Abstract
AIM
To identify the risk factors and clarify the subsequent clinical courses.
METHODS
This study retrospectively analyzed consecutive patients with esophageal squamous cell carcinoma (ESCC) treated using endoscopic submucosal dissection (ESD) between April 2008 and October 2012. We divided the ESCC lesions into perforation cases and non-perforation cases, and compared characteristics and endoscopic findings between the two groups. "Intraoperative perforation" was defined as the detection of a perforation site during ESD and the presence of mediastinal emphysema.
RESULTS
In total, 147 patients with 156 ESCC lesions were treated by ESD. Intraoperative perforation was recorded for nine lesions (5.8%) from nine patients. Multivariate analysis identified mucosal deficiency larger than 75% of the circumference of the esophagus as an independent risk factor for intraoperative perforation (OR = 7.37, 95%CI: 1.45-37.4, P = 0.016). The predominant site of perforation was the left wall [6/9 (67%)]. Six of nine perforation sites were successfully closed by clips during the procedures. Two of nine cases required drainage for pleural effusions; however, all nine cases recovered with conservative treatment and without surgical intervention. At the median follow up of 42 mo after ESD, no cases of local recurrence or distant organ metastasis had been observed.
CONCLUSION
This study suggests that mucosal deficiency larger than 75% of the luminal circumference is a risk factor for intraoperative perforation during ESD for ESCC.
Keywords: Endoscopic submucosal dissection, Risk factor, Esophageal carcinoma, Perforation
Core tip: Perforation is the major complication during endoscopic submucosal dissection (ESD), with a frequency of 0%-6.9%. The risk factors for intraoperative perforation during ESD for esophageal squamous cell carcinoma (ESCC) are largely unknown. In this study, we assessed the differences in perforation and non-perforation groups regarding the characteristics and endoscopic findings. Mucosal deficiency larger than 75% of the luminal circumference was a risk factor for intraoperative perforation during ESD for ESCC.
INTRODUCTION
An increasing number of esophageal squamous cell carcinoma (ESCC) lesions are being detected because of recent technological advances in endoscopy[1]. Therefore, we can now detect ESCC more frequently at the early stage. Endoscopic mucosal resection (EMR) has been accepted widely as the standard procedure for superficial ESCC. However, it is difficult to resect lesions larger than 20 mm in diameter by an en bloc manner in EMR. Therefore, endoscopic submucosal dissection (ESD) was developed as a new technique to resolve the problem.
ESD has the advantage over EMR of enabling ESCC resection in an en bloc manner, regardless of tumor size, and to provide a reduction in the local recurrence rate[2]. However, ESD is technically more difficult and has a higher rate of complications than EMR[3], because the esophagus has a narrow lumen and a thin wall without a serous membrane. Perforation is the major complication during ESD, and the frequency is reported to be 0%-6.9%[2,4-6]. However, little is known regarding the risk factors for intraoperative perforation and the subsequent clinical courses. The aim of this study was to identify the risk factors for intraoperative perforations and to clarify the clinical courses after perforation during ESD for superficial ESCC.
MATERIALS AND METHODS
Patients
This study analyzed retrospectively consecutive patients with ESCC treated using ESD at the National Cancer Center Hospital East in Japan between April 2008 and October 2012. The indication criteria of ESD for ESCC were as follows: (1) clinical depth invasion was limited within submucosal 1 (SM1)[7]; (2) absence of lymph node or distant metastasis; (3) histologically confirmed ESCC with biopsy specimens before ESD; and (4) provision of written informed consent. Lesions of ESD for cervical ESCC that required general anesthesia in the operation room were excluded.
Macroscopic type was classified using the Paris classification[8]. All cases were divided into two groups: intraoperative perforation cases and non-perforation cases. "Intraoperative perforation" was defined as the detection of a perforation site during ESD, and the presence of mediastinal emphysema as observed on computed tomography (CT) or radiography.
All information was collected from medical records, including endoscopic images in filing systems, radiological images, and pathological reports. The institutional review board of our institution approved the study protocol in September 2014 (2014-119). The study was performed according to the ethical principles of the Declaration of Helsinki.
ESD procedure
All ESD procedures were performed using a single-channel upper gastrointestinal endoscope (GIF-Q260J; Olympus Medical Systems, Tokyo, Japan), a water-jet system (OFP; Olympus), and a high frequency generator (ICC200 or VIO300D; Erbe Elektromedizin Ltd., Germany). The transparent attachment (disposable distal attachment; Olympus) was fitted on to the tip of the endoscope.
The outline of the lesion was identified by staining with 2% iodine solution, and marking spots were made on the entire circumference outside of the tumor margins. The mucosa around the lesion was cut circumferentially with a dual knife (Olympus) or an insulation-tipped diathermic knife (IT knife; Olympus), after injection into the submucosal layer of 0.4% sodium hyaluronate (MucoUp®; Johnson and Johnson, Tokyo, Japan) diluted with normal saline solution to create a submucosal cushion. The dual knife was used in most ESD procedures in all cases. We used the IT knife adjunctively, with which a cut was by drawing the knife in the direction of the long axis in cases with a long lesion. All patients underwent ESD using carbon dioxide (CO2) insufflation.
The patients were placed in the left lateral decubitus position and put under sedation with an intravenous injection of 2-3 mg midazolam and 35 mg pethidine hydrochloride. Sedative drugs were added as required to keep the patients calm, and the patients were monitored with pulse oximeters and administered with oxygen via a cannula when their saturation became low.
Cases with no complications were allowed to drink water on the day after surgery, and gradually converted to solid food.
Treatment for perforation cases
When a perforation was detected during the ESD procedure, an operator tried to close the perforation with through-the-scope clips (HX-610; Olympus); however, this was only performed in cases where the operator predicted that the intervention would lead to interruption of the remaining ESD procedure. If the patient's vital status, such as oxygen saturation, blood pressure, or subcutaneous emphysema worsened despite the best supportive treatment during the procedure, ESD was immediately discontinued. If the patient's vital status was stable, the ESD procedure was continued, or the operator switched from ESD to performing endoscopic piecemeal mucosal resection (EPMR), using a snare to reduce the procedure time. After the procedure, the patient was assessed with CT or radiography to evaluate the degree of subcutaneous or mediastinal emphysema, or the presence of a pneumothorax or pleural effusion. Cases with perforation were fasted and treated by intravenous administration of antibiotics under continual drainage of saliva at the perforation site through a nasal tube until the confirmation of closure. Closure of the perforation was confirmed by esophagography under endoscopic observation. In cases of massive pleural effusion, a chest drain was inserted as required.
Statistical analysis
We analyzed the data comparatively between the perforation group and non-perforation group using Fisher's exact tests or t-tests for the following variables: age, sex, history of treatment for esophageal carcinoma, maximum dimension of lesion, rate of 75% or larger circumference of mucosal deficiency after ESD, lesion location, depth of invasion, predominant site, operator, procedure time, and en bloc resection rate. Operators were divided into two cohorts, instructor and novice. The instructor in this study was defined as the Japan Gastroenterological Endoscopy Society board certified instructor.
Risk factors for perforation were determined using a logistic regression model. Factors that might influence intraoperative perforation were analyzed by univariate logistic regression analysis, and factors with a P value < 0.2 were analyzed by multiple logistic regression under the forced entry method. Multicollinearity was assessed using the Pearson product moment correlation coefficient or Spearman's rank correlation coefficient. A correlation coefficient > 0.5 was regarded as a strong correlation.
A P value < 0.05 was considered statistically significant. All analyses were performed using SPSS version 22 (SPSS Japan Inc., Tokyo, Japan).
RESULTS
Clinicopathological characteristics and procedure outcomes
A total of 147 consecutive patients with 156 ESCC lesions treated with ESD were enrolled. Male sex was predominant, and the median age of patients was 68 years (range, 46-88). The median maximum lesion dimension was 29 mm (range, 6-68), and most tumors were located in the middle (53.2%) or lower (41.6%) thoracic esophagus.
Of the 156 ESCC, 131 (84.0%) were intramucosal carcinomas and 25 (16.0%) were submucosal carcinomas. The macroscopic types of almost all tumors were type 0-IIc (151 lesions, 96.8%). The mean procedure time was 107.1 ± 50.2 min. The en block resection rate was 90.4%. Intraoperative perforations were recorded as having occurred in nine lesions (5.8%) in nine patients. There was no case of late perforation in this study (Table 1).
Table 1.
Clinicopathological characteristics and procedure outcomes of the 156 esophageal squamous cell carcinomas in 147 patients n (%)
| Patients = 147, ESCC = 156 | |
| Age, median (range) | 68 (46-88) |
| Sex | |
| Male | 131 (89.1) |
| Female | 16 (10.9) |
| Lesion | |
| Maximum dimension, median (range), mm | 29 (6-68) |
| Location | |
| Upper | 8 (5.1) |
| Middle | 83 (53.2) |
| Lower | 65 (41.7) |
| Depth of invasion | |
| EP | 50 (32.1) |
| LPM | 44 (28.2) |
| MM | 37 (23.7) |
| SM1 | 8 (5.1) |
| SM2 | 17 (10.9) |
| Macroscopic type | |
| 0-IIc | 151 (96.8) |
| 0-IIa | 3 (1.9) |
| 0-I + IIc | 2 (1.3) |
| Predominant site | |
| Right | 102 (65.4) |
| Left | 54 (34.6) |
| Operator | |
| Instructor | 100 (64.1) |
| Novice | 56 (35.9) |
| Procedure time, mean ± SD, min | 107.1 ± 50.4 |
| En block resection | 141 (90.4) |
| Perforation | 9 (5.8) |
| Late perforation | 0 (0.0) |
ESCC: Esophageal squamous cell carcinoma.
Clinicopathological findings associated with intraoperative perforation
No significant differences were observed between the perforation group (n = 9) and the non-perforation group (n = 148) in terms of age, sex, history of treatment for prior esophageal carcinoma, lesion location, depth of invasion, predominant site, or operator. In the perforation group, however, the mean maximum dimension of the lesion was significantly larger (42.9 mm vs 30.8 mm, P = 0.016), and the frequency of having a circumference of mucosal deficiency of 75% or larger after ESD was significantly higher (77.8% vs 30.6%, P = 0.007). Furthermore, the mean procedure time was significantly longer (183.8 min vs 102.4 min, P < 0.001). In contrast, the en block resection rate was significantly lower (33.3% vs 93.9%, P < 0.001) in ESD for patients who developed a perforation during the procedure (Table 2).
Table 2.
Clinicopathological findings associated with intraoperative perforation during endoscopic submucosal dissection n (%)
|
Perforated group |
Non-perforated group |
P value | |
| n = 9 | n = 147 | ||
| Age, mean ± SD | 67.9 ± 9.7 | 68.1 ± 7.5 | 0.929 |
| Sex | |||
| Male | 9 (100) | 131 (89.1) | |
| Female | 0 (0) | 16 (10.9) | 0.599 |
| History of treatment for esophageal carcinoma, n (%) | |||
| Yes | 0 (0) | 17 (11.6) | |
| No | 9 (100) | 130 (88.4) | 0.599 |
| Maximum dimension of lesion, mean ± SD, mm | 42.9 ± 19.3 | 30.8 ± 14.1 | 0.016 |
| Mucosal deficiency ≥ 75% circumference, n (%) | |||
| Yes | 7 (77.8) | 45 (30.6) | |
| No | 2 (22.2) | 102 (69.4) | 0.007 |
| Location | |||
| Upper | 0 (0) | 8 (5.4) | |
| Middle | 4 (44.4) | 79 (53.7) | |
| Lower | 5 (55.6) | 60 (40.8) | 0.697 |
| Depth of invasion | |||
| M | 6 (66.7) | 125 (85.0) | |
| SM | 3 (33.3) | 22 (15.0) | 0.158 |
| Predominant site | |||
| Right | 4 (44.4) | 98 (66.7) | |
| Left | 5 (55.6) | 49 (33.3) | 0.277 |
| Operator | |||
| Instructor | 6 (66.7) | 94 (63.9) | |
| Novice | 3 (33.3) | 53 (36.1) | 1.00 |
| Procedure time, mean ± SD, min | 183.8 ± 48.7 | 102.4 ± 46.7 | < 0.001 |
| En bloc resection | 3 (33.3) | 138 (93.9) | < 0.001 |
Risk factors for perforation by univariate and multivariate analysis
Upon univariate analysis, the maximum lesion dimension (10 mm increments; OR = 1.64, 95%CI: 1.07-2.52, P = 0.025) and a mucosal deficiency corresponding to more than 75% of the circumference (OR = 7.93, 95%CI: 1.59-39.7, P = 0.012) were risk factors for intraoperative perforation (Table 3). There was a strong correlation between maximum lesion dimension and mucosal deficiency (correlation coefficient = 0.598), thus the maximum lesion dimension was excluded from the multivariate analysis. Multiple logistic regression analysis identified only a mucosal deficiency larger than 75% of the lumen circumference as an independent risk factor for intraoperative perforation (OR = 7.37, 95%CI: 1.45-37.4, P = 0.016; Table 4).
Table 3.
Risk factors for perforation by univariate analysis
| Factor | Odds ratio1 (95%CI) | P value |
| Age (10 yr increments) | 0.96 (0.40-2.32) | 0.931 |
| Maximum dimension of lesion (10 mm increments) | 1.64 (1.07-2.52) | 0.025 |
| Mucosal deficiency (< 75% vs ≥ 75% circumference) | 7.93 (1.59-39.7) | 0.012 |
| Location (upper + middle vs lower) | 1.86 (0.48-7.23) | 0.368 |
| Depth of invasion (M vs SM) | 3.00 (0.69-12.9) | 0.140 |
| Predominant site (right vs left) | 2.50 (0.64-9.72) | 0.186 |
| Operator (novice vs instructor) | 1.13 (0.27-4.69) | 0.869 |
Univariate logistic regression analysis. CI: Confidence interval.
Table 4.
Risk factors for perforation by multivariate analysis
| Factor | Odds ratio1 (95%CI) | P value |
| Mucosal deficiency (< 75% vs ≥ 75% circumference | 7.37 (1.45-37.4) | 0.016 |
| Depth of invasion (M vs SM) | 2.63 (0.57-12.2) | 0.218 |
| Predominant site (right vs left) | 2.33 (0.57-9.57) | 0.240 |
Multivariate logistic regression analysis.
Outcomes of ESD and clinical courses in perforation cases
While ESD was withdrawn and switched to EPMR after confirmation of perforation during the procedure in five of nine cases, endoscopic resection was completed in all cases. Although ESD was continued in the remaining four cases, it was not possible to complete en block resection in one of the four cases, whereas the procedure was completed in the other three cases.
The left wall was the predominant site of perforation (67%; Table 5). Six of nine perforation sites were closed successfully by clips during the procedures. The other three perforation sites were too large to be closed. Subcutaneous emphysema was detected in eight cases (89%) during the procedures. After the ESD procedures, five cases had nasogastric tubes inserted.
Table 5.
Clinicopathological characteristics and outcomes of endoscopic submucosal dissection in perforation cases (n = 9)
| Case | Age | Sex | Location | Predo-minant site | Circum-ference of tumor | Circum-ference of mucosal deficiency | Maximum dimension (mm) | Macroscopic type | Procedure time (mm) | En bloc resection | Swiching to EPMR after perforation | Depth of invasion | Perforation site |
| 1 | 70 | M | Middle | Right | 2/3 | 3/4 | 54 | 0-IIc | 240 | No | Yes | SM2 | Left |
| 2 | 59 | M | Lower | Left | 1/2 | 7/8 | 50 | 0-IIc | 240 | Yes | No | M3 | Left |
| 3 | 61 | M | Lower | Left | 3/4 | 7/8 | 38 | 0-IIc | 150 | No | Yes | M2 | Left |
| 4 | 58 | M | Middle | Right | 1/3 | 1/2 | 30 | 0-IIc | 140 | No | Yes | SM2 | Posterior > left |
| 5 | 77 | M | Lower | Left | 3/4 | 7/8 | 67 | 0-IIc | 210 | Yes | No | M3 | Anterior > left |
| 6 | 61 | M | Middle | Right | 1/2 | 7/8 | 40 | 0-IIc | 210 | No | No | M2 | Left |
| 7 | 77 | M | Lower | Left | 1/2 | 3/4 | 32 | 0-IIc | 150 | No | Yes | M3 | Left |
| 8 | 85 | M | Middle | Left | Circ | Circ | 68 | 0-IIc | 210 | Yes | No | SM2 | Left |
| 9 | 63 | M | Lower | Right | 1/8 | 1/2 | 7 | 0-IIc | 104 | No | Yes | M1 | Right |
Circ: Whole circumference. EPMR: Endoscopic piecemeal mucosal resection.
While no case developed a pneumothorax, pleural effusion was detected by CT in four cases (44%). Two of the four cases had chest drains inserted because of a massive pleural effusion. Five cases (56%) required continuous oxygen administration for hypoxia after ESD. Seven cases (78%) became feverish (body temperature ≥ 37.0 °C) on the day after ESD. Median peak of fever was on postoperative day 1 (range, 1-2). Mean maximal C-reactive protein (CRP) level was 9.4 mg/dL (range, 1.9-26.5). Median fasting duration and hospitalization after the procedure were six days (range, 5-22) and 12 d (range, 7-41), respectively. All nine cases recovered with conservative treatment and without surgical intervention. A representative case that suffered perforation during ESD is shown in Figure 1.
Figure 1.
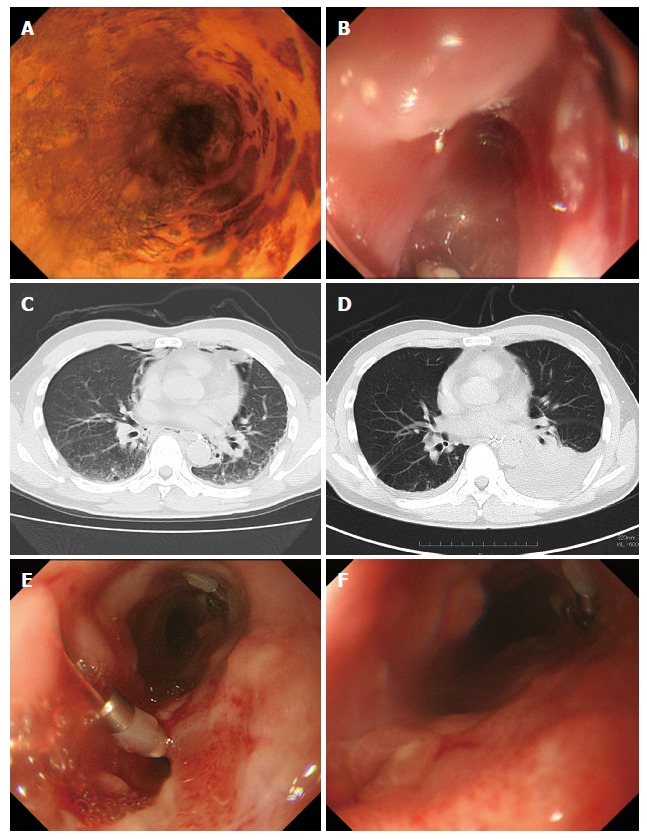
Clinical course of a patient who suffered intraoperative perforation during endoscopic submucosal dissection. A: Type 0-IIc tumor located in the right wall of the middle thoracic esophagus; B: During ESD, a perforation site was detected on the left wall (this image was rotated); C: Following perforation, the presence of mediastinal emphysema was observed on CT; D: This patient required a chest drain because of a massive pleural effusion on postoperative day (POD) 4; E: The closure of the perforation was not confirmed on POD 11; F: After confirmation of the closure on POD 18, oral intake was initiated. ESD: Endoscopic submucosal dissection; CT: Computed tomography.
Three of the nine cases underwent additional treatments, including chemoradiotherapy or esophagectomy because of submucosal infiltration. At a median follow-up of 42 mo (range, 28-59) after ESD, no case of local recurrence or metastasis had been observed (Table 6).
Table 6.
Clinical courses and treatment after perforations (n = 9)
| Case | Fasting duration (d) | Hospitali-zation (d) | Maximal CRP (mg/dL) |
Complication |
Treatment |
Local recurrence | Follow-up (mo) | |||||||
| Pneumo-derma | Pneumo-thorax | Pleural effusion | Hypoxia | Fever ( °C) | Closing by clips | ABx | NG tube | Chest drain | ||||||
| 1 | 8 | 20 | 26.5 | − | − | + | − | 38.1 | Possible | + | − | + | −1 | 59 |
| 2 | 8 | 13 | 6.7 | + | − | − | − | 37.7 | Possible | + | − | − | − | 50 |
| 3 | 6 | 12 | 5.7 | + | − | − | + | 37.6 | Possible | + | + | − | − | 41 |
| 4 | 5 | 8 | 1.9 | + | − | − | − | 37.2 | Possible | + | − | − | −2 | 46 |
| 5 | 5 | 7 | 6.9 | + | − | + | − | < 37.0 | Possible | + | + | − | − | 42 |
| 6 | 19 | 22 | 16.7 | + | − | + | + | 38.3 | Impossible | + | + | + | − | 40 |
| 7 | 22 | 41 | 6.3 | + | − | − | + | 37.6 | Impossible | + | + | − | − | 30 |
| 8 | 6 | 9 | 5.5 | + | − | + | + | < 37.0 | Impossible | + | − | − | −2 | 28 |
| 9 | 6 | 9 | 8.8 | + | − | − | + | 37.8 | Possible | + | + | − | − | 53 |
Additional chemoradiotherapy because of submucosal infiltration;
Additional curative surgery because of submucosal infiltration. CRP: C-Reactive protein; ABx: Antibiotics; NG tube: Nasogastric tube.
DISCUSSION
This study is the first to report the risk factors for intraoperative perforation during ESD of superficial ESCC. It showed that mucosal deficiency corresponding to more than 75% of the lumen circumference was an independent risk factor for intraoperative perforation.
In contrast, previous reports regarding ESD for gastric carcinomas demonstrated risk factors for perforation such as long procedure time, location of the lesion (body), and piecemeal resection[9-11]. Meanwhile, in colorectal ESD, large tumor size, fibrosis, and laterally spreading tumor type have been reported as risk factors[12-15]. Hence, various risk factors for perforation have been demonstrated in different organs despite identical ESD. In the present study, while the procedure times were significantly longer in cases with perforation compared with those with no perforation, a long procedure time may not only be a risk factor for perforation, but also might have been influenced by the perforation itself. In addition, in some cases, we switched to EPMR to reduce the procedure time. Therefore, we excluded the procedure time and the rate of en bloc resection from the univariate logistic regression analysis. In this study, there was a strong correlation between maximum lesion dimension and mucosal deficiency, because the circumference of the tumor and mucosal deficiency become more extensive as the tumor expands.
In this study, six of nine cases of perforations occurred on left side of the wall. The reason may be that lesions on the left side are affected by the direction of gravity, so that water, blood, and small fragments collect in the left wall[16,17]. Patients maintain a left lateral decubitus position during ESD procedure; therefore, it is difficult to make full use of counter traction when dissecting the left side of the lesion. These data suggested that the location of a mucosal deficiency on the left wall is associated with ESD difficulties. To overcome this problem, endoscopists should shorten the range of mucosal deficiency as narrowly as possible, while maintaining oncological curability, and changing the position of the patient may enable the use of gravity to help control the movements of the endoscope[18]. Furthermore, these are methods to improve counter traction. The clip-with-line method and the outer route method make the endoscopic view clearer and counter traction easier[19,20].
All cases with a perforation were treated successfully with conservative management. In general, the mortality of esophageal perforation from any cause is high; it is reported to be 11.9%, based on results from a meta-analysis[21]. The favorable outcome after perforation without surgical intervention in the present study might reflect the occurrence of early detection immediately after perforation and the fasting period before and after ESD. Furthermore, all cases were monitored not only by endoscopists, but also by surgeons to avoid any delay in the surgical intervention. A meta-analysis of the outcome of esophageal perforation reported that treatment starting within 24 h after the event resulted in a mortality rate of 7.4% compared with 20.3% in patients treated later (RR = 2.279, 95%CI: 1.63-3.18)[21]. Therefore, it is very important to detect the perforation during ESD and start treatment immediately. In this study, pneumoderma was identified in eight of nine cases. Pneumoderma extends into the cervical subcutaneous tissue beyond the mediastinum, so that the operators are able to perceive it by palpation during the procedure. If there is a suspicion of an intraoperative perforation, the operators or assistants should carefully palpate the patient's neck to detect this complication as quickly as possible. Although CT also helps to detect emphysema caused by a perforation, mediastinal emphysema observed on CT does not always indicate a perforation. Mediastinal emphysema was found on CT in 62.9% of the patients treated, even in those without a perforation, because of the lack of serosa[22].
The present study indicated that perforation does not necessarily worsen patient prognosis if the perforation is managed appropriately. In particular, careful follow-up is required during hospitalization. If the patient is unstable after the perforation, consultation with a surgeon has to be performed quickly.
ESD was switched to EPMR and the rate of piecemeal resection was high in most perforation cases. Piecemeal resection is a risk factor for local recurrence in ESCC treated using EMR[23]. In addition, esophageal perforation in ESD for ESCC may lead to pleural and mediastinal dissemination. Although no cases of local recurrence or metastasis have been observed so far, perforation cases require careful long-term observation, not only using endoscopy, but also CT scan.
The present study has several limitations associated with a retrospective single center study. The number of perforations was small because the frequency of perforation was low. A larger number of cases are required to confirm our results. There is a possibility that the device used could be a factor related to intraoperative perforation. In this study, we used a dual knife in most of the ESD procedures. An IT knife was used adjunctively according to physician's judgment, as mentioned in the Methods. Therefore, it is very difficult to compare the risk of perforation between each device. In this study, procedure time was excluded from the logistic regression analysis, because we only recorded the entire duration of the ESD procedure and did not separately record the procedure time before and after the perforation. However, a longer procedure time might cause operator fatigue and worsen the patient's condition; therefore, we speculated that procedure time could be a risk factor.
In conclusion, the present study suggested that mucosal deficiency corresponding to 75% of the circumference or larger is an independent risk factor for intraoperative perforation during ESD for ESCC. Although perforations in the esophagus represent a potentially deadly complication, this study suggested that most instances of perforation, if appropriately managed and detected during ESD, might allow the patients to recover with conservative treatment under careful observation.
COMMENTS
Background
Endoscopic submucosal dissection (ESD) was developed to improve the curability in endoscopic resection. However, ESD is technically difficult and carries a risk of intraoperative perforation. Little is known regarding the risk factors for intraoperative perforation and the subsequent clinical courses.
Research frontiers
The risk factors for intraoperative perforation in gastric or colonic ESD were reported as follows; long procedure time, location of the lesion, piecemeal resection, large tumor size, fibrosis, and laterally spreading tumor type.
Innovations and breakthroughs
This study is the first report of the risk factors for intraoperative perforation during ESD of superficial esophageal squamous cell carcinoma. It showed that mucosal deficiency corresponding to more than 75% of the lumen circumference was an independent risk factor for intraoperative perforation. Additionally, this study clarified the clinical courses after intraoperative perforations during ESD.
Applications
Endoscopists should shorten the range of mucosal deficiency as narrowly as possible, while maintaining oncological curability. In addition, we changed the position of the patient or used the clip-with-line method during the procedure to improve the intraoperative visualization.
Terminology
Perforation is the major complication during ESD, and "Intraoperative perforation" was defined as the detection of a perforation site during ESD, and the presence of mediastinal emphysema as observed on computed tomography or radiography.
Peer-review
In this study, the authors aimed to identify the risk factors for intraoperative perforation during ESD for esophageal squamous cell carcinoma and to clarify the subsequent clinical courses using 147 patients with 156 esophageal squamous cell carcinoma lesions. The incidence rate of intraoperative perforation was 5.8% and in multivariate analysis, mucosal deficiency larger than 75% of the circumference of the esophagus was an independent risk factor for intraoperative perforation.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was conducted in accordance with the principles of the Declaration of Helsinki, and was reviewed ethically and approved by Institutional Review Board of National Cancer Center Hospital (2014-119).
Informed consent statement: Patient informed consent was waived due to the retrospective design of the study.
Conflict-of-interest statement: Authors declare no conflict of interest relevant to this article.
Data sharing statement: No additional data are available.
Peer-review started: September 18, 2016
First decision: October 10, 2016
Article in press: November 16, 2016
P- Reviewer: Li HC, Sugimoto M S- Editor: Qi Y L- Editor: Stewart G E- Editor: Liu WX
References
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video) Gastrointest Endosc. 2010;72:255–264, 264.e1-e2. doi: 10.1016/j.gie.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20:5540–5547. doi: 10.3748/wjg.v20.i18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Tanaka K. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:960–966. doi: 10.1016/j.gie.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–866. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Isomoto H, Yamaguchi N, Minami H, Nakao K. Management of complications associated with endoscopic submucosal dissection/endoscopic mucosal resection for esophageal cancer. Dig Endosc. 2013;25 Suppl 1:29–38. doi: 10.1111/j.1443-1661.2012.01388.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 9.Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30–36. doi: 10.1007/s00535-009-0137-4. [DOI] [PubMed] [Google Scholar]
- 10.Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, Yamasaki T, Okugawa T, Ikehara H, Oshima T, et al. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: A prospective pilot study. World J Gastrointest Endosc. 2013;5:281–287. doi: 10.4253/wjge.v5.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Seo HE, Chung YJ, Kwon JG, Jung JT, et al. Predictive risk factors of perforation in gastric endoscopic submucosal dissection for early gastric cancer: a large, multicenter study. Surg Endosc. 2013;27:1372–1378. doi: 10.1007/s00464-012-2618-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy. 2011;43:573–578. doi: 10.1055/s-0030-1256339. [DOI] [PubMed] [Google Scholar]
- 13.Lee EJ, Lee JB, Choi YS, Lee SH, Lee DH, Kim DS, Youk EG. Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc. 2012;26:1587–1594. doi: 10.1007/s00464-011-2075-5. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima T, Kato M, Iwanaga I, Sato F, Kubo K, Ehira N, Uebayashi M, Ono S, Nakagawa M, Mabe K, et al. Technical difficulty according to location, and risk factors for perforation, in endoscopic submucosal dissection of colorectal tumors. Surg Endosc. 2015;29:133–139. doi: 10.1007/s00464-014-3665-9. [DOI] [PubMed] [Google Scholar]
- 15.Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679–683. doi: 10.1055/s-0029-1214979. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol. 2005;3:S74–S76. doi: 10.1016/s1542-3565(05)00254-5. [DOI] [PubMed] [Google Scholar]
- 17.Ono S, Fujishiro M, Koike K. Endoscopic submucosal dissection for superficial esophageal neoplasms. World J Gastrointest Endosc. 2012;4:162–166. doi: 10.4253/wjge.v4.i5.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori H, Kobara H, Nishiyama N, Fujihara S, Matsunaga T, Ayaki M, Yachida T, Tsushimi T, Masaki T. Efficient and safe esophageal endoscopic submucosal dissection using inverted overtube after changing patient position. Endoscopy. 2014;46 Suppl 1 UCTN:E88–E89. doi: 10.1055/s-0033-1344874. [DOI] [PubMed] [Google Scholar]
- 19.Ota M, Nakamura T, Hayashi K, Ohki T, Narumiya K, Sato T, Shirai Y, Kudo K, Yamamoto M. Usefulness of clip traction in the early phase of esophageal endoscopic submucosal dissection. Dig Endosc. 2012;24:315–318. doi: 10.1111/j.1443-1661.2012.01286.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohata K, Fu K, Shouzushima M, Hamanaka J, Ono A, Ito T, Tsuji Y, Chiba H, Matsuhashi N. A novel traction system for esophageal endoscopic submucosal dissection. Endoscopy. 2012;44 Suppl 2 UCTN:E410–E411. doi: 10.1055/s-0032-1325735. [DOI] [PubMed] [Google Scholar]
- 21.Biancari F, D'Andrea V, Paone R, Di Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg. 2013;37:1051–1059. doi: 10.1007/s00268-013-1951-7. [DOI] [PubMed] [Google Scholar]
- 22.Maeda Y, Hirasawa D, Fujita N, Suzuki T, Obana T, Sugawara T, Ohira T, Harada Y, Noda Y. Mediastinal emphysema after esophageal endoscopic submucosal dissection: its prevalence and clinical significance. Dig Endosc. 2011;23:221–226. doi: 10.1111/j.1443-1661.2010.01085.x. [DOI] [PubMed] [Google Scholar]
- 23.Ishihara R, Iishi H, Takeuchi Y, Kato M, Yamamoto S, Yamamoto S, Masuda E, Tatsumi K, Higashino K, Uedo N, et al. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc. 2008;67:799–804. doi: 10.1016/j.gie.2007.08.018. [DOI] [PubMed] [Google Scholar]


