Abstract
Although chronic eosinophilic inflammation is a common feature in patients with asthma, some patients have neutrophil-dominant inflammation, which is known to be associated with severe asthma.Human mesenchymal stem cells (hMSCs) have shown promise in treating various refractory immunological diseases. Thus, hMSCs may represent an alternative therapeutic option for asthma patients with neutrophil-dominant inflammation, in whom current treatments are ineffective. BALB/c mice exposed to ovalbumin and polyinosinic:polycytidylic acid (Poly I:C) to induce neutrophilic airway inflammation were systemically treated with hMSCs to examine whether the hMSCs can modulate neutrophilic airway inflammation. In addition, cytokine production was evaluated in co-cultures of hMSCs with either anti-CD3/CD28-stimulated peripheral blood mononuclear cells (PBMCs) obtained from asthmatic patients or cells of the human bronchial epithelial cell line BEAS-2B to assess the response to hMSC treatment. The total number of immune cells in bronchoalveolar lavage fluid (BALF) showed a dramatic decrease in hMSC-treated asthmatic mice, and, in particular, neutrophilic infiltration was significantly attenuated. This phenomenon was accompanied by reduced CXCL15 production in the BALF. BEAS-2B cells co-cultured with hMSCs showed reduced secretion of IL-8. Moreover, decreased secretion of IL-4, IL-13 and IFN-γ was observed when human PBMCs were cultured with hMSCs, whereas IL-10 production was greatly enhanced. Our data imply that hMSCs may have a role in reducing neutrophilic airway inflammation by downregulating neutrophil chemokine production and modulating T-cell responses.
Introduction
Asthma, which is characterized by airway inflammation and hyper-responsiveness, affects more than 300 million people worldwide, and its prevalence is increasing.1, 2 Airway inflammation is a fundamental element of asthma,3 and chronic eosinophilic inflammation is typical in most asthmatic patients, usually showing a favorable response to inhaled corticosteroids. However, some asthmatic patients show lower responsiveness or even resistance to corticosteroids, which is critically linked to severe refractory asthma. Although the precise pathogenesis of severe asthma remains to be clarified, steroid-insensitive airway inflammation is associated with irreversibly decreased lung function and uncontrolled symptoms despite administration of currently available anti-asthma therapies. Steroid-insensitive airway inflammation in severe asthma is a critical unmet problem in asthma management, and novel drugs for this subtype of asthma are urgently needed.4
Although chronic eosinophilic inflammation is a common feature of airway inflammation, patterns of inflammation in asthma vary. Neutrophils, which are observed in the airways of poorly controlled asthma, have been linked to the development of severe asthma. Indeed, increasing evidence indicates that neutrophilic inflammation is correlated with poor lung function and airflow obstruction in asthma.5, 6, 7, 8 Currently, there is no known effective therapy for neutrophilic airway inflammation.
Human mesenchymal stem cells (hMSCs) are multipotent cells that can differentiate into a variety of mesenchymal cell types. MSCs were initially regarded as providing a new therapeutic strategy for tissue repair and regeneration;9, 10 however, they also appear to be effective in refractory inflammatory diseases, and increasing evidence indicates that they have immunomodulatory properties.11, 12, 13, 14 At sites of inflammation, MSCs become activated and secrete high levels of indoleamine 2,3-dioxygenase, prostaglandin E2, and nitric oxide, which suppress T-cell proliferation and polarize T cells toward a regulatory phenotype.15 B cells, dendritic cells and NK cells can also be suppressed by interactions with MSCs.16, 17 Some reports have shown a potential therapeutic effect of MSCs on many refractory immunologic diseases, such as rheumatologic diseases, atopic dermatitis and asthma.18, 19, 20 Moreover, recent studies have suggested that intravenously administered stem cells are initially trapped in the lungs,21 and MSCs appear to migrate in response to inflammatory mediators.22, 23 These findings imply that MSCs may attenuate airway inflammation through the production of various immunosuppressive mediators and through interactions with immune and non-immune cells in the lungs.
With regard to asthma, current understanding of the immunomodulatory properties of MSCs is mostly based on the ‘classical' model of ovalbumin-induced eosinophilic airway inflammation.15, 24, 25, 26, 27 These studies have demonstrated that MSCs ameliorate airway inflammation in lung histopathology and decrease Th2-type cytokine levels in bronchoalveolar lavage fluid (BALF); however, the effect of hMSCs on neutrophilic airway inflammation is largely unknown.
In the present study, we used a murine model of Poly(I:C)-induced neutrophilic airway inflammation to investigate the effect of hMSCs on this inflammation. In addition, we evaluated the effects of hMSCs on human peripheral blood mononuclear cells (PBMCs) obtained from severe asthma patients and on human bronchial epithelial cells.
Materials and methods
Preparation of hMSCs: heterogeneous mixture
After obtaining informed consent from the mother, a sample of neonatal umbilical cord blood was collected from the umbilical vein when the baby is born. Mononuclear cells were isolated by layering cord blood on a Ficoll–Hypaque gradient (Sigma, St Louis, MO, USA) followed by centrifugation at 300 g at 25 °C. The separated mononuclear cells were suspended in α-minimum essential medium (α-MEM, Gibco BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA). The cells were seeded in a culture plate at a density of 5 × 105 cells cm−2 and incubated at 37 °C in a humidified atmosphere containing 5% CO2. The culture medium was replaced with fresh medium twice a week, and after 1–3 weeks of growth, the monolayer colonies reached 80% confluence. Cells were detached using 0.25% trypsin containing EDTA (HyClone), then washed and resuspended in culture medium (α-MEM supplemented with 10% FBS) and subcultured at a density of 5 × 104 cells cm−2.
Animal preparation and experimental protocol
Wild-type BALB/c mice (aged 6 weeks) were purchased from OrientBio (Gapyong, Korea). To generate the Poly(I:C)-induced asthma model, mice were intranasally sensitized with 75 μg ovalbumin (Sigma) and 10 μg Poly(I:C) (Calbiochem, Darmstadt, Germany) on days 0, 1, 2, 3 and 7. The mice were challenged with 50 μg ovalbumin and 10 μg Poly(I:C) via the intranasal route on days 14, 21, 22 and 23. On day 15, 3 × 105 (low dose) or 6 × 105 (high dose) hMSCs were administered in 200 μl sterile PBS via tail vein injection (Figure 1a). The control group received no treatment. BALF, lymph nodes and lung tissues were obtained from the mice 24 h after the last immunization. The animal protocols were approved by the institutional Animal Care and Use Committee.
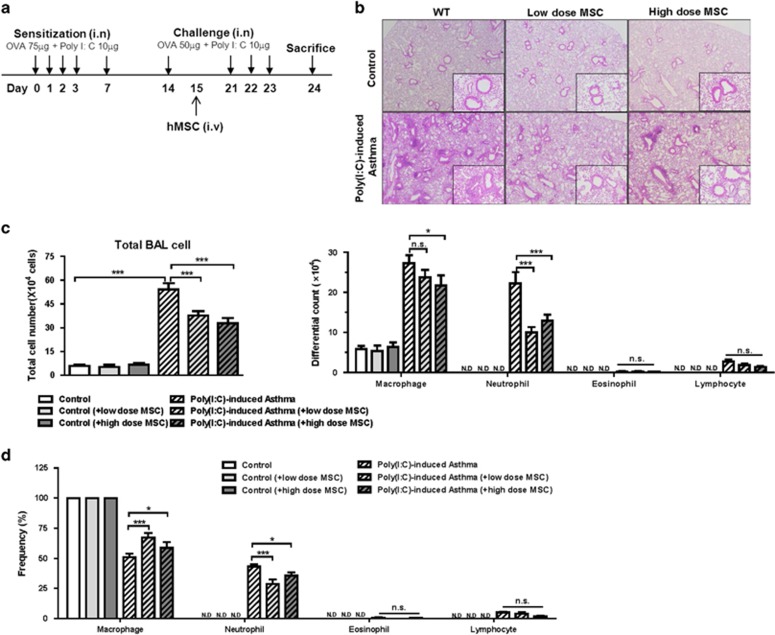
Figure 1.
hMSCs reduce airway inflammation in a Poly(I:C)-induced mouse model of asthma. (a) Protocol for the Poly(I:C)-induced mouse model of asthma. Mice were systemically treated with 3 × 105 (low dose) or 6 × 105 (high dose) hMSCs. (b) Histology of lung sections after hematoxylin and eosin staining (original magnification: × 40, × 200). (c) BALF differential cell count. (d) Cellular frequencies in each group of mice. Similar results were obtained in three independent experiments. n⩾9 for every group *P<0.05, **P<0.001 and ***P<0.0001.
Lung histology
Mouse lungs were perfused with 5 ml PBS through the right ventricle and fixed with 10% neutral buffered formalin. Fixed lungs were embedded in paraffin and sectioned at 4 μm. To examine the magnitude of inflammation around the bronchial and vascular areas, lung sections were stained with hematoxylin and eosin. For immunofluorescence staining, sections were stained with anti-human β-microglobulin monoclonal antibody (Santa Cruz Biotechnology, Heidelberg, Germany) and Alexa Fluor 488 conjugated secondary antibody (Abcam, Cambridge, UK). All slides were mounted with medium containing DAPI (Vector Laboratories Inc., Burlingame, CA, USA).
Analysis of BALF
Samples (2 ml) of mouse BALF were obtained after tracheostomy by lavage with PBS. Cells were collected by centrifugation at 400 g for 10 min at 4 °C, and the pellets were resuspended in PBS. The total number of cells and differential cell counts were determined after cyto-centrifugation (StatSpin CytoFuge 12, Iris International Inc., Westwood, MA, USA) at 14 g for 5 min at room temperature followed by Diff-Quik staining (Sysmex Co., Kobe, Japan) and fixation with synthetic mounting medium (Histomount, Ted Pella, Inc., Redding, CA, USA). At least 300 cells in each preparation of BALF were counted to measure numbers of monocytes, eosinophils, neutrophils and lymphocytes. The concentrations of various cytokines in the BALF were measured by enzyme-linked immunosorbent assays (ELISAs).
Determination of cytokines secreted from cells cultured from lung-draining lymph nodes
Lung-draining lymph nodes were excised from the mice and teased apart with forceps in RPMI containing penicillin-streptomycin (Welgene, Daegu, Korea) before filtration through a 40-μm filter. Cells were then centrifuged at 250 g for 5 min, plated at a density of 5 × 105 cells per well in a 96-well plate, and re-stimulated with 1 mg ml−1 ovalbumin and 50 μg ml−1 Poly(I:C) for 3 days. Concentrations of IL-5, IL-10, IFN-γ and IL-17 in the supernatant were measured with Duoset ELISA development kits (R&D Systems, Minneapolis, MN, USA).
Co-culture of hMSCs with human bronchial epithelial cells
To test whether hMSCs could inhibit production of IL-8 by BEAS-2B human bronchial epithelial cells, BEAS-2B cells were treated with 10 μg ml−1 Poly(I:C) for 24 h, and 2 × 104 hMSCs were plated. The BEAS-2B cells were detached using trypsin and added to the hMSCs (BEAS-2B to hMSC ratio 10:1). The ELISA for measuring IL-8 in culture supernatant was as described above.
Co-culture of hMSCs with PBMCs from patients with severe asthma
PBMCs were obtained from severe asthma patients attending the outpatient clinic at a university hospital and from healthy control subjects. All the study subjects gave informed consent, and the study was approved by the institutional review board.
hMSCs were seeded in 24-well plates at a density of 2 × 104 cells per well and cultured for 12 h in RPMI medium (Welgene) containing 1% penicillin–streptomycin and 10% FBS. PBMCs from healthy and asthmatic patients (n=3, each) were isolated by density gradient centrifugation and added to the hMSCs (PBMC to hMSC ratio, 10:1). Wells were set up in duplicate. Anti-CD3 (1 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies were added to stimulate the PBMCs for 3 days. ELISAs for measuring IL-4, IL-13, IFN-γ and IL-10 in the culture supernatant were as described above.
Statistical analysis
Results are presented as the means±s.e.m.'s. One- or two-way analysis of variance was used to determine differences between multiple groups, with the Bonferroni method for post hoc comparisons. Student's t-test was used for comparisons between two groups (GraphPad Prism; GraphPad Software, Inc., La Jolla, CA, USA). Values of P<0.05 were considered statistically significant.
Results
hMSCs attenuate airway inflammation in a neutrophil-dominant Poly(I:C)-induced murine model of asthma
A histopathological examination of lung tissue showed massive inflammatory cells around the peribronchial and perivascular area in the ovalbumin and Poly(I:C)-challenged mice; however, the inflammation was remarkably reduced in both low- and high-dose hMSC-treated groups (Figure 1b). The cells that accumulated in the BALF were mainly a mixture of macrophages and neutrophils. The frequency and absolute numbers of neutrophils were significantly reduced in both hMSC-treated groups (Figures 1c and d).
Mobilization of hMSCs to the lungs in the murine model of asthma
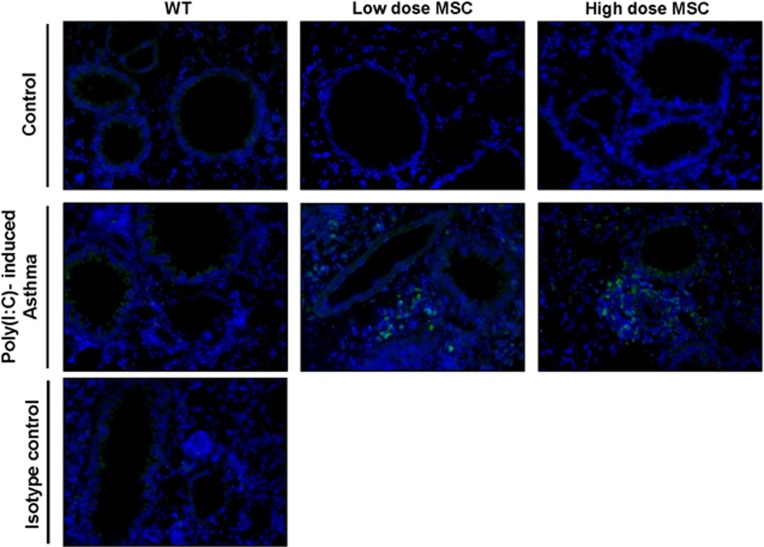
Immunofluorescence analysis was used to examine the localization of hMSCs and confirm their effect on airway inflammation. Accordingly, mouse lung sections were stained with human β-microglobulin, which is expressed in all human nucleated cells. As expected, hMSCs were present close to blood vessels and the bronchial epithelium within immune cell aggregates in the asthmatic mice treated with both low and high doses of hMSCs (Figure 2).
Figure 2.
Location of hMSCs in mouse lungs. Representative fluorescence images of lung sections stained with human β-microglobulin (green) and DAPI (blue). hMSCs and other immune cells accumulated close to bronchial tissues and blood vessels in asthmatic mice treated with both low and high doses of hMSCs. Original magnification × 200.
The effect of hMSC treatment on levels of various cytokines in mouse BALF
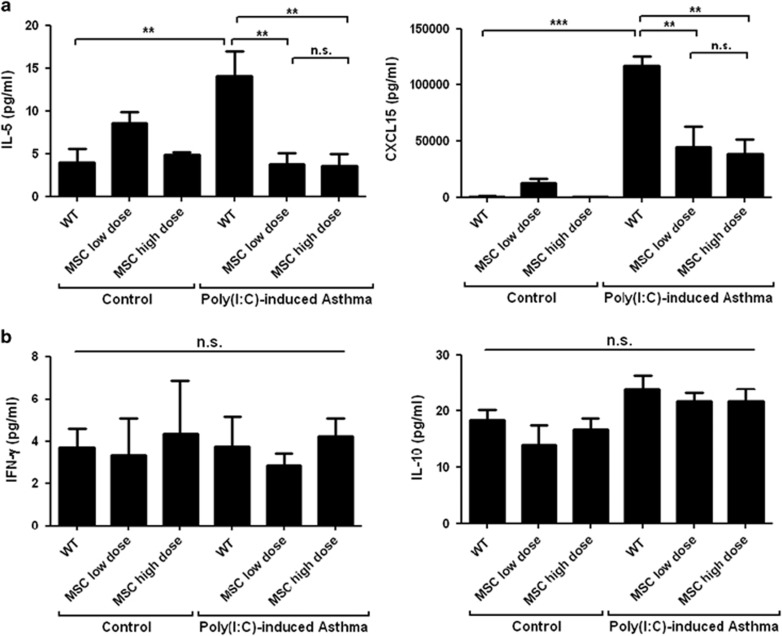
Levels of Th2-type cytokines and chemokines in mouse BALF were measured to confirm whether the hMSCs affected neutrophil infiltration and Th2 activation through effects on these chemokines. The levels of IL-5 and CXCL15 were significantly reduced in the BALF of asthmatic mice treated with both low and high doses of hMSCs (Figure 3a). Levels of IFN-γ and IL-10 were unaffected (Figure 3b), and IL-17 was not detected in the BALF.
Figure 3.
The effect of hMSCs on cytokine production in BALF in a Poly(I:C)-induced mouse model of asthma. (a) Levels of Th2 cytokines IL-5 and IL-8 in BALF. (b) Levels of IFN-γ and IL-10. n⩾9 for every group; *P<0.05, **P<0.001 and ***P<0.0001.
The effect of hMSC treatment on immune cells from mouse drainage lymph nodes
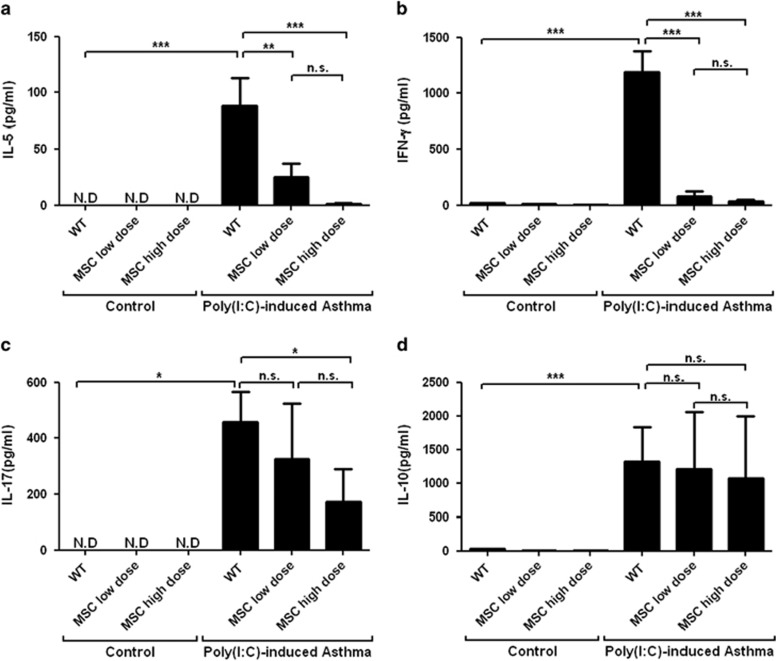
The immune cells from lung drainage lymph nodes of the hMSC-treated asthmatic mice secreted less IL-5, IL-17 and IFN-γ than those from lung drainage lymph nodes of the control mice following ex vivo stimulation with ovalbumin and Poly(I:C) (Figures 4a–c). There was no difference in IL-10 expression between the two sets of cells (Figure 4d).
Figure 4.
The effect of hMSCs on cytokine production in culture supernatant from draining lymph nodes of mice. Lymph nodes were excised, filtered and cultured in the presence or absence of 1 mg ml−1 ovalbumin and 50 μg ml−1 Poly(I:C) for 3 days. Levels of IL-5 (a), IFN-γ (b), IL-17 (c) and IL-10 (d) in culture supernatant. Similar results were obtained from three independent experiments. n⩾4 for every group; *P<0.05, **P<0.001 and ***P<0.0001.
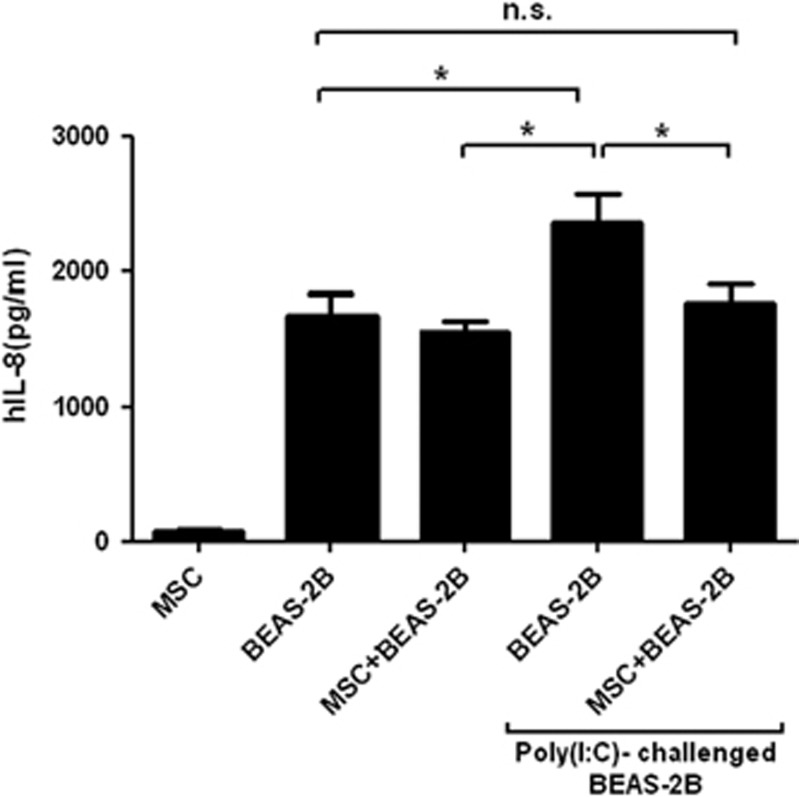
Effect of hMSCs on IL-8 production by BEAS-2B human bronchial cells
As bronchial epithelial cells are the first barrier and major source of IL-8 production in the human lung, we tested whether hMSCs interacted with BEAS-2B cells to regulate IL-8 production. As shown in Figure 5, hMSCs had no effect on IL-8 production by the BEAS-2B cells in the steady state. However, when hMSCs were co-cultured with Poly(I:C)-stimulated BEAS-2B cells, IL-8 secretion was reduced.
Figure 5.
Effect of hMSCs on Poly(I:C)-induced IL-8 production by human bronchial epithelial BEAS-2B cells. IL-8 level after co-culture of activated BEAS-2B cells with hMSCs. *P<0.05.
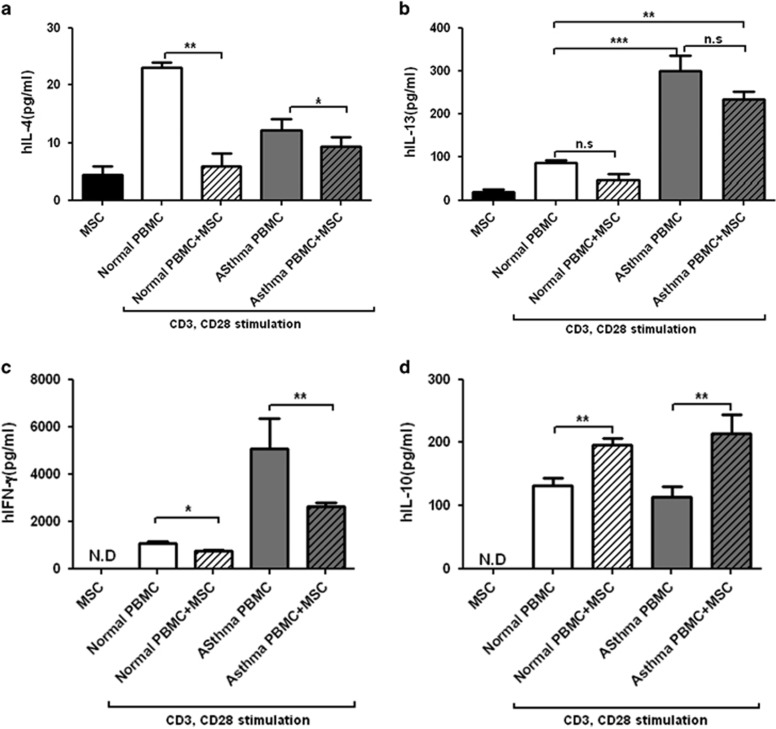
Effect of hMSCs on cytokine production by human PBMCs from asthmatic patients
hMSCs were co-cultured with PBMCs from severe asthma patients and healthy control persons. The presence of hMSCs resulted in significant reductions in the levels of IL-4 and IFN-γ in the supernatant of CD3/CD28-stimulated PBMCs from both healthy controls and asthma patients (Figures 6a–c), whereas IL-10 secretion was enhanced (Figure 6d). IL-13 production was slightly decreased, but not by a significant amount (Figure 6b).
Figure 6.
Effect of hMSCs on cytokine secretion by human PBMCs. Human PBMCs were isolated from asthma patients (n=3) and healthy controls (n=3). Human PBMCs were added to hMSCs (PBMCs to hMSC ratio, 10:1) and stimulated with anti-CD3 (1 μg ml−1) and anti-CD28 (5 μg ml−1) antibodies. Levels of IL-4 (a), IL-13 (b), IFN-γ (c) and IL-10 (d) in the supernatant were measured. *P<0.05, **P<0.001 and ***P<0.0001.
Discussion
In this study, we demonstrated a suppressive effect of hMSCs on neutrophil-dominant airway inflammation in a murine model of neutrophilic asthma. We also showed that hMSCs exert an immunomodulatory effect on human PBMCs obtained from severely asthmatic patients. On the basis of these findings, we propose that hMSCs attenuate airway inflammation by modulating immune responses in asthma that is refractory to conventional treatment. Although further studies are needed to evaluate the precise effects of hMSCs on asthmatic inflammation, these cells may provide a novel method of managing severe refractory asthma.
Asthma is a complex and heterogeneous disease in which a variety of immune cells play pathogenic roles. Accumulation of eosinophils in the lungs and expansion of Th2-type immune responses are regarded as defining features of allergic asthma.28, 29 Although it is unclear whether neutrophilic inflammation represents a unique feature of asthma, it has been reported that some asthmatics have prominent neutrophilic inflammation.8, 30 Furthermore, there is increasing evidence to indicate that neutrophils are associated with asthma severity.5, 6, 31, 32 In the current treatments for asthma, glucocorticoids are used to suppress airway inflammation; however, neutrophils are fundamentally resistant to steroids and such treatment can prolong neutrophil survival.33, 34 Thus, glucocorticoids alone may not be sufficient to manage severe asthma characterized by neutrophil-dominated inflammation, and new therapeutic strategies are urgently needed.
Previous studies have highlighted the immunoregulatory activities of MSCs, which include inhibition of T-cell proliferation, dendritic cell maturation and neutrophil function.12, 14, 35, 36 Indeed, MSCs have been considered as a treatment for inflammatory diseases such as graft-versus-host disease,37 multiple sclerosis38 and myocardial ischemia.39 A potential benefit of MSCs in asthma has been assumed from their immunosuppressive activities and their ability to accumulate at sites of inflammation in filtering organs such as the lungs, liver and spleen, in particular following intravenous injection. MSC therapy for asthma has not yet undergone clinical trials, but recent studies have reported benefits in animal models.40 However, these studies appear to have focused on eosinophilic airway inflammation, and there have been few trials in which MSCs were used in neutrophilic airway inflammation.
In the present study, a beneficial effect of hMSCs on Poly(I:C)-induced neutrophilic airway inflammation has been demonstrated for the first time. The administration of hMSCs decreased inflammation, as observed in both lung histopathology and BALF analysis. In particular, neutrophil numbers were markedly reduced among the infiltrating immune cells in BALF. Moreover, decreased BALF levels of the Th2-type inflammatory cytokines IL-5 and IL-8 were observed along with reduced levels of IL-5, IL-17 and IFN-γ secretion. These findings clearly show that hMSCs have therapeutic potential in neutrophilic inflammation comparable to that in eosinophilic inflammation. Given the lack of effective treatment for severe refractory asthma, particularly neutrophilic inflammation, a role for hMSCs as a novel therapeutic strategy for severe asthma appears promising, although further clinical studies are warranted.
Although the precise pathogenic mechanism underlying neutrophilic airway inflammation in severe refractory asthma is largely unknown, IL-8 is believed to be the pivotal cytokine responsible for recruiting neutrophils.41 Many studies have shown that various cytokines, including IL-8, can attract hMSCs to sites of inflammation,22, 23, 42 and in the present study hMSCs were found to be present in the asthmatic airways. We suggest that the decrease in IL-8 levels in the airways is the critical mechanism underlying the suppression of neutrophilic airway inflammation. Elevated IL-8 expression was observed in the BALF, and hMSC administration was followed by a significant decrease. In agreement with these in vivo studies, human bronchial epithelial cells (BEAS-2B) produced less IL-8 after Poly(I:C) stimulation in the presence of the hMSCs. Thus, it is probable that the decreased number of neutrophils in the lungs was closely related to the reduced IL-8 levels. Nevertheless, the underlying mechanism of the action of the MSCs on bronchial epithelial cells is largely unknown and should be further investigated.
Many groups have described effects of hMSCs on immune cells, and we also demonstrated above that hMSCs modulated the production of pro-inflammatory cytokines, including IL-13, IL-4 and IFN-γ, by PBMCs derived from patients with severe asthma. In this study, three patients who had both severe asthma and neutrophilic airway inflammation were enrolled. All met the criteria for severe asthma according to the international ERS/ATS guidelines on severe asthma.43 All of them showed mixed granulocytic phenotype in induced sputum with neutrophils constituting more than 60%. The patients had uncontrolled asthma but were not in flare-up state when the PBMCs were collected.
Interestingly, IL-10, a typical anti-inflammatory cytokine, increased when PBMCs were cultured with hMSCs. This finding may explain how hMSCs reduce asthmatic inflammation: the effects of hMSCs on the production of various chemokines by immune cells seem to be critical. Recent findings have suggested that MSCs have both pro-inflammatory and anti-inflammatory properties. MSCs express Toll-like receptors on their surface, and hMSCs and their supernatant appear to contribute to pro-inflammatory responses in some studies. However, in the presence of an inflammatory environment, hMSCs display an anti-inflammatory phenotype.14, 44 Indeed, co-culture with activated human bronchial epithelial cells or PBMCs tends to reduce the production of pro-inflammatory cytokines. This effect of MSCs may be a response to the inflammatory environment, as MSCs actively interact with immune cells before changing phenotype.14, 45
A limitation of this study is that the number of severe asthma patients enrolled to study the suppressive effect of stem cells on PBMCs was too small. In addition, sex and age were not matched with the controls, and clinical tests were not performed consistently to enable a comparison of clinical characteristics in the studied subjects. Further prospective large-scale studies are required to confirm our pilot experiment.
In conclusion, hMSCs may be beneficial in neutrophil-dominant airway inflammation by reducing neutrophil infiltration and Th2 activation. New biological therapies are needed for severe asthma, and our findings imply that the administration of hMSCs may be a useful strategy.
Acknowledgments
We acknowledge the financial support from the Korean Health Technology R&D Project (grants HI13C1962 and HI14C2628) of the Ministry of Health, Welfare and Family Affairs and by the ASAN Institute for Life Sciences, Seoul, Korea. This study was supported by the Korean Health Technology R&D Project (grants HI13C1962 and HI14C2628) of the Ministry of Health, Welfare and Family Affairs and by the ASAN Institute for Life Sciences, Seoul, Korea.
Author contributions
GH Hong and YS Cho coordinated the study, contributed to the study design, carried out the laboratory procedures and drafted the manuscript and figures. HS Kwon, SW Kim, TB Kim and HB Moon contributed to the coordination of the study. W Oh was responsible for MSC preparation. KY Lee, EH Ha and KA Moon helped with the animal experiments. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
References
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31: 143–178. [DOI] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120: S94–S138. [DOI] [PubMed] [Google Scholar]
- Berair R, Brightling CE. Asthma therapy and its effect on airway remodelling. Drugs 2014; 74: 1345–1369. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012; 18: 684–692. [DOI] [PubMed] [Google Scholar]
- Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis 1993; 148: 713–719. [DOI] [PubMed] [Google Scholar]
- Ordoñez CL ST, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma. Am J Respir Crit Care Med 2000; 161: 1185–1190. [DOI] [PubMed] [Google Scholar]
- Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010; 125: 1028–1036 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteseirin J. Neutrophils and asthma. J Investig Allergol Clin Immunol 2009; 19: 340–354. [PubMed] [Google Scholar]
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology 2016; 62: 216–225. [DOI] [PubMed] [Google Scholar]
- Tevlin R, Walmsley GG, Marecic O, Hu MS, Wan DC, Longaker MT. Stem and progenitor cells: advancing bone tissue engineering. Drug Deliv Transl Res 2016; 6: 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noel D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther 2010; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011; 6: 457–478. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C et al. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol 2010; 10: 1496–1500. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013; 13: 392–402. [DOI] [PubMed] [Google Scholar]
- Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol 2013; 91: 12–18. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Ren H, Han Z. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother 2016; 2: 3–20. [Google Scholar]
- Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol 2011; 164: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Jung M, Kim JH, Yoon NY, Choi EH. The effect of adipose-derived stem cell-cultured media on oxazolone treated atopic dermatitis-like murine model. Ann Dermatol 2012; 24: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol 2009; 5: 392–399. [DOI] [PubMed] [Google Scholar]
- Lee SH, Jang AS, Kwon JH, Park SK, Won JH, Park CS. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res 2011; 3: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009; 18: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol 2007; 83: 241–247. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009; 4: 206–216. [DOI] [PubMed] [Google Scholar]
- Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 2012; 30: 2692–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HK, Cho KS, Park HY, Shin DH, Kim YK, Jung JS et al. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev 2010; 19: 1811–1818. [DOI] [PubMed] [Google Scholar]
- Ou-Yang HF, Huang Y, Hu XB, Wu CG. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood) 2011; 236: 1461–1467. [DOI] [PubMed] [Google Scholar]
- Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy 2011; 66: 523–531. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 2010; 10: 838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers J, Branco Madeira F, Hammad H. Innate immune cells in asthma. Trends Immunol 2013; 34: 540–547. [DOI] [PubMed] [Google Scholar]
- Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc 2009; 6: 256–259. [DOI] [PubMed] [Google Scholar]
- Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133: 1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Tobin MC, Thomas LL. Neutrophil-like low-density granulocytes are elevated in patients with moderate to severe persistent asthma. Ann Allergy Asthma Immunol 2014; 113: 635–640 e2. [DOI] [PubMed] [Google Scholar]
- Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol 1995; 154: 4719–4725. [PubMed] [Google Scholar]
- Saffar AS, Ashdown H, Gounni AS. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr Drug Targets 2011; 12: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014; 21: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 2008; 26: 151–162. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441. [DOI] [PubMed] [Google Scholar]
- Auletta JJ, Bartholomew AM, Maziarz RT, Deans RJ, Miller RH, Lazarus HM et al. The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis. Immunotherapy 2012; 4: 529–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayyum AA, Haack-Sorensen M, Mathiasen AB, Jorgensen E, Ekblond A, Kastrup J. Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): study design. Regen Med 2012; 7: 421–428. [DOI] [PubMed] [Google Scholar]
- Srour N, Thebaud B. Stem cells in animal asthma models: a systematic review. Cytotherapy 2014; 16: 1629–1642. [DOI] [PubMed] [Google Scholar]
- Singer M, Sansonetti PJ. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of shigella-induced colitis. J Immunol 2004; 173: 4197–4206. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim JH, Lee JK, Choi SJ, Kim JS, Jeun SS et al. Overexpression of CXC chemokine receptors is required for the superior glioma-tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev 2009; 18: 511–519. [DOI] [PubMed] [Google Scholar]
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 2010; 5: e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell 2012; 10: 709–716. [DOI] [PubMed] [Google Scholar]