Abstract
In the last decade, single pulse electrical stimulation (SPES) has been used as an investigational tool in the field of epilepsy surgery. Direct cortical stimulation applied at a frequency of ∼1 Hz can probe cortico-cortical connections by averaging electrocorticogram time-lock to the stimuli (2 x 20–30 trials). These evoked potentials that emanate from adjacent and remote cortices have been termed cortico-cortical evoked potentials (CCEPs). Although limited to patients undergoing invasive presurgical evaluations with intracranial electrodes, CCEP provides a novel way to explore inter-areal connectivity in vivo in the living human brain to probe functional brain networks such as language and cognitive motor networks. In addition to its impact on systems neuroscience, this method, in combination with 50 Hz electrical cortical stimulation, could contribute clinically to map the functional brain systems by tracking the cortico-cortical connections among the functional cortical regions in each individual patient. This approach may help identify the normal cortico-cortical network within pathology as well as reveal connections that might arise from neural plasticity. Because of its high practicality, it has been recently applied for intraoperative monitoring of the functional brain networks for patients with brain tumor. With regard to epilepsy, SPES has been used for the two major purposes, one to probe cortical excitability of the focus, namely, epileptogenicity, and the other to probe seizure networks. Both early (i.e., CCEP) and delayed responses, and probably their high frequency oscillation counterparts, are regarded as a surrogate marker of epileptogenicity. With regards to its impact on the human brain connectivity map, worldwide collaboration is warranted to establish the standardized CCEP connectivity map as a solid reference for non-invasive connectome researches.
Keywords: Cortico-cortical evoked potential, single pulse electrical stimulation, effective connectivity, electrocorticogram, electrical cortical stimulation, functional system mapping
Introduction
In order to better understand the workings of the brain systems, a detailed knowledge of neuronal connectivity between these functional cortical regions is essential. The knowledge of how our brains are wired also helps us better understand the seizure networks involved in generation of seizures and functional deficits. Until very recently, very little was known regarding human inter-areal or cortico-cortical neuronal connectivity. This is mostly due to the fact that there is a limited repertoire of suitable anatomic techniques that can be used in the living human brain. In the 20th century, knowledge of human white matter connectivity such as cortico-cortical and cortico-subcortical connections has mainly come from extrapolation from invasive tract-tracing studies performed in non-human primates by means of retro- and anterograde tracers. It is noteworthy to state in this review that a technique based on tracing the spread of experimentally induced seizures, namely strychnine neuronography, was used to study these pathways before the advent of these modern tract tracing techniques [1].
It was only in the advent of 21st century that we have obtained methods to probe ‘in vivo’ connectivity in humans. These methods are usually classified into two categories: anatomic and functional brain connectivity. Anatomic connectivity is illustrated by diffusion tensor imaging (DTI) using MRI. Diffusion tensor tractography enables us to visualize ‘in vivo dissections’ of association and commissural fibers, and has confirmed the presence of major white matter fasciculi in the living human brain [2]. These pathways, however, are solely determined by mathematical calculations of anisotropy of water molecules. Despite recent technological advancements [3], it is still difficult to entirely trace the white matter tracts beyond fiber-crossings or kissing and into the specific grey matters due to the signal-to-noise ratio.
Functional brain connectivity in a broad sense is further divided into functional connectivity and effective connectivity. Functional connectivity is inferred when spatially disparate neurophysiological events appear to be temporally related, whereas effective connectivity refers to the influence of one neural system over another [4]. Functional connectivity can be defined by non-invasive neurophysiological (EEG, MEG) and functional neuroimaging (PET, fMRI) approaches. Because of its high practicality, resting-state fMRI has been widely used by calculating correlations of the ultraslow fluctuation of BOLD activity. It certainly provides a resolution of few millimeters and serves as a promising non-invasive research tool. It, however, is still too early to rely on its connectivity findings alone for presurgical evaluation since the generator mechanism still remains elusive.
Effective connectivity refers to the causal influence between brain regions. There are two approaches to probe effective connectivity -non-interventional and interventional. The non-interventional approaches are observational and infer causality indirectly by analyzing simultaneous recordings of neural activities to quantify the directionality of functional connections using measures such as Granger causality and dynamic causal modeling [5, 6]. In contrast, interventional approaches use perturbations to directly infer effective connectivity. An electrical stimulus is applied either directly by cortical stimulation or indirectly by transcranial magnetic stimulation, which is precisely defined in time and space. Then the evoked responses are recorded directly by electrical activities (EEG or electrocorticogram (ECoG)) or indirectly by fMRI in order to assess its effects on other brain regions.
As one of the clinical neurophysiologists who engaged in the development and establishment of cortico-cortical evoked potentials (CCEPs), we introduce CCEP as an invasive interventional approach to probe effective connectivity. We review its academic impact on understanding the functional brain networks, and clinical utility for functional ‘system’ mapping and probing epileptogenicity and seizure networks.
CCEP methodology
Historical review
The initial attempts to probe connections by single pulse electrical stimulation (SPES) in living human brains date back to early 1990’s. Goldring and coworkers applied SPES to the sensorimotor cortices to record very adjacent Direct Cortical Responses (DCRs), which were originally explored in animals by Adrian (1936) [7] [8]. These early attempts to map cortical responses focused on defining the wave morphology of DCR (N1 counterpart of CCEP) characteristic to the primary motor, primary sensory or premotor cortex. Wilson and coworkers were the first to apply SPES to probe the adjacent and remote cortical responses [9, 10]. By means of depth electrodes, they applied SPES to mesial temporal structures through the depth electrodes and recorded cortical evoked responses in the ipsilateral and contralateral mesial temporal regions.
Around the beginning of the 21st century, groups at the Cleveland Clinic [11, 12], University of Iowa [13], and King’s College of London [14] independently developed their own methods using SPES to probe effective connectivity or cortical excitability. Since then SPES has been employed in the field of epilepsy surgery to probe functional and seizure networks as well as to probe epileptogenicity (Table 1). The term cortico-cortical evoked potential (CCEP) is widely used for its clarity, which was originally introduced by the Cleveland group (Matsumoto, Nair, Lüders and their colleagues) [11, 12]. The term SPES is also used in particular when probing epileptogenicity[14]. This technique is ‘old’ in a sense that the original attempt was performed in early 20th century, and ‘new’ in that its clinical application has expanded with multichannel intracranial recording in the last decade owing to the development of digital EEG equipment. CCEP cannot directly identify the actual anatomical pathway of the circuit and in this regard it may well be regarded as ‘functional tractography’ as compared with ‘anatomical fiber tractography’ by diffusion tractography.
Table 1.
CCEP connectivity studies to probe functional brain networks
| Direct Cortical Responses | |
|---|---|
| Functional cortical characterization | Goldring et al. 1994 |
| Language system | |
| Dorsal Language Network |
Matsumoto et al. 2004, Conner et al. 2011, Keller et al. 2011, Enatsu et al. 2013 David et al., 2013, Yamao et al. 2014*, Saito et al. 2014*, Tamura et al. 2016* |
| Ventral Language Network | Matsumoto et al. 2004, Umeoka et al. 2009, Koubeissi et al. 2012, Araki et al. 2014 |
| Cognitive motor system | |
| preSMA/SMA-lateral PM/MI | Matsumoto et al. 2007, Kikuchi et al. 2012*, Swann et al. 2012 |
| Negative motor network | Enatsu et al. 2013 |
| Interhemispheric connections | Terada et al. 2008, 2012 |
| Fontal Lobe Network | |
| IFG connectivity | Greenlee et al. 2004, 2007, Garell et al. 2014 |
| Fronto-Parietal connectivity | Matsumoto et al. 2012 |
| Fronto-Temporal connectivity | Lacruz et al. 2007 |
| Limbic Network | |
| Limbic pathways |
Wilson et al. 1990, Catenoix et al., 2005, Kubota et al. 2013, Koubeissi et al. 2013, Lacuey et al. 2014, Enatsu et al. 2014 |
| Interhemispheric connections | Wilson et al. 1991, Umeoka et al. 2009, Jimenez-Jimenez-Jimenez-Jimenez et al. 2015 |
| Insular connectivity | Almashaikhi et al. 2014a, b |
| Auditory system | |
| A1-pSTG connectivity | Howard et al. 2000, Brugee et al. 2003, Oya et al. 2007 |
| Visual system | |
| V1-higher visual cortices | Matsuzaki et al. 2014 |
| Thalamo-cortical network | |
| Pulvinar-Cortices | Rosenberg et al. 2009 |
| Connectivity maps | |
| BA parcellation map | Entz et al. 2014 |
| Comparison with ECoG broadgamma envelope | Keller et al. 2014 |
| Comparison with resting state fMRI | Keller et al. 2011 |
Intraoperative studies
SMA = supplementary motor area; PM = premotor area; MI = primary motor area; IFG = inferior frontal gyrus; A1 = primary auditory cortex; V1 = primary visual cortex; BA = Brodmann’s area
[Those highlighted by yellow is not cited in the maintext, the following numbers are from endnote]
Procedure
In order to avoid possible seizure induction, CCEP study is usually performed after the seizures have been recorded and the antiepileptic medications are restored to the baseline dosage for functional cortical mapping with 50 Hz electrical stimulation. From our experience, seizure induction is extremely rare. SPES is applied to the cortex through intracranial electrodes such as subdural electrodes or depth electrodes. The electrical stimulus is given in a bipolar fashion through a pair of adjacent electrodes to deliver localized current. Electrical stimulus that consists of a constant-current square-wave pulse (pulse width of 0.1–1 ms) is employed typically at a fixed frequency of 1 Hz (some use 0.2, 0.5 or 2 Hz). Either a monophasic pulse with an alternating polarity or a biphasic pulse is used to 1) reduce the stimulus artifacts, 2) avoid electrical charges building up at the cortex (a safety consideration), and 3) avoid polarization of platinum electrodes which can decrease the current density over time. The current is given at 80–100% of the intensity that produces either clinical signs or afterdischarges (ADs) during the standard 50 Hz stimulation. In our setting (monophasic square wave pulse, alternating polarity, 0.3 ms duration, subdural electrodes), the intensity is set at 10–12 mA if no clinical sign or ADs are present at 15 mA, which is usually the case with association cortices. For depth electrodes, the intensity is usually lowered according to the size of contact surface to adjust the total amount of electric charge per unit area (mm2) [1–3 mA (pulse width of 1 ms or 3 ms[15, 16]) to 4–8 mA (pulse width of 1 ms[14, 17] or 0.2–0.3 ms [18, 19])]. In cases in which the stimulus artifact, namely, the excessive baseline drift becomes larger beyond the dynamic range, the intensity should be lowered stepwise by 1 mA until artifacts become small enough to visualize the raw ECoG traces.
CCEP is either on-line or off-line averaged by using the multi-channel intraoperative evoked potential machine or digital EEG machine, respectively. Usually ECoG is recorded with a sampling rate of 1000–5000 Hz. Low frequency filter is usually set between 0.08 to 1 Hz. All the subdural electrodes are referenced to a scalp electrode placed on the skin over the mastoid process contralateral to the side of electrode implantation. CCEPs are obtained by averaging ECoG with a time window of 200–500 ms, time-locked to the stimulus. In each session, at least two trials of 20–30 responses are averaged separately to confirm the reproducibility of the responses.
Initial studies found that CCEP generally consists of an early sharp negative potential (N1: peak 10–50 ms) and a later slow-wave like potential (N2: peak 50–300 ms) [11, 12, 20]. A small positive deflection preceding N1, namely, the onset of N1, is termed P1 by some authors since this could reflect the very first volley to the target cortex [21, 22]. As many CCEP studies have been reported in the last decade, it is now known that CCEP waveform has some variations. Some reports describe variety of polarities and latencies of these potentials[23, 24]. N1 could be recorded as a positive potential, potentially reflecting the positive end of the dipolar activity in the sulcus[11, 12, 25]. CCEP responses occasionally have a very small N1 activity, followed by a relatively large N2 potential. In some case, N1 peak latency could exceed 50 ms [23], and the N2 potential (peak latency of >100 ms) may occur alone. It is natural that CCEP waveforms vary according to the cytoarchitechure of the response site as addressed by human DCR recordings in the primary and secondary sensori-motor cortices[8]. CCEP recording is highly practical because 1) the subjects are not requested to perform any specific task, just lying or sitting on a bed and 2) cortico-cortical connectivity can be probed with directionality information from one stimulus site within a minute. Because of its high practicality and feasibility during general anesthesia, CCEPs are now applied in the intraoperative setting to monitor functional brain networks such as language[26] [27, 28].
It should be mentioned here that advancements of neuroimaging techniques, in particular, coregistration and nagivation techniques, brought great progress in precise anatomical localization of each electrodes both in the extraoperative and intraoperative settings[12, 29]. Together with anatomical precision provided by neuroimaging studies, CCEP method has an excellent temporal resolution and fairly well spatial resolution of interelectrode distance of 5 mm-1 cm.
Generator Mechanism of CCEP
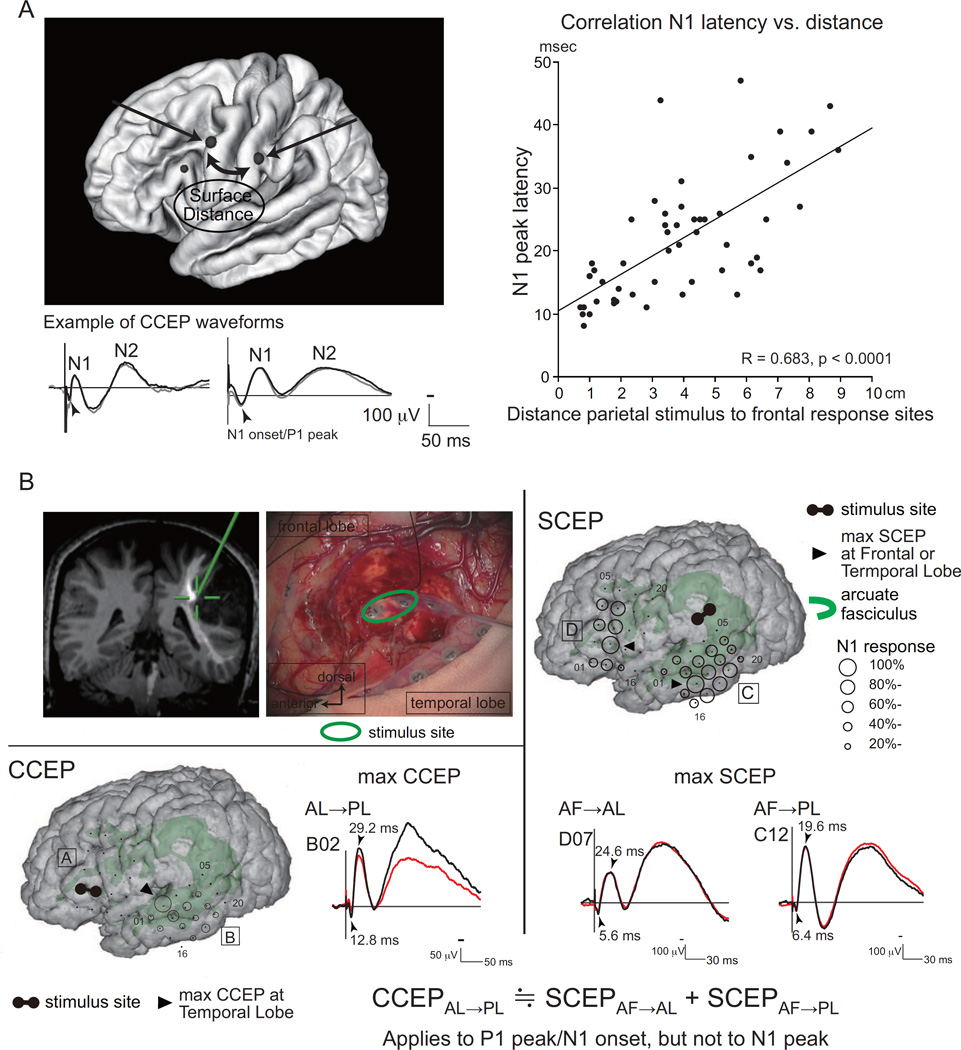
The generator mechanism of CCEP is not precisely known. Two possible modes of impulse propagation have been proposed: direct cortico-cortical propagation through white matter tracts, and indirect cortico-subcortico-cortical propagation via subcortical structures. Our recent studies favor the direct cortico-cortical connection. The parieto-frontal CCEP connectivity study showed a linear correlation between the N1 peak latency and the surface distance from the parietal stimulus site to the frontal maximum response site (Figure 1A) [30]. This observation favors the direct cortico-cortical white matter pathway, because the longer the surface distance is, the proportionally longer the actual white matter pathway connecting the two cortical sites and, accordingly, its traveling time is. This hypothesis was further strengthened by an intraoperative CCEP study. Yamao and his coworkers applied SPES to the anterior perisylvian language area (AL) and recorded CCEPs from the posterior perisylvian language area (PL) during awake tumor surgery [26]. In addition, they also applied SPES to the arcuate fasciculus at the floor of the removal cavity and recorded subcortico-cortical evoked potentials (SCEPs) from AL and PL. Comparison between SCEP and CCEP latencies demonstrated that the very first volley, as reflected by the first positive deflection, namely N1 onset or P1 peak, was directly conveyed through the arcuate faciculus (Figure 1B). Regarding the relatively long N1 peak latency (∼10 ms even at the adjacent cortex), oligo- or multi- synaptic responses, i.e., local jitter of synaptic activity at the site of stimulation and at the target cortex, could account for the relatively long-latency, blunt negative peak of the N1 potential. After the excitation of the cortex giving rise to the N1, the N2 potential could then be generated in and immediately surrounding the cortex via either a local cortico-cortical or a cortico-subcortico-cortical reverberating cirtuit.
Figure 1. Evidence favoring direct cortico-cortical connections in generation of CCEPs.
A. Lateral parieto-frontal connectivity was explored by stimulating the parietal pairs and recording CCEPs from the frontal lobe. Representative CCEP waveforms (two subaverages each, N1 and N2 potentials) are shown (left, bottom). The vertical bar corresponds to the time of singe pulse electrical stimulation. Surface distance from the parietal stimulus sites (midpoint of the pair is used for calculation) to the maximum frontal CCEP response was measured. A positive correlation was observed between the surface distance and the N1 peak latency of the maximum response. When the regression line is extrapolated to the zero distance, there still remains ∼10 ms. This likely corresponds to the relatively long peak latency (∼10 ms) observed in the locally evoked DCR in humans [8]. Namely, direct cortical stimulation produces oligo- or multi- synaptic responses in the local cortical circuits. Adapted with permission from Ref. [30].
B. Subcortico-cortical evoked potentials (SCEPs) in a representative case. Left upper panel: Stimulation site (electrode pair, green circle) at the deep white matter at the floor of the tumor removal cavity. The site (green cross) was attached to the AF tract in the coregistered image on the neuro-navigation system.
Right panel: SPES (1 Hz, pulse width 0.3 ms, alternating polarity, 15 mA, 2 x 30 trials) was delivered to the stimulus site, and SCEPs were recorded both from the AL (SCEPAF→AL, D plate) and PL (SCEPAF→PL,C plate) at and around the terminations of the AF tract. The diameter of the circle at each electrode represented the percentile to the largest amplitude (N1) at the maximum SCEP response site.
Left lower panel: SPES of the AL (confirmed by 50 Hz electrical stimulation) produced CCEPs in the temporal lobe (presumed PL). The diameter of the circle at each electrode represented the percentile to the largest amplitude (N1) at the maximum CCEP response site. At the maximum response sites, the summation of P1 peak/N1 onset latencies of SCEPs (SCEPAF→AL + SCEPAF→PL, 12.0 ms) was very close to the P1 peak/N1 onset latency of CCEPAL→PL (12.8 ms). Adapted with permission from [26].
AF = arcuate fasciculus, AL = anterior language area, DCR = direct cortical response, PL = posterior language area, SPES = single pulse electrical stimulation
The mode of activation is likely to be orthodromic, but a possibility of an antidromic activation of the presynaptic axonal terminals of the association fibers remains. However, compared with highly structured pyramidal neurons or interneurons, poorly organized arrangements of these small axon terminals seem to be less favorable for effective direct activation.
Probing functional brain networks by CCEP
Although limited to the invasive presurgical evaluations with chronic implantation of intracranial electrodes, CCEP has been widely applied to probe functional brain systems in vivo since the introduction of CCEP methodology at the beginning of this century (Table 1).
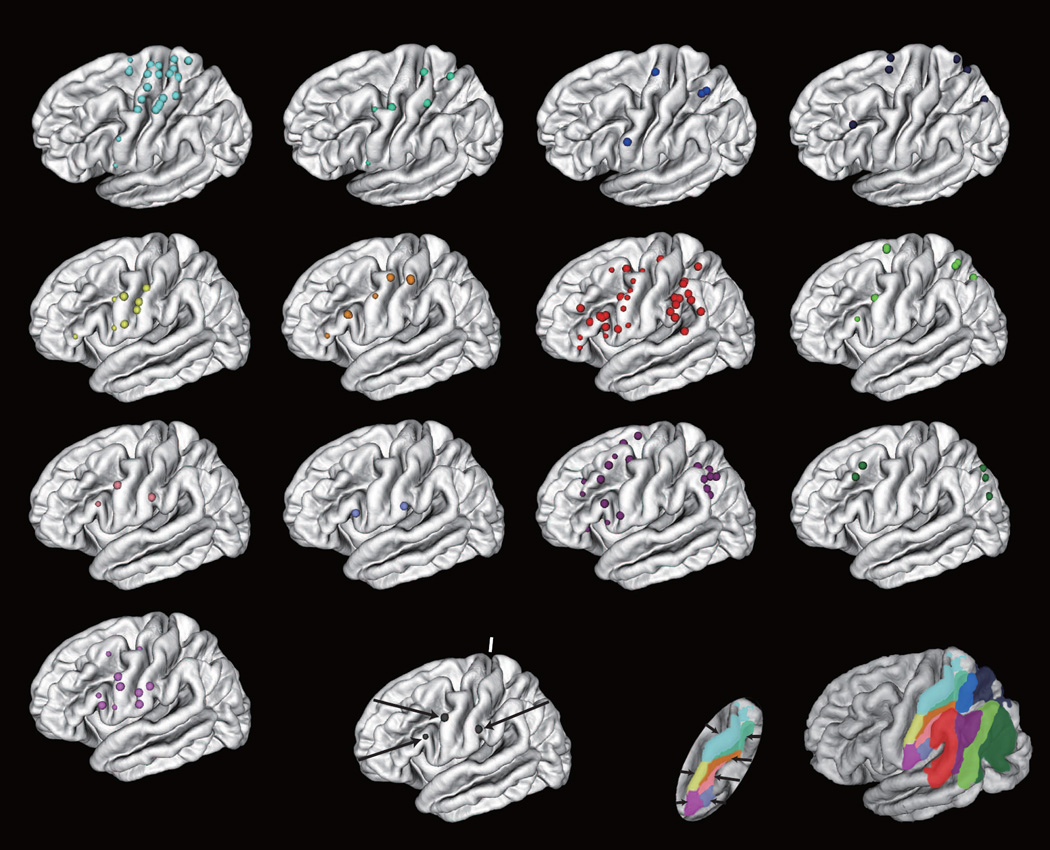
The language network has been extensively studied using CCEP since this function is unique to human and the findings from monkey tracer studies are largely not relevant. We first explored the classical language network [12], which is now regarded as the dorsal language stream that engages mainly in auditory-to-motor mapping (Figure 2A) [31, 32]. The AL or Broca’s area, which was defined by 50 Hz stimulation, had connections not only to the classical Wernicke’s area (posterior part of the superior temporal gyrus), but also to the adjacent middle temporal gyrus and the inferior parietal lobule. The CCEP distribution was generally larger than the core language area defined by 50 Hz stimulation (Figure 2B). The connection was site specific: stimulation of the adjacent face motor area showed entirely different CCEP distribution mainly in the postcentral gyrus. The CCEP connectivity pattern from Broca’s area to both the lateral inferior parietal and temporal areas was immediately confirmed by the diffusion tensor tractography study (Figure 4C)[33]. Both effective and anatomical connectivity studies complemented each other to establish the contemporary connections within the dorsal language stream. The CCEP connectivity pattern in the dorsal language stream has been consistently reported by other groups [15, 24, 27, 34, 35] [28]. In addition, a series of language CCEP studies demonstrated bidirectional connections between AL and PL through AF, which has been traditionally considered as a unidirectional pathway from PL to AL (Wernicke-Geschwind model) in the 20th century. Effective connectivity demonstrated here supports the contemporary concepts of language organization, namely that neuronal groups participate as components of a network by means of feed-forward and feed-back projections [36].
Figure 2.
A. Representative CCEPAL→PL in the extraoperative setting. SPES was delivered to the AL defined by 50 Hz electrical stimulation (red electrode pair) and CCEPs were recorded from the posterior language area (plate A). Two subaverage waveforms are plotted in superimposition for each electrode. Evoked responses were mainly recorded from the posterior part of the superior temporal gyrus and the adjacent portion of the middle temporal gyrus in and surrounding the language electrode defined by 50 Hz cortical stimulation (A18: highlighted with a dotted circle). STS: superior temporal sulcus, Sylv: sylvian fissure, na: CCEP not available due to high impedance in the recording electrode. From [12] with permission.
B. Circle maps (N2 amplitude) for CCEPAL→PL in other patients. Language electrodes (red) are defined by 50 Hz electrical stimulation. Major sulci are highlighted by white lines (CS: central sulcus). CCEP connectivity pattern from the AL indicates a large posterior language network distributed over the posterior part of the superior temporal gyrus, adjacent portion of the middle temporal gyrus and the supramarginal gyrus in and immediately surrounding the language electrode. Adapted with permission from [12].
C. Deterministic diffusion tensor tractography complemented the CCEP connectivity pattern by providing the anatomical white matter pathways between AL and PL (both the inferior parietal lobule and the posterior part of the superior and middle temporal gyri). From [33] with permission.
Other conventions are the same as for Figure 1.
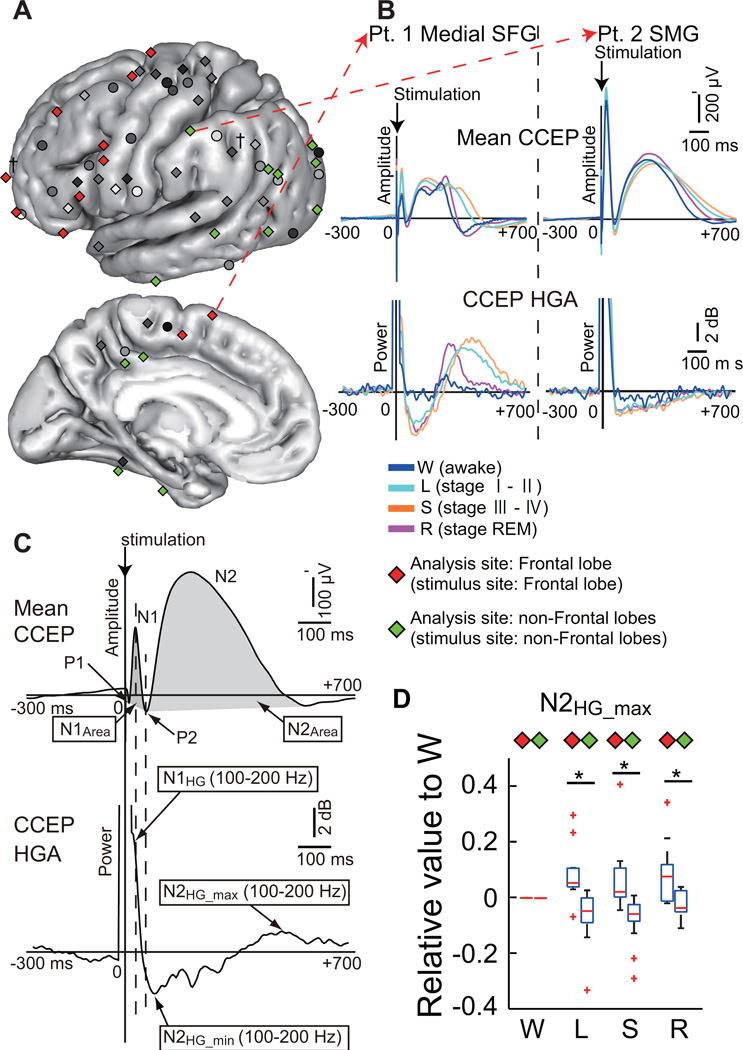
Figure 4. Modulation of intralobar connectivity and excitability during sleep.
A: The modulation mode was compared between the frontal lobe (N = 11) and other lobes (N = 13). Stimulus ( ) and analysis (Frontal lobe
) and analysis (Frontal lobe  , other lobes
, other lobes  ) sites from all patients were coregistered onto the MNI standard space (all in the left hemisphere for display purposes). B: Representative CCEP waveform and its HGA counterpart in the frontal (superior frontal gyrus [SFG] of Pt. 1) and parietal (the supramarginal gyrus [SMG] of Pt. 2) lobes. C: Five indices were used for statistical analysis. The troughs or positive sharp reflections before and after the N1 peak were termed P1 and P2, respectively. Each index is highlighted by a rectangle: CCEP sizes (area under the curve) of N1 and N2 (N1Area and N2Area); their high-gamma activity (HGA) counterparts (N1HG, N2HG_min, and N2HG_max). D: Values relative to W were calculated in the sleep stages (L: light sleep, S: slow wave sleep, R: REM sleep) for five indices, and these values were compared between the Frontal lobe and the non- Frontal lobes. In each sleep stage, the left column denotes values of the Frontal sites and the right column denotes values of the non-Frontal sites. A significant difference was observed in N2HG_max (L, S, R) between Frontal and non-frontal sites. The direction of the change was opposite: increase in frontal sites and decrease in non-Frontal sites. †Recorded from the contralateral hemisphere. *Statistically significant at P < 0.05, Mann–Whitney U test, corrected by FDR = 0.05. Adapted with permission from Ref. [56].
) sites from all patients were coregistered onto the MNI standard space (all in the left hemisphere for display purposes). B: Representative CCEP waveform and its HGA counterpart in the frontal (superior frontal gyrus [SFG] of Pt. 1) and parietal (the supramarginal gyrus [SMG] of Pt. 2) lobes. C: Five indices were used for statistical analysis. The troughs or positive sharp reflections before and after the N1 peak were termed P1 and P2, respectively. Each index is highlighted by a rectangle: CCEP sizes (area under the curve) of N1 and N2 (N1Area and N2Area); their high-gamma activity (HGA) counterparts (N1HG, N2HG_min, and N2HG_max). D: Values relative to W were calculated in the sleep stages (L: light sleep, S: slow wave sleep, R: REM sleep) for five indices, and these values were compared between the Frontal lobe and the non- Frontal lobes. In each sleep stage, the left column denotes values of the Frontal sites and the right column denotes values of the non-Frontal sites. A significant difference was observed in N2HG_max (L, S, R) between Frontal and non-frontal sites. The direction of the change was opposite: increase in frontal sites and decrease in non-Frontal sites. †Recorded from the contralateral hemisphere. *Statistically significant at P < 0.05, Mann–Whitney U test, corrected by FDR = 0.05. Adapted with permission from Ref. [56].
In order to understand the rapid spread of epileptic discharges through the cortico-cortical networks involved in ictal motor manifestation, it is important to know cortico-cortical connections between the lateral and medial motor cortices. This network is also important to understand various cognitive motor controls for which lateral and medial premotor cortices coordinate with each other. By means of CCEP, we demonstrated a human cortico-cortical network connecting 1) anatomical homologous areas of the lateral and medial motor cortex along the rostro-caudal cognitive-motor gradient [37–39] (e.g., supplementary motor area (SMA) to the caudal lateral premotor/primary motor (MI) area, pre-SMA to the rostral lateral premotor area), and 2) the somatotopically homologous regions in the lateral and medial motor cortices (e.g., the face SMA to the precentral hand face area) in a reciprocal manner [20]. Clinically, these circuits could account for the propagation of epileptic discharge from/into SMA [40] and the atypical motor responses infrequently seen in standard cortical stimulation: tonic and clonic responses in MI and SMA stimulation, respectively [41]. Functionally, it was not only the dorsal premotor area (mainly the caudal superior frontal gyrus and adjacent precentral gyrus) but also the ventral premotor area (mainly the caudal inferior frontal gyrus and adjacent precentral gyrus) that connected to the pre-SMA and SMA. The latter CCEP connectivity finding accounts for a recent diffusion tractography finding of the frontal aslant tract that connects the rostral SMA and the caudal part of the ventrolateral frontal area [42].
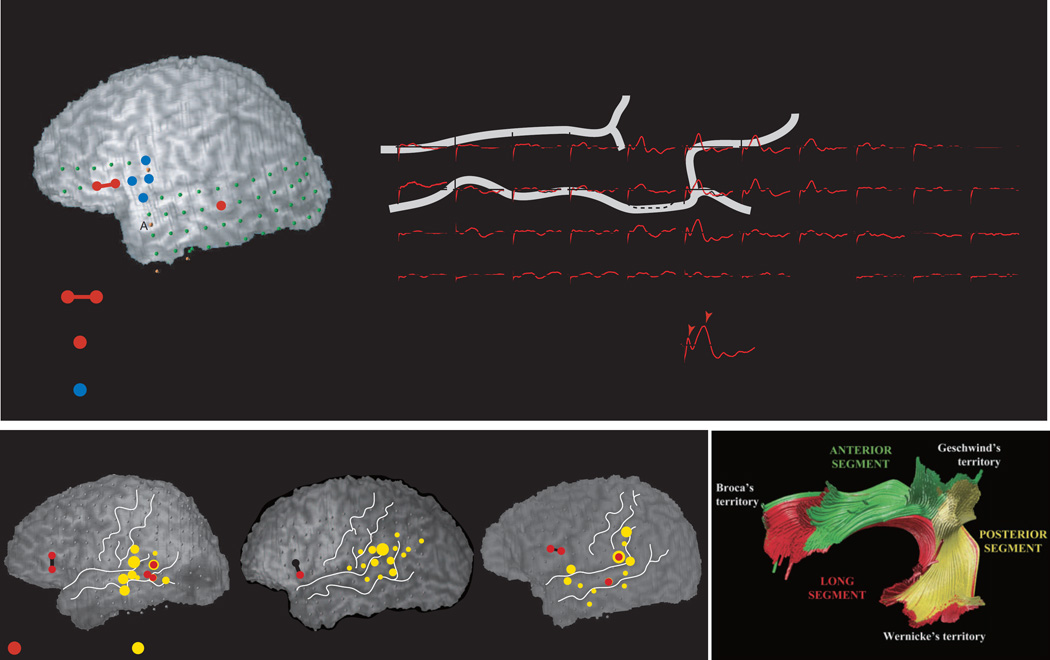
Detailed knowledge of the parieto-frontal network is important for understanding spike propagation from the parietal to the frontal lobe in parieto-occipital lobe epilepsy. From the viewpoint of behavioral neurology, this network is essential in sensori-motor integration for various complex behaviors, and its disruption is associated with the pathophysiology of apraxia and visuo-spatial disorders. Inter-areal connections of the lateral parieto-frontal network were investigated by means of CCEP [30]. The lateral parieto-frontal network was characterized by 1) a near-to-near and distant-to-distant, mirror symmetric configuration across the central sulcus, 2) preserved dorso-ventral organization (the inferior parietal lobule to the ventral premotor area and the superior parietal lobule to the dorsal premotor area), and 3) projections to more than one frontal cortical sites in 56% of explored connections (Figure 3). These CCEP connectivity findings provided an anatomical blueprint underlying the lateral parieto-frontal network and demonstrated a connectivity pattern similar to non-human primates in the newly developed inferior parietal lobule in humans. From the viewpoint of seizure propagation, the mirror symmetric configuration across the central sulcus could account for the rapid spread of ictal discharges from the posterior parietal cortex into the frontal lobe. This would lead to ictal elementary or complex motor manifestations in patients with parieto-occipital lobe epilepsy.
Figure 3. Standardized parieto-frontal CCEP connectivity map.
3D display of the stimulus and response sites in the MNI standard space. The stimulus and response sites are accumulated from all the 7 patients and coregistered into the MNI standard space. All the stimulus and response sites are collapsed onto the left convexity for a display purpose. Stimulus sites are labeled and displayed together according to the Jülich cytoarchitectonic atlas on the same 3D brain (see the label in the left upper corner of each 3D brain). Regarding the frontal CCEP responses, a large sphere represents the location of the electrode showing the maximum CCEP response, i.e., the target site of the predominant connection from the stimulus site. A small sphere represents the electrode showing the maximum response of the additional, separate CCEP field, i.e., the target site of the additional divergent connection from the stimulus site. Note the location of the maximum and additional response sites may be overlapped within the lateral frontal area. Also note that the parieto-frontal connections were not equally distributed along the central sulcus partly due to the less electrode coverage in the dorsal part than the ventral part. The lateral view of the 3D brain in the right lower corner indicates the parietal segmentations of the Jülich atlas where electrical stimuli were applied. Two general frameworks are deduced from the CCEP connectivity findings: a near-to-near and distant-to-distant, mirror symmetric configuration across the central sulcus, and preserved dorso-ventral organization (the inferior parietal lobule to the ventral premotor area and the superior parietal lobule to the dorsal premotor area).
Limbic network has been extensively investigated since this network is involved in the seizure network of mesial temporal lobe epilepsy as well as important brain functions such as memory. Pioneered by Wilson and coworkers in early 1990’s [9, 10], its connectivity has been extensively investigated over the last several years. The Cleveland group studied intra-limbic networks by means of SEEG and found that various regions within the limbic network are intimately connected in reverberating circuits and linked to specific ipsilateral and contralateral regions, which may reflect distinct functional roles [18] [19, 43, 44]. In patients with posterior cingulate epilepsy, CCEP investigations revealed effective connectivity from the posterior cingulate gyrus to parietal, temporal, mesial occipital and medial frontal areas. Patients indeed presented different semiologies (motor manifestation vs. dialeptic/automotor seizure) depending upon the seizure spread patterns through the network revealed by CCEP.
Although limited to rare opportunities in patients with intractable partial epilepsy who undergo invasive evaluation, CCEP can provide one of the most solid connectivity maps by taking advantage of its ability to probe effective connectivity with high spatiotemporal resolution [30, 45] [15, 46]. The CCEP connectivity map can be used as a reference for non-invasive connectivity studies. Although the number of enrolled patients are still small, a general correspondence was observed between the CCEP connectivity and functional (resting-state fMRI) or anatomic (diffusion tractography) findings in the dorsal language and parieto-frontal networks[26, 34, 47] [24]. In order to make a large-scale standardized CCEP connectivity map over hundreds of patients, a worldwide collaboration is highly important to gather the CCEP data both from subdural electrodes and SEEG.
Clinical system mapping by combined high and low frequency electrical stimulation
In order to map the functionally relevant cortices such as language and motor cortices, it is important to know whether the function coexists or not within the pathology. Focal cortical dysplasia (FCD) is one of the common causes of intractable partial epilepsy. While functional reorganization is presumed to occur at and around FCD, cortical functions can co-localize at ‘MRI-negative’ FCD where cortical dyslamination and columnar disorganization are noted in the absence of balloon cells [48]. CCEP investigation successfully delineated the presence of the motor and language networks within ‘MRI-negative’ FCD, while CCEPs were occasionally recorded partly within ‘MRI-positive’ FCD [12, 20]. In these circumstances, it would be ideal to map the whole functional system, the cortical regions and inter-regional connections, to distinguish whether the cortical region around or at the pathology is functional and constitutes a part of the functional network. Thus, when altered functional configuration is presumed at and around the focus, combined 50 Hz and 1 Hz stimulation would help identify the cortico-cortical network of a given function within the context of pathology and any resultant plasticity of brain systems. Our preliminary investigation indicates the utility of system mapping in probing the praxis subnetwork within the ventral parieto-frontal network [49].
CCEP investigation can be applicable to intraoperative mapping and monitoring since it takes only <1 min to probe cortico-cortical connections from one stimulus site. We have applied CCEP to monitor the cortical motor network and dorsal language pathway. SPES to the primary motor area of the hand can help probe the candidate area of the hand SMA so that we can limit the 5 train stimulation to the candidate electrodes [50].
As pioneered by Duffau and colleagues, high frequency white matter stimulation can probe eloquent fibers, such as those related with motor and language functions[51]. Disconnection of these eloquent fibers results in motor or language impairment. By analogy to the motor evoked potential monitoring, we attempted to monitor the integrity of AF by stimulating AL and recording CCEPs from PL in our pilot study [26]. CCEPs were reproducibly recorded during general anesthesia. The CCEP connectivity pattern, taken together with preoperative non-invasive anatomical and functional MRI, successfully probed the dorsal language pathway. The frontal stimulus site, when 50 Hz stimulation was performed after the patients were awakened, produced language impairment in all patients, indicating that the CCEP connectivity pattern during general anesthesia can locate the core AL. Integrity of the language network was monitored by stimulating AL and by recording CCEPs from PL consecutively during both general anesthesia and awake condition. Despite an amplitude decline (<32%) in two patients, CCEP monitoring successfully prevented persistent language impairment. Intraoperative CCEP monitoring is clinically feasible for evaluating the integrity of the dorsal language network. This intraoperative method has been introduced successfully in other institutes[27, 28] and was combined with other methods such as high gamma activity mapping during receptive language tasks[28]. Enrollment of a larger number of patients is warranted to establish its clinical utility. Similar attempts could be applicable to intraoperative mapping and monitoring of other systems such as the cortical motor network, praxis network and ventral language stream [50].
SPES to probe epileptogenicity and seizure networks
With regard to epilepsy, SPES has been used for the two major purposes, one to probe cortical excitability of the focus, namely, epileptogenicity, and the other to probe seizure networks. Alarcon and coworkers are the first to apply SPES to probe epileptogenictiy. By applying SPES in a lower frequency such as 0.1 or 0.2 Hz, they found that two groups of cortical responses occur – the early and delayed responses. The early response corresponds to CCEP and can be recorded both from normal and epileptic cortices. Delayed responses, which usually occur 100 ms to 1.5 sec after the electrical stimuli, appear to be specific to the epileptogenic zone and could be considered as a surrogate marker of epileptogenicity[14, 52, 53]. Removal of areas involved in generating delayed responses was associated with good seizure control[53].
The Cleveland group focused on the change of the waveforms of CCEPs/early responses based upon the notion that CCEP would reflect the cortical excitability at the stimulus and/or response site. By comparing the large adjacent CCEP response presumably generated through the dense short U-fibers, they found that stimulation of the ictal onset zone results in a larger N1 amplitude than when the control normal cortex is stimulated [54]. Additionally, ictal onset patterns characterized by repetitive spiking show larger CCEP amplitudes than those by focal paroxysmal fast activity [55]. These early or delayed responses could be used as an interictal surrogate marker of epileptogenicity.
By analogy to interictal epileptic high frequency oscillations (HFO) overriding on the epileptic spike, attempts have been made to investigate high frequency activities (HFA) induced by SPES. In order to understand the properties of SPES-induced HFA, Usami and his coworkers focused on HFA (100–200 Hz) at the timing of N1 (HFA-CCEPN1) in the non-epileptic cortices [56]. They found that HFA-CCEPN1 power correlated with the N1 amplitude or size. HFA-CCEPN1 became larger in non-REM sleep than the awake state, while that in REM sleep was in the intermediate state between them. This physiological change of cortical excitability to the exogenous input (cortico-cortical input by SPES) likely underlies increased occurrence of epileptic spikes/HFOs during non-REM sleep. During non-REM sleep, HGA increase at the N1 phase was immediately followed by HFA decrease or inhibition at the timing of N2 onset or ascending slope. The HGA decrease was then temporally followed by HGA re-increase at or after N2 peak during non-REM sleep. This HFA rebound or re-increase of neuronal synchrony was largest in the frontal lobe compared with the other lobes. This neuronal property of the frontal lobe may account for frequent nocturnal seizures in frontal lobe epilepsy.
Regarding induced HFA in the epileptic focus, the Netherland group found that induced HFA in the period of early and delayed responses were larger in the seizure-onset zone compared with non seizure-onset zone [57] [58]. We focused on the HFA (ripple and fast ripple range) at the timing of N1 (very early HFA) and found that, similar to the features of spontaneous epileptic HFOs, HFA-CCEPN1 power was greater in the seizure onset zone than outside the seizure onset zone, particularly in MTLE [59].
SPES/CCEP has been employed to probe the seizure network in individual patients. By applying single pulse stimulation to the ictal onset zone, we could delineate the cortico-cortical network involved in interictal spike propagation in each individual patient. This would be clinically useful to differentiate “green” spread spikes from “red” spikes originating from the epileptogenic focus. Attempts have also been made to probe ictal propagation pattern. The topography of cortico-cortical networks as determined by CCEPs is not always consistent with the seizure spread. Enatsu and coworkers compared the ictal EEG spread pattern and CCEP connectivity pattern by the focus stimulation, and found general correspondence in two out of three patients with posterior cingulate epilepsy [18]. Furthermore, in cases where focal epilepsy was associated with secondary generalization, the discrepancy between the CCEP distribution and ictal propagation pattern was larger in patients with secondary generalization than in those without [60]. Ictal propagation to regions not generating CCEPs by the focus stimulation supports the notion of step-wise seizure propagation to regions that are not under the direct influence of the ictal onset zone.
It has not yet been solved whether the connections within the seizure networks are different from those in the normal or physiological networks. Lacruz and coworkers found that ipsi- and contra-lateral frontal and temporal connections between normal and epileptogenic hemispheres were similar [61]. A later graph theoretical analysis of CCEP connectivity confirmed similar network topology between the seizure onset zone and the control cortex [62]. Taken together with the interictal N1 amplitude increase within the seizure network [54, 60], it is likely that the strength of the functional connections, but not the distribution, is affected by the epileptic condition. Indeed, our incidental CCEP recording by the focus stimulation during an aura showed increased CCEP amplitudes both within the ictal onset zone and the spread areas, but the distribution remained the same (see Figure 3 of [63]). In agreement with this notion, a recent combined diffusion tractography and FDG-PET study revealed that the white matter integrity change, namely FA decrease, in mesial temporal lobe epilepsy was evident in the seizure propagation pathway (through the existing fasciculi such as the uncinate fasciculus) that connect the focus and remote functional deficit zone [64]. No aberrant white matter pathway was detected by probabilistic diffusion tractography. The degree of FA decrease was significantly more intense in the seizure propagation pathway than other major white matter fasciculi.
Future perspectives
Although limited to patients undergoing invasive presurgical evaluations, CCEP provides a new way to explore inter-areal connectivity in vivo in the living human brain. In addition to its impact on the systems neuroscience, this method, in combination with 50 Hz electrical cortical stimulation, could contribute clinically to map the functional brain systems by tracking the cortico-cortical connections among the functional cortical regions in each individual patient. This approach may help identify the normal cortico-cortical network within the pathology or the plasticity of brain systems in conjunction with pathology. Because of its high practicality, it is also applicable to intraoperative monitoring of the functional brain networks for patients with brain tumor. Further studies with large patient cohorts would establish its clinical utility for mapping functional brain networks as a part of presurgical evaluations. Worldwide collaboration is also necessary to make a CCEP effective connectivity map in the standard space such as the MNI space with a large patient cohort. The common connectome framework warrants multidisciplinary approaches to establish the human standardized connectivity map by incorporating both invasive and non-invasive connectivity findings.
In relation to epileptogenicity, CCEP helps us study the cortical excitability as well as locate the network involved in the spread of epileptic discharges. In particular, pilot studies indicated the clinical utility of SPES induced very early (CCEP N1), early and delayed responses and its HFO counterparts as interictal surrogate markers of epileptogenicity. Inter-institutional collaboration warrants establishment of its clinical efficacy.
COI
The authors have nothing to declare for the contents of this manuscript.
Acknowledgments
The authors would like to thank Professors Hiroshi Shibasaki, Hans Lüders, and Akio Ikeda for their long-standing supports and advices for establishment of CCEP methodology. A series of CCEP studies have been partly supported by the Advanced International Clinical Fellowship Award from the Cleveland Clinic Foundation, KAKENHI 17790578, 20591022, 23591273, 26282218, 26560465, 15H01664, 15H05874, 15K10361 from the Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Research Grants from the Japan Epilepsy Research Foundation, SPIRITS (Supporting Program for Interaction-based Initiative Team Studies) from Kyoto University, and NIH RO1 Grant (RNS089212A_A7886P1).
Abbreviations
- AL
anterior language area
- AF
arcuate fascisulus
- CCEP
cortico-cortical evoked potential
- ECoG
electrocorticogram
- FCD
focal cortical dysplasia
- HFA
high frequency activity
- HFO
high frequency oscillation
- MI
primary motor area
- PL
posterior language area
- SCEP
subcortico-cortical evoked potential
- SEEG
stereoelectroencephalogram
- SMA
supplementary motor ara
- SPES
single pulse electrical stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bailey P, von Bonin G, Garol H, McCulloch W. Long association fibers in cerebral hemispheres of monkey and chimpanzee. J Neurophysiol. 1943;6:129–134. [Google Scholar]
- 2.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 3.Dell’acqua F, Scifo P, Rizzo G, Catani M, Simmons A, Scotti G, Fazio F. A modified damped Richardson-Lucy algorithm to reduce isotropic background effects in spherical deconvolution. Neuroimage. 2010;49:1446–1458. doi: 10.1016/j.neuroimage.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Lee L, Harrison L, Mechelli A. A report of the functional connectivity workshop, Dusseldorf 2002. Neuroimage. 2003;19:457–465. doi: 10.1016/s1053-8119(03)00062-4. [DOI] [PubMed] [Google Scholar]
- 5.Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiebel SJ, Garrido MI, Moran R, Chen CC, Friston KJ. Dynamic causal modeling for EEG and MEG. Hum Brain Mapp. 2009;30:1866–1876. doi: 10.1002/hbm.20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adrian E. The spread of activity in the cerebral cortex. J Physiol (Lond) 1936;88:127–161. doi: 10.1113/jphysiol.1936.sp003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldring S, Harding GW, Gregorie EM. Distinctive electrophysiological characteristics of functionally discrete brain areas: a tenable approach to functional localization. J Neurosurg. 1994;80:701–709. doi: 10.3171/jns.1994.80.4.0701. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CL, Isokawa M, Babb TL, Crandall PH. Functional connections in the human temporal lobe. I. Analysis of limbic system pathways using neuronal responses evoked by electrical stimulation. Exp Brain Res. 1990;82:279–292. doi: 10.1007/BF00231248. [DOI] [PubMed] [Google Scholar]
- 10.Wilson CL, Isokawa M, Babb TL, Crandall PH, Levesque MF, Engel J., Jr Functional connections in the human temporal lobe. II. Evidence for a loss of functional linkage between contralateral limbic structures. Exp Brain Res. 1991;85:174–187. doi: 10.1007/BF00229999. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Luders HO. Cortico-cortical evoked potentials. In: Luders HO, editor. Deep brain stimulation and epilepsy. London: Martin Dunitz; 2004. pp. 105–111. [Google Scholar]
- 12.Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- 13.Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Valentin A, Anderson M, Alarcon G, Seoane JJ, Selway R, Binnie CD, Polkey CE. Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain. 2002;125:1709–1718. doi: 10.1093/brain/awf187. [DOI] [PubMed] [Google Scholar]
- 15.David O, Job AS, De Palma L, Hoffmann D, Minotti L, Kahane P. Probabilistic functional tractography of the human cortex. Neuroimage. 2013;80:307–317. doi: 10.1016/j.neuroimage.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg DS, Mauguiere F, Catenoix H, Faillenot I, Magnin M. Reciprocal thalamocortical connectivity of the medial pulvinar: a depth stimulation and evoked potential study in human brain. Cereb Cortex. 2009;19:1462–1473. doi: 10.1093/cercor/bhn185. [DOI] [PubMed] [Google Scholar]
- 17.Lacruz ME, Valentin A, Seoane JJ, Morris RG, Selway RP, Alarcon G. Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience. 2010;170:623–632. doi: 10.1016/j.neuroscience.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Enatsu R, Gonzalez-Martinez J, Bulacio J, Kubota Y, Mosher J, Burgess RC, Najm I, Nair DR. Connections of the limbic network: A corticocortical evoked potentials study. Cortex. 2015;62:20–33. doi: 10.1016/j.cortex.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Kubota Y, Enatsu R, Gonzalez-Martinez J, Bulacio J, Mosher J, Burgess RC, Nair DR. In vivo human hippocampal cingulate connectivity: a corticocortical evoked potentials (CCEPs) study. Clin Neurophysiol. 2013;124:1547–1556. doi: 10.1016/j.clinph.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain. 2007;130:181–197. doi: 10.1093/brain/awl257. [DOI] [PubMed] [Google Scholar]
- 21.Terada K, Umeoka S, Usui N, Baba K, Usui K, Fujitani S, Matsuda K, Tottori T, Nakamura F, Inoue Y. Uneven interhemispheric connections between left and right primary sensori-motor areas. Hum Brain Mapp. 2012;33:14–26. doi: 10.1002/hbm.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terada K, Usui N, Umeoka S, Baba K, Mihara T, Matsuda K, Tottori T, Agari T, Nakamura F, Inoue Y. Interhemispheric connection of motor areas in humans. J Clin Neurophysiol. 2008;25:351–356. doi: 10.1097/WNP.0b013e31818f4fec. [DOI] [PubMed] [Google Scholar]
- 23.Araki K, Terada K, Usui K, Usui N, Araki Y, Baba K, Matsuda K, Tottori T, Inoue Y. Bidirectional neural connectivity between basal temporal and posterior language areas in humans. Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Keller CJ, Bickel S, Entz L, Ulbert I, Milham MP, Kelly C, Mehta AD. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Natl Acad Sci U S A. 2011;108:10308–10313. doi: 10.1073/pnas.1019750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller CJ, Honey CJ, Megevand P, Entz L, Ulbert I, Mehta AD. Mapping human brain networks with cortico-cortical evoked potentials. Philos Trans R Soc Lond B Biol Sci. 2014:369. doi: 10.1098/rstb.2013.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamao Y, Matsumoto R, Kunieda T, Arakawa Y, Kobayashi K, Usami K, Shibata S, Kikuchi T, Sawamoto N, Mikuni N, Ikeda A, Fukuyama H, Miyamoto S. Intraoperative dorsal language network mapping by using single-pulse electrical stimulation. Hum Brain Mapp. 2014;35:4345–4361. doi: 10.1002/hbm.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito T, Tamura M, Muragaki Y, Maruyama T, Kubota Y, Fukuchi S, Nitta M, Chernov M, Okamoto S, Sugiyama K, Kurisu K, Sakai KL, Okada Y, Iseki H. Intraoperative cortico-cortical evoked potentials for the evaluation of language function during brain tumor resection: initial experience with 13 cases. J Neurosurg. 2014;121:827–838. doi: 10.3171/2014.4.JNS131195. [DOI] [PubMed] [Google Scholar]
- 28.Tamura Y, Ogawa H, Kapeller C, Prueckl R, Takeuchi F, Anei R, Ritaccio A, Guger C, Kamada K. Passive language mapping combining real-time oscillation analysis with cortico-cortical evoked potentials for awake craniotomy. J Neurosurg. 2016:1–9. doi: 10.3171/2015.4.JNS15193. [DOI] [PubMed] [Google Scholar]
- 29.Dykstra AR, Chan AM, Quinn BT, Zepeda R, Keller CJ, Cormier J, Madsen JR, Eskandar EN, Cash SS. Individualized localization and cortical surface-based registration of intracranial electrodes. Neuroimage. 2012;59:3563–3570. doi: 10.1016/j.neuroimage.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto R, Nair DR, Ikeda A, Fumuro T, Lapresto E, Mikuni N, Bingaman W, Miyamoto S, Fukuyama H, Takahashi R, Najm I, Shibasaki H, Luders HO. Parieto-frontal network in humans studied by cortico-cortical evoked potential. Hum Brain Mapp. 2012;33:2856–2872. doi: 10.1002/hbm.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 33.Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 34.Conner CR, Ellmore TM, DiSano MA, Pieters TA, Potter AW, Tandon N. Anatomic and electro-physiologic connectivity of the language system: a combined DTI-CCEP study. Comput Biol Med. 2011;41:1100–1109. doi: 10.1016/j.compbiomed.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garell PC, Bakken H, Greenlee JD, Volkov I, Reale RA, Oya H, Kawasaki H, Howard MA, Brugge JF. Functional connection between posterior superior temporal gyrus and ventrolateral prefrontal cortex in human. Cereb Cortex. 2013;23:2309–2321. doi: 10.1093/cercor/bhs220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damasio A, Damasio H. Aphasia and neural basis of language. In: Mesulam M, editor. Principles of Behavioral and Cognitive Neurology. New York: Oxford Press; 2000. pp. 294–315. [Google Scholar]
- 37.Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, Nagamine T, Taki W, Kimura J, Shibasaki H. Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain. 1999;122(Pt 5):915–931. doi: 10.1093/brain/122.5.915. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto R, Ikeda A, Ohara S, Matsuhashi M, Baba K, Yamane F, Hori T, Mihara T, Nagamine T, Shibasaki H. Motor-related functional subdivisions of human lateral premotor cortex: epicortical recording in conditional visuomotor task. Clin Neurophysiol. 2003;114:1102–1115. doi: 10.1016/s1388-2457(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 39.Usami K, Matsumoto R, Kunieda T, Shimotake A, Matsuhashi M, Miyamoto S, Fukuyama H, Takahashi R, Ikeda A. Pre-SMA actively engages in conflict processing in human: a combined study of epicortical ERPs and direct cortical stimulation. Neuropsychologia. 2013;51:1011–1017. doi: 10.1016/j.neuropsychologia.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Baumgartner C, Flint R, Tuxhorn I, Van Ness PC, Kosalko J, Olbrich A, Almer G, Novak K, Luders HO. Supplementary motor area seizures: propagation pathways as studied with invasive recordings. Neurology. 1996;46:508–514. doi: 10.1212/wnl.46.2.508. [DOI] [PubMed] [Google Scholar]
- 41.Lim S, Dinner D, Lüders H. Comparison of contralateral upper extremity movements elicited from stimulation of the supplementary and primary motor areas. Epilepsia. 1991;(suppl 3):22. [Google Scholar]
- 42.Catani M, Dell’acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Lacuey N, Zonjy B, Kahriman ES, Kaffashi F, Miller J, Luders HO. Functional connectivity between right and left mesial temporal structures. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0810-0. [DOI] [PubMed] [Google Scholar]
- 44.Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol. 2013;74:223–231. doi: 10.1002/ana.23915. [DOI] [PubMed] [Google Scholar]
- 45.Entz L, Toth E, Keller CJ, Bickel S, Groppe DM, Fabo D, Kozak LR, Eross L, Ulbert I, Mehta AD. Evoked effective connectivity of the human neocortex. Hum Brain Mapp. 2014;35:5736–5753. doi: 10.1002/hbm.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donos C, Maliia MD, Mindruta I, Popa I, Ene M, Balanescu B, Ciurea A, Barborica A. A connectomics approach combining structural and effective connectivity assessed by intracranial electrical stimulation. Neuroimage. 2016;132:344–358. doi: 10.1016/j.neuroimage.2016.02.054. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto R, Sawamoto N, Urayama S, Mikuni N, Hanakawa T, Behrens T, Ikeda A, Takahashi R, Fukuyama H. In vivo tracking of cortico-cortiocal connections in humans: a combined study of CCEP and probabilistic diffusion tractography. Neuroimage. 2008;41:146. [Google Scholar]
- 48.Marusic P, Najm IM, Ying Z, Prayson R, Rona S, Nair D, Hadar E, Kotagal P, Bej MD, Wyllie E, Bingaman W, Luders H. Focal cortical dysplasias in eloquent cortex: functional characteristics and correlation with MRI and histopathologic changes. Epilepsia. 2002;43:27–32. doi: 10.1046/j.1528-1157.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 49.Shimotake A, Matsumoto R, Fumuro T, Inouchi M, Matsuhashi M, Mikuni N, Miyamoto S, Fukuyama H, Takahashi R, Ikeda A. Parieto-frontal network in praxis of human: a combined study of high frequency cortical stimulation and CCEP study. Clinical Neurophysiology. 2010;121:S198. (abstract) [Google Scholar]
- 50.Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, Fukuyama H, Miyamoto S, Hashimoto N. Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol. 2012;123:324–334. doi: 10.1016/j.clinph.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, Bitar A, Fohanno D. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain. 2002;125:199–214. doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- 52.Flanagan D, Valentin A, Garcia Seoane JJ, Alarcon G, Boyd SG. Single-pulse electrical stimulation helps to identify epileptogenic cortex in children. Epilepsia. 2009;50:1793–1803. doi: 10.1111/j.1528-1167.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 53.Valentin A, Alarcon G, Honavar M, Garcia Seoane JJ, Selway RP, Polkey CE, Binnie CD. Single pulse electrical stimulation for identification of structural abnormalities and prediction of seizure outcome after epilepsy surgery: a prospective study. Lancet Neurol. 2005;4:718–726. doi: 10.1016/S1474-4422(05)70200-3. [DOI] [PubMed] [Google Scholar]
- 54.Iwasaki M, Enatsu R, Matsumoto R, Novak E, Thankappen B, Piao Z, O’Connor T, Horning K, Bingaman W, Nair D. Accentuated cortico-cortical evoked potentials in neocortical epilepsy in areas of ictal onset. Epileptic Disord. 2010;12:292–302. doi: 10.1684/epd.2010.0334. [DOI] [PubMed] [Google Scholar]
- 55.Enatsu R, Piao Z, O’Connor T, Horning K, Mosher J, Burgess R, Bingaman W, Nair D. Cortical excitability varies upon ictal onset patterns in neocortical epilepsy: a cortico-cortical evoked potential study. Clin Neurophysiol. 2012;123:252–260. doi: 10.1016/j.clinph.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 56.Usami K, Matsumoto R, Kobayashi K, Hitomi T, Shimotake A, Kikuchi T, Matsuhashi M, Kunieda T, Mikuni N, Miyamoto S, Fukuyama H, Takahashi R, Ikeda A. Sleep modulates cortical connectivity and excitability in humans: Direct evidence from neural activity induced by single-pulse electrical stimulation. Hum Brain Mapp. 2015;36:4714–4729. doi: 10.1002/hbm.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van ‘t Klooster MA, Zijlmans M, Leijten FS, Ferrier CH, van Putten MJ, Huiskamp GJ. Time-frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain. 2011;134:2855–2866. doi: 10.1093/brain/awr211. [DOI] [PubMed] [Google Scholar]
- 58.Mouthaan BE, van ‘t Klooster MA, Keizer D, Hebbink GJ, Leijten FS, Ferrier CH, van Putten MJ, Zijlmans M, Huiskamp GJ. Single Pulse Electrical Stimulation to identify epileptogenic cortex: Clinical information obtained from early evoked responses. Clin Neurophysiol. 2016;127:1088–1098. doi: 10.1016/j.clinph.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi K, Matsumoto R, Matsuhashi M, Usami K, Shimotake A, Kunieda T, Mikuni N, Miyamoto S, Fukuyama H, Takahashi R, Ikeda A. HFO correlates of cortico-cortical evoked potentials reveal altered excitability in the human epileptic focus. American Epilepsy Society Annual Meeting. 2012 http://www.aesnet.org Abst. A.06. [Google Scholar]
- 60.Enatsu R, Jin K, Elwan S, Kubota Y, Piao Z, O’Connor T, Horning K, Burgess RC, Bingaman W, Nair DR. Correlations between ictal propagation and response to electrical cortical stimulation: a cortico-cortical evoked potential study. Epilepsy Res. 2012;101:76–87. doi: 10.1016/j.eplepsyres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Lacruz ME, Garcia Seoane JJ, Valentin A, Selway R, Alarcon G. Frontal and temporal functional connections of the living human brain. Eur J Neurosci. 2007;26:1357–1370. doi: 10.1111/j.1460-9568.2007.05730.x. [DOI] [PubMed] [Google Scholar]
- 62.Keller CJ, Honey CJ, Entz L, Bickel S, Groppe DM, Toth E, Ulbert I, Lado FA, Mehta AD. Corticocortical evoked potentials reveal projectors and integrators in human brain networks. J Neurosci. 2014;34:9152–9163. doi: 10.1523/JNEUROSCI.4289-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsumoto R, Kinoshita M, Taki J, Hitomi T, Mikuni N, Shibasaki H, Fukuyama H, Hashimoto N, Ikeda A. In vivo epileptogenicity of focal cortical dysplasia: a direct cortical paired stimulation study. Epilepsia. 2005;46:1744–1749. doi: 10.1111/j.1528-1167.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 64.Imamura H, Matsumoto R, Takaya S, Nakagawa T, Shimotake A, Kikuchi T, Sawamoto N, Kunieda T, Mikuni N, Miyamoto S, Fukuyama H, Takahashi R, Ikeda A. Network specific change in white matter integrity in mesial temporal lobe epilepsy. Epilepsy Res. 2016;120:65–72. doi: 10.1016/j.eplepsyres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Brugge JF, Volkov IO, Garell PC, Reale RA, Howard MA., 3rd Functional connections between auditory cortex on Heschl’s gyrus and on the lateral superior temporal gyrus in humans. J Neurophysiol. 2003;90:3750–3763. doi: 10.1152/jn.00500.2003. [DOI] [PubMed] [Google Scholar]
- 66.Enatsu R, Kubota Y, Kakisaka Y, Bulacio J, Piao Z, O’Connor T, Horning K, Mosher J, Burgess RC, Bingaman W, Nair DR. Reorganization of posterior language area in temporal lobe epilepsy: a cortico-cortical evoked potential study. Epilepsy Res. 2013;103:73–82. doi: 10.1016/j.eplepsyres.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Catenoix H, Magnin M, Guenot M, Isnard J, Mauguiere F, Ryvlin P. Hippocampal-orbitofrontal connectivity in human: an electrical stimulation study. Clin Neurophysiol. 2005;116:1779–1784. doi: 10.1016/j.clinph.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Greenlee JD, Oya H, Kawasaki H, Volkov IO, Kaufman OP, Kovach C, Howard MA, Brugge JF. A functional connection between inferior frontal gyrus and orofacial motor cortex in human. J Neurophysiol. 2004;92:1153–1164. doi: 10.1152/jn.00609.2003. [DOI] [PubMed] [Google Scholar]
- 69.Greenlee JD, Oya H, Kawasaki H, Volkov IO, Severson MA, 3rd, Howard MA, 3rd, Brugge JF. Functional connections within the human inferior frontal gyrus. J Comp Neurol. 2007;503:550–559. doi: 10.1002/cne.21405. [DOI] [PubMed] [Google Scholar]
- 70.Matsuzaki N, Juhasz C, Asano E. Cortico-cortical evoked potentials and stimulation-elicited gamma activity preferentially propagate from lower- to higher-order visual areas. Clin Neurophysiol. 2013;124:1290–1296. doi: 10.1016/j.clinph.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oya H, Poon PW, Brugge JF, Reale RA, Kawasaki H, Volkov IO, Howard MA., 3rd Functional connections between auditory cortical fields in humans revealed by Granger causality analysis of intra-cranial evoked potentials to sounds: comparison of two methods. Biosystems. 2007;89:198–207. doi: 10.1016/j.biosystems.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 72.Enatsu R, Matsumoto R, Piao Z, O’Connor T, Horning K, Burgess RC, Bulacio J, Bingaman W, Nair DR. Cortical negative motor network in comparison with sensorimotor network: a cortico-cortical evoked potential study. Cortex. 2013;49:2080–2096. doi: 10.1016/j.cortex.2012.08.026. [DOI] [PubMed] [Google Scholar]