INTRODUCTION and DISCUSSION
It has been well known that chronic kidney disease (CKD) is associated with increased risk of cardiovascular disease. In fact studies have shown that cardiovascular event rates and all-cause mortality increase as a function of CKD as determined by decrease in estimated glomerular filtration rate (eGFR) and/or presence of microalbuminuria 1, 2.
In recent years the term cardiorenal syndrome (CRS) has been proposed to define the relationship between cardiac disease and renal disease. Ronco et al has defined cardiorenal syndrome as a pathophysiologic disorder of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other 3–6. CRS Type 1: Acute Cardiorenal Syndrome: is defined as sudden worsening of cardiac function (acute cardiogenic shock or acutely decompensated heart failure) leading to acute kidney injury. CRS Type 2: Chronic Cardiorenal Syndrome: is defined as chronic abnormalities in cardiac function (chronic congestive heart failure) causing progressive and potentially permanent chronic kidney disease. CRS Type 3: Acute Renocardiac Syndrome: is defined as sudden worsening of kidney function (AKI or Acute GN) causing acute cardiac disorder (heart failure, arrhythmia, or ischemia). CRS Type 4: Chronic Renocardiac Syndrome: is defined as chronic kidney disease contributing to decreased cardiac function, cardiac hypertrophy and/or increased risk of adverse cardiovascular events. CRS Type 5: Secondary Cardiorenal Syndrome: is defined as systemic condition (obesity, diabetes mellitus, sepsis) causing simultaneous cardiac and renal dysfunction.
In this review I will be discussing mainly the type 5 Cardiorenal Syndrome associated with obesity and diabetes and models of atherosclerosis and vascular calcification. In addition to discussing renal disease I will also discuss vascular disease as vascular dysfunction is a well characterized complication of renal disease and vascular dysfunction is also a leading cause of cardiac disease.
Role for altered renal lipid metabolism in the pathogenesis of kidney disease in obesity and diabetes
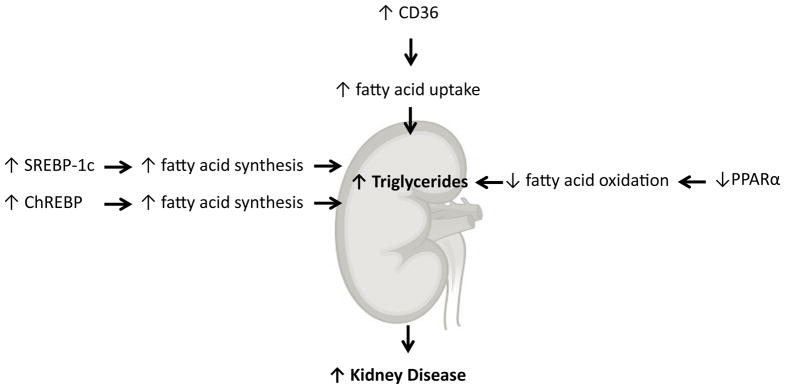
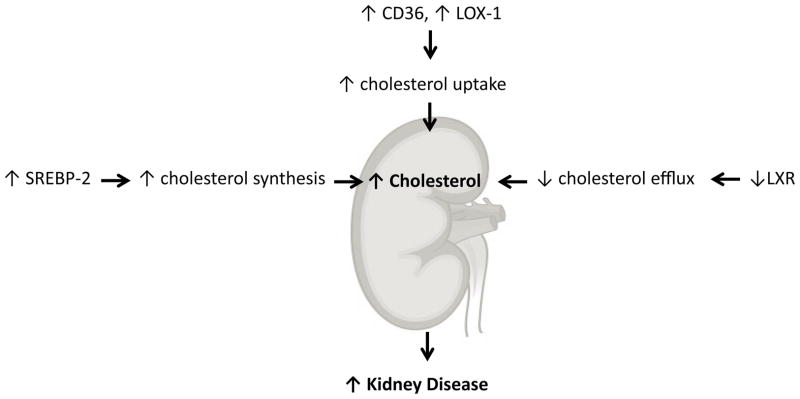
Our work early on determined an important role for altered renal lipid metabolism in the pathogenesis of kidney disease in obesity and diabetes. In a series of studies using rodent models of type I diabetes 7–9, high fat diet induced obesity and insulin resistance 10, 11, type 2 diabetes mellitus 12, 13, and aging 14, 15 we determined that abnormal lipid metabolism mediated by increased expression and activity of the sterol regulatory element binding proteins (SREBPs) and carbohydrate regulatory element binding protein (ChREBP) and decreased activity of the peroxisome proliferator activated receptor alpha (PPARα) and liver X receptor (LXR) played an important role in kidney disease (Figures 1 and 2).
Figure 1.
Regulation of triglyceride metabolism in the kidney.
Figure 2.
Regulation of cholesterol metabolism in the kidney.
In streptozotocin-induced diabetes in the rat, we found marked increases in SREBP-1 and fatty acid synthase (FAS) expression, resulting in increased triglyceride (TG) accumulation. Treatment of diabetic rats with insulin prevented the increased renal expression of SREBP-1 and the accumulation of TG. The role of hyperglycemia in the up-regulation of SREBP-1 was confirmed in renal cells cultured in a high glucose media. High glucose induced increased expression of SREBP-1a and -1c mRNA, SREBP-1 protein, and FAS, resulting in increased TG content. To determine a direct role for SREBP in mediating the increase in renal lipids and glomerulosclerosis, we studied SREBP-1a transgenic mice with increased renal expression of SREBP-1. The increase in SREBP-1 was associated with increased expression of FAS and acetyl CoA carboxylase, resulting in increased TG content, increased expression of transforming growth factor beta1 and vascular endothelial growth factor, mesangial expansion, glomerulosclerosis, and proteinuria. Our study therefore indicated that renal SREBP-1 expression is increased in diabetes and that SREBP-1 plays an important role in the increased lipid synthesis, TG accumulation, mesangial expansion, glomerulosclerosis, and proteinuria by increasing the expression of transforming growth factor beta and vascular endothelial growth factor 7.
Additional studies were performed in Akita and OVE26 mice which are two genetic models of type 1 diabetes. Diabetic nephropathy was characterized by mesangial expansion and loss of podocytes, resulting in glomerulosclerosis and proteinuria, and is associated with increased expression of profibrotic growth factors, proinflammatory cytokines, and increased oxidative stress. We had also found significant increases in renal triglyceride and cholesterol content. The increase in renal triglyceride content was associated with 1) increased expression of sterol regulatory element-binding protein (SREBP)-1c and carbohydrate response element-binding protein (ChREBP), which collectively results in increased fatty acid synthesis, 2) decreased expression of peroxisome proliferator-activated receptor PPAR-alpha and PPAR-delta, which results in decreased fatty acid oxidation, and 3) decreased expression of farnesoid X receptor (FXR) and small heterodimer partner (SHP). The increase in cholesterol content was associated with 1) increased expression of SREBP-2 and 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase, which results in increased cholesterol synthesis, and 2) decreased expression of liver X receptor (LXR)-alpha, LXR-beta, and ATP-binding cassette transporter-1, which results in decreased cholesterol efflux. Our results had further indicated that in type 1 diabetes, there is altered renal lipid metabolism favoring net accumulation of triglycerides and cholesterol, which are driven by increases in SREBP-1, ChREBP, and SREBP-2 and decreases in FXR, LXR-alpha, and LXR-beta, which may also play a role in the increased expression of profibrotic growth hormones, proinflammatory cytokines, and oxidative stress 9.
In addition to diabetes, obesity and metabolic syndrome are associated with glomerulosclerosis and proteinuria. Using a model of diet induced obesity and insulin resistance, C57BL/6J mice that were fed a high fat, 60 kcal % saturated (lard) fat diet (HFD) developed obesity, hyperglycemia, and hyperinsulinemia compared with those that were fed a low fat, 10 kcal % fat diet (LFD). In contrast, A/J mice were resistant when fed the same diet. C57BL/6J mice with HFD exhibited significantly higher levels of renal SREBP-1 and SREBP-2 expression than those mice with LFD, whereas in A/J mice there were no changes with the same treatment. The increases in SREBP-1 and SREBP-2 expression in C57BL/6J mice resulted in renal accumulation of triglyceride and cholesterol. There were also significant increases in the renal expression of plasminogen activator inhibitor-1 (PAI-1), vascular endothelial growth factor (VEGF), type IV collagen, and fibronectin, resulting in glomerulosclerosis and proteinuria. To determine a role for SREBPs per se in modulating renal lipid metabolism and glomerulosclerosis we performed studies in SREBP-1c (−/−) mice. In contrast to control mice, in the SREBP-1c (−/−) mice with HFD the accumulation of triglyceride was prevented, as well as the increases in PAI-1, VEGF, type IV collagen, and fibronectin expression. Our results therefore indicated that diet-induced obesity causes increased renal lipid accumulation and glomerulosclerosis in C57BL/6J mice via an SREBP-1c-dependent pathway 10.
db/db mice on the FVB genetic background with loss-of-function mutation of the leptin receptor (FVB-Lepr(db) mice or FVB db/db) develop severe diabetic nephropathy, including glomerulosclerosis, tubulointerstitial fibrosis, increased expression of type IV collagen and fibronectin, and proteinuria, which is associated with increased renal mRNA abundance of transforming growth factor-beta, plasminogen activator inhibitor-1, and vascular endothelial growth factor. Electron microscopy demonstrated increases in glomerular basement membrane thickness and foot process (podocyte) length. We found that there is a marked increase in neutral lipid deposits in glomeruli and tubules by oil red O staining and biochemical analysis for cholesterol and triglycerides. We also detected a significant increase in the renal expression of adipocyte differentiation-related protein (adipophilin), a marker of cytoplasmic lipid droplets. We examined the expression of sterol regulatory element-binding protein (SREBP)-1 and -2, transcriptional factors that play an important role in the regulation of fatty acid, triglyceride, and cholesterol synthesis. We found significant increases in SREBP-1 and -2 protein levels in nuclear extracts from the kidneys of FVB db/db mice, with increases in the mRNA abundance of acetyl-CoA carboxylase, fatty acid synthase, and 3-hydroxy-3-methylglutaryl-CoA reductase, which mediates the increase in renal triglyceride and cholesterol content. Our results indicate that in FVB db/db mice, renal triglyceride and cholesterol accumulation is mediated by increased activity of SREBP-1 and -2 12. Of note when the above mice originally generated in Dr. Streamson Chu’s lab at Columbia University were re-derived at Jackson Labs, the resulting phenotype was quite different 16. This may have been due to different FVB strains, diets, and barrier (pathogen free conditions, i.e. microbiome). We therefore also performed studies with db/db mice obtained from Jackson Labs in the BKS genetic background. Our results essentially confirmed our findings with the FVB db/db mice 13.
Parallel studies in aging mice 14, 15 also indicated a significant role for altered lipid metabolism in modulating renal disease.
Role for bile acid activated receptors in the pathogenesis of kidney disease in obesity and diabetes
The above findings of increased SREBP and ChREBP activity and decreased PPARα activity in obesity and diabetes led to the investigation of the role of the bile acid activated nuclear hormone receptor the farnesoid X receptor (FXR) in modulation of lipid metabolism and renal disease. There was indeed emerging evidence in the liver that FXR is an important negative regulator of SREBP-1 and ChREBP, and positive regulator of PPARα activity17–19.
In diabetic humans there is decreased FXR expression in the kidney. In support for FXR playing an important role in diabetic kidney disease we found that there is accelerated renal injury in diabetic FXR knockout mice 8. In contrast, treatment of the STZ diabetic mice 8, diet induced obesity mice 11, or db-db mice 13 with the FXR agonist INT-747 (6α-ethyl-3α,7α-dihydroxy-5β-cholan-24-oic acid) improved renal injury by decreasing proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis, and modulating renal lipid metabolism, macrophage infiltration, and renal expression of SREBPs, profibrotic growth factors, and oxidative stress enzymes.
Additional work from other labs has also confirmed our results. Administration of chenodeoxycholic acid, a FXR agonist, prevents kidney fibrosis, inflammation, and oxidative stress in high fructose fed rats 20. FXR activation also prevents cisplatin-induced kidney injury through it anti-fibrotic, anti-inflammatory, and anti-apoptotic effects 21.In addition FXR agonist protects against kidney disease induced by high fat feeding in uninephrectomized mice 22, 23.
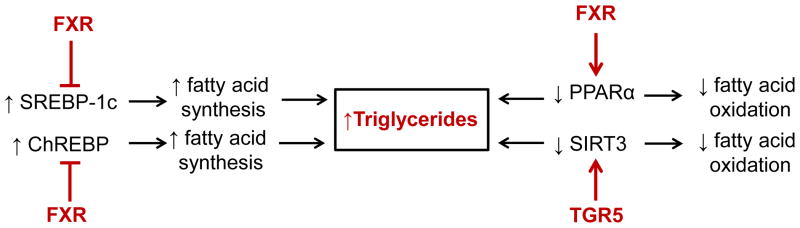
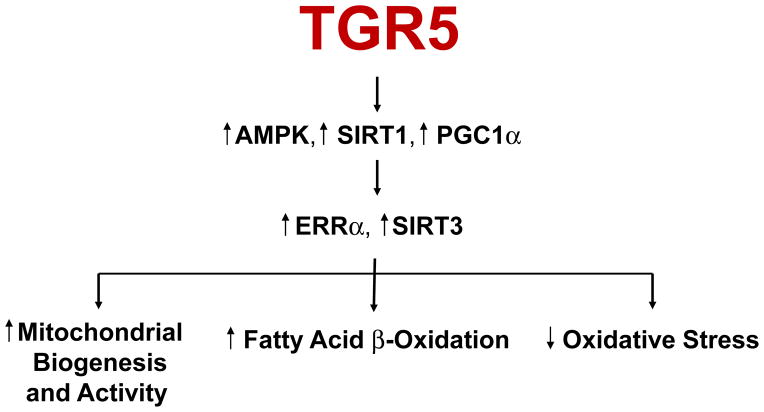
In addition to FXR, a bile acid activated G protein coupled receptor TGR5 has also been identified 24–26. We found that treatment of diabetic db/db mice with the selective TGR5 agonist INT-777 (6α-ethyl-23(S)-methyl-3α,7α,12α-trihydroxy-5β-cholan-24-oic acid) decreased proteinuria, podocyte injury, mesangial expansion, fibrosis, and CD68 macrophage infiltration in the kidney. INT-777 also induced renal expression of master regulators of mitochondrial biogenesis, inhibitors of oxidative stress, and inducers of fatty acid β-oxidation, including sirtuin 1 (SIRT1), sirtuin 3 (SIRT3), and Nrf-1. Increased activity of SIRT3 was evidenced by normalization of the increased acetylation of mitochondrial superoxide dismutase 2 (SOD2) and isocitrate dehydrogenase 2 (IDH2) observed in untreated db/db mice. Accordingly, INT-777 decreased mitochondrial H2O2 generation and increased the activity of SOD2, which associated with decreased urinary levels of H2O2 and thiobarbituric acid reactive substances. Furthermore, INT-777 decreased renal lipid accumulation. INT-777 also prevented kidney disease in mice with diet-induced obesity. In human podocytes cultured with high glucose, INT-777 induced mitochondrial biogenesis, decreased oxidative stress, and increased fatty acid β-oxidation. Compared with normal kidney biopsy specimens, kidney specimens from patients with established ORG or DN expressed significantly less TGR5 mRNA, and levels correlated with disease progression. Our results indicate that TGR5 activation induces mitochondrial biogenesis and prevents renal oxidative stress and lipid accumulation, establishing a role for TGR5 in inhibiting kidney disease in obesity and diabetes 27 (Figures 3 and 4).
Figure 3.
Role of farnesoid X receptor (FXR) and TGR5 in regulation of triglyceride metabolism in the kidney.
Figure 4.
Role of TGR5 in regulating mitochondrial biogenesis and activity, fatty acid beta-oxidation, and oxidative stress in the kidney.
In view of important roles for FXR and TGR5 in modulating kidney disease in obesity and in diabetes in collaboration with the Intercept scientists we have also characterized a dual FXR-TGR5 agonist INT-767 (6α-ethyl-3α,7α,23-trihydroxy-24-nor-5β-cholan-23-sulfate sodium salt) 28. We found that INT-767 is a potent agonist for both FXR (mean EC(50), 30 nM by PerkinElmer AlphaScreen assay) and TGR5 (mean EC(50), 630 nM by time resolved-fluorescence resonance energy transfer), the first compound described so far to potently and selectively activate both BA receptors.
In preliminary studies in streptozotocin diabetic mice and db-db mice we have found that treatment of mice INT-767 has significant effects to prevent development of albuminuria and glomerular disease.
Role for bile acid activated receptors in the pathogenesis of atherosclerosis and vascular calcification in ApoE KO and LDLR KO mice with chronic kidney disease
We found that FXR also play an important role on vascular calcification on apolipoprotein E (ApoE) knockout mice (KO) with 5/6 nephrectomy induced CKD 29. We found that FXR was highly induced during osteogenic differentiation of bovine calcifying vascular cells (CVCs) and in the aorta of ApoE KO mice with CKD which are common tissue culture and mouse model, respectively, for aortic calcification. FXR activation by a synthetic FXR agonist, 6alpha-ethyl chenodeoxycholic acid (INT-747) inhibited phosphate induced-mineralization and triglyceride accumulation in CVCs. FXR dominant negative expression augmented mineralization of CVCs and blocked the anticalcific effect of INT-747 whereas VP16FXR that is a constitutively active form reduced mineralization of CVCs. INT-747 treatment also increased phosphorylated c-Jun N-terminal kinase (JNK). SP600125 (specific JNK inhibitor) significantly induced mineralization of CVCs and alkaline phosphatase expression, suggesting that the anticalcific effect of INT-747 is attributable to JNK activation. We also found that INT-747 ameliorates CKD-induced vascular calcification in ApoE KO mice with 5/6 nephrectomy without affecting the development of atherosclerosis 29.
In additional studies we have treated ApoE KO mice and LDLR KO mice with the FXR-TGR5 dual agonist INT-767. INT-767 treatment drastically reduced serum cholesterol levels and significantly reduced atherosclerotic plaque formation in both ApoE KO and LDLRKO mice 30. INT-767 decreased the expression of pro-inflammatory cytokines and chemokines in the aortas of ApoE KO mice through the inactivation of NF-κB. In addition, J774 macrophages treated with INT-767 had significantly lower levels of active NF-κB, resulting in cytokine production in response to LPS through a PKA dependent mechanism. This study demonstrates that concurrent activation of FXR and TGR5 attenuates atherosclerosis by reducing both circulating lipids and inflammation.
Summary
In summary our studies indicate an important role for altered lipid metabolism mediated by significant alterations in nuclear hormone receptors, transcription factors, and G Protein Coupled Receptors in modulating kidney disease in diabetes, obesity and aging and atherosclerosis and vascular calcification in LDLR KO and ApoE KO mice with 5/6 nephrectomy induced CKD. Studies discussed in this review indicate that in these conditions FXR and TGR5 agonists play an important role in preventing progression of kidney disease, atherosclerosis and vascular calcification. Future studies will also need to determine if FXR and TGR5 agonists have similar beneficial effects in preventing cardiac dysfunction in chronic kidney disease.
Acknowledgments
It is a great honor for me to have been selected for the KCVD Donald Seldin Lecturer Award by the Council for Kidney in Cardiovascular Disease of the American Heart Association. From the time I joined the faculty at the University of Texas Southwestern Medical Center in 1983 and until I moved to the University of Colorado in 2002 Dr. Seldin was my Chair of Medicine and then my mentor, colleague, and friend. He was an exemplary leader who was encouraged vertical and creative thinking. His influence and friendship continued even after my move to the University of Colorado where I continued to see him and seek his advice on a regular basis.
I thank Dr. Tao Jiang for overseeing the studies with SREBPs, Dr. Xiaoxin Wang for overseeing the studies with FXR and TGR5, and Dr. Makoto Miyazaki for overseeing the studies with atherosclerosis and vascular calcification. Our progress in this area would not have been possible without their dedicated and excellent work.
The studies have been supported by grants from NIH, VA, AHA, JDRF, and Intercept Pharmaceuticals. Special thanks to Dr. Mark Pruzanski and Dr. Luciano Adorini who continue to collaborate with us with the FXR and TGR5 related projects.
Grant Support
The studies discussed here were supported by grant support from NIH, VA, and Intercept to Moshe Levi, MD.
Footnotes
Disclosures
Moshe Levi, MD has received a medical school administered research grant from Intercept.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Moody WE, Ferro CJ, Edwards NC, Chue CD, Lin EL, Taylor RJ, Cockwell P, Steeds RP, Townend JN. Cardiovascular effects of unilateral nephrectomy in living kidney donors. Hypertension. 2016;67:368–77. doi: 10.1161/HYPERTENSIONAHA.115.06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015;20:259–272. doi: 10.1007/s10741-014-9460-9. [DOI] [PubMed] [Google Scholar]
- 4.Husain-Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, Ronco C. Cardio-pulmonary-renal interactions: A multidisciplinary approach. J Am Coll Cardiol. 2015;65:2433–2448. doi: 10.1016/j.jacc.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Palazzuoli A, Lombardi C, Ruocco G, Padeletti M, Nuti R, Metra M, Ronco C. Chronic kidney disease and worsening renal function in acute heart failure: Different phenotypes with similar prognostic impact? Eur Heart J Acute Cardiovasc Care. doi: 10.1177/2048872615589511. Published Online: June 4, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Clementi A, Virzi GM, Brocca A, de Cal M, Pastori S, Clementi M, Granata A, Vescovo G, Ronco C. Advances in the pathogenesis of cardiorenal syndrome type 3. Oxid Med Cell Longev. 2015;2015:148082. doi: 10.1155/2015/148082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919–18927. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 8.Wang XX, Jiang T, Shen Y, Caldas Y, Miyazaki-Anzai S, Santamaria H, Urbanek C, Solis N, Scherzer P, Lewis L, Gonzalez FJ, Adorini L, Pruzanski M, Kopp JB, Verlander JW, Levi M. Diabetic nephropathy is accelerated by farnesoid x receptor deficiency and inhibited by farnesoid x receptor activation in a type 1 diabetes model. Diabetes. 2010;59:2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in akita and ove26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 10.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M. Diet-induced obesity in c57bl/6j mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 11.Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, Scherzer P, Lewis L, Miyazaki-Anzai S, Levi M. The farnesoid x receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297:F1587–1596. doi: 10.1152/ajprenal.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in fvbdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 13.Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M. Farnesoid x receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 14.Jiang T, Liebman SE, Lucia MS, Li J, Levi M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005;68:2608–2620. doi: 10.1111/j.1523-1755.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 15.Jiang T, Liebman SE, Lucia MS, Phillips CL, Levi M. Calorie restriction modulates renal expression of sterol regulatory element binding proteins, lipid accumulation, and age-related renal disease. J Am Soc Nephrol. 2005;16:2385–2394. doi: 10.1681/ASN.2004080701. [DOI] [PubMed] [Google Scholar]
- 16.Brosius FC, 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T Animal Models of Diabetic Complications C. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor fxr/bar impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving fxr, shp, and srebp-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 20.Hu Z, Ren L, Wang C, Liu B, Song G. Effect of chenodeoxycholic acid on fibrosis, inflammation and oxidative stress in kidney in high-fructose-fed wistar rats. Kidney Blood Press Res. 2012;36:85–97. doi: 10.1159/000341485. [DOI] [PubMed] [Google Scholar]
- 21.Bae EH, Choi HS, Joo SY, Kim IJ, Kim CS, Choi JS, Ma SK, Lee J, Kim SW. Farnesoid x receptor ligand prevents cisplatin-induced kidney injury by enhancing small heterodimer partner. PLoS One. 2014;9:e86553. doi: 10.1371/journal.pone.0086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gai Z, Hiller C, Chin SH, Hofstetter L, Stieger B, Konrad D, Kullak-Ublick GA. Uninephrectomy augments the effects of high fat diet induced obesity on gene expression in mouse kidney. Biochim Biophys Acta. 2014;1842:1870–1878. doi: 10.1016/j.bbadis.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Gai Z, Gui T, Hiller C, Kullak-Ublick GA. Farnesoid x receptor protects against kidney injury in uninephrectomized obese mice. J Biol Chem. 2016;291:2397–2411. doi: 10.1074/jbc.M115.694323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A g protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 25.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through tgr5 in a murine enteroendocrine cell line stc-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 27.Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, Lucia S, Adorini L, D’Agati VD, Levi J, Rosenberg A, Kopp JB, Gius DR, Saleem MA, Levi M. G protein-coupled bile acid receptor tgr5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol. doi: 10.1681/ASN.2014121271. Published Online: September 30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, Levi M, Pruzanski M, Adorini L. Functional characterization of the semisynthetic bile acid derivative int-767, a dual farnesoid x receptor and tgr5 agonist. Mol Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazaki-Anzai S, Levi M, Kratzer A, Ting TC, Lewis LB, Miyazaki M. Farnesoid x receptor activation prevents the development of vascular calcification in apoe−/− mice with chronic kidney disease. Circ Res. 2010;106:1807–1817. doi: 10.1161/CIRCRESAHA.109.212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki-Anzai S, Masuda M, Levi M, Keenan AL, Miyazaki M. Dual activation of the bile acid nuclear receptor fxr and g-protein-coupled receptor tgr5 protects mice against atherosclerosis. PLoS One. 2014;9:e108270. doi: 10.1371/journal.pone.0108270. [DOI] [PMC free article] [PubMed] [Google Scholar]