Abstract
The THIN-B metallo-β-lactamase, a subclass B3 enzyme produced by the environmental species Janthinobacterium lividum, was overproduced in Escherichia coli by means of a T7-based expression system. The enzyme was purified (>95%) by two ion-exchange chromatography steps and subjected to biochemical analysis. The native THIN-B enzyme is a monomeric protein of 31 kDa. It exhibits the highest catalytic efficiencies with carbapenem substrates and cephalosporins, except for cephaloridine, which acts as a poor inactivator. Individual rate constants for inactivation by chelators were measured, suggesting that inactivation occurred by a mechanism involving formation of a ternary complex.
Metallo-β-lactamases (MBLs) are the focus of increasing investigation both as resistance determinants and as model enzymes. As resistance determinants their relevance depends on their functional features (broad substrate specificity, efficient carbapenemase activity, resistance to the so-called “mechanism-based” β-lactamase inactivators) and on the recent emergence of MBLs encoded by genes associated with mobile DNA among major bacterial pathogens (19, 21, 23, 24). The interest in MBLs as model enzymes arises from the as yet superficial understanding of their catalytic mechanism and structure-function relationships, which could be essential to the development of new β-lactams and enzyme inhibitors. On the other hand, the MBL fold is conserved within a large protein superfamily that includes a growing number of proteins which do not hydrolyze β-lactams (2, 6, 8).
MBLs belong to molecular class B (1) and constitute a family of very diverse enzymes. Based on structural relatedness, they can be grouped into three different subclasses: B1, B2, and B3 (14, 23). Subclass B3, originally represented by the L1 enzyme from Stenotrophomonas maltophilia (4, 26), has recently expanded to include several enzymes from primarily environmental bacteria (FEZ from Legionella gormanii [5], GOB from Chryseobacterium meningosepticum [3], THIN-B from Janthinobacterium lividum [25], and CAU from Caulobacter crescentus [9]), some of which can occasionally behave as opportunistic pathogens. The MBLs of subclass B3 are highly divergent from those of subclass B1 at the sequence level (14) and, although they retain a three-dimensional fold that is similar overall, exhibit an organization of the metal-binding sites that differs significantly from that of enzymes of subclass B1 (15, 28).
The THIN-B enzyme from J. lividum was identified following an environmental screening of MBL-producing bacteria (25) and currently is the only known MBL from a member of the β-Proteobacteria class. Compared to the other enzymes of subclass B3, THIN-B is quite divergent and exhibits some unique structural features, including a larger size and a higher number of cysteine residues (25). However, the biochemical properties of this enzyme have not been investigated.
In this paper we describe a system for overproduction of the THIN-B enzyme in Escherichia coli, the protocol for purification of the recombinant protein, and the biochemical and kinetic characterization of THIN-B.
MATERIALS AND METHODS
Bacterial strains.
E. coli XL-1 Blue (Stratagene, Inc., La Jolla, Calif.) was used as a host for recombinant plasmids. E. coli strains BL21(DE3) (Stratagene), BL21-SI (Invitrogen, Carlsbad, Calif.), and MCT236(DE3) {E. coli CGSC6159 [lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBA-DAH33 ΔrhaBADLD78 ΔpyrC (λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1)]} (constructed in our laboratory) were used as hosts for T7 promoter-based expression plasmids for blaTHIN-B overexpression experiments.
Media and culture conditions.
Bacteria were always grown aerobically at 37°C unless otherwise specified. Luria-Bertani medium (27) was routinely used for the propagation of E. coli strains. SB medium (20 g of yeast extract/liter, 35 g of tryptone/liter, and 5 g of NaCl/liter; buffered with 50 mM sodium phosphate buffer [pH 7.0]) was used in overexpression experiments with BL21(DE3) and MCT236(DE3) strains. LBON (5 g of yeast extract/liter and 10 g of tryptone/liter) was used in overexpression experiments with BL21-SI.
Recombinant DNA methodologies.
The open reading frame encoding THIN-B was amplified by PCR using primers THIN-B-EXP/f (5′-CAT ATG ACA CTA TTG GCG AAG TTG ATG CTG), which added an NdeI linker (boldfaced) to the 5′ end, and THIN-B-EXP/r (5′-GGA TCC TAG TGC GCG TGC TGG G), which added a BamHI linker (boldfaced) to the 3′ end. PCR was performed in a volume of 50 μl, with 3.5 U of the Expand High Fidelity PCR system (Roche Biochemicals, Mannheim, Germany) in the buffer provided by the manufacturer, 200 μM deoxynucleoside triphosphates, 50 pmol of each primer, and 10 ng of plasmid pBCIRO-K (25) as the template for the blaTHIN-B gene. The following cycling conditions were used: initial denaturation at 94°C for 3 min; denaturation at 94°C for 60 s, annealing at 58°C for 60 s, and extension at 72°C for 90 s, repeated for 30 cycles; and a final extension step at 72°C for 10 min. The amplified DNA was cloned into the SmaI site of plasmid pUC-18 by using the Sure Cloning kit (Amersham Biosciences, Uppsala, Sweden), yielding the recombinant plasmid pUC-CIRO. Finally, the 0.95-kb NdeI-BamHI fragment of pUC-CIRO, containing the blaTHIN-B gene, was subcloned into the pET-9a expression vector to produce the recombinant plasmid pET-THIN-B. The cloned blaTHIN-B gene was sequenced to rule out the presence of any PCR-generated mutations.
Expression experiments.
THIN-B production was tested by using three different expression systems, obtained by transformation of E. coli BL21(DE3), BL21-SI, or MCT236(DE3) with pET-THIN-B. With each system MBL production was monitored, over a 24 h time course, in both supernatants and cell extracts of cultures growing in SB medium containing 50 μg of kanamycin/ml at either 25 or 37°C. Individual cultures were incubated until the A600 reached 0.8 and then were split into two identical flasks, to one of which was added 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) [for the BL21(DE3) and MCT236(DE3) hosts] or 0.3 M NaCl (for the BL21-SI host). Aliquots (1 ml) were sampled at different times and centrifuged (12,000 × g, 5 min, 4°C), and the culture supernatant was stored at 4°C. The bacterial pellet was resuspended in 1 ml of 10 mM HEPES-NaOH (pH 7.5) supplemented with 50 μM ZnSO4 and was disrupted by sonication (5 cycles, for 20 s each cycle, at 45 W) using a B. Braun (Melsungen, Germany) Labsonic L sonicator. The supernatant obtained after centrifugation at 10,000 × g for 15 min, to remove cell debris, represented the cell extract. MBL activities in supernatants and cell extracts were determined spectrophotometrically at 30°C by using 150 μM imipenem as the substrate (wavelength, 300 nm; Δɛ, −9,000 M−1 · cm−1) in 10 mM HEPES-NaOH buffer (pH 7.5) (20). The reaction volume was 500 μl.
Purification of the THIN-B enzyme.
The THIN-B MBL was purified from a culture of E. coli MCT236(DE3)(pET-THIN-B) grown in 0.5 liter of SB medium at 25°C. The culture was induced with 1 mM IPTG when the A600 was equal to 0.8. Cells were collected, 24 h after induction, by centrifugation (10,000 × g, 15 min, 4°C), resuspended in 20 ml of 20 mM ethanolamine-NaOH buffer (pH 9.8) containing 1 mM MgCl2 and 50 μM ZnSO4, and disrupted by sonication as described above. Cellular debris was removed by centrifugation (12,000 × g, 60 min, 4°C). The cleared supernatant was desalted by using a HiPrep 26/10 desalting column (Amersham Biosciences) and loaded (flow rate, 2 ml/min) onto an HR column (1.6 by 5 cm) packed with 10 ml of Source 15Q (Amersham Biosciences), equilibrated with 20 mM ethanolamine-NaOH buffer (pH 9.8) (buffer A). Proteins were eluted (flow rate, 4 ml/min) by using a linear gradient of NaCl (0 to 0.2 M, in 40 ml) in buffer A. Fractions exhibiting imipenemase activity were pooled and concentrated by ultrafiltration using a Centriprep YM10 filter unit (Millipore, Bedford, Mass.), and the buffer was changed to 50 mM sodium acetate buffer (pH 5.0) (buffer B) by using a PD-10 disposable column (Amersham Biosciences). The sample was then loaded (flow rate, 1 ml/min) onto a Resource S column (6.4 by 30 mm; Amersham Biosciences) preequilibrated with buffer B and was eluted by using a linear NaCl gradient in buffer B (0 to 1 M, in 20 ml). Fractions exhibiting the highest imipenemase activity were pooled, and the buffer was changed to 50 mM HEPES-NaOH (pH 7.5) containing 50 μM ZnSO4 (HBZ buffer), as above. The purified enzyme was stored in HBZ buffer at −80°C until use. During purification, imipenemase activity was assayed as described for expression experiments (see preceding section).
Protein analysis techniques.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (18) by using a 12% acrylamide concentration in the resolving gel. After electrophoresis, protein bands were stained with Coomassie brilliant blue. Analytical isoelectric focusing (IEF) of the purified protein and zymographic detection of β-lactamase activity were carried out as described previously (21). The molecular mass of native THIN-B was estimated by size exclusion chromatography using a Superdex 75 HR 10/30 column (Amersham Biosciences) as described previously (9, 10). The column was calibrated by using a mixture of bovine serum albumin (0.2 mg), ovalbumin (0.2 mg), chymotrypsinogen A (0.2 mg), and RNase A (0.5 mg). The protein concentration in solution was determined by using a commercial kit (Bio-Rad [Richmond, Calif.] protein assay) with bovine serum albumin as the standard. The BBL numbering scheme (14) is used throughout this paper.
Mass spectrometry.
The mass of the purified THIN-B protein was measured by using an Ettan MALDI-TOF Pro mass spectrometer (Amersham Biosciences). The enzyme preparation had previously been desalted by using a ZipTip C4 (Millipore Corporation). Protein identification by mass spectrometry was achieved by peptide mass fingerprinting (PMF) (17). Briefly, the purified THIN-B protein was digested in 50 mM NH4HCO3 buffer (pH 7.0) by using trypsin (Promega, Madison, Wisc.) at a final protease/protein ratio of 1:50; the reaction took place at 37°C for 1 h. The mass spectra of the peptides in the mixture were acquired by using an Ettan MALDI-TOF Pro mass spectrometer (Amersham Biosciences), and the experimental data were compared to theoretical PMFs calculated for all the sequences in DNA and protein sequence databases by using the database search engine MASCOT, available online (http://www.matrixscience.com).
Determination of kinetic parameters.
The steady-state kinetic parameters for β-lactam hydrolysis were measured in HBZ buffer at 30°C as described previously (9, 10). Competition experiments with aztreonam were carried out by using 200 μM imipenem as the reporter substrate and aztreonam concentrations up to 1 mM. Inactivation rates (ki) with divalent metal chelators were determined as described previously (10) in 50 mM HEPES-NaOH buffer (pH 7.5) (HB buffer) at 30°C by using 1 mM meropenem as the reporter substrate. Data were analyzed as described previously (16) according to the following scheme:
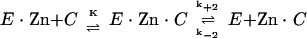 |
where E · Zn is the metalloenzyme, E is the inactive apoenzyme, C is the metal chelator, Zn · C is the metal-chelator complex, E · Zn · C is the ternary metal-enzyme-chelator complex, K is the dissociation constant for the ternary complex, k+2 is the rate constant for dissociation of the ternary complex, and k−2 is the second-order rate constant for formation of the ternary complex. The individual constants K, k+2, and k−2′ (the pseudo-first-order rate constant for formation of the ternary complex from the apoenzyme and the metal-chelator complex) were calculated by use of a nonlinear regression by fitting the equation 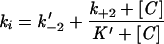 to the experimental data, where k−2′ is k−2 · [Zn · C] and K′ is
to the experimental data, where k−2′ is k−2 · [Zn · C] and K′ is 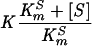 , where KmS is the Km for the reporter substrate (16). The enzyme concentration in kinetic assays ranged between 3.5 and 95 nM.
, where KmS is the Km for the reporter substrate (16). The enzyme concentration in kinetic assays ranged between 3.5 and 95 nM.
Determination of free sulfhydryl groups in the protein.
The free sulfhydryl groups were titrated in unfolded purified protein samples (after addition of 6 M guanidinium chloride), in either unreduced or reduced forms, by using Ellman's assay, as described previously (7). The reduced form was obtained after incubation of the protein in the presence of 10 mM dithiothreitol for 1 h at 20°C and subsequent removal of the excess reducer by desalting the sample by use of a PD-10 column (Amersham Biosciences). The final enzyme concentration in the assay was 0.1 mg/ml.
RESULTS AND DISCUSSION
THIN-B production in E. coli.
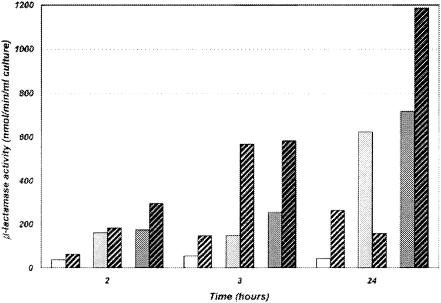
The blaTHIN-B gene was cloned in the T7-based expression vector pET-9a, and enzyme production was tested by using three different E. coli hosts producing T7 RNA polymerase. Pilot expression experiments, in which cultures were grown at 37°C, yielded poor β-lactamase activity both in culture supernatants and in cell extracts (specific imipenemase activity was always lower than 100 nmol/min/ml) (data not shown). Lowering the incubation temperature to 25°C significantly increased the β-lactamase yield (approximately 3- to 14-fold, depending on the host). Comparative analysis of β-lactamase production in the three different expression systems showed that addition of the inducer generally improved the yield of recombinant protein and that the highest activity was observed in the cell extracts after 24 h (Fig. 1). β-Lactamase activity was also measured in the culture supernatants at different times, but no trace of enzyme was found in the corresponding samples, except with the BL-21-SI-based system, for which significant cell lysis was observed when the inducer (NaCl at 0.3 M) was added to the culture (data not shown). The highest yield was obtained with MCT236(DE3)(pET-THIN-B) after induction with 1 mM IPTG, and this system was adopted for larger-scale THIN-B production and purification.
FIG. 1.
Time-dependent production of the THIN-B enzyme in cell lysates with different expression systems. E. coli BL21(DE3)(pET-THIN-B), white; E. coli BL21-SI(pET-THIN-B), light grey; E. coli MCT236(DE3)(pET-THIN-B), dark grey. Hatched bars represent samples from cultures to which an inducer (1 mM IPTG, or 0.3 M NaCl for BL21-SI) was added.
THIN-B purification.
THIN-B was purified from a cell extract of E. coli MCT236(DE3)(pET-THIN-B), obtained from a 0.5-liter culture induced with IPTG and grown at 25°C for 24 h, by means of two ion-exchange chromatography steps, an anion exchange at pH 9.8 followed by a cation exchange at pH 5.0. No detectable changes in enzyme activity were registered within 2 h of exposure at such pH values. A summary of the purification process is shown in Table 1. Higher activity was observed after desalting of the crude extract, presumably due to removal of low-molecular-weight inhibitors whose nature was not specifically investigated. The yield of purified protein was approximately 30 mg per liter of culture. The purity of the protein preparation was estimated to be >95%, according to SDS-PAGE analysis (data not shown). Recovery was relatively low (30%), mainly because, after the cation-exchange step, only the purest fractions were retained.
TABLE 1.
Summary of a typical purification procedure of the THIN-B enzyme from E. coli MCT236(DE3)(pET-THIN-B)
| Product of purification step | Vol (ml) | Total amt of protein (mg) | Total activitya (U) | Sp act (U/mg of protein) | Recov- eryc (%) | Purifi- cation (fold) |
|---|---|---|---|---|---|---|
| Cell extract | 20 | 680 | 330 | 0.49 | ||
| Sephadex G-25 eluate | 36 | 580 | 880b | 1.51 | 100 | 3.12 |
| Source 15Q eluate | 27 | 38 | 390 | 10.3 | 44 | 21 |
| Resource S eluate | 8.8 | 14.6 | 300 | 20.5 | 34 | 41 |
One unit of activity was defined as the amount of enzyme able to hydrolyze 1 μmol of substrate in 1 min under the conditions described in Materials and Methods.
The higher activity was presumably due to buffer change and/or the elimination of inhibitors potentially present in the crude extract.
Calculated on the basis of the total activity obtained after buffer exchange (Sephadex G-25 eluate).
Matrix-assisted laser desorption ionization—time-of-flight (MALDI-TOF) mass spectrometry yielded an average mass of 30,915 ± 45 Da for the purified THIN-B protein. The mass spectrum of the enzyme tryptic digest, obtained by using a MALDI-TOF spectrometer, yielded nine peaks, all at m/z ratios in agreement with those predicted by computing analysis, and covering 43% of the protein sequence (Table 2). This confirmed the authenticity of the enzyme preparation.
TABLE 2.
MALDI-TOF mass spectrum data obtained for the THIN-B protein preparation after digestion with trypsina
| Observed m/z value | Predicted mass (Da) | Mass difference (Da) | Peptide sequence |
|---|---|---|---|
| 1,381.708 | 1,381.7334 | 0.025 | G310VALPAAAPAAQHAH |
| 1,382.730 | 1,382.7096 | −0.019 | A292YAATADAMLTKRb |
| 1,506.764 | 1,506.7434 | −0.02 | G236DGPDISASFAASIAK |
| 1,530.716 | 1,530.6754 | −0.04 | S277GEHNPFIDANACR |
| 1,666.870 | 1,666.8771 | 0.007 | E308RGVALPAAAPAAQHAHb |
| 1,673.740 | 1,673.7509 | 0.011 | T21PAPKPDTPVDCDSCK |
| 1,678.930 | 1,678.8911 | −0.038 | D152DPQFQAKPVVHVAK |
| 2,263.138 | 2,263.2002 | 0.062 | V252AALPCDIILSVHPDSTGVLDK |
| 2,515.120 | 2,515.1333 | 0.013 | C213LDVVYADSLNPYSSGDFTYTGK |
The observed m/z values are compared to the predicted monoisotopic masses of the different trypsin-generated peptides, whose sequences are shown and which covered 43% of the protein sequence.
Peptides obtained with one trypsin miscleavage.
Structural features of THIN-B.
Analytical IEF of the purified protein preparation revealed a pI of 6.3 ± 0.3 (data not shown), in good agreement with the calculated value for the mature protein (pI 6.22).
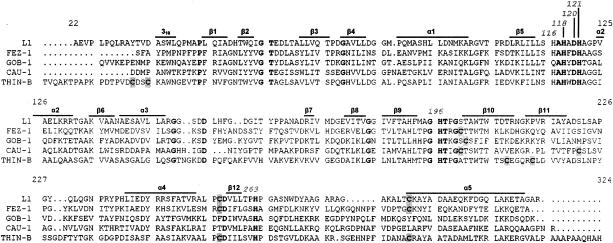
Size exclusion chromatography yielded a molecular mass of 32 ± 2 kDa, indicating that, under the experimental conditions adopted, the native enzyme is monomeric, unlike L1 but like most other MBLs (3, 4, 9, 22). The absence of oligomerization in THIN-B, despite its longer N-terminal portion, is consistent with the absence of the residues that are known to be involved in oligomerization of L1 (28). In particular, the methionine residue at position 175, which is responsible for hydrophobic intersubunit interactions involved in formation of the L1 dimer, is replaced in THIN-B by a glycine whose side chain lacks any possibility of interacting with the hydrophobic pocket of another subunit. In addition, of the three hydrophobic residues (Leu154, Pro198, Tyr236) that form the L1 hydrophobic pocket where Met175 is accommodated, only Pro198 is conserved in THIN-B (Fig. 2).
FIG. 2.
Amino acid sequence alignment of the THIN-B MBL in comparison with the other subclass B3 enzymes (L1 from S. maltophilia IID1275 [29], FEZ-1 from L. gormanii ATCC 33297 [5], GOB-1 from Chryseobacterium meningosepticum PINT [3], and CAU-1 from Caulobacter crescentus DSM4727 [9]). Residues that are identical in all sequences are boldfaced. The structural elements of L1 are indicated above the sequences (α and 310, helices; β, strands). Cysteine residues are shaded.
The THIN-B enzyme contains six cysteine residues, remarkably more than are found in the mature GOB-1 (one cysteine residue), L1 or CAU-1 (two cysteine residues), and FEZ-1 (three cysteine residues) proteins (Fig. 2). Determination of the free sulfhydryl groups yielded free cysteine/enzyme ratios of 2.1 ± 0.3 and 5.6 ± 0.4 for the unreduced and reduced proteins, respectively. This suggested that, in THIN-B, four cysteines might be involved in the formation of two disulfide bridges. One of these is likely formed by the Cys256 and Cys290 residues, which in L1 and FEZ-1 are known to form a disulfide bridge that links the last strand of the β-sheet in the C-terminal domain with the terminal α-helix (15, 28), and which are conserved in THIN-B. The other disulfide bridge would most likely involve residues 208 and 213, which are located at the extremities of the β11 and β12 strands, respectively (15). Alternatively, the disulfide bridge could involve the two cysteine residues at positions 32 and 35, which are located close to the N terminus of the protein (Fig. 2).
Another notable feature of THIN-B, compared to other enzymes of subclass B3, is its larger protein size (Fig. 2). THIN-B is 29 and 33 amino acids longer, respectively, than L1 and FEZ-1, the two subclass B3 enzymes whose three-dimensional structures have been solved. Sequence comparison and consideration of structural data indicate that most of those extra amino acids are likely to be part of a longer N-terminal coil and of a longer C-terminal β-helix, with no important modifications of internal elements (Fig. 2).
Kinetic properties of the THIN-B enzyme.
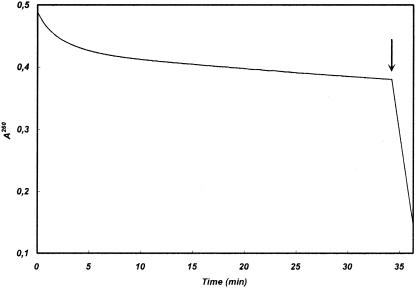
Determination of the kinetic parameters of THIN-B with several substrates representative of different β-lactam families, including penicillins, narrow- to expanded-spectrum cephems, carbapenems, and aztreonam, revealed that all the compounds tested, except aztreonam, were consistently hydrolyzed by the enzyme, although with a remarkable variability of kcat/Km ratios (from 8 × 103 to 5 × 106 M−1 · s−1) (Table 3). The highest catalytic efficiencies (kcat/Km ratios, >106 M−1 · s−1) were observed with carbapenems, cefuroxime, and cefotaxime, while cefepime appeared to be a poor substrate. Comparison of Km values indicated a higher apparent affinity for carbapenems and cephalosporins (except cefepime) than for penicillins. The highest turnover rate was observed with ampicillin (kcat, 480 s−1), a substrate for which the enzyme also exhibited the highest Km (1.3 mM), resulting in a relatively low catalytic efficiency compared to those of expanded- and broad-spectrum cephalosporins and carbapenems (Table 3). Aztreonam was not hydrolyzed and did not interact with the enzyme (Table 3), as has also been observed with other MBLs (3, 9-13, 20, 22, 23). With cephaloridine (at a substrate concentration of 100 μM), the hydrolysis kinetics presented a notable though slow inactivation pattern until an apparent steady state was reached (rate, 650 μmol/min/mg of enzyme) (Fig. 3). Although the inactivation mechanism has not been further investigated, the most reliable hypothesis possibly explaining a similar behavior could be the existence of two enzyme-substrate complex forms with significantly different turnover rates, leading to accumulation of the enzyme-substrate complex with minor activity. It would be interesting to further investigate this point by mass spectrometry experiments.
TABLE 3.
Kinetic parameters of the purified THIN-B metallo-β-lactamase and comparison with catalytic efficiencies of other subclass B3 enzymesa
| Substrate | kcat (s−1) | Km (μM) |
kcat/Km (M−1 · s−1)
|
||||
|---|---|---|---|---|---|---|---|
| THIN-B | L1 | GOB-1 | FEZ-1 | CAU-1 | |||
| Ampicillin | 480 | 1,300 | 3.7 × 105 | 4.4 × 106 | —b | 1.1 × 104 | 5 × 105 |
| Piperacillin | 100 | 500 | 2 × 105 | 7 × 106 | 1.66 × 106 | 1.2 × 104 | 5.7 × 105 |
| Cefuroxime | 140 | 50 | 2.8 × 106 | 2.7 × 106 | 9.8 × 105 | 6.6 × 106 | 1.4 × 104 |
| Cefotaxime | 80 | 40 | 2 × 106 | 2.6 × 106 | 8.5 × 105 | 2.4 × 106 | — |
| Ceftazidime | 20 | 140 | 1.4 × 105 | — | 7.6 × 105 | 4 × 103 | 2.0 × 103 |
| Cefepime | >2.3 | >300 | 7.9 × 103 | 1.9 × 104 | 2 × 105 | 6 × 103 | — |
| Imipenem | 120 | 80 | 1.5 × 106 | 7.5 × 105 | 6.6 × 105 | 2 × 105 | 2 × 105 |
| Meropenem | 200 | 40 | 5 × 106 | 4.5 × 106 | 5.34 × 106 | 5 × 105 | 2.6 × 105 |
| Aztreonam | NHc | >1,000 | — | — | — | — | — |
Data are means from three independent measurements; standard deviations were below 10%. Data for other enzymes are from the work of Felici and colleagues (11, 12) (L1), Bellais et al. (3) (GOB-1), Mercuri et al. (22) (FEZ-1), and Docquier et al. (9) (CAU-1).
—, data not available.
NH, no hydrolysis detected.
FIG. 3.
Time course for cephaloridine hydrolysis in the presence of 13 nM THIN-B. A steady state was reached after approximately 10 min. Cephaloridine was normally hydrolyzed when 8 nM VIM-2 (10) was added to the reaction mixture, as indicated by the arrow.
THIN-B exhibits a broad substrate profile, similar to that of other MBLs of subclass B3 (3, 9, 11, 12, 22). Comparison of the kinetic parameters of these enzymes reveals both common features and functional differences. The carbapenemase activity tends to be quite efficient in all cases, with an overall preference for meropenem, while cefepime tends to behave as a relatively poor substrate in most cases. On the other hand, large differences in catalytic efficiency can be observed with penicillins and some cephems (Table 3). The slow inactivation of THIN-B by cephaloridine is certainly an uncommon feature.
Inactivation of THIN-B by metal chelators.
The THIN-B enzyme was efficiently inactivated by EDTA, o-phenanthroline, and dipicolinic acid, allowing the determination of pseudo-first-order inactivation rates (ki).
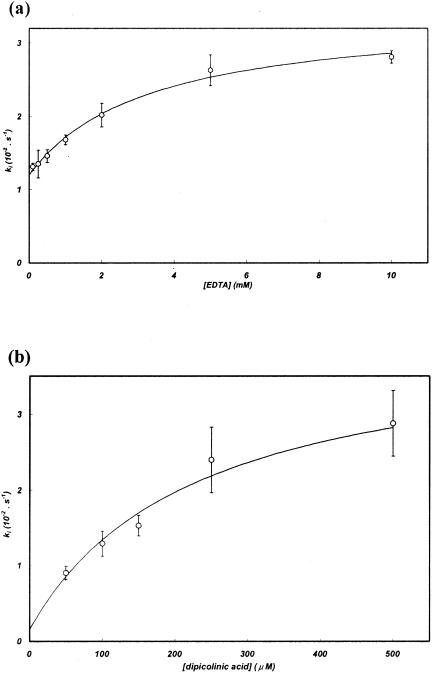
With EDTA and dipicolinic acid, the inactivation rates varied with the inactivator concentration, following a hyperbolic dependence, and individual inactivation constants (K, k+2, k−2′) could be calculated (Fig. 4; Table 4). This behavior suggests that the inactivator does not act by simply scavenging the free metal from the buffer but that a ternary enzyme-metal-inactivator complex is formed during inactivation. No similar behavior has been observed for other enzymes of subclass B3 (9, 22), and THIN-B represents the first example of a subclass B3 enzyme for which formation of the ternary complex could be hypothesized.
FIG. 4.
Dependence of the pseudo-first-order inactivation rate (ki) of THIN-B on the concentration of the metal chelator EDTA (a) or dipicolinic acid (b). The line in each graph represents the best fit resulting from the nonlinear regression used to calculate individual inactivation parameters (K, k+2, and k−2′).
TABLE 4.
Inactivation parameters for the THIN-B metallo-β-lactamase with various chelating agentsa
| Chelating agent | k−2′ (s−1) | k+2 (s−1) | K (μM) | k+2/K (M−1 · s−1) |
|---|---|---|---|---|
| EDTA | 1.2 × 10−2 | 2.2 × 10−2 | 3,140 | 7.0 × 103 |
| o-Phenanthroline | <10−2 | 1.2 × 10−2 | <50 | >2.4 × 102 |
| Dipicolinic acid | 1.5 × 10−3 | 3.9 × 10−2 | 215 | 1.8 × 102 |
Standard deviations were below 10%.
With o-phenanthroline, the measured inactivation rate [ki, (1.22 ± 0.06) × 10−2 s−1] was independent of the inactivator concentration (in the range of 50 to 500 μM) and corresponded to the k+2 constant, indicating that formation of the ternary complex was rather efficient (K, <50 μM) (Table 4).
The inactivation efficiencies of chelators were generally high, with values similar to those observed for subclass B1 enzymes (10, 13, 20). Interestingly, with THIN-B, EDTA was a better inactivator than dipicolinic acid or o-phenanthroline (Table 4), at variance from what has generally been observed for MBLs (9, 10, 13, 16, 20, 22).
Concluding remarks.
Subclass B3 of MBLs, which until recently included only the L1 enzyme from S. maltophilia, currently includes several members of notable structural and functional diversity. Although produced by a nonpathogenic bacterium, the THIN-B enzyme exhibits some peculiar structural and functional features and could be an interesting model for further structural studies. Moreover, the distribution of MBLs among an increasing number of bacterial species typically associated with environmental niches that might act as a reservoir for efficient resistance determinants might offer an important clue for MBL evolution.
Acknowledgments
This work was supported by the European Research Network on metallo-β-lactamases (contract HPRN-CT-2002-00264) and by a grant from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2003). J.-D.D. is funded by the “Fonds National de la Recherche Scientifique” of Belgium as a “Collaborateur Scientifique F.N.R.S.”
REFERENCES
- 1.Ambler, R. P. 1980. The structure of β-lactamases. Philos. Trans. R. Soc. Lond. B 289:321-331. [DOI] [PubMed] [Google Scholar]
- 2.Aravind, L. 1999. An evolutionary classification of the metallo-β-lactamase fold proteins. In Silico Biol. 1:69-91. [PubMed] [Google Scholar]
- 3.Bellais, S., D. Aubert, T. Naas, and P. Nordmann. 2000. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 44:1878-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bicknell, R., E. L. Emanuel, J. Gagnon, and S. G. Waley. 1985. The production and molecular properties of the zinc β-lactamase of Pseudomonas maltophilia IID 1275. Biochem. J. 229:791-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boschi, L., P. S. Mercuri, M. L. Riccio, G. Amicosante, M. Galleni, J. M. Frère, and G. M. Rossolini. 2000. The Legionella (Fluoribacter) gormanii metallo-β-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 β-lactamases. Antimicrob. Agents Chemother. 44:1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callebaut, I., D. Moshous, J. P. Mornon, and J. P. de Villartay. 2002. Metallo-β-lactamase fold within nucleic acids processing enzymes: the β-CASP family. Nucleic Acids Res. 30:3592-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creighton, T. E. 1989. Disulfide bonds between cysteine residues, p. 156-168. In T. E. Creighton (ed.), Protein structure, a practical approach. IRL Press, Oxford, United Kingdom.
- 8.Daiyasu, H., K. Osaka, Y. Ishino, and H. Toh. 2001. Expansion of the zinc metallo-hydrolase family of the β-lactamase fold. FEBS Lett. 503:1-6. [DOI] [PubMed] [Google Scholar]
- 9.Docquier, J. D., F. Pantanella, F. Giuliani, M. C. Thaller, G. Amicosante, M. Galleni, J. M. Frère, K. Bush, and G. M. Rossolini. 2002. CAU-1, a subclass B3 metallo-β-lactamase of low substrate affinity encoded by an ortholog present in the Caulobacter crescentus chromosome. Antimicrob. Agents Chemother. 46:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docquier, J. D., J. Lamotte-Brasseur, M. Galleni, G. Amicosante, J. M. Frère, and G. M. Rossolini. 2003. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 51:257-266. [DOI] [PubMed] [Google Scholar]
- 11.Felici, A., and G. Amicosante. 1995. Kinetic analysis of extension of substrate specificity with Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob. Agents Chemother. 39:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felici, A., G. Amicosante, A. Oratore, R. Strom, P. Ledent, B. Joris, L. Fanuel, and J. M. Frère. 1993. An overview of the kinetic parameters of class B β-lactamases. Biochem. J. 291:151-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschini, N., B. Caravelli, J. D. Docquier, M. Galleni, J. M. Frère, G. Amicosante, and G. M. Rossolini. 2000. Purification and biochemical characterization of the VIM-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:3003-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, and J. M. Frère. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Saez, I., P. S. Mercuri, C. Papamicael, R. Kahn, J. M. Frère, M. Galleni, G. M. Rossolini, and O. Dideberg. 2003. Three-dimensional structure of FEZ-1, a monomeric subclass B3 metallo-β-lactamase from Fluoribacter gormanii, in native form and in complex with d-captopril. J. Mol. Biol. 325:651-660. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Valladeres, M., A. Felici, G. Weber, H. W. Adolph, M. Zeppezauer, G. M. Rossolini, G. Amicosante, J. M. Frère, and M. Galleni. 1997. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry 36:11534-11541. [DOI] [PubMed] [Google Scholar]
- 17.James, P., M. Quadroni, E. Carafoli, and G. Gonnet. 1994. Protein identification in DNA databases by peptide mass fingerprinting. Protein Sci. 3:1347-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Laraki, N., M. Galleni, I. Thamm, M. L. Riccio, G. Amicosante, J. M. Frère, and G. M. Rossolini. 1999. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob. Agents Chemother. 43:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. de Pauw, G. Amicosante, J. M. Frère, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercuri, P. S., F. Bouillenne, L. Boschi, J. Lamotte-Brasseur, G. Amicosante, B. Devreese, J. Van Beeumen, J. M. Frère, G. M. Rossolini, and M. Galleni. 2001. Biochemical characterization of the FEZ-1 metallo-β-lactamase of Legionella gormanii ATCC 33297T produced in Escherichia coli. Antimicrob. Agents Chemother. 45:1254-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 24.Osano, E., Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura, and N. Kato. 1994. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossolini, G. M., M. A. Condemi, F. Pantanella, J. D. Docquier, G. Amicosante, and M. C. Thaller. 2001. Metallo-β-lactamase producers in environmental microbiota: new molecular class B enzyme in Janthinobacterium lividum. Antimicrob. Agents Chemother. 45:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saino, Y., F. Kobayashi, M. Inoue, and S. Mitsuhashi. 1982. Purification and properties of inducible penicillin β-lactamase isolated from Pseudomonas maltophilia. Antimicrob. Agents Chemother. 22:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Ullah, J. H., T. R. Walsh, I. A. Taylor, D. C. Emery, C. S. Verma, S. J. Gamblin, and J. Spencer. 1998. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 Å resolution. J. Mol. Biol. 284:125-136. [DOI] [PubMed] [Google Scholar]
- 29.Walsh, T. R., L. Hall, S. J. Assinder, W. W. Nichols, S. J. Cartwright, A. P. MacGowan, and P. M. Bennett. 1994. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta 1218:199-201. [DOI] [PubMed] [Google Scholar]