Abstract
A combination of biochemical and genetic experiments were performed in order to better understand the mechanism of expression of high-level vancomycin resistance in Staphylococcus aureus. The transcription of pbp2 of the highly vancomycin- and oxacillin-resistant strain COLVA200 and its mutant derivative with inactivated mecA were put under the control of an inducible promoter, and the dependence of oxacillin and vancomycin resistance and cell wall composition on the concentration of the isopropyl-β-d-thiogalactopyranoside inducer was determined. The results indicate that mecA—the genetic determinant of oxacillin resistance—while essential for oxacillin resistance, is not involved with the expression of vancomycin resistance. Penicillin binding protein 2A, the protein product of mecA, appears to be unable to utilize the depsipeptide cell wall precursor produced in the vancomycin-resistant cells for transpeptidation. The key penicillin binding protein essential for vancomycin resistance and for the synthesis of the abnormally structured cell walls characteristic of vancomycin-resistant S. aureus (A. Severin, K. Tabei, F. Tenover, M. Chung, N. Clarke, and A. Tomasz, J. Biol. Chem. 279:3398-3407, 2004) is penicillin binding protein 2.
In the highly vancomycin-resistant Staphylococcus aureus (VRSA) strains that were recently recovered from clinical specimens, both the β-lactam resistance gene mecA and the vancomycin resistance gene complex vanA coexist in the same S. aureus cell (6, 22). In a recent communication (17) it was shown that the VRSA strain COLVA grown in the presence of vancomycin undergoes a major shift in the chemical composition of the cell wall precursor pool and produces a cell wall peptidoglycan of unusual chemical composition. It was also shown that inactivation of the key genetic determinant of the β-lactam resistance mechanism, mecA, did not reduce the vancomycin MIC for strain COLVA, implying that penicillin binding protein (PBP) 2A, the protein product of mecA, is not needed for the biosynthesis of the cell wall produced in the vancomycin-resistant cells. The cell wall peptidoglycan of strain COLVA grown in the presence of vancomycin was shown to have an abnormal chemical composition: all stem pentapeptides were replaced by tetrapeptides, and most muropeptide monomers and some muropeptide oligomers were deficient in the pentaglycine branches, which are characteristic of the S. aureus cell wall (17). The main purpose of the studies described here was to identify the key S. aureus PBP(s) essential for the expression of high-level vancomycin resistance and the synthesis of the abnormally structured cell wall. We chose to examine first the possible role of PBP2 in this process. PBP2 is an essential protein in cell wall biosynthesis; it has both a transglycosylase (TGase) and a transpeptidase (TPase) domain, as well as an experimental system which allows the controlled transcription of pbp2 is available (14). Our data indicate that S. aureus PBP2 plays a critical role in the expression of vancomycin resistance and cell wall biosynthesis in S. aureus growing in the presence of vancomycin.
MATERIALS AND METHODS
Isolation of COLVAORG and COLVA200.
Strain COLVAORG (vancomycin MIC, 512 μg/ml) was constructed by transferring plasmid pLW1043 from the clinical VRSA isolate HIP11714 to the methicillin-resistant S. aureus (MRSA) strain COL by filter mating (17). Plasmid pLW1043 carries the entire vanA gene complex of the enterococcal transposon Tn1546 (22). A mutant derivative of COLVAORG capable of expressing high-level resistance to vancomycin without prior induction was isolated in the following manner. One-hundred colonies of COLVAORG (named for COLVA original) were picked at random from tryptic soy agar (TSA) plates containing no antibiotics, and the colonies were streaked on plates that contained gradually increasing concentrations of vancomycin (5, 10, and 200 μg/ml). All colonies grew on agar containing 5 and 10 μg of vancomycin/ml, but only one out of four (25%) colonies also grew on agar containing 200 μg of vancomycin/ml. In order to test the stability of this high-level resistance, each of the 25 colonies that grew on agar containing 200 μg of vancomycin/ml were passaged serially by streaking on drug-free TSA for 20 consecutive times, after which they were retested for vancomycin resistance. Each of the 25 colonies had retained their capacity to grow on agar containing 200 μg of vancomycin/ml. One of these colonies, called COLVA200 (vancomycin MIC, 1,000 μg/ml), was used in all subsequent experiments. Comparison of the composition of cell wall precursor pools of COLVAORG and COLVA200 suggests that the expression of the vanA gene complex has become partially constitutive in COLVA200 (see Fig. 1 and 2). The nature of the genetic alteration responsible for this change in gene expression is under investigation. COLVAORG and COLVA200 grew with identical rates in tryptic soy broth (TSB) in the absence of antibiotics.
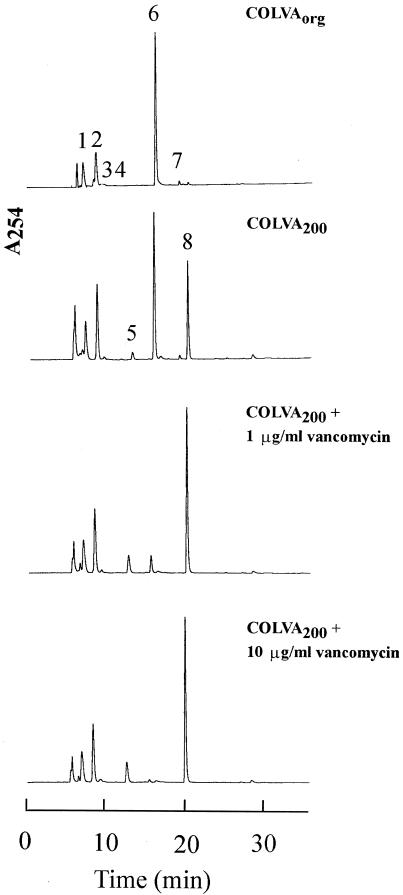
FIG. 1.
Composition of the cell wall precursor pool in strain COLVA. Strains COLVAORG and COLVA200 were grown and cell wall precursor extracts were prepared and analyzed for the composition of the precursors pool as described in Materials and Methods. The numbers refer to the following UDP-MurNAc precursors: 1, UDP-MurNAc; 2, UDP-MurNAc-l-Ala; 3, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys; 4, UDP-MurNAc-l-Ala-γ-d-Glu; 5, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala; 6, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala; 7, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys(Gly)-d-Ala-d-Ala; 8, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Lac. The relative amounts of all UDP-linked cell wall precursors in the precursor pool are listed in Table 1.
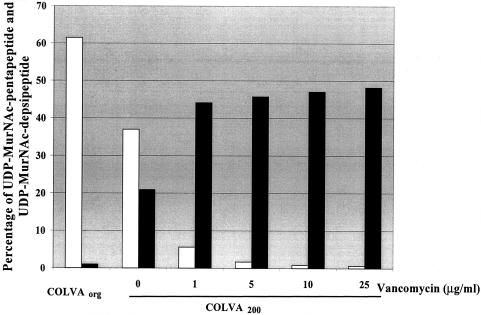
FIG. 2.
Effect of different concentrations of vancomycin on the proportion of the UDP-MurNAc-pentapeptide and UDP-MurNAc-depsipeptide in the cell wall precursor pool. Strain COLVA200 was grown in antibiotic-free medium and with medium supplemented with different concentrations of vancomycin. The analysis of the cell wall precursor pool was performed as described in Materials and Methods. For comparison, the composition of the cell wall precursor pool was also determined in COLVAORG. UDP-MurNAc-pentapeptide, empty bars; UDP-MurNAc-depsipeptide, solid bars.
Introduction of the construct of Spc-controlled pbp2 into COLVA200 and COLVA200Tn551::mecA by transduction.
The phage 80α lysate of the strain COLpPBP2iC, in which the promoter for pbp2 was replaced with the Escherichia coli Pspc promoter (13), was used to introduce the Pspc construct into strains COLVA200 and COLVA200Tn551::mecA. The transductants COLVA200pPBP2iC and COLVA200Tn551::mecApPBP2iC were selected on agar containing 20 μg of chloramphenicol/ml (for the Pspc construct), 10 μg of vancomycin/ml (for the vanA-carrying plasmid), and 500 μM isopropyl-β-d-thiogalactopyranoside (IPTG) at 30°C for 96 h. The success of replacement of the pbp2 promoter with Pspc was confirmed by growing the transductants on TSA with and without IPTG, and the presence of vanA-carrying plasmid was examined by extraction and restriction digestion of the plasmid.
Bacterial strains and growth conditions.
S. aureus strains were grown in TSB (Difco, Detroit, Mich.) at 37°C with aeration. Growth was monitored by measuring the optical density at 600 nm with an LKB spectrophotometer (Pharmacia LKB Biotechnology, Inc., Uppsala, Sweden). In some experiments, peptidoglycan and cell wall precursor analyses were performed on bacteria grown in the presence of vancomycin. In these experiments, the overnight cultures grown in TSB supplemented with the antibiotic were diluted into fresh TSB medium containing the same antibiotic concentration.
Testing antibiotic susceptibility.
The vancomycin resistance level was determined by E-test, following the recommendations of the manufacturer (Ab Biodisk, Solna, Sweden), and by the method of population analysis (21).
Preparation and analysis of peptidoglycan.
Cell wall peptidoglycan was prepared, purified, and solubilized by enzymatic hydrolysis with mutanolysin (M1) according to previously described methods (4, 7, 12). The muropeptides liberated by enzymatic hydrolysis were reduced by borohydride and separated on a 250- by 4.6-mm reversed-phase column (ODS-Hypersil, 3 μm; Thermo Hypersil-Keystone, Bellefonte, Pa.) by high-performance liquid chromatography (RP-HPLC) using a Shimadzu LC-10A HPLC system as described before (7). The column was eluted at a flow rate of 0.5 ml/min, with a linear gradient starting with 5% (vol/vol) methanol in 100 mM NaH2PO4 (pH 2.5) up to 30% (vol/vol) methanol in the same buffer for 150 min.
Preparation of UDP-linked peptidoglycan precursors and analysis by HPLC.
Cytoplasmic pools of the UDP-linked precursors were extracted by a method previously described (8), with some modification (18). In strains grown in antibiotic-free medium, bacitracin (100 μg/ml or 2× MIC) was added to the cultures 30 min before harvesting the cells in order to amplify the amounts of cell wall precursors. It was shown before (17) that bacitracin, an inhibitor of the regeneration of bactoprenylphosphate in the membrane transfer reaction of cell wall synthesis (19, 20), can be successfully used instead of vancomycin (3) for the amplification of the cell wall precursor pool. Analysis was performed with a Shimadzu LC-10A HPLC system. Samples were applied to a 250- by 4.6-mm RP-HPLC column (ODS-Hypersil, 3 μm; Thermo Hypersil-Keystone). The column was eluted at a flow rate of 0.5 ml/min, with a linear gradient of 5% (vol/vol) methanol in 100 mM ammonium formate (pH 2.5) to 30% (vol/vol) methanol in 100 mM ammonium formate (pH 3.5) for 30 min. The pH of buffers was adjusted by formic acid. Column temperature was 40°C. The eluted compounds were detected by absorption at 254 nm. The structure of the precursors was identified by coinjection with the precursors of known structure and by mass spectrometer analysis, as described earlier (17).
RESULTS
Figure 1 shows the results of the chemical analysis of the cell wall precursor pool in COLVAORG and COLVA200 with or without growth in the presence of various concentrations of vancomycin. The major component of the precursor pool in COLVAORG was UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala (UDP-MurNAc-pentapeptide) (peak 6 in Fig. 1); peak 8, representing UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Lac (UDP-MurNAc-depsipeptide), was only present in small amounts. In COLVA200 the proportion of peak 8 increased, and growth of COLVA200 in the presence of increasing concentrations of vancomycin caused further major shifts in the direction of the UDP-MurNAc-depsipeptide component (Fig. 2). The chemical structures and relative amounts of the various cell wall precursors are shown in Table 1.
TABLE 1.
Precursor pool composition of vancomycin-resistant S. aureus strain COLVA
| Peak no.a | Precursor | % of composition
|
|||||
|---|---|---|---|---|---|---|---|
| COLVAORGb | COLVA200b | COLVA200 + (1 μg/ml) vancomycin | COLVA200 + (5 μg/ml) vancomycin | COLVA200 + (10 μg/ml) vancomycin | COLVA200 + (25 μg/ml) vancomycin | ||
| 1 | UDP-MurNAc | 13.8 | 11.8 | 14.9 | 14.3 | 14.8 | 14.2 |
| 2 | UDP-MurNAc-Ala | 17.7 | 20.7 | 22.7 | 21.4 | 21.8 | 20.9 |
| 3c | UDP-MurNAc-Ala-Glu-Lys+ | ||||||
| 4c | UDP-MurNAc-Ala-Glu | 2.6 | 0.8 | 1.2 | 2.2 | 1.8 | 1.9 |
| 5 | UDP-MurNAc-Ala-Glu-Lys-Ala | 0.0 | 2.3 | 7.3 | 8.0 | 8.9 | 10.4 |
| 6 | UDP-MurNAc-Ala-Glu-Lys-Ala-Ala | 61.5 | 37.1 | 5.6 | 1.7 | 0.9 | 0.7 |
| 7 | UDP-MurNAc-Ala-Glu-Lys(Gly)-Ala-Ala | 1.3 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| 8 | UDP-MurNAc-Ala-Glu-Lys-Ala-Lac | 1.0 | 20.7 | 43.8 | 45.7 | 47.7 | 48.2 |
| Total | 98 | 94 | 96 | 93 | 96 | 96 | |
Peak numbers as in Fig. 1.
In order to accumulate cytoplasmic precursor, cells were exposed for the last 30 min of growth to 100 μg of bacitracin/ml.
The peaks 3 and 4 were integrated together.
Figure 3 and Table 2 show the composition of cell wall peptidoglycan produced in COLVAORG, COLVA200, and COLVA200Tn551::mecA, a derivative of COLVA200 in which the mecA gene was insertionally inactivated (11). Each strain produced a peptidoglycan of mixed muropeptide composition containing normal COL-type and abnormal vancomycin specific COLVA-type muropeptides (Fig. 4). The proportion of abnormal muropeptide components characteristic of cells expressing the vanA gene complex (17) roughly reflected the relative proportions of the UDP-MurNAc-pentapeptide and UDP-MurNAc-depsipeptide in the wall precursor pool. Thus, the vancomycin-specific, i.e., abnormal, muropeptides were only present in minute amounts in the peptidoglycan of COLVAORG, mirroring the trace amount of the UDP-MurNAc-depsipeptide in the wall precursor pool of this strain (see Fig. 1). In contrast, cell walls of mixed composition with substantial proportion of the abnormal muropeptides were present in COLVA200 and its derivative with inactivated mecA (Table 2). The prototypes of the major abnormal and normal muropeptide structural types are shown in Fig. 4, which illustrates the most important structural abnormalities of the muropeptides, produced by the vancomycin-resistant staphylococci. One of these abnormalities is the complete lack of pentapeptide stem peptides, which in vancomycin-susceptible S. aureus are present in all monomeric and oligomeric components of the cell wall (7). Another frequent deficiency is the complete lack or incomplete chain length of the pentaglycine branches that decorate the great majority of the muropeptides in S. aureus cell walls (7).
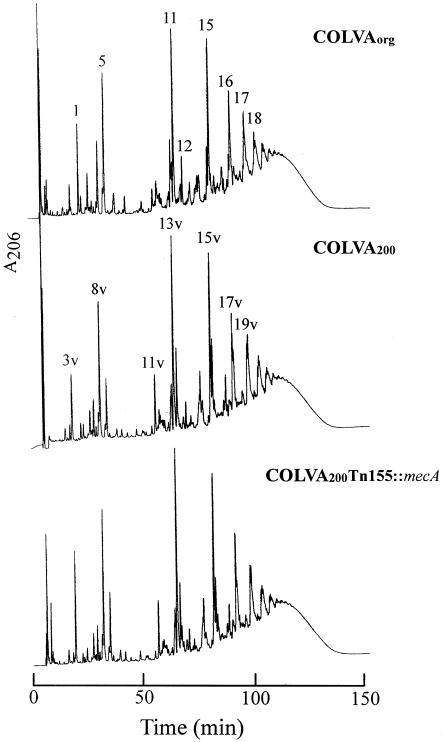
FIG. 3.
The muropeptide composition of strain COLVAORG and two of its mutant derivatives. Cell wall peptidoglycan was isolated and the muropeptide components were separated by HPLC as described in Materials and Methods. Muropeptide species typical of S. aureus strain COL are identified by numbers, and the muropeptide species with abnormal structures characteristic of S. aureus expressing the vanA gene complex are identified by a number followed by the letter v. The major structural types of normal and vancomycin-specific muropeptides are shown in Fig. 4, and the complete structural characterizations are described in reference 17.
TABLE 2.
Muropeptide composition of major peaks of vacomycin-resistant S. aureus strain COLVA
| Major peaka | % of compositionb
|
|||||
|---|---|---|---|---|---|---|
| COLVAORG | COLVAORG + (10 μg/ml) vancomycin | COLVA200 | COLVA200 + (10 μg/ml) vancomycin | COLVA200Tn551::mecA | COLVA200Tn551::mecA + (10 μg/ml) vancomycin | |
| 1 | 1.7 | 0.0 | 0.4 | 0.0 | 2.1 | 0.0 |
| 5 | 2.9 | 0.0 | 1.3 | 0.0 | 0.5 | 0.0 |
| 11 | 4.3 | 0.0 | 1.9 | 0.0 | 1.6 | 0.0 |
| 15 | 4.2 | 0.0 | 2.3 | 0.0 | 1.8 | 0.0 |
| 16 | 3.2 | 0.0 | 2.1 | 0.0 | 1.9 | 0.0 |
| 17 | 3.0 | 0.0 | 3.5 | 0.0 | 2.9 | 0.0 |
| 3v | 0.5 | 10.0 | 1.7 | 10.0 | 0.4 | 12.4 |
| 8v | 1.7 | 2.3 | 3.1 | 2.3 | 3.3 | 8.9 |
| 11v | 0.5 | 1.9 | 1.2 | 1.9 | 1.2 | 3.1 |
| 13v | 1.7 | 3.4 | 4.9 | 3.4 | 5.2 | 7.7 |
| 14v | 0.6 | 1.9 | 1.3 | 1.9 | 1.2 | 2.7 |
| 15v | 1.2 | 3.4 | 4.4 | 3.4 | 4.4 | 5.7 |
| 16v | 0.0 | 1.4 | 1.2 | 1.4 | 0.9 | 1.3 |
| 17v | 0.8 | 2.4 | 2.6 | 2.4 | 2.6 | 2.8 |
| 19v | 0.0 | 1.4 | 1.2 | 1.4 | 1.2 | 1.8 |
| Monomers | 13.9 | 21.9 | 14.2 | 21.9 | 14.0 | 35.3 |
| Oligomers | 47.6 | 40.4 | 51.6 | 40.4 | 46.4 | 48.8 |
| Hump | 37.6 | 38.3 | 32.8 | 38.3 | 39.0 | 15.8 |
Muropeptide peak numbers as in Fig. 2.
Relative amounts of muropeptide species are expressed as the percent of total UV-absorbing material recovered from the HPLC column.
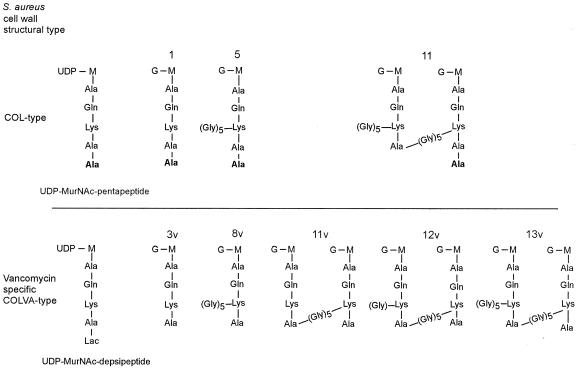
FIG. 4.
Major structural types of muropeptide species identified in S. aureus and in S. aureus expressing the vanA gene complex. Structures of the UDP-linked cell wall precursors and the major monomeric and cross-linked muropeptides species recovered from the peptidoglycan of S. aureus strain COL and S. aureus strain COLVA200 expressing the vanA gene complex are shown.
A genetic construct that puts the transcription of pbp2 under the control of an IPTG-inducible spac promoter was introduced into these strains, generating COLVA200pPBP2iC and COLVA200Tn551::mecApPBP2iC. Table 3 shows that in COLVA200pPBP2iC the level of both oxacillin and vancomycin resistance depended on the concentration of IPTG in the medium. Upon increasing the IPTG concentration from 0 through 25, 90, and up to 500 μM, the vancomycin MIC increased from 1.5 through 12 μg/ml, and 90 up to 400 μg/ml. The oxacillin MIC increased from 1 (at 0 IPTG) to 4 (at 50 μM IPTG) and finally to >256 μg/ml at the highest IPTG concentration. As expected, in strain COLVA200Tn551::mecApPBP2iC there was no growth in the absence of IPTG in the medium (13), and resistance to oxacillin remained low regardless of the concentration of IPTG in the medium. In sharp contrast, increasing the IPTG concentration of the growth medium from 25 to 50 and then 500 μM increased the vancomycin MIC for this oxacillin-susceptible mutant from less than 1.5 to 12 μg/ml and then up to 200 μg/ml.
TABLE 3.
Effect of pbp2 expression on vancomycin and oxacillin resistance in S. aureus strain COLVA200
| Strain | IPTG concn (μM) | MIC (μg/ml)
|
|
|---|---|---|---|
| Vancomycin | Oxacillin | ||
| COLVA200 | 1,000 | 800 | |
| COLVA200pPBP2iC | 0 | 1 | 1.0 |
| 25 | 12 | 8.0 | |
| 50 | 90 | 24 | |
| 500 | 400 | >256 | |
| COLVA200Tn551::mecApPBP2iC | 0 | No growth | No growth |
| 25 | <1.5 | 1.5-3 | |
| 50 | 12 | 1.5-3 | |
| 500 | 200 | 1.5-3 | |
| COLVA200RUSA130a | 5 | 6 | |
COLVA200 carrying a Tn551 insert in pbpB (14).
Table 3 also shows the drastic reduction of both oxacillin and vancomycin MICs for COLVA200 in which pbp2 was partially inactivated by insertion of Tn551 into the transglycosylase (TGase) domain of the gene (see COLVA200RUSA130) (14).
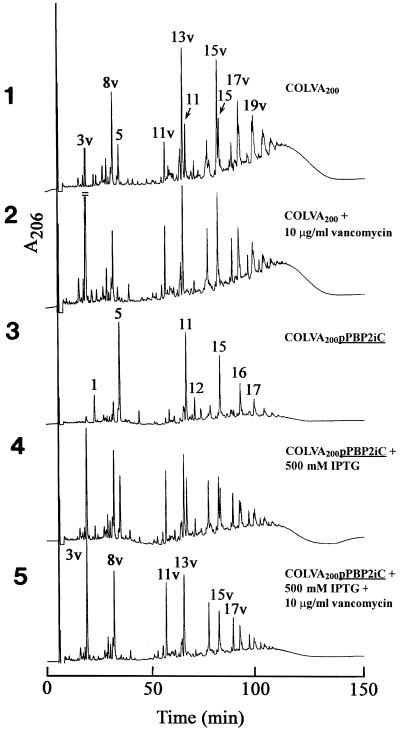
The composition of cell wall peptidoglycan in strain COLVA200pPBP2iC grown with or without IPTG in the medium and also in the presence of both IPTG and vancomycin are shown in Fig. 5, and quantitative data on the representation of various muropeptides identified in the peptidoglycan of these bacteria are summarized in Table 4. Strain COLVA200 produced a peptidoglycan of mixed composition in which both normal muropeptides and abnormal muropeptides (i.e., muropeptides characteristic of cells utilizing the UDP-MurNAc-depsipeptide wall precursor) were present. A similar (mixed) muropeptide composition was apparent in COLVA200pPBP2iC grown with 500 μM IPTG in the medium. Shifts in cell wall composition, in different directions, were observed in COLVA200pPBP2iC grown without IPTG or with 500 mM IPTG plus 10 μg of vancomycin/ml in the medium. The former growth condition produced peptidoglycan from which the abnormally structured muropeptides were virtually absent. The opposite change was seen in cells grown with both IPTG and vancomycin. In these bacteria, the normal muropeptides disappeared and were replaced quantitatively by the family of vancomycin-specific components (muropeptides 3v through 19v) in the peptidoglycan (see Table 4 and Fig. 4).
FIG. 5.
Effect of the transcription of pbp2 on the composition of cell wall peptidoglycan produced in S. aureus strain COLVA200. COLVA200pPBP2iC, in which pbp2 was put under the control of the inducible promoter spac, was grown without and with an optimal concentration of IPTG inducer (500 μM). Additional variants included the presence of vancomycin in the growth medium. The top two panels show the HPLC profile of muropeptides in the peptidoglycan of COLVA200 and in the peptidoglycan of the same strain grown in the presence of 10 μg of vancomycin/ml. Panels 3, 4, and 5 show the effect of IPTG and vancomycin on the structure of the cell wall peptidoglycan.
TABLE 4.
Effect of pbp2 expression on cell wall muropeptide composition of S. aureus strain COLVA200pPBP2iC
| Major peaka | % of compositionb
|
|||
|---|---|---|---|---|
| COLVA200 | COLVA200pPBP2iC without IPTG | COLVA200pPBP2iC + 500 μM IPTG | COLVA200pPBP2iC + 500 μM IPTG + (10 μg/ml) vancomycin | |
| 1 | 0.4 | 3.3 | 0.8 | 0.0 |
| 5 | 1.3 | 12.3 | 3.6 | 0.0 |
| 11 | 1.9 | 10.9 | 4.3 | 0.0 |
| 15 | 2.3 | 7.9 | 3.2 | 0.0 |
| 16 | 2.1 | 4.3 | 2.4 | 0.0 |
| 17 | 3.5 | 3.5 | 0.8 | 0.0 |
| 3v | 1.7 | 0.8 | 7.6 | 11.2 |
| 8v | 3.1 | 2.7 | 5.1 | 6.8 |
| 11v | 1.2 | 0.7 | 3.9 | 5.6 |
| 13v | 4.9 | 0.0 | 6.3 | 7.6 |
| 14v | 1.3 | 0.0 | 3.8 | 4.0 |
| 15v | 4.4 | 0.0 | 3.8 | 5.1 |
| 16v | 1.2 | 0.0 | 2.2 | 2.6 |
| 17v | 2.6 | 0.0 | 1.6 | 2.9 |
| 19v | 1.2 | 0.0 | 0.3 | 0.7 |
| Monomers | 14.2 | 36.2 | 31.1 | 34.8 |
| Oligomers | 51.6 | 60.1 | 54.4 | 54.8 |
| Hump | 32.8 | 3.4 | 13.8 | 6.8 |
Muropeptide peak numbers as in Fig. 2.
Relative amounts of muropeptide species are expressed as the percent of total UV-absorbing material recovered from the HPLC column.
DISCUSSION
The recent emergence of vancomycin-resistant clinical isolates of methicillin-resistant S. aureus has created, for the first time in clinical history, highly VRSA strains (6). VRSA strains carry both the chromosomally located mecA and a plasmid-borne vanA gene complex (22). These resistance mechanisms are targeted on the bacterial cell wall by distinct mechanisms. The essential component of the mecA-dependent β-lactam resistance is penicillin-binding protein 2A (PBP2A), a transpeptidase of very low reactivity for all β-lactam antibiotics (9, 10). PBP2A was proposed to function as a surrogate transpeptidase (TPase) in peptidoglycan synthesis in the presence of β-lactam antibiotics, which inactivate all the native PBPs of the bacterium (7). The vanA-dependent mechanism activates an alternative cell wall biosynthetic pathway that avoids the vancomycin-sensitive step by producing an abnormal cell wall precursor in which the carboxy-terminal dipeptide d-alanyl-d-alanine is replaced by the vancomycin-insensitive depsipeptide composed of d-alanyl-d-lactate (1, 2, 5).
While these two mechanisms coexist in the same VRSA strain and are capable of providing high-level resistance against each class of antibiotics, the mechanisms of expression of the resistant phenotypes are independent and even mutually antagonistic (17). In a previous communication it was shown that expression of high-level vancomycin resistance does not depend on an intact mecA, because selective inactivation of the gene did not reduce the vancomycin MIC for the bacteria (17). Furthermore, vancomycin resistance was suppressed by adding oxacillin to the growth medium under conditions where bacterial growth and cell wall synthesis depends on the TPase activity of PBP2A (7). Observations described with the VRSA strain COLVAORG suggest that the mechanism of this antagonistic effect is the inability of PBP2A to utilize the depsipeptide-containing precursor as a substrate for transpeptidation reaction. Under conditions when both the UDP-MurNAc-depsipeptide and the UDP-MurNAc-pentapeptide precursors were available in the cell wall precursor pool, strain COLVAORG produced a cell wall of mixed muropeptide composition. The majority of components were those of the typical S. aureus cell wall accompanied by a family of muropeptides with the unusual structural features identified in the cell wall of COLVAORG grown in the presence of vancomycin (17). When the same COLVAORG strain was grown in the presence of 10 μg of oxacillin/ml, the bacteria produced a cell wall peptidoglycan from which the abnormal muropeptide components were missing (17), and the HPLC profile of muropeptides was indistinguishable from that of strain COL (free of the vanA transposon) grown in the presence of similar concentrations of oxacillin. A typical feature of staphylococcal cell walls grown in the presence of sub-MIC oxacillin is the drastic reduction in highly oligomeric muropeptides and enrichment for monomeric and dimeric components (7).
The apparent restriction of the catalytic activity of PBP2A to the UDP-MurNAc-pentapeptide cell wall precursors was also confirmed in the experimental system that we introduced in the present communication. A derivative of strain COLVAORG, called COLVA200, produces a cell wall precursor pool in which the UDP-MurNAc-pentapeptide and the UDP-MurNAc-depsipeptide cell wall precursors are both present in substantial amounts (Fig. 1, Fig. 2, and Table 1) even when the bacteria were grown in vancomycin-free medium, presumably reflecting a partial constitutive expression of the vanA gene complex.
COLVA200 and COLVA200Tn551::mecA produced cell walls of identical composition. They were both made up of normally and abnormally structured muropeptides which were present in roughly equal proportion.
When the vanA gene complex was fully induced in COLVA200 and COLVA200Tn551::mecA by vancomycin, the composition of the cell wall precursor pool shifted: the amount of the UDP-MurNAc-pentapeptide was diminished and the overwhelming majority of cell wall precursors had the UDP-MurNAc-depsipeptide structure. The composition of the cell wall produced under these conditions shifted in the direction of the abnormal muropeptides, which now represented the great majority of the muropeptide components of the cell wall (Fig. 5, panels 1 and 2, and Table 4). These observations further confirm that PBP2A does not take part in the assembly of cell walls in S. aureus expressing vancomycin resistance.
If PBP2A cannot participate in the synthesis of staphylococcal cell wall from the UDP-MurNAc-depsipeptide precursor, which of the staphylococcal PBPs catalyze cell wall biosynthesis in the vancomycin-resistant bacteria? To obtain an answer to this question, we introduced an experimental system in which pbp2, one of the essential PBPs of S. aureus, was put under the control of an inducible promoter in the strain COLVA200 (15, 16). It was shown earlier that the growth of methicillin-susceptible strains of S. aureus containing such constructs have an absolute requirement for the IPTG inducer in the medium, demonstrating that PBP2 is an essential protein (15). It was also shown that PBP2 is no longer essential for growth in the background of methicillin-resistant strains, apparently because the surrogate transpeptidase PBP2A can replace the essential TPase function of PBP2 in the resistant cells (15).
Table 3 shows the effect of various IPTG concentrations on growth and vancomycin and oxacillin resistance in such constructs introduced into strains COLVA200 and COLVA200Tn551::mecA. The findings summarized in Table 3 document several important observations. In COLVA200pPBP2iC, both vancomycin and oxacillin MICs were drastically reduced in the absence of IPTG, and resistance levels increased for both antibiotics in parallel with the increasing concentrations of IPTG in the medium. For strain COLVA200Tn551::mecApPBP2iC the findings were different. Increasing concentrations of IPTG were accompanied by parallel increases in vancomycin MICs. However, oxacillin MICs remained low (1.5 to 3 μg/ml) and did not change with the IPTG concentration. Furthermore, these bacteria could not grow in the absence of IPTG, as expected from the absence of PBP2A as well as PBP2. The high MICs for vancomycin and for oxacillin shown by strain COLVA200 were not reached by COLVA200pPBP2iC even at the highest concentration of IPTG, consistent with the superior strength of the native promoter of pbp2 (13).
Our data clearly demonstrate that PBP2 is essential for the expression of both oxacillin and vancomycin resistance in the VRSA strain COLVA. However, the mechanisms involved are completely different. The dependence of oxacillin resistance on a functioning PBP2 was shown earlier to involve only the TGase domain of this bifunctional protein, while the transpeptidase activity required for wall synthesis may be provided by PBP2A. It is the functional cooperation between the TPase and TGase domains of these two proteins which is essential for the expression of β-lactam resistance in S. aureus (13, 15).
The dependence of the vancomycin MIC on the transcription of pbp2 appears to involve both the TPase and TGase activities of PBP2. In COLVA200pPBP2iC the vancomycin MIC increases as a function of the IPTG concentration in the medium, even in the absence of an intact mecA. Thus, the native PBP2 TPase domain must be actively participating in the biosynthesis of cell walls both in the presence and absence of a functional mecA.
The essential role of PBP2 in the biosynthesis of cell wall in vancomycin-resistant cells is shown in Fig. 5. Panel 3 of Fig. 5 shows the composition of the cell wall in bacteria grown in the absence of IPTG in the medium, i.e., under conditions where PBP2 was not present and the essential function of this protein was replaced by PBP2A (15). In these bacteria the HPLC profile of muropeptides was typical of the vancomycin-susceptible parental strain COL (without the vanA transposon) grown in the presence of oxacillin. There was enrichment of the cell wall for the major monomeric muropeptide (muropeptide 5) and dimeric muropeptide (muropeptide 11), and there was virtual elimination of the highly oligomeric muropeptides eluting after 100 min from the HPLC column. There were no abnormal muropeptide species detectable. Analysis of the cell wall precursor pool indicated that the UDP-MurNAc-pentapeptide and UDP-MurNAc-depsipeptide precursors were both present in substantial amounts (data not shown). The composition of the cell wall indicates that peptidoglycan synthesis occurred virtually exclusively through the use of the UDP-MurNAc-pentapeptide precursor, consistent with PBP2A being the primary transpeptidase involved with the synthesis of this particular cell wall. These findings are similar to the ones reported from a previous communication, in which the cell wall of strain COLVAORG was synthesized through the activity of PBP2A because the native PBPs were inactivated by the presence of oxacillin in the medium (17).
Panel 4 of Fig. 5 shows that growing COLVA200pPBP2iC in the presence of 500 μM IPTG completely changed the peptidoglycan composition from the profile seen in panel 3 of the figure. The bacteria now produced a peptidoglycan composed of a roughly equal mixture of both normal and vancomycin-specific muropeptide species. We interpret this finding as an indication that PBP2 took over the catalysis of cell wall biosynthesis and that this protein was able to use with equal efficiency both the pentapeptide and depsipeptide containing cell wall precursors.
Still, additional and dramatic shifts in cell wall composition occurred when COLVA200pPBP2iC was grown in the presence of an optimal concentration of IPTG (500 μM) and 10 μg of vancomycin/ml. Figures 1 and 2 and Table 1 show that the presence of vancomycin caused a full induction of the vanA gene complex, leading to a virtually complete replacement of the UDP-MurNAc-pentapeptide precursor by the UDP-MurNAc-depsipeptide. The cell wall composition produced under these conditions (Fig. 5, bottom panel) shows a complete shift in the direction of the abnormal muropeptides that become the exclusive components of the COLVA cell wall (Table 4).
Our findings confirm the conclusions of a previous communication (17). The two major antibiotic resistance mechanisms encoded by mecA and vanA residing in the same S. aureus isolate use different sets of enzymes for the synthesis of cell walls. The key component of cell wall synthesis that allows bacterial growth and survival in the presence of β-lactam antibiotics is PBP2A collaborating with the TGase activity of PBP2. In bacteria growing in the presence of vancomycin, the activity of PBP2A is irrelevant and the native penicillin-binding protein PBP2 assumes a key role. The possible roles of other PBPs are presently under investigation. Apparently, PBP2A is unable to utilize the UDP-MurNAc-depsipeptide cell wall precursors, while PBP2 can catalyze cell wall synthesis by using either the pentapeptide- or depsipeptide-containing cell wall precursors. One prediction of this finding is that selective inhibitors of PBP2 should block the expression of vancomycin resistance in S. aureus. However, a combination of vancomycin plus β-lactam antibiotics is unlikely to represent a therapeutic option. Strain COLVA grown in the presence of sub-MIC oxacillin produced a heterogeneous vancomycin resistance phenotype which contained, with various frequencies, subpopulations of bacteria that were still capable of expressing high-level resistance (up to 100 μg/ml) to vancomycin (17).
Acknowledgments
Partial support for these investigations came from a grant from the U.S. Public Health Service (1 RO1 AI45738).
REFERENCES
- 1.Arthur, M., F. Depardieu, L. Cabanie, P. Reynolds, and P. Courvalin. 1998. Requirement of the VanY and VanX D, D-peptidases for glycopeptide resistance in enterococci. Mol. Microbiol. 30:819-830. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, M., and R. Quintiliani, Jr. 2001. Regulation of VanA- and VanB-type glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 45:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billot-Klein, D., L. Gutmann, D. Bryant, D. Bell, J. van Heijenoort, J. Grewal, and D. M. Shlaes. 1996. Peptidoglycan synthesis and structure in Staphylococcus haemolyticus expressing increasing level of resistance to glycopeptide antibiotics. J. Bacteriol. 178:4696-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boneca, I. G., Z.-H. Huang, D. A. Gage, and A. Tomasz. 2000. Characterization of Staphylococcus aureus cell wall glycan strands, evidence for a new beta-N-acetylglucosaminidase activity. J. Biol. Chem. 275:9910-9918. [DOI] [PubMed] [Google Scholar]
- 5.Bugg, T. D. H., G. D. Wright, S. Dutka-Malen, M. Arthur, P. Courvalin, and C. T. Walsh. 1991. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 30:10408-10415. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. Pouch-Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge, B. L., Y.-S. Chang, D. Gage, and A. Tomasz. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 267:11248-11254. [PubMed] [Google Scholar]
- 8.Flouret, B., D. Mengin-Lecreulx, and J. van Heijenoort. 1981. Reverse-phase high-pressure liquid chromatography of uridine diphosphate N-acetylmuramyl peptide precursors of bacterial cell wall peptidoglycan. Anal. Biochem. 114:59-63. [DOI] [PubMed] [Google Scholar]
- 9.Hartman, B. J., and A. Tomasz. 1984. Low-affinity penicillin binding protein associated with β-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim, D., and N. C. J. Strynadka. 2002. Structural basis for the beta-lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Biol. 9:870-876. [DOI] [PubMed] [Google Scholar]
- 11.Matthews, P., and A. Tomasz. 1990. Insertional inactivation of the mec gene in a transposon mutant of a methicillin-resistant clinical isolate of Staphylococcus aureus. Antimicrob. Agents Chemother. 34:1777-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ornelas-Soares, A., H. de Lencastre, B. de Jonge, D. Gage, Y.-S. Chang, and A. Tomasz. 1993. The peptidoglycan composition of a Staphylococcus aureus mutant selected for reduced methicillin resistance. J. Biol. Chem. 268:26268-26272. [PubMed] [Google Scholar]
- 13.Pinho, M. G., S. Filipe, H. de Lencastre, and A. Tomasz. 2001. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein (PBP) 2 by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 183:6525-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinho, M. G., H. de Lencastre, and A. Tomasz. 1998. Transcriptional analysis of the Staphylococcus aureus penicillin binding protein 2 gene. J. Bacteriol. 180:6077-6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinho, M. G., H. de Lencastre, and A. Tomasz. 2001. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. USA 98:10886-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinho, M., A. M. Ludovice, S. Wu, and H. de Lencastre. 1997. Massive reduction in methicillin resistance by transposon inactivation of the normal PBP2 in a methicillin resistant strain of Staphylococcus aureus. Microb. Drug Resist. 3:409-413. [DOI] [PubMed] [Google Scholar]
- 17.Severin, A., K. Tabei, F. Tenover, M. Chung, N. Clarke, and A. Tomasz. 2004. High level oxacillin and vancomycin resistance and altered cell wall composition in Staphylococcus aureus carrying the staphylococcal mecA and the enterococcal vanA gene complex. J. Biol. Chem. 279:3398-3407. [DOI] [PubMed] [Google Scholar]
- 18.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone, K. J., and J. L. Strominger. 1971. Mechanism of action of bacitracin: complexation with metal ion and C 55-isoprenyl pyrophosphate. Proc. Natl. Acad. Sci. USA 68:3223-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storm, D. R. 1974. Mechanism of bacitracin action: a specific lipid-peptide interaction. Ann. N. Y. Acad. Sci. 235:387-398. [DOI] [PubMed] [Google Scholar]
- 21.Tomasz, A., S. Nachman, and H. Leaf. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 35:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigel, L. M., D. B. Clewell, S. R. Gill, N. C. Clark, L. K. McDougal, S. E. Flannagan, J. F. Kolonay, J. Shetty, G. E. Killgore, and F. C. Tenover. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569-1571. [DOI] [PubMed] [Google Scholar]