Abstract
AIM
To investigate the impact of inflammatory bowel disease (IBD) on α2-Heremans-Schmid Glycoprotein (AHSG/fetuin A) and potential associations with disease and patient characteristics.
METHODS
AHSG serum levels were determined in treatment-naïve newly-diagnosed patients, 96 with ulcerative colitis (UC), 84 with Crohn's disease (CD), 62 with diarrhea-predominant or mixed irritable bowel syndrome (IBS, D- and M- types) and 180 healthy controls (HC), by an enzyme linked immunosorbent assay (ELISA). All patients were followed for a minimum period of 3 years at the Gastroenterology Department of the University Hospital of Larissa, Greece. C-reactive protein (CRP), anti-glycan antibodies, anti-Saccharomyces cerevisiae mannan antibodies IgG, anti-mannobioside carbohydrate antibodies IgG, anti-laminariobioside carbohydrate antibodies IgG and anti-chitobioside carbohydrate antibodies IgA were also determined via immunonephelometry and ELISA, respectively.
RESULTS
The mean ± SE of serum AHSG, following adjustment for confounders, was 0.32 ± 0.02 g/L in IBD, 0.32 ± 0.03 g/L in CD and 0.34 ± 0.03 g/L in UC patients, significantly lower than in IBS patients (0.7 ± 0.018 g/L) and HC (0.71 ± 0.02 g/L) (P < 0.0001, in all cases). AHSG levels were comparable between the CD and UC groups. Based on AHSG levels IBD patients could be distinguished from HC with about 90% sensitivity and specificity. Further adjusted analysis verified the inverse association between AHSG and penetrating, as well as stricturing CD (partial correlation coefficient: -0.45 and -0.33, respectively) (P < 0.05). After adjusting for confounding factors, inverse correlations between AHSG and CRP and the need for anti-TNFα therapy or surgery, were found (partial correlation coefficients: -0.31, -0.33, -0.41, respectively, P < 0.05, in all cases). Finally, IBD individuals who were seropositive, for at least one marker, had AHSG levels falling within the two lower quartiles (OR = 2.86, 95%CI: 1.5-5.44, P < 0.001) while those with at least two serological markers positive exhibited AHSG concentrations within the lowest quartile (OR = 5.03, 95%CI: 2.07-12.21, P < 0.001), after adjusting for age, sex and smoking.
CONCLUSION
AHSG can be used to distinguish between IBD and IBS patients or HC while at the same time "predicting" complicated disease behavior, need for therapy escalation and surgery. Moreover, AHSG may offer new insights into the pathogenesis of IBD, since it is involved in key processes.
Keywords: Inflammatory bowel disease, Irritable bowel syndrome, Fetuin A
Core tip: α2-Heremans-Schmid Glycoprotein (AHSG/fetuin A) a multi-task negative acute-phase protein is downregulated in patients with inflammatory bowel disease (IBD). Based on this significant decrease a discrimination between IBD patients with either Crohn's disease or ulcerative colitis and those with irritable bowel syndrome or healthy controls can be made. A more robust AHSG decrease correlates well with and predicts complicated disease behavior, need for biological therapy and surgery, within three years from diagnosis. These associations are supported by additional links between AHSG and acute-phase markers i.e., C-reactive protein or serological markers linked to IBD course i.e., anti-glycan antibodies.
INTRODUCTION
Inflammatory bowel disease (IBD) has been long ago recognized as a systemic inflammatory entity and as such, it is anticipated to induce changes exceeding the boundaries of bowel mucosa, being reflected in a broader spectrum of tissues, including blood[1-7]. Examples of such changes are the fluctuations in the levels of C-reactive protein (CRP), tumour necrosis factor alpha (TNF-α), interleukins, S100 proteins, metalloproteinases, angiogenins, etc[4-7]. Since a different task is carried out by each of these biomarkers, it has been proposed that IBD induces multifarious responses which in turn, may affect the levels of different compounds via feedback mechanisms, consumption or reprioritisation of synthesis[4-8]. These observations along with the inflammatory nature of IBD itself seem to encourage the study of novel proteins that tend to become affected by the establishment of systemic inflammation[3,8]. Such a candidate is fetuin A or α2-Heremans-Schmid glycoprotein (AHSG), a substance synthesized in the liver, bone marrow and fetal organs, exhibiting properties similar to those possessed by negative acute-phase proteins[9]. AHSG has been shown to carry out various immunologic tasks, including regulation of macrophage-related lipopolysaccharide-triggered opsonisation, TNF-α and transforming growth factor beta (TGF-β) levels[10-13]. AHSG is also an inhibitor of ectopic tissue calcification such as that taking place in coronary arteries and heart valves, while at the same time promoting mineralization within fibrils and subsequently, proper bone formation[13-15]. Other recently discovered properties of AHSG include a binding ability to insulin receptor, modifying its sensitivity, participation in wound healing as well as tumor growth processes, through the induction of a more effective cell migration[8,9]. Increased AHSG levels have been recorded in fetal organs (probably reflecting an additional role for AHSG in normal organ development), in adults with either metabolic syndrome or increased insulin resistance alone, patients infected with the severe acute respiratory syndrome-coronavirus as well as individuals at high risk for future cardiovascular events: stroke and acute myocardial infarction[8,9,16]. Low levels of AHSG have been recorded in patients on hemodialysis, those with cirrhosis, hepatoma or rheumatoid arthritis (RA), an entity sharing common inflammatory pathways with IBD, and have been linked to vascular - excessive valvular and coronary artery calcification, ischemic events- and skeletal disorders - osteopenia[14,17,18]. Moreover, similar phenomena: upregulation of TNF-α and TGF-β, remodelling in intestinal microvessels, both on an acute and chronic basis, as well as manifestations linked to mineral homeostasis i.e., osteoporosis and urolithiasis, are all exerted in IBD. When bearing in mind these characteristics, the idea of examining AHSG levels in IBD, seems more than tempting[4,19-23].
MATERIALS AND METHODS
Patients
Among several patients visiting ER wards, outpatient clinics or being hospitalised for chronic diarrhea, a total of 242 patients were recruited: 96 diagnosed with ulcerative colitis (UC), 84 with Crohn's disease (CD) and 62 with irritable bowel syndrome (IBS). All patients were followed for a minimum period of 3 years at the Department of Gastroenterology at the University Hospital of Larissa, Greece. Another group consisting of 180 healthy individuals was also formed (HC). Study groups were age and sex matched (P > 0.05) with one exception: the CD and UC groups, differed significantly (P = 0.002) as anticipated, since CD is often associated with younger age. All individuals participating in the study lacked any known disease i.e., end-stage renal disease, RA, cirrhosis, hepatoma, liver metastases potentially affecting AHSG levels, with the exception of diabetes mellitus (DM), which was treated as a confounding factor and was embedded in the models used for multivariate testing. The demographic and clinical characteristics of patients and HC are presented in Table 1.
Table 1.
Patient/control and disease characteristics
| UC | CD | IBS | HC | |
| No | 96 | 84 | 62 | 180 |
| Age, yr (mean ± SD) | 50.2 ± 13.4 | 42.3 ± 15.6 | 49 ± 19.4 | 46.7 ± 12.3 |
| Age at onset | ||||
| ≥ 40 yr | - | 15 | - | - |
| < 40 yr | - | 69 | - | - |
| Sex | ||||
| Male | 60 | 45 | 36 | 114 |
| Female | 36 | 39 | 26 | 66 |
| Current smoking | ||||
| Yes | 24 | 57 | 39 | 93 |
| No | 72 | 27 | 23 | 87 |
| Disease extent (UC) | ||||
| Proctitis | 16 | - | - | - |
| Left-sided colitis | 28 | - | - | - |
| Pancolitis | 52 | - | - | - |
| Disease location (CD) | ||||
| Upper GI involvement | - | 11 | - | - |
| Ileum | - | 32 | - | - |
| Colon | - | 10 | - | - |
| Ileocolon | - | 41 | - | - |
| Disease behavior (CD) | ||||
| NS/NP | - | 49 | - | - |
| Stricturing | - | 20 | - | - |
| Penetrating | - | 15 | - | - |
| Extraintestinal manifestations | ||||
| None | 69 | 33 | - | - |
| ≥1 | 27 | 51 | - | - |
| Treatment for remission | ||||
| 5-ASA | 94 | 82 | - | - |
| Corticosteroids | 45 | 60 | - | - |
| Immunosuppressants | 18 | 30 | - | - |
| Anti- TNFα | 3 | 24 | - | - |
| Surgery | 3 | 12 | - | - |
UC: Ulcerative colitis; CD: Crohn’s disease; IBS: Irritable Bowel Syndrome; HC: Healthy controls; GI: Gastrointestinal; NS/NP: Non stricturing/non penetrating; ASA: Aminosalicylates; TNFα: Tumor necrosis factor-alpha.
The diagnosis of IBD and IBS was established upon the co-evaluation of findings originating from clinical and endoscopic procedures, imaging studies, histopathology and laboratory analyses. IBS patients were also diagnosed and classified as D-IBS or M-IBS (40 and 22 individuals, respectively) according to the Rome III criteria[24]. Disease activity in the IBD group was documented using conventional indices: Crohn's Disease Activity Index (CDAI) and the Clinical Activity Index (CAI), for UC[25,26]. A CDAI score greater than 150 and a CAI score exceeding 4, on a 0-16 scale, were considered as active CD and active UC, respectively. Disease location and behavior, in CD, were determined using the Vienna classification whereas for disease extent, in UC, the Montreal classification was used[27,28]. No animals were used for the present study.
Sample collection and preparation
Blood samples were collected upon presentation of patients in our hospital, in serum separator tubes and were allowed to clot for 30 min. All samples were then centrifuged and the obtained serum was stored at -25 °C for later analysis. The pre-analytical phase, including sampling and handling methods (sampling tubes, storage conditions etc.) was identical in all cases.
Laboratory assays
AHSG assay: For AHSG determinations, a two-site "sandwich" enzyme-linked immunosorbent assay (ELISA) was performed, using a commercially available human Fetuin A ELISA kit (BioSource Europe SA, Belgium). Assay calibrators, controls and prediluted patient serum samples (10 μL initially) containing human AHSG were added to microplate wells, coated with a high affinity polyclonal goat anti-human AHSG antibody. During incubation period, the antibody could capture human AHSG in the sample. Unbound proteins were then washed away and a horseradish peroxidase (HRP) conjugated polyclonal anti-human AHSG antibody was added to each well, so that a "sandwich" of "capture antibody-human AHSG-HRP conjugated detecting antibody" could be formed. After additional washing, incubation with a substrate solution took place, the reaction was stopped and the developed colour was quantified spectrophotometrically. The enzymatic activity of the detecting antibodies, bound to the AHSG on the wall of the microwells, was directly proportional to the amount of AHSG in the sample. A calibration curve which was generated by plotting the absorbance vs the respective human AHSG concentration for each calibrator, allowed sample AHSG determination.
C-reactive protein assay: For the determination of C-reactive protein (CRP), immunonephelometry was performed using the Behring Nephelometer Analyzer II, as well as the N High Sensitivity commercially available kit (Dade Behring Gmbh, Germany). The control and standard sera were provided by the same company and used according to the manufacturer's instructions.
Anti-glycan antibodies assay: Serum levels of anti-Saccharomyces cerevisiae mannan antibodies (gASCA) IgG, anti-mannobioside carbohydrate antibodies (AMCA) IgG, anti-laminariobioside carbohydrate antibodies (ALCA) IgG and anti-chitobioside carbohydrate antibodies (ACCA) IgA were also determined in 108 IBD (56 CD and 52 UC) patients, using commercially available ELISA kits (IBDX, Glycominds Ltd., Israel). Cut-off levels for positivity were set at 50, 100, 60, and 90 U/mL for gASCA IgG, AMCA IgG, ALCA IgG, and ACCA IgA, respectively, as instructed by the manufacturer.
Statistical analysis
Normality (Kolmogorov-Smirnov) test was initially carried out and since the normality assumption was satisfied for the comparison of means between two groups, Student's t-tests were used. For comparisons between multiple groups, one-way ANOVA and Tukey's post-hoc tests were applied. Variables are expressed as mean ± SD or mean ± SE. For variables without comparable variations, Welch's correction has been applied. AHSG was tested for its ability to predict IBD, UC and CD, separately, using receiver operating characteristic (ROC) curves, while area under the curve (AUC) and cut-off values, with the optimal sensitivity and specificity, were also calculated. For the simple correlation studies, Pearson's rank test was used. Statistical significance was set at P < 0.05. Whenever statistical significance or trend (0.05 < P < 0.1) was recorded in univariate analysis, multivariate testing was also performed. Using multiple linear regression and a backward selection process independent variables affecting AHSG levels - confounders - were identified. As candidate confounding factors were initially considered age, sex, smoking, DM, treatment modalities, disease duration and behaviour, age at onset. For the associations originating from multiple linear regression, partial correlation coefficients - quantifying the relationship between two variables while controlling for other factors- are reported. Whenever a categorical parameter was treated as dependent variable, logistic regression analyses, simple and multiple, were applied and odds ratios (ORs) as well as 95%CIs, unadjusted/adjusted for confounding, were calculated. Adjusted means were also calculated using analysis of covariance. Statistical analyses were conducted using GraphPad Prism (4.0 and 7.0) and the MedCalc 10.2.0.0 statistical softwares. Statistical review of the study was performed by a biostatistician.
Ethical considerations
The study was approved by the University of Thessaly Medical School Ethics Committee. Informed consent was obtained from all study participants, along with a verbal permission for the use of the acquired samples for scientific research.
RESULTS
AHSG levels with regard to disease characteristics
The mean ± SE of AHSG in serum was 0.33 ± 0.01 g/L for IBD, 0.73 ± 0.02 g/L for IBS patients and 0.7 ± 0.02 g/L for HC. The recorded difference between the IBD and control groups was statistically significant (P < 0.0001) and this was also the case when CD and UC were compared separately with IBS patients and HC. AHSG levels in the CD group were 0.31 ± 0.01 g/L, significantly lower than those of IBS patients and HC (P < 0.0001). Likewise, UC patients also exhibited lower AHSG levels (0.34 ± 0.01 g/L) compared to IBS patients and HC (P < 0.001). These differences remained significant between IBD (0.32 ± 0.02 g/L), CD (0.32 ± 0.03 g/L) or UC patients (0.34 ± 0.03 g/L), IBS patients (0.7 ± 0.018 g/L) and HC (0.71 ± 0.02 g/L), after adjustment for age and sex (P < 0.001, for all comparisons). When AHSG levels were compared between UC and CD patients or between IBS patients and HC, no significant differences were observed (P > 0.05, in both cases) (Figure 1).
Figure 1.
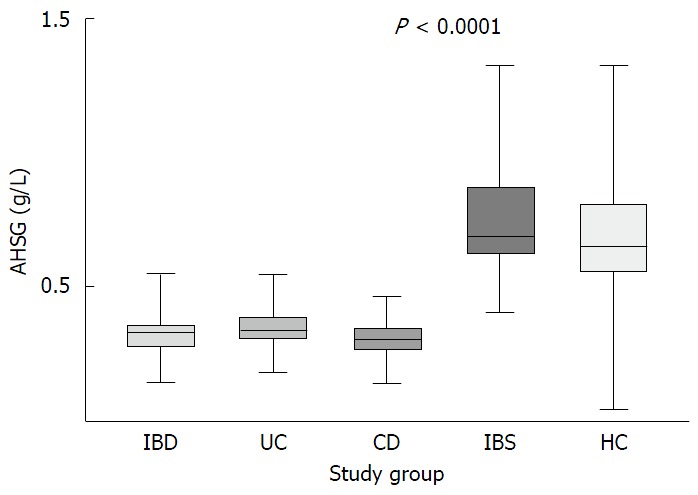
α2-Heremans-schmid glycoprotein levels in patients with inflammatory bowel disease, ulcerative colitis, Crohn's disease, irritable bowel syndrome patients and healthy controls. AHSG: α2-Heremans-schmid glycoprotein; IBD: Inflammatory bowel disease; UC: Ulcerative colitis; CD: Crohn's disease; IBS: Irritable bowel syndrome; HC: Healthy controls.
ROC curve analysis showed that the optimal cut-off of AHSG for the prediction of IBD was 0.44 g/L (90% sensitivity and specificity). Similarly, an AHSG value of 0.44 g/L could distinguish CD patients from non-IBD individuals (IBS and HC) with a sensitivity of 90% and a specificity of 90.6%, while a value of 0.42 g/L could discriminate UC patients and non-IBD subjects with 91.7% sensitivity and 89.3% specificity. The AUC was 0.94 (95%CI: 0.91-0.97), 0.94 (95%CI: 0.91-0.97) and 0.95 (95%CI: 0.92-0.98) for the prediction of IBD, UC and CD, respectively (P < 0.0001, in all cases) (Figure 2).
Figure 2.
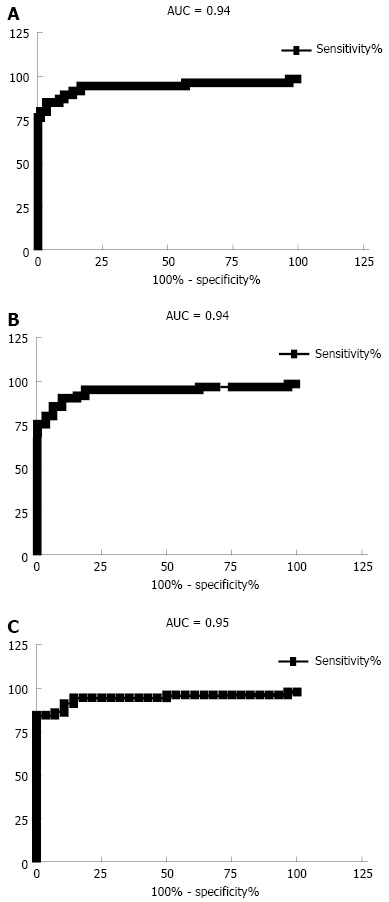
Receiver operating characteristic curves for the discrimination. A: inflammatory bowel disease (IBD) and non-IBD group -irritable bowel syndrome and healthy controls; B: ulcerative colitis; C: Crohn's disease and non-IBD group. AUC: Area under curve.
AHSG levels with regard to disease characteristics
All IBD patients exhibited active disease when blood was drawn for later analysis. In our study, in patients with active disease the AHSG levels were marginally associated with CDAI and CAI scores (r = -0.24, P = 0.08). When disease location (CD) and extent (UC) were taken under consideration no statistically significant differences were observed (P > 0.05 in all cases).
Another part of the present study included the comparison of AHSG serum concentrations among CD patients with diverse disease behavior: stricturing, penetrating and non-stricturing non-penetrating (ns/np). The performed analysis showed that patients with stricturing or penetrating disease had lower AHSG levels (0.26 ± 0.07 g/L and 0.26 ± 0.06 g/L, respectively), compared to patients in the ns/np subgroup (0.33 ± 0.07 g/L). These differences were statistically significant between CD patients with stricturing and ns/np (P < 0.01) or between the penetrating and ns/np disease subgroup (P < 0.001) but not between the stricturing and penetrating subgroups (P > 0.05) (Figure 3). Further analysis verified the inverse association between AHSG and penetrating, as well as stricturing CD, both before (r = -0.44 and -0.32, respectively, P < 0.05), as well as after adjustment (partial correlation coefficient: -0.45 and -0.33, respectively, P < 0.05) for age, sex and smoking status. In order to perform additional testing of the link between lower AHSG levels and complicated disease behavior, logistic regression was applied, while considering penetrating or stricturing CD, as dependent, and AHSG concentrations, in quartiles - lowest, low, high, highest - as independent variables. The results originating from this analysis showed that AHSG levels, in the lowest quartile, were associated with both penetrating as well as stricturing disease, before (OR = 4.25, 95%CI: 1.54-11.8 and OR = 1.23, 95%CI: 1.1-8.31, respectively) and after adjustment (OR = 8.36, 95%CI: 2.57-27.17 and OR = 3.5, 95%CI: 1.47-12.9, respectively) for age, sex and smoking status (P < 0.01, in all cases).
Figure 3.
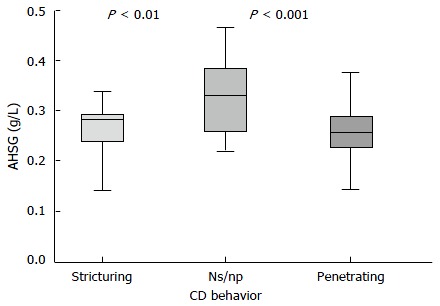
α2-Heremans-schmid glycoprotein levels in Crohn's disease patients exhibiting distinct disease behavior patterns: stricturing, penetrating or non stricturing non penetrating (ns/np). AHSG: α2-Heremans-schmid glycoprotein; CD: Crohn's disease.
AHSG levels were also examined with respect to the presence of one or more IBD-related extraintestinal manifestations. IBD patients exhibiting extraintestinal manifestations had comparable AHSG levels (0.32 ± 0.01 g/L) to the IBD subgroup without such disorders (0.33 ± 0.01 g/L, P = 0.55). Interestingly, 11 IBD patients with a history of recurrent urolithiasis had AHSG levels at the lowest quartile, this result, however, did not reach statistical significance. All data on AHSG variations according to the already described disease characteristics are presented in Table 2.
Table 2.
α2-Heremans-schmid glycoprotein levels (g/L) with respect to disease characteristics
| IBD | UC | CD | P value | |
| Extent (UC) | ||||
| Proctitis | - | 0.35 ± 0.1 | - | > 0.05 |
| Left-sided colitis | - | 0.34 ± 0.12 | - | |
| Pancolitis | - | 0.35 ± 0.10 | - | |
| Location (CD) | ||||
| Upper GI | - | - | 0.29 ± 0.09 | > 0.05 |
| Ileum | - | - | 0.32 ± 0.08 | |
| Colon | - | - | 0.31 ± 0.09 | |
| Ileocolon | - | - | 0.29 ± 0.07 | |
| Behavior (CD) | ||||
| NS/NP (a) | - | - | 0.33 ± 0.07 | a vs b: < 0.01 |
| Stricturing (b) | - | - | 0.26 ± 0.07 | a vs c: < 0.001 |
| Penetrating (c) | - | - | 0.26 ± 0.06 | b vs c: > 0.05 |
| Extraintestinal manifestations | ||||
| None | 0.33 ± 0.01 | 0.33 ± 0.09 | 0.33 ± 0.07 | > 0.05 |
| ≥ 1 | 0.32 ± 0.01 | 0.36 ± 0.1 | 0.30 ± 0.08 |
IBD: Inflammatory bowel disease; UC: Ulcerative colitis; CD: Crohn’s disease; GI: Gastrointestinal; NS/NP: Non stricturing/Non penetrating.
AHSG levels with focus on patients' characteristics
The levels of serum AHSG were studied with respect to gender of IBD patients, so that potential differences could be highlighted. Both male and female IBD patients exhibited comparable AHSG levels (0.32 ± 0.01 g/L and 0.33 ± 0.01 g/L, respectively) (P = 0.53). Likewise, AHSG levels were similar between IBD patients, while taking into account age at onset and smoking habits (Table 3).
Table 3.
Variations of α2-Heremans-schmid glycoprotein (g/L) according to patient’s characteristics
| UC | P | CD | P value | |
| Sex | ||||
| Male | 0.34 ± 0.07 | > 0.05 | 0.30 ± 0.08 | > 0.05 |
| Female | 0.34 ± 0.12 | 0.33 ± 0.07 | ||
| Current smoking | ||||
| Yes | 0.31 ± 0.02 | > 0.05 | 0.30 ± 0.01 | > 0.05 |
| No | 0.32 ± 0.01 | 0.28 ± 0.01 | ||
| Age at onset | ||||
| ≥ 40 | NA | - | 0.29 ± 0.01 | > 0.05 |
| < 40 | NA | 0.32 ± 0.02 |
UC: Ulcerative colitis; CD: Crohn’s disease; NA: Not applicable.
Serum AHSG concentrations were examined with regard to treatment modalities adequate for inducing and maintaining remission during the 3-year follow up period: 5-aminosalicylates (5-ASA), corticosteroids, immunosuppressants, anti-TNFα agents or surgery. During comparison, IBD patients requiring surgical intervention or the use of anti-TNFα therapy exhibited lower AHSG concentrations (0.26 ± 0.07 g/L and 0.3 ± 0.05 g/L), compared to those adequately treated with 5-ASA (0.33 ± 0.05 g/L) or corticosteroids (0.35 ± 0.1 g/L) (P < 0.05, in both cases). Further evaluation of the recorded associations, using simple linear regression analysis, showed that AHSG levels were inversely associated with the need for anti-TNFα treatment (r = -0.31, P < 0.05) and surgery (r = -0.36, P < 0.05). After multivariate analysis - also considering age, sex, activity, duration, smoking status- the inverse association between AHSG and need for anti-TNFα therapy or surgery remained statistically significant (partial correlation coefficients: -0.33 and -0.41, respectively - P < 0.05, in both cases). Since these results are suggestive of a link between a more profound downregulation of AHSG levels and the need for anti-TNFα treatment or surgical intervention, AHSG concentrations were classified into quartiles. Using logistic regression, AHSG levels in the lowest quartile were found to be an independent predictor of the need for anti-TNFα treatment, in a model adjusted for other treatment modalities, age, sex, smoking status and disease duration (OR = 5.22, 95%CI: 1.58-17.3, P < 0.01). Similarly, by applying the same adjusted model, it was shown that need for surgery could be independently predicted by the presence of AHSG levels within the lowest quartile (OR = 5.51, 95%CI: 1.11-27.3, P < 0.01).
AHSG and its association with IBD markers
IBD patients had higher CRP levels (median: 3.2 mg/dL, range: 0.9-29.3 mg/dL) compared to IBS (median: 1.05 mg/dL, range: 0-4.3 mg/dL) and HC groups (median: 0.9 mg/dL, range: 0-2.8 mg/dL) (P < 0.001). A correlation study of AHSG with the levels of the inflammatory marker CRP was performed revealing marginal association (r = -0.28, P = 0.07). Multivariate analysis, considering as confounding variables age, sex and smoking on the other hand, revealed a closer association between the two substances with a -0.31 partial correlation coefficient and a P = 0.02 level of significance.
Positivity rates for serological markers were 18% for gASCA, 25.6% for ALCA, 5% for AMCA and ALCA while actual median concentrations are presented in Table 4. Additional associations were investigated by examining positivity for these serological markers and AHSG (in quartiles). Interestingly, an inverse association between serology and AHSG levels was recorded. IBD individuals who were seropositive, for at least one marker, had AHSG levels falling within the two lower quartiles (OR = 2.87, 95%CI: 1.51-5.45, P < 0.001) while those with at least two serological markers positive exhibited AHSG concentrations within the lowest quartile (OR = 5.12, 95%CI: 2.17-12.08, P < 0.001). Further analysis also considering potential confounding factors such as age, sex and smoking did not alter the reported associations significantly: OR = 2.86 (95%CI: 1.5-5.44) for single and OR = 5.03 (95%CI: 2.07-12.21) for multiple seropositivity (P < 0.001, in both cases).
Table 4.
Concentrations of anti-glycan antibodies in inflammatory bowel disease patients
|
Median(range) |
P value | ||
| UC | CD | ||
| gASCA | 25.5 (18-27.2) | 49.5 (28.5-111) | < 0.0001 |
| ALCA | 46.9 (31-77.5) | 66 (30.4-94.5) | > 0.05 |
| AMCA | 67 (50.1-114.6) | 45.8 (37.7-75) | 0.0001 |
| ACCA | 65 (48-102) | 63 (51.1-105.3) | > 0.05 |
UC: Ulcerative colitis; CD: Crohn’s disease; ASCA: Anti-Saccharomyces cerevisiae mannan antibodies; AMCA: Anti-mannobioside carbohydrate antibodies; ALCA: Anti-laminariobioside carbohydrate antibodies; ACCA: Anti-chitobioside carbohydrate antibodies.
DISCUSSION
In accordance with a preliminary report from our team in 2010, AHSG levels were downregulated in patients with IBD thus, allowing discrimination from IBS and HC individuals[29]. Furthermore, a more profound downregulation of AHSG was very well associated with complicated disease behavior and the need for biological anti-TNFα treatment or surgery. Additional associations with CRP and anti-glycan antibodies offered better insight into AHSG's link with acute-phase response and IBD course.
The finding of an IBD-induced downregulation of AHSG is nothing but surprising since, AHSG levels decrease in the presence of robust inflammation[9,11,18]. Moreover, an interplay had already been documented between AHSG and the "notorious", in IBD, TNF-α and TGF-β[11-13]. An additional finding further confirming the tight link of AHSG with TNFα, was the discovery of a binding site for TNFα, within the AHSG gene. As a consequence of TNFα's binding on this region, the expression of AHSG gene is suppressed, leading to decreased AHSG production while other substances are favored-repriorisation of liver synthesis[8,11-13]. Due to the magnitude and rather IBD-selective character of this suppression, however, an evaluation of AHSG's ability to differentiate between entities has been performed. When a cut-off of 0.44 g/L was used, AHSG levels were shown to discriminate IBD from IBS and HC with a sensitivity and specificity of 90%.
When magnitude of disease activity, as expressed through CDAI and CAI scores, was taken into consideration marginal differences were detected. According to the study by Ma et al[30] significant differences in AHSG levels exist between IBD patients with active or inactive disease. On the other hand, a correlation between AHSG and CRP was confirmed, in our study. This may be, at least in part, due to limitations of the indices themselves although one should bear in mind that apart from the negative acute phase protein properties attributed to AHSG, its downregulation is also a part of a more complex liver deregulation, resulting from an excessive uptake and processing of signals[8-18]. Perhaps, possible associations of AHSG with other indices should also be evaluated[31-32]. At this point, however, an asset of the present study has to be underlined: the AHSG levels recorded in IBD patients correspond to a prior-to-therapy status and therefore, could not have been altered by any of the known drugs used to treat IBD.
A different set of results was obtained while examining AHSG levels with respect to UC extent and CD location. The recorded AHSG levels in this study could not predict disease location or extent and therefore, could not be used for this purpose.
This was not the case when disease behavior was taken into account. A further downregulation of AHSG was observed, in CD patients with stricturing or penetrating disease, compared to those with the ns/np subtype. This is not surprising as a more diffuse transmural inflammation would exert a stronger proinflammatory effect[33]. Moreover, in many organs inflammatory stress leads to fibrosis and subsequently tissue calcification[34]. From another pathogenetic perspective, a reason for the observed link between AHSG and stricturing disease could also be the reduced AHSG-induced counteraction of TGF-β's fibrogenic and antiproliferative potential[12,13,19]. Likewise, microvascular calcification, reduced blood flow and microthrombosis, exerted phenomena in the presence of low AHSG levels are also major findings during a chronic phase in the intestinal vasculature of IBD patients, leading to diminished intestinal perfusion and in turn to ulceration and fibrosis[21,35].
Another scopus of the present study was to test whether there is a link between AHSG levels and the presence of extraintestinal manifestations. The performed analysis did not reveal any associations between AHSG serum concentration and the presence of any extraintestinal manifestations. In this case, a limitation exists: the rather small number of IBD patients manifesting specific disorders i.e., urolithiasis.
A study focusing on the characteristics of IBD patients and AHSG levels was also performed. AHSG concentrations were compared among patients of different gender, smoking status and age at onset. None of these characteristics - male or female sex, current smoking or non-smoking habit and age at onset- could be linked with significant AHSG variations.
This was not the case when AHSG levels were evaluated with respect to treatment modalities adequately inducing and sustaining disease remission during the three-year follow-up period. A robust downregulation of AHSG levels correlated with the need for anti-TNFα therapy. This finding is within reason since, a more profound and persisting upregulation of TNFα, requiring subsequently the use of an anti-TNFα agent, would result in a greater AHSG decrease. As far as the link between AHSG levels and need for surgery, is concerned, one should also bear in mind that this subgroup predominantly consisted of CD patients with stricturing and penetrating disease. In the case of a refractory UC, requiring colectomy, safe conclusions could not be drawn due to the small number of colectomised patients, in our study.
The evidence mentioned above seems to make rather appealing the idea of AHSG's use as a diagnostic or even "predictive" tool rather tightly linked to IBD behavior as well as with markers related to it, such as the anti-glycan antibodies[36]. A prerequisite for this type of use is that its lack of a disease-specific nature, with similar fluctuations also reported in other disorders, is taken into account[9,14,17,18]. A combined clinical and AHSG-based algorithm for the diagnosis of IBD, on the other hand, could help overcome this limitation.
Setting aside the already-performed diagnostically-oriented interpretation of current results, a careful examination of AHSG's potential implication in the pathogenesis and complications of IBD, should also be performed. As already mentioned, AHSG is an inhibitor of unwanted, ectopic, tissue calcification and its decreased levels, as in this case, have been linked with excessive valvular and arterial calcification[4]. Although, initial reports originated from animal studies and those recruiting dialysis patients, similar results were obtained in patients with ischemic heart disease, alone[37]. Since a decrease in circulating AHSG is present in IBD, it would be logical to hypothesize that an accompanying reduced inhibition of vascular and valvular calcification would also be present. As this study was not designed to "tackle" these matters, the actual contribution of low AHSG levels in vascular changes and related acute or chronic ischemic events in IBD[38], remains to be clarified.
Apart from the implication of AHSG in vascular pathology, another role, that of bone mineralisation has been identified[13-15]. Under the influence of AHSG, a "reliable", stress-resistant bone structure is formed, while in the absence of AHSG, minerals deposit outside fibrils, leading to defective osseous formation and finally osteoporosis[15]. As IBD patients are at high risk for osteoporosis, resulting from corticosteroids, malnutrition and inflammation-induced osteopenia[22] the examination of possible contribution of AHSG suppression for the onset of osteopenic manifestations might prove fruitful. In a rather similar manner, a defective coordination in mineral use, predominantly that of calcium, due to decreased AHSG levels, may lead to urolithiasis. Indeed, in the study of Stejskal et al[39] lower levels of AHSG in urine have been associated with the presence of urolithiasis. Although, in the case of IBD, well-established mechanisms- i.e., hyperoxaluria, reduced citric acid etc[18] - for urinary compications exist, when bearing in mind that still 80% of urinary stones consist of calcium salts it is within reason to test for additional candidates involved in calcium homeostasis and urinary manifestations, such as AHSG[40].
Conclusively, AHSG seems to emerge as a molecule of potential diagnostic and perhaps predictive value in IBD, as its downregulation shows a tight link with acute-phase. As well as with chronic inflammatory responses in both CD and UC. This link also encompasses the more challenging cases, those with complicated disease behavior, as well as those requiring advanced treatment strategies such as biological agents or surgery. As far as the pathogenetic role of AHSG in IBD is concerned, further studies designed to assess the impact and the association of low AHSG levels with respect to micro- and macro-vascular changes, skeletal and urinary complications recorded in patients with CD and UC, are needed.
ACKNOWLEDGMENTS
The authors would like to thank the mathematician/statistician Ms Aikaterini Nikolaidou, MSc for checking and verifying statistical analysis data.
COMMENTS
Background
α2-Heremans-schmid glycoprotein (AHSG/fetuin A), a negative acute-phase protein with multiple functions, becomes downregulated in the presence of inflammation.
Research frontiers
An AHSG downregulation in inflammatory bowel disease (IBD) patients has been initially reported by our research team in 2010. Another independent study, later verified this result. A major confounding factor, that of IBD-specific treatment existed in both studies.
Innovations and breakthroughs
This is the first study assessing AHSG levels in treatment-naive patients. The low levels of AHSG found in IBD patients are not recorded in control subjects-IBS patients and healthy controls- and are well-associated with complicated disease behavior, as well as the need for anti-TNFα treatment or surgery. Additional links with the acute phase protein C-reactive protein and serological markers linked to IBD course, the anti-glycan antibodies, have been found.
Applications
AHSG could serve as an additional marker for IBD diagnosis and prediction of a more challenging course requiring treatment with biological agents or surgery, during the three-year period following initial diagnosis.
Peer-review
The paper is valuable. It finds AHSG can be used to distinguish between IBD and irritable bowel syndrome, and may predict complicated disease behavior, and foretell the need for intensified therapy and surgery. Moreover, AHSG may offer new insights into the pathogenesis of IBD.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the University of Thessaly Medical School Ethics Committee.
Informed consent statement: Informed consent was obtained from all study participants, along with a verbal permission for the use of the acquired samples for scientific research.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Data sharing statement: No additional data are available.
Peer-review started: September 23, 2016
First decision: November 9, 2016
Article in press: December 21, 2016
P- Reviewer: Lakatos PL, Wen DG S- Editor: Qi Y L- Editor: A E- Editor: Liu WX
References
- 1.Wong A, Bass D. Laboratory evaluation of inflammatory bowel disease. Curr Opin Pediatr. 2008;20:566–570. doi: 10.1097/MOP.0b013e32830d3aaf. [DOI] [PubMed] [Google Scholar]
- 2.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Plevy S. The immunology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:77–92. doi: 10.1016/s0889-8553(01)00006-1. [DOI] [PubMed] [Google Scholar]
- 4.Niederau C, Backmerhoff F, Schumacher B, Niederau C. Inflammatory mediators and acute phase proteins in patients with Crohn's disease and ulcerative colitis. Hepatogastroenterology. 1997;44:90–107. [PubMed] [Google Scholar]
- 5.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 6.Ravi A, Garg P, Sitaraman SV. Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm Bowel Dis. 2007;13:97–107. doi: 10.1002/ibd.20011. [DOI] [PubMed] [Google Scholar]
- 7.Kapsoritakis A, Sfiridaki A, Maltezos E, Simopoulos K, Giatromanolaki A, Sivridis E, Koukourakis MI. Vascular endothelial growth factor in inflammatory bowel disease. Int J Colorectal Dis. 2003;18:418–422. doi: 10.1007/s00384-003-0495-y. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Emoto M, Inaba M. Fetuin-A: a multifunctional protein. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:124–146. doi: 10.2174/187221411799015372. [DOI] [PubMed] [Google Scholar]
- 9.Arnaud P, Kalabay L. Alpha2-HS glycoprotein: a protein in search of a function. Diabetes Metab Res Rev. 2002;18:311–314. doi: 10.1002/dmrr.315. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhang M, Bianchi M, Sherry B, Sama A, Tracey KJ. Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci USA. 1998;95:14429–14434. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ombrellino M, Wang H, Yang H, Zhang M, Vishnubhakat J, Frazier A, Scher LA, Friedman SG, Tracey KJ. Fetuin, a negative acute phase protein, attenuates TNF synthesis and the innate inflammatory response to carrageenan. Shock. 2001;15:181–185. doi: 10.1097/00024382-200115030-00004. [DOI] [PubMed] [Google Scholar]
- 12.Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 13.Szweras M, Liu D, Partridge EA, Pawling J, Sukhu B, Clokie C, Jahnen-Dechent W, Tenenbaum HC, Swallow CJ, Grynpas MD, et al. alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem. 2002;277:19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 14.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 15.Price PA, Toroian D, Lim JE. Mineralization by inhibitor exclusion: the calcification of collagen with fetuin. J Biol Chem. 2009;284:17092–17101. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manolakis AC, Tiaka EK, Kapsoritakis AN, Georgoulias P, Tsiopoulos F, Valotassiou V, Potamianos SP. Increased fetuin A levels in Helicobacter pylori infection: a missing link between H. pylori and insulin resistance? Diabetologia. 2011;54:472–474. doi: 10.1007/s00125-010-1995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalabay L, Jakab L, Prohászka Z, Füst G, Benkö Z, Telegdy L, Lörincz Z, Závodszky P, Arnaud P, Fekete B. Human fetuin/alpha2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur J Gastroenterol Hepatol. 2002;14:389–394. doi: 10.1097/00042737-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Kazama JJ, Wada Y, Kuroda T, Narita I, Gejyo F, Gao P, Yamashita H. Decreased levels of circulating alpha2-Heremans-Schmid glycoprotein/Fetuin-A (AHSG) in patients with rheumatoid arthritis. Intern Med. 2007;46:1685–1691. doi: 10.2169/internalmedicine.46.6269. [DOI] [PubMed] [Google Scholar]
- 19.Burke JP, Mulsow JJ, O'Keane C, Docherty NG, Watson RW, O'Connell PR. Fibrogenesis in Crohn's disease. Am J Gastroenterol. 2007;102:439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 20.Danese S. Nonimmune cells in inflammatory bowel disease: from victim to villain. Trends Immunol. 2008;29:555–564. doi: 10.1016/j.it.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Hatoum OA, Binion DG, Gutterman DD. Paradox of simultaneous intestinal ischaemia and hyperaemia in inflammatory bowel disease. Eur J Clin Invest. 2005;35:599–609. doi: 10.1111/j.1365-2362.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- 22.Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122:599–604. doi: 10.1016/j.amjmed.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caudarella R, Rizzoli E, Pironi L, Malavolta N, Martelli G, Poggioli G, Gozzetti G, Miglioli M. Renal stone formation in patients with inflammatory bowel disease. Scanning Microsc. 1993;7:371–379; discussion 371-379;. [PubMed] [Google Scholar]
- 24.Rome Foundation. Guidelines--Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J Gastrointestin Liver Dis. 2006;15:307–312. [PubMed] [Google Scholar]
- 25.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 26.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 29.Manolakis AC, Kapsoritakis AN, Georgoulias P, Tiaka EK, Oikonomou KA, Christodoulidis G, Valotassiou VJ, Potamianos SP. Inflammatory bowel disease-induced changes in circulating levels of a2-Heremans-Schmid Glycoprotein (Fetuin A) JCC. 2010;4:S37. doi: 10.3748/wjg.v23.i3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma P, Feng YC. Decreased serum fetuin-A levels and active inflammatory bowel disease. Am J Med Sci. 2014;348:47–51. doi: 10.1097/MAJ.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 31.Jørgensen LG, Fredholm L, Hyltoft Petersen P, Hey H, Munkholm P, Brandslund I. How accurate are clinical activity indices for scoring of disease activity in inflammatory bowel disease (IBD)? Clin Chem Lab Med. 2005;43:403–411. doi: 10.1515/CCLM.2005.073. [DOI] [PubMed] [Google Scholar]
- 32.Turner D, Seow CH, Greenberg GR, Griffiths AM, Silverberg MS, Steinhart AH. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1081–1088. doi: 10.1016/j.cgh.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Peng CF, Murphy ML, Straub KD. Effects of early reperfusion on the mechanical and biochemical characteristics of ischemic myocardium. J Surg Res. 1986;41:493–502. doi: 10.1016/0022-4804(86)90167-8. [DOI] [PubMed] [Google Scholar]
- 34.Stelow EB, Bardales RH, Lai R, Mallery S, Linzie BM, Crary GS, Stanley MW. The cytological spectrum of chronic pancreatitis. Diagn Cytopathol. 2005;32:65–69. doi: 10.1002/dc.20160. [DOI] [PubMed] [Google Scholar]
- 35.Redberg RF, Shaw LJ. A review of electron beam computed tomography: implications for coronary artery disease screening. Prev Cardiol. 2002;5:71–78. doi: 10.1111/j.1520-037x.2002.0576.x. [DOI] [PubMed] [Google Scholar]
- 36.Papp M, Altorjay I, Dotan N, Palatka K, Foldi I, Tumpek J, Sipka S, Udvardy M, Dinya T, Lakatos L, et al. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am J Gastroenterol. 2008;103:665–681. doi: 10.1111/j.1572-0241.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 37.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Häring HU, Boeing H, Fritsche A. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 38.Ha C, Magowan S, Accortt NA, Chen J, Stone CD. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol. 2009;104:1445–1451. doi: 10.1038/ajg.2009.81. [DOI] [PubMed] [Google Scholar]
- 39.Stejskal D, Karpisek M, Vrtal R, Student V, Solichova P, Fiala R, Stejskal P. Urine fetuin-A values in relation to the presence of urolithiasis. BJU Int. 2008;101:1151–1154. doi: 10.1111/j.1464-410X.2007.07432.x. [DOI] [PubMed] [Google Scholar]
- 40.Devuyst O, Pirson Y. Genetics of hypercalciuric stone forming diseases. Kidney Int. 2007;72:1065–1072. doi: 10.1038/sj.ki.5002441. [DOI] [PubMed] [Google Scholar]


