg/ml vs. 254.8 pg/ml) but not IFNγ, TNFα, IL-2 or IL-10 were significantly lower in TBL compared to LTB individuals. Finally, as shown in Figure 3C, the mitogen stimulated concentrations of IL-1β (GM of 2345 pg/ml vs. 3267 pg/ml), IL-18 (GM of 178.6 pg/ml vs. 286 pg/ml) and IFNγ (GM of 1737 pg/ml vs. 2650 pg/ml) but not TNFα, IL-2 or IL-10 were significantly lower in TBL compared to LTB individuals Thus, TBL is associated with reduced concentrations of TB antigen stimulated IL-1β and IL-18.
Figure 3. TBL is associated with reduced baseline, mycobacterial antigen and mitogen stimulated concentrations of IL-1β and IL-18.



(A) The baseline or (B) mycobacterial antigen – stimulated or (C) mitogen stimulated concentrations of IFNγ, TNFα, IL-2, IL-1β, IL-18 and IL-10 were measured in TBL (n=31) and LTB (n=31) individuals. The results are shown as scatter plots with each circle representing a single individual and the bar representing the GM. The antigen or mitogen stimulated values are shown as net cytokine concentrations with the baseline subtracted. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons.
Treatment of TBL is associated with increased baseline, TB antigen and mitogen stimulated concentrations of IL-1β and IL-18
To determine the effect of treatment on mycobacterial antigen stimulated cytokine profile in TBL, we measured the concentrations of these cytokines following stimulation of whole blood with no antigen or a cocktail of TB antigens (ESAT-6, CFP-10, TB 7.7) or mitogen in TBL individuals before (pre-T) and after (post-T) treatment (Figure 4). As shown in Figure 4A, the spontaneously produced concentrations of IL-1β (GM of 135.5 pg/ml vs. 409.7 pg/ml), IL-18 (GM of 183 pg/ml vs. 232.4 pg/ml) and TNFα but not IFNγ, IL-2 or IL-10 were significantly higher in TBL individuals post-treatment compared to pre-treatment concentrations. Similarly, as shown in Figure 4B, the TB antigen stimulated concentrations of IL-1β (GM of 326.6 pg/ml vs. 871.3 pg/ml) and IL-18 (GM of 188.9 pg/ml vs. 222.4 pg/ml) but not IFNγ, TNFα, or IL-10 were significantly higher in TBL individuals post-treatment compared to pre-treatment concentrations. IL-2 concentrations were significantly lower post-treatment compared to pre-treatment concentrations. Finally, as shown in Figure 4C, the mitogen stimulated concentrations of IL-1β (GM of 2345 pg/ml vs. 2953 pg/ml), IFNγ, TNFα and IL-10 but not IL-2 and IL-18 were significantly higher in TBL individuals post-treatment compared to pre-treatment concentrations. Thus, treatment of TBL is associated with elevated concentrations of TB antigen stimulated IL-1β and IL-18.
Figure 4. Treatment modifies the baseline, mycobacterial antigen and mitogen stimulated concentrations of IL-1β and IL-18 in TBL.
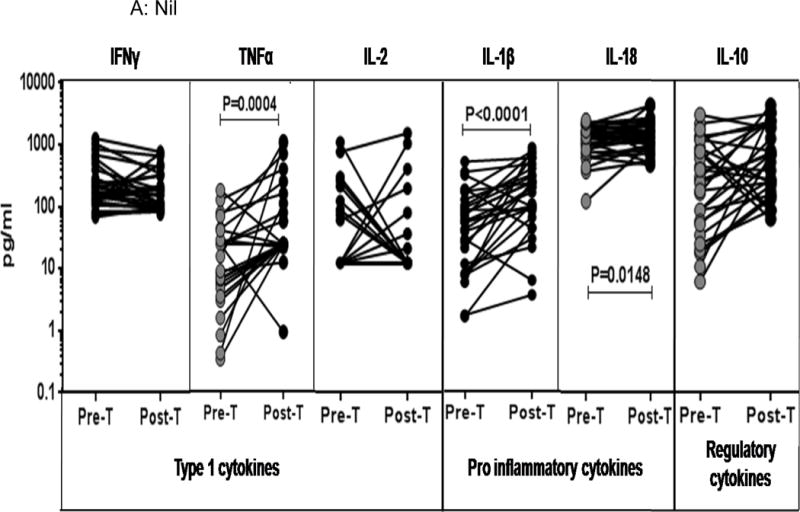


(A) The baseline or (B) mycobacterial antigen – stimulated or (C) mitogen stimulated concentrations of IFNγ, TNFα, IL-2, IL-1β, IL-18 and IL-10 were measured in TBL (n=31) individuals before (pre-T) and 6 months after (post-T) anti-TB treatment. The results are shown as line diagrams with each line representing a single individual. The antigen or mitogen stimulated values are shown as net cytokine concentrations with the baseline subtracted. P values were calculated using the Wilcoxon signed rank test with Holm’s correction for multiple comparisons.
DISCUSSION
The correlates of protective immunity in pulmonary TB is still not well understood [7]. However, there is at least a road map of the immune response that confers protection versus susceptibility in this form of TB, mainly using animal models [8]. Thus, Type 1, Type 17 and other pro-inflammatory cytokines (especially of the IL-1 family) are known to play an important role in protection while Type 2 and regulatory cytokines and Type 1 interferons are known to be detrimental in host immunity to TB [4]. However, very little is known about the association of these cytokines in the immune responses engendered in TBL. Previous studies have shown that TBL is characterized by an expansion of mono- and multi-functional Th1 and Th17 cells in response to TB antigens and that CD8+ T cells expressing Type 1 and Type 17 cytokines are also increased in comparison to pulmonary TB [9, 10]. Similarly, enhanced concentrations of circulating IL-10, IL-8 and TNFβ have been described as potential blood biomarkers in TBL [11], while level of IFNγ, TNFα, GM-CSF, IL-1β, IL-2, IL-4 and IL-6 have been shown to be elevated in adult TBL compared to children with TBL [12]. Finally, in our previous study on cytokine responses in TBL compared to pulmonary TB, we have shown that TBL individuals exhibit significantly increased production of Type 1, Type 2 and Type 17 cytokines in response to TB antigens [13]. However, the data regarding cytokine response in TBL compared to LTB is scant.
Our study on cytokine responses, both systemic and antigen – induced, reveal three major features. First, amongst the variety of cytokine examined, only IL-1β and IL-18 exhibit significant differences in their systemic concentrations when comparing TBL with LTB individuals. This suggests that deficiency in the IL-1β – IL-18 axis is associated with TBL disease. Second, examination of antigen or mitogen stimulated responses also reveals that IL-1β and IL-18 are the two major cytokines that are induced at significantly lower concentrations in TBL individuals. This again reiterates the important association of lack of these cytokines with TBL disease. Third, our data on the treatment induced modulation of cytokines also reveals that IL-1β and IL-18 are the only cytokines that exhibit significant treatment induced reversal after TB antigen stimulation. There is growing evidence for a role for IL-1 in the innate response to TB and its central role in host resistance in mouse models has been established using IL-1β or IL-1R knockout mice [14, 15]. Similarly, IL-18 another member of the IL-1 family is also necessary for host resistance since IL-18 knockout mice are more susceptible to TB infection [16]. Interestingly, both IL-1β and IL-18 share a common pathway of production with both of these cytokines requiring cleavage by caspase −1, in a process dependent on the NLRP3 inflammasome [17]. Thus, future studies to explore the role of the inflammasome complex and the caspase family should yield valuable insights into the pathogenesis of TBL.
Defects in the IL-12-IFNγ pathway is an important component of disseminated mycobacterial infection [18], while regulatory cytokines play an important role in down modulation of antigen – specific T cell responses in pulmonary TB [5]. Thus, a lack of significant association of these cytokines in the comparison of TBL versus LTB individuals is surprising. So is the lack of association observed with Type 17 cytokines. Our study suffers from the limitation of having a limited sample size and mainly examining associations, nevertheless, our data clearly delineate an important role for IL-1β and IL-18 in TBL disease. We have also consistently observed differences in the plasma concentrations of cytokines and the concentrations in the media control of the QFT supernatants, although the reason behind this needs to be determined. In addition, we did not have a PTB group of individuals as a comparator group and hence the need to be interpreted with caution. Our data also show some major differences in terms of the significant biomarkers in the periphery of TBL from a previous study by Abhimanyu et al [11]. They found significant increased serum levels of IL-10, IL-8 and TNF-β as markers of TBL but not IL-1β. It is possible that differences in the study population (north versus south Indian), diagnostic criterion (FNAC versus lymph node biopsy) or other confounding factors could have accounted for these differences.
TB lymphadenitis is the commonest form of extrapulmonary TB and, in the face of HIV/AIDS, is becoming a major health problem worldwide [1]. In addition, TBL adds a layer of complexity in the field of TB due to the difficulty in diagnosis and treatment [3]. In this study, we examined a large panel of cytokines, encompassing Type 1, Type 17, IL-1 family and regulatory cytokines, in TBL individuals in comparison to LTB. Our goal was to elucidate correlates of protective immunity that would be reflected by significant differences between TBL and LTB both systemically and in an antigen-specific manner. Moreover, we reasoned that important correlates of protective immunity would not only be different at baseline but that the difference would be reversible upon successful chemotherapy. Indeed, our data reveal IL-1β and IL-18 as two major candidates that fulfill both criteria for protective immunity. Future studies should aim at validating these results in larger sample sizes and different populations.
Highlights.
Reduced circulating concentrations of IL-1β and IL-18 in tuberculous lymphadenitis in comparison to latent tuberculosis infection
Reduced baseline and antigen – specific concentrations of IL-1β and IL-18 in tuberculous lymphadenitis
This reduction is reversible upon standard anti-tuberculous treatment of lymphadenitis individuals
Acknowledgments
We thank V. Rajesh Kumar of NIH-NIRT-ICER and the staff of the Department of Clinical Research, NIRT, and Government Stanley Hospital, Government General Hospital and Government Kilpauk Medical Hospital, Chennai for valuable assistance in recruiting the patients for this study; N. Pavan Kumar, R. Anuradha and Jovvian George of the NIH-NIRT-ICER for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohapatra PR, Janmeja AK. Tuberculous lymphadenitis. J Assoc Physicians India. 2009;57:585–90. [PubMed] [Google Scholar]
- 2.Handa U, Mundi I, Mohan S. Nodal tuberculosis revisited: a review. J Infect Dev Ctries. 2012;6:6–12. doi: 10.3855/jidc.2090. [DOI] [PubMed] [Google Scholar]
- 3.Fontanilla JM, Barnes A, von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis. 2011;53:555–62. doi: 10.1093/cid/cir454. [DOI] [PubMed] [Google Scholar]
- 4.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 5.Mayer-Barber KD, Sher A. Cytokine and lipid mediator networks in tuberculosis. Immunol Rev. 2015;264:264–75. doi: 10.1111/imr.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellner JJ. Immunoregulation in TB: observations and implications. Clin Transl Sci. 2010;3:23–8. doi: 10.1111/j.1752-8062.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt K, Verma S, Ellner JJ, Salgame P. Quest for correlates of protection against tuberculosis. Clin Vaccine Immunol. 2015;22:258–66. doi: 10.1128/CVI.00721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar NP, Sridhar R, Banurekha VV, et al. Expansion of pathogen-specific mono- and multifunctional Th1 and Th17 cells in multi-focal tuberculous lymphadenitis. PLoS One. 2013;8:e57123. doi: 10.1371/journal.pone.0057123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar NP, Sridhar R, Hanna LE, et al. Altered CD8(+) T cell frequency and function in tuberculous lymphadenitis. Tuberculosis (Edinb) 2014;94:482–93. doi: 10.1016/j.tube.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abhimanyu, Bose M, Varma-Basil M, et al. Establishment of Elevated Serum Levels of IL-10, IL-8 and TNF-beta as Potential Peripheral Blood Biomarkers in Tubercular Lymphadenitis: A Prospective Observational Cohort Study. PLoS One. 2016;11:e0145576. doi: 10.1371/journal.pone.0145576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustafa T, Brokstad KA, Mfinanga SG, Wiker HG. Multiplex Analysis of Pro- or Anti-Inflammatory Serum Cytokines and Chemokines in relation to Gender and Age among Tanzanian Tuberculous Lymphadenitis Patients. Tuberc Res Treat. 2015;2015:561490. doi: 10.1155/2015/561490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar NP, Gopinath V, Sridhar R, et al. IL-10 dependent suppression of type 1, type 2 and type 17 cytokines in active pulmonary tuberculosis. PLoS One. 2013;8:e59572. doi: 10.1371/journal.pone.0059572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juffermans NP, Florquin S, Camoglio L, et al. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis. 2000;182:902–8. doi: 10.1086/315771. [DOI] [PubMed] [Google Scholar]
- 15.Mayer-Barber KD, Andrade BB, Barber DL, et al. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–34. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–9. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 18.Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15:968–80. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]


