Abstract
Dimeric derivatives (compounds 7 to 9) of the influenza virus neuraminidase inhibitor zanamivir (compound 2), which have linking groups of 14 to 18 atoms in length, are approximately 100-fold more potent inhibitors of influenza virus replication in vitro and in vivo than zanamivir. The observed optimum linker length of 18 to 22 Å, together with observations that the dimers cause aggregation of isolated neuraminidase tetramers and whole virus, indicate that the dimers benefit from multivalent binding via intertetramer and intervirion linkages. The outstanding long-lasting protective activities shown by compounds 8 and 9 in mouse influenza infectivity experiments and the extremely long residence times observed in the lungs of rats suggest that a single low dose of a dimer would provide effective treatment and prophylaxis for influenza virus infections.
Multivalent interactions involve the simultaneous binding of multiple ligands on one molecule to multiple receptors on another. There are numerous examples of natural multivalent interactions, many of which involve the binding of multiple copies of a carbohydrate or oligosaccharide ligand to protein receptors on a cell surface (2, 3, 19). Because of the importance of multivalency in biological systems, there has been a growing research effort to explore and rationalize the effects of multivalency (8, 16, 18) and also to make use of multivalent ligands as potentially more effective new drugs (10). Thus, a number of multivalent compounds and particularly dimeric forms of known small-molecule therapeutic agents are being investigated as drug candidates (31). However, to date no such molecules have been approved for clinical use, suggesting that in most cases multimeric forms of existing drugs will not give superior biological activity. Indeed, it is likely that multivalent forms of existing drugs will show superior properties only if several necessary requirements are met (15). First, and in keeping with nearly all reported successful multivalent interactions involving either natural or synthetic multimers, the target protein should be located on the surface of cells, bacteria, or viruses (19). Second, a reasonable surface density of the target receptor molecule is required so that binding sites are not too far apart, and ideally, the protein itself should be multimeric. Third, a key requirement is that a covalently bound tether can be attached to the monomeric ligand without significant interference with the binding of the ligand with the receptor. One target protein which meets all of these prerequisites is influenza virus neuraminidase (NA).
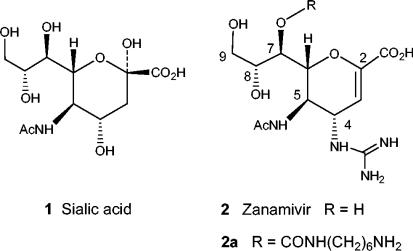
Influenza viruses A and B have two major surface glycoproteins, hemagglutinin and the enzyme NA, both of which are essential for infectivity. Hemagglutinin binds to the terminal sialic acid groups on cell surface glycoproteins, thereby attaching the virus to the cell. NA cleaves terminal sialic acids from cell surface glycoconjugates and is thought to be necessary for release of the virus from cell surfaces and thus for the movement of virus through mucus (17). With the aid of the X-ray crystal structure of influenza virus NA complexed with sialic acid (compound 1) or 2,3-dehydro-sialic acid, the nanomolar inhibitor zanamivir (compound 2) was designed as an enzyme substrate mimic (Fig. 1) (29). Because of its highly polar structure, zanamivir has very low oral bioavailability, but when it is used as an inhaled powder, it has been demonstrated to have clinical efficacy and has been approved for use for the treatment of influenza virus infections (5).
FIG. 1.
Structures of sialic acid, zanamivir, and 7-carbamate derivative.
The surface of an influenza virus typically has about 50 tetrameric NA spikes (22), with each spike displaying rotational symmetry, and the NA active site has been characterized as a strain-invariant cavity on the upper surface of each NA subunit (28). When zanamivir is bound to influenza virus NA, the 7-hydroxy group lies close to the surface of the protein and points out and away from the active site. Thus, derivatives of zanamivir functionalized at the 7 position, such as the aminohexyl carbamate derivative (compound 2a), retain good activity against all influenza virus strains tested (1). A conjugate of compound 2a and biotin has also been used to capture influenza virus virions onto a surface (21).
It has recently been reported that high-molecular-weight multivalent zanamivir conjugates are able to inhibit NA and show promising anti-influenza virus activities in vitro and in vivo (13, 26). We now report that certain dimeric derivatives of zanamivir show remarkable antiviral activities which we attribute to the effects of bivalent binding.
MATERIALS AND METHODS
Chemical reagents.
Research samples of zanamivir are available from GlaxoSmithKline. All chemical reagents used for the synthesis of compounds 4 to 12 were purchased from commercial suppliers (e.g., Aldrich) and were used without further purification. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Aldrich. Nonidet P-40 and 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA) were purchased from Sigma.
Cells.
Madin-Darby canine kidney (MDCK) cells were obtained from the European Collection of Cell Cultures, Salisbury, United Kingdom [ECACC; ECACC reference no. MDCK CB2784; lot number 98/F/0020(02/07/98); ECACC catalogue number 84121903; http://www.ecacc.org.uk].
Viruses.
Influenza viruses A/Sydney/5/97, B/Harbin/7/95, A/Victoria/3/75, B/Victoria/1/67, and A/Singapore/1/57 were obtained from the World Health Organization Collaborating Centre for Reference and Research on Influenza, Parkville, Melbourne, Victoria, Australia.
Chemical synthesis.
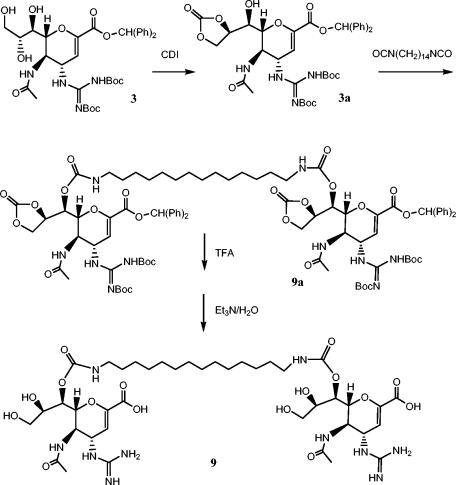
Compound 2a was synthesized by published procedures (1). The synthesis of compound 9 is outlined in Fig. 2, and the other dimers were prepared in an analogous manner (6, 14). Thus, intermediate 3 (27) was converted into compound 9 in a four-step sequence: (i) carbonyl diimidazole in acetonitrile (CH3CN) (86% yield); (ii) 1,14-diisocyanatotetradecane, 4-dimethylaminopyridine, 4-Å sieves in dichloromethane (50% yield); (iii) CF3CO2H (82% yield); and (iv) triethyl in dichloromethane methanol, and H2O (40% yield). Compounds 4 to 12 were all purified by preparative high-pressure liquid chromatography and showed 1H nuclear magnetic resonance and liquid chromatography (LC)-mass spectrometry (MS) parameters which were consistent with the expected structures.
FIG. 2.
Synthetic route used for the preparation of dimer 9. Abbreviations: Ph, phenyl; CDI, carbonyldiimidazole; Boc, t-butyloxycarbonyl; TFA, trifluoroacetic acid; Et3N, triethylamine.
Inhibition of influenza virus replication by compounds 2 and 2a and dimers 4 to 12.
Cytopathic effect (CPE) assays were performed by a previously published method (30). MDCK cells were infected with a defined inoculum of virus (determined by experimentation to be the minimum inoculum sufficient to cause an adequate CPE in 72 h and to be susceptible to the control compounds at concentrations considered consistent with published norms). This equated to a multiplicity of infection of approximately 0.0001 PFU per cell in the presence of triplicate serial (9 × half log10) dilutions of the compounds. Cultures were incubated for up to 72 h at 37°C in a 5% CO2 atmosphere in modified Eagle's medium with 0.02% bovine serum albumin (BSA) and 2.5 μg of trypsin per ml plus glutamine and insulin-transferrin-selenium mixtures for cell culture supplementation (the supplements were added according to the directions of the manufacturer [Gibco]). The extent of the CPE and, hence, viral replication was determined by measurement of the metabolism of the vital dye (MTT) by published methods. The compound concentration that inhibited the CPE by 50% (EC50) was calculated by using a computer program for curve fitting. Influenza viruses A/Sydney/5/97 and B/Harbin/7/95 were assayed. The cytotoxicities of all compounds were determined in parallel with the antiviral assays and by an identical protocol, with the exception that the viruses were omitted.
Determination of Kd values.
The inhibition of influenza virus NA activity was determined for compound 2 and dimers 8 to 10 by using the synthetic substrate MUNANA (25). The Kd values for the compounds were obtained by using two representative influenza virus strains (A/Aichi/2/68 and B/Beijing/1/87) in two ways: by determination of the effect of the compound on progress plots and by titration. Progress plot assays were performed as described previously (12). Experiments were carried out with purified NA and in some cases with NA solubilized by treating the virions with Nonidet P-40. Entirely similar results were obtained with NA solubilized by the two different methods. For the titrations, samples of enzyme and various concentrations of compound were incubated at room temperature for up to 24 h, with the length of incubation dependent on the values for kon determined from the progress plot assays. Residual enzyme activity was then determined by using MUNANA as the substrate. Data were fitted to an equation describing tight-binding inhibition.
Gel filtration experiments.
Gel filtration runs were performed at 4°C on a column of Superdex 200 10/30. The buffer used was 32.5 mM morpholineethanesulfonic acid-NaOH (pH 6.5) containing 4 mM MgCl2 and 0.1 M NaCl. The column was calibrated with Bio-Rad molecular weight standards. Fractions were collected, and samples were assayed in the standard way with MUNANA as the substrate. For samples in which inhibitor was present, the rates were measured after 2 to 2.5 h, once the inhibitor had dissociated and a steady-state rate was obtained.
Plaque reduction assay.
MDCK cells were seeded into six-well tissue culture plates and grown to confluence by standard methods. Influenza viruses were diluted in a minimal volume of phosphate-buffered saline (PBS) supplemented with 0.2% BSA to yield an estimated titer of 50 to 100 PFU per well. After adsorption onto the MDCK cells for 1 h at 37°C in a 5% CO2 atmosphere, the viral inocula were aspirated and replaced with viral growth medium (minimal Eagle's medium supplemented with BSA, trypsin, and insulin-transferrin-selenium at optimal concentrations) containing agar or agarose at an amount (generally 1 to 2%) sufficient to cause the medium to gel at room temperature. The plates were incubated at 37°C in a CO2 atmosphere until plaques developed (generally 2 to 4 days). The plaques were visualized with a suitable stain (e.g., 0.4% crystal violet in formal saline) before they were counted. Antiviral potency (EC50) was determined as the concentration of the compound in the medium that reduced the plaque numbers to 50% of the value for the untreated control.
Determination of compound retention in rat lungs.
Male Sprague-Dawley rats (weight, 220 to 280 g) were anesthetized with isoflurane. The rats were then placed in a supine position on a board inclined at an angle of 45° from the horizontal and were secured in position by hooking the upper incisors to a band secured to the upper part of the board. A halogen-fiber light was positioned close to the neck to guide the intubation. When the lower jaw was pulled down and the tongue was pulled to one side of the muzzle, the trachea was brought into view and a tracheal cannula was inserted until it was just above the bifurcation point. Compound was administered at a concentration of 0.5 mg/ml in PBS (pH 7.4) as a bolus at a dose of 0.8 ml/kg of body weight. The cannula was immediately withdrawn after dosing, and the animal was held in the upright position until full recovery from the anesthetic was achieved. This prevented the dose from refluxing back up the trachea. At 48 and 168 h postdosing, animals (two per time point) were placed under terminal anesthesia, and the lungs were then removed for sample analysis.
Lung tissue was homogenized in 5 ml of CH3CN-H2O (5/95) by using a PRO200 homogenizer with 10-mm cutting tools attached. A 200-μl aliquot of the homogenate was placed into a 1.5-ml Eppendorf tube, and protein was precipitated by the addition of 600 μl of CH3CN and 100 μl of 3% acetic acid. Samples were then vortex mixed and centrifuged at high speed in a Microfuge for 10 min. A 700-μl aliquot of the resulting supernatant was transferred to a 96-well plate by using a Tecan Genesis robotic system. The samples were then evaporated to dryness under nitrogen heated to 40°C and reconstituted in 200 μl of CH3CN-H2O (5/95). Prior to analysis, the sample plate was centrifuged at 2,000 × g for 15 min. For quantitative analysis, 50 μl of the sample was injected onto an LC-MS/MS system. The LC system consisted of a CTC autosampler, an HP series 1100 quaternary pump, and a Luna C18 analytical column (4.6 cm by 2.1 mm [inner diameter]; particle size, 5 μm). The mobile phases were 1% aqueous formic acid and 1% formic acid in CH3CN. Analysis was performed by gradient elution starting from a 5% organic phase to a 95% organic phase over 5 min at a flow rate of 0.4 ml/min. Mass detection was performed with a Sciex API365 detector with a turbo-ion spray interface, with the voltage set at 5 kV and the temperature set to 350°C. Positive multiple reaction monitoring transitions were monitored after optimization by infusion, and quantification was performed with MacQuan (Sciex) software.
Seven-day mouse influenza prevention assay.
The mouse influenza prevention assay has been fully described previously (7). Strain Y mice were challenged with 50 μl of a 104 50% tissue culture infective dose of stock influenza virus per ml via the external nares while they were under light ether anesthesia. A single dose of compound was administered as an aqueous solution 7 days prior to infection with a nonlethal strain of influenza virus. Efficacy was assessed by determining reductions in virus titers in the lungs. The lungs were removed 1 day following infection, and homogenized lung samples were assayed for the virus by established methods. The titers of the virus were estimated and compared to the titers of virus in the lungs of vehicle (PBS)-treated mice. Groups of 8 to 10 mice each were used for each dose and for calculation of the mean virus titer.
Electron micrograph images.
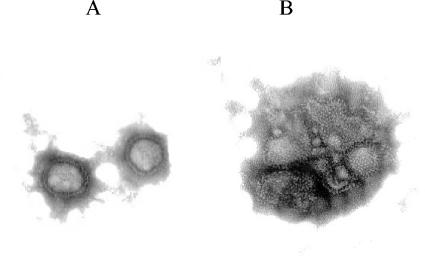
Influenza virus A/Sydney/97 grown in MDCK cells and purified by established methods was incubated for 30 min at 37°C in the presence of 200 ng of zanamivir per ml, 2 ng of compound 9 per ml, or no compound as a control. The samples were immediately transferred to ice and examined by negative-stain (3% phosphotungstic acid [pH 6.8 to 7.2]) transmission electron microscopy (JEDL JEM 1200 EX transmission electron microscope). Representative fields are shown in Fig. 4. The fields for samples from mice treated with 200 ng of zanamivir per ml and no compound were indistinguishable.
FIG. 4.
Electron microscopic images of influenza virus alone (A) and after incubation with 2 ng of compound 9 per ml (B).
RESULTS
Preparation and in vitro testing of dimeric NA inhibitors.
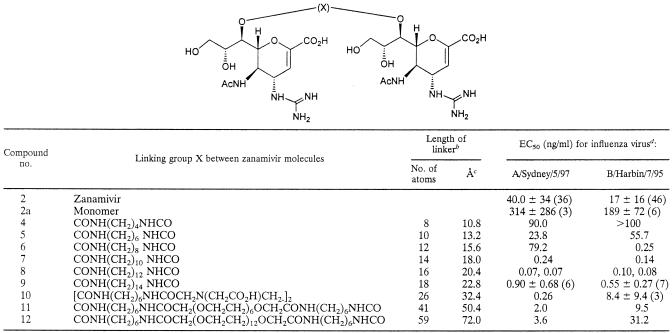
To explore whether dimeric forms of zanamivir would display observable bivalent effects, we prepared (Fig. 2) and tested zanamivir dimers connected via 7-carbamate groups to a wide range of linker lengths and types (6). Some representative dimers are shown in Table 1, including compounds 4 to 9 with straight alkyl chain cores and compounds 10 to 12 with longer linkers comprising EDTA and polyethylene glycol central groups coupled to zanamivir derivative 2a.
TABLE 1.
Structures, linker lengths, and antiviral activities of zanamivir dimers 4 to 12a
In the chemical structure, Ac represents acetyl.
Fully extended length (in angstroms) (number of bonds) × 1.2 Å (average bond contribution).
The decrease in activity observed with the longer dimers (dimers 11 and 12) may partially reflect the entropic penalty required to uncoil the polyethylene glycol linker.
Average EC50 (±standard deviations) are provided for assays performed three or more times (number in parentheses). No evidence of cytotoxicity was observed at concentrations up to 100 ng/ml for compounds 4 to 12 and at concentrations up to 1,000 ng/ml for compounds 2 and 2a.
Dimers 4 to 12 were tested for their antiviral activities by an influenza virus CPE assay with representative influenza A and B virus strains (Table 1). Three clear trends were found in the antiviral data: (i) dimers with linkers of 10 atoms or less were inactive or less active than zanamivir; (ii) compounds with linkers of about 12 to 18 atoms showed remarkably high potencies, with dimer 8 being at least 100 times more active than zanamivir; and (iii) when the linker length was extended beyond 18 atoms, the potency steadily dropped so that dimer 12, with a 59-atom linker, only showed activity similar to that of zanamivir.
Three of the most active dimers (dimers 8 to 10), plus zanamivir (compound 2) and derivative 2a, were tested for their inhibition of the enzyme activity of isolated influenza virus NA tetramers (Table 2). For this study we chose two NAs that have been well characterized previously (12) and which were available in sufficient amounts for detailed study, one from an influenza A virus and one from an influenza B virus. We believe that it is unlikely that the results would have differed significantly if NAs from other influenza virus strains had been chosen. NAs from two other strains are inhibited by zanamivir in a manner entirely similar to that in which the NAs used in this study were inhibited (11, 20), and the 50% inhibitory concentrations of zanamivir are very similar for NAs from a range of different influenza viruses (4). The results with zanamivir obtained in this study are similar to those reported previously (12). The Kd values for the dimers were 0.5 to 1.5 orders of magnitude greater than those for zanamivir, with the concentrations of the dimers expressed in terms of zanamivir equivalents. The Kd values were obtained from the ratio of koff/kon. In all cases, kon values for the dimer were lower than those for zanamivir, indicating that derivatization of zanamivir with a large substituent at the 7 position slows somewhat the association of the enzyme with the compound. The variation between repeat determinations of kon was less than 20%. The koff values were somewhat variable, with repeat determinations sometimes varying by as much as twofold, showing the difficulty of accurately determining intercept values when koff values are low, but they are generally not very different from the values for zanamivir.
TABLE 2.
Inhibition of NA
| Compound no. | NA from influenza virus A/Aichi/2/68a
|
NA from influenza virus B/Beijing/1/87a
|
Kd (nN) of NA from influenza virus A/Aichi/2/68b | ||||
|---|---|---|---|---|---|---|---|
| koff (s−1 [104]) | kon (N−1 s−1 [10−5]) | Kd (nN) | koff (s−1 [104]) | kon (N−1 s−1 [10−5]) | Kd (nN) | ||
| 2 | 1.1 | 1.1 | 1.0 | 0.80 | 0.34 | 2.4 | <1.0 |
| 2a | 1.6 | 0.066 | 24 | 0.48 | 0.080 | 6.0 | NDc |
| 8 | 6.0 | 0.20 | 30 | 4.4 | 0.13 | 34 | <1.0-2.8 |
| 9 | 0.90 | 0.090 | 10 | 2.0 | 0.065 | 31 | 5.6 |
| 10 | 1.5 | 0.13 | 11 | 0.33 | 0.048 | 6.9 | <1.0-2.8 |
Data were obtained from progress plot analysis, as described previously (12).
Data were obtained by titration, as described in the text.
ND, not determined.
An alternative method of estimating potency is to incubate samples of enzyme with various concentrations of compound for an amount of time sufficient for equilibrium to be reached and then to measure residual activity without disruption of the equilibrium. By this titration approach, zanamivir (compound 2) and dimers 8 and 10 bound too tightly to the enzyme for precise values of Kd to be determined, i.e., the Kd values against NA from influenza A virus were <1 nN. Simulations showed that a Kd value of 1 nM could be determined with confidence, and while a Kd value of 0.5 nM would generally be able to be determined, titration plots for Kd values lower than this would become indistinguishable from each other. The Kd values for dimers 8 and 10 obtained by titration are much lower than those obtained by the kinetics approach. By contrast, a Kd value for dimer 9 of 5.6 nN was obtained by titration. This is not very different from the Kd value obtained by the kinetics approach.
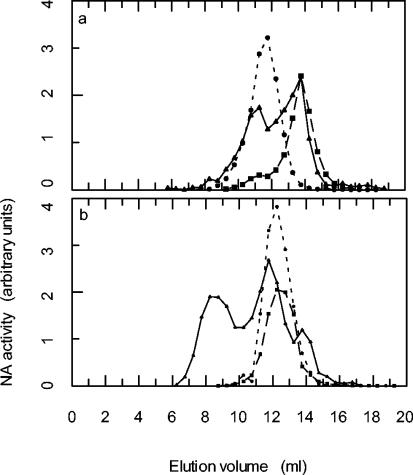
Gel filtration experiments were conducted to determine whether dimer 10 caused any alteration of the influenza virus NA elution profile (Fig. 3). Incubation of dilute solutions (20 to 30 nM) of NA that had been proteolytically cleaved from virions and purified led to some dissociation of tetramers to lower-molecular-weight species. This was found to be true for NAs from both influenza A and influenza B viruses. However, if the incubations were performed in the presence of dimer 10 (at a relative concentration, based on zanamivir equivalents of 1:1 or 2:1 dimer/enzyme) the elution profiles differed. The NA from influenza A virus showed much less dissociation, and there was evidence for the formation of some high-molecular-weight aggregates (Fig. 3a). The NA from influenza B virus formed an even larger amount of high-molecular-weight material (data not shown). When Nonidet P-40-solubilized NA from influenza A virus was used, high-molecular-weight aggregates were observed when NA was run through the column immediately after dilution. Following incubation in dilute solution, there was much dissociation of aggregates to species whose elution volumes are about those expected for NA tetramers, dimers, and monomers. When Nonidet P-40-solubilized NA was incubated with dimer 10 (relative concentration, 1/1), very little NA that was not in high-molecular-weight aggregates was present (Fig. 3b). Direct evidence for the formation of virus aggregates by zanamivir dimers was obtained by electron microscopy. Thus, in contrast to the images of virus alone (Fig. 4A) or when virus was treated with monomer 2 (data not shown), after incubation of virus with dimer 9, the images showed significant clumping of virions (Fig. 4B).
FIG. 3.
Gel filtration experiments with influenza virus NA and dimer 10. (a) NA purified from influenza virus A/Aichi/2/68. Dotted line, NA diluted to 20 nM and immediately gel filtered; dashed line, NA diluted to 20 nM and gel filtered after 25 h at room temperature; solid line, NA (20 nM) and dimer 10 (20 nN in zanamivir equivalents) gel filtered after 26 h at room temperature. (b) NA solubilized from influenza virus A/Aichi/2/68 with Nonidet P-40. Dotted line, NA diluted to 20 nM and immediately gel filtered; dashed line, NA diluted to 20 nM and gel filtered after 23 h at room temperature; solid line, NA (20 nM) and dimer 10 (20 nN in zanamivir equivalents) gel filtered after 24 h at room temperature. Fractions from the column were assayed for NA activity as described in the text. At least two column runs were performed for each experimental condition, and the elution volumes were entirely reproducible.
Plaque reduction assays were carried out with compounds 8 to 10 by standard methods with representative influenza virus strains, and the results were confirmed by the remarkably high antiviral potencies of the dimers relative to that of zanamivir (Table 3).
TABLE 3.
Plaque reduction assay and lung retention for compound 2 and compounds 8 to 10
| Compound no. | EC50 (pM)a
|
Lung retentionb
|
||
|---|---|---|---|---|
| A/Vic/3/75 | B/Vic/1/67 | 48 h | 168 h | |
| 2 | 11,000, 12,000, 50,000 | 50,000, 50,000 | 1.0 | 1.0 |
| 8 | 55, 24 | 55, 9, 10 | 33.7 | 113 |
| 9 | 283, 19 | 95, 47 | 34.5 | 160 |
| 10 | 160 | 330 | 1.5 | 3.7 |
EC50 were determined by the plaque reduction assay. Influenza virus was added to MDCK cells, incubated for 1 h, replaced with viral growth medium, and incubated until the plaques developed (2 to 4 days). After visualization, the plaques were counted. Potency was determined as the compound concentration needed to reduce plaque numbers by 50% of the control plaque number. Values are from separate experiments. Insufficient replicate data were available for meaningful standard deviations to be quoted.
Rats were treated with an intratracheal dose of compound (0.4 mg/kg), followed by removal of the lungs at set times and then determination of the compound concentration. The data are relative to the results for compound 2.
In vivo lung retention and efficacies of dimers 8 to 10.
The in vivo retentions of dimers 8 to 10 in lung tissue were compared with that of zanamivir by treating rats with an intratracheal dose of an aqueous solution of compound, followed by removal of the lungs at 48 and 168 h postdosing (n = 2). The concentrations of each compound in the lung tissue were measured by MS, and the results are presented relative to those for zanamivir (Table 3). The dimers showed retention profiles longer than those of zanamivir; and compounds 8 to 9, the hydrocarbon-linked dimers, displayed particularly long retention profiles, being present at more than 100-fold greater concentrations than compound 2 at 168 h.
The in vivo efficacies of intranasally administered dimers 8 to 10 were compared with that of zanamivir (compound 2) in an established influenza virus-infected mouse model (7). This involved administration of a single intranasal dose 7 days prior to infection of the animals with influenza virus. The effectiveness of the compounds in preventing virus replication was determined by measuring the amount of virus remaining in the lungs 24 h after the viral infection and comparing this to the level of virus in the lungs of placebo-treated mice (Table 4). All three dimeric compounds (compounds 8 to 10) showed outstanding in vivo activities, being more effective than zanamivir at a fraction of the dose, with compound 9, for example, being 100-fold more active than zanamivir. Notably, compound 8 was tested against both the A/Singapore and the A/Victoria strains by identical procedures, and similar efficacies against both strains were observed.
TABLE 4.
In vivo mouse efficacya data for compounds 8 to 10
| Compound no. and dose (mg/kg) | nb | No. of virus-free animals | % Reduction in virus titer | Significance (P) | ED90 (mg/kg) |
|---|---|---|---|---|---|
| 2 | |||||
| 5 | 10 | 1 | 93.69 | <0.01 | |
| 1 | 10 | 0 | 74.88 | NSe | 2.92c |
| 0.2 | 10 | 0 | 33.17 | NS | |
| 8 | |||||
| 1 | 10 | 0 | 97.34 | <0.01 | |
| 0.2 | 10 | 1 | 97.02 | <0.05 | 0.079c |
| 0.04 | 10 | 0 | 78.87 | <0.05 | |
| 8 | |||||
| 0.4 | 8 | 2 | 96.70 | <0.05 | |
| 0.1 | 8 | 0 | 55.97 | NS | 0.17d |
| 0.025 | 8 | 0 | 80.05 | NS | |
| 9 | |||||
| 0.4 | 8 | 4 | 99.27 | <0.01 | |
| 0.1 | 8 | 0 | 92.17 | <0.05 | 0.03d |
| 0.025 | 8 | 2 | 91.99 | NS | |
| 9 | |||||
| 1 | 8 | 6 | 99.30 | <0.01 | |
| 0.25 | 8 | 4 | 99.00 | <0.01 | 0.026d |
| 0.0625 | 8 | 1 | 91.34 | NS | |
| 0.0156 | 8 | 1 | 87.59 | NS | |
| 10 | |||||
| 1 | 10 | 0 | 97.44 | <0.01 | |
| 0.1 | 10 | 0 | 81.16 | <0.05 | 0.12d |
| 0.01 | 10 | 0 | 73.39 | <0.05 |
Efficacy was determined by using the 7-day mouse model. Compounds were dosed 7 days prior to infection with a non lethal strain of virus. Efficacy was determined by measurement of the reductions in virus titers in the lungs. The lungs were removed 1 day following infection, and viral loads were compared to the virus titers in the lungs of vehicle-treated mice.
n, number of animals per group.
Influenza virus A/Singapore/1/57 was used.
Influenza virus A/Victoria/3/75 was used.
NS, not significant.
DISCUSSION
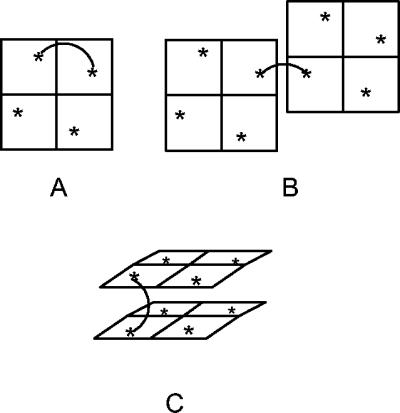
We have demonstrated that dimers of zanamivir that exhibit remarkably increased potencies compared with that of zanamivir can be synthesized both in vitro and in vivo. We hypothesized that such increased potencies could be attributed to bivalent binding, and we considered that at least three types of bivalent binding could occur: binding to two active sites within a single tetramer (intratetramer) (Fig. 5A), binding between sites on different tetramers on the same virion (intravirion) (Fig. 5B), and head-to-head binding between NA sites, which would most likely occur between separate virions (intervirion) (Fig. 5C). If the potential contribution of glycosyl moieties is ignored, the X-ray structure of influenza virus NA reveals that the distance between the position-7 oxygen atoms of zanamivir molecules bound at neighboring active sites within a tetramer is approximately 50 Å (29). Because the active sites lie toward the edge of each NA subunit, when two separate NA tetramers come into close proximity, the intermolecular distance between active sites on different tetramers could be as little as 25 to 30 Å. For head-to-head binding of two NA tetramers, we estimate that the minimum separation of the 7-position oxygen atoms in opposing NA sites is approximately 16 Å, but this distance may vary from influenza virus strain to influenza virus strain due to differences in the residues on the outer surface of the protein and also the degree of glycosylation of the NA surface (29). The dramatic increase in antiviral activities of the zanamivir dimers described above when the linker length is increased from 10 atoms to 12 to 14 atoms corresponds to the spacing between the zanamivir groups reaching about 16 to 18 Å, if it is assumed that the linker is fully extended (9). From the estimated distances between NA sites for the different modes of binding mentioned above, this optimum spacing suggests that intertetramer and, probably, intervirion binding are taking place.
FIG. 5.
Two-dimensional representation of the exposed top surface of influenza virus NA tetramers and three possible modes of binding for a dimeric inhibitor: intratetrameric binding (spans approximately 50 Å) (A), dimer binding to neighboring NA tetramers (25 to 30Å) (B), and dimer binding to NA tetramers on different virions (16 Å) (C).
The apparently different Kd values for the two types of NA assays with dimers 8 and 10 may be reconciled when due consideration is given to the nature of the two assays. In the progress plot assays, low concentrations of enzyme were used and the concentrations of inhibitor were 102 to 104 times greater than the enzyme concentration. Statistically, the chance that an NA active site will bind to a zanamivir unit of a dimer that has another NA molecule bound to the other end is extremely low. In this situation, we measured a true dissociation constant for the interaction between an NA active site and a zanamivir unit which has a modification to the 7 position. In the titration experiment the NA active sites and dimer molecules were present at approximately equal concentrations. Thus, when an NA active site binds to a zanamivir unit of a dimer, there is a very high probability that the zanamivir unit at the other end is already bound to another NA active site. As NA molecules are mostly present as tetramers, large matrices can build up. In such a matrix, when an individual zanamivir unit dissociates from an NA active site, neither NA nor the zanamivir unit can move far because they are held in a matrix. They can very easily reassociate, and so in this situation the apparent affinity will be very much higher than that in a progress plot experiment. Consistent with the inhibition of NA kinetic observations, the gel filtration experiments with NA tetramers isolated from representative influenza A and B virus strains show that dimer 10 is able to cross-link NA, leading to the formation of higher-molecular-weight aggregates. A big difference in Kd values between titration and progress plot analysis was not found for compound 9, indicating that little matrix formation occurred. This suggests that the distance between zanamivir groups and/or their relative orientation is important if a large matrix is to be built up from dimer and an isolated enzyme, and this is determined by the nature of the linking group.
The remarkable antiviral potencies of dimeric compounds 8 to 10 observed in the CPE assay were confirmed when the compounds were tested for inhibition of whole-virus replication in plaque reduction assays with representative influenza A and B virus strains. For example, compound 8 was found to be about a 1,000-fold more active than zanamivir (Table 3), and we believe that the high antiviral activity is a result of the ability of the dimers to cross-link and aggregate virus particles. These conclusions are supported by gel filtration data, and direct evidence of dimer-mediated intervirion binding was provided by electron microscopy. It has previously been shown (23) that NA inhibitors, by blocking the removal of sialic acid from glycoproteins, can cause influenza virus particles to remain immobilized on the host cell surface; and this is considered the reason for their antiviral effects. Presumably, the dimeric NA inhibitors likewise cause virus particles to remain attached to the cell surface, and through their ability to form linkages between different virus particles, they would further aggregate and immobilize the virus, providing an enhanced antiviral effect.
In addition to their increased potencies, evidence was obtained for a substantial increase in the lung residence times of the zanamivir dimers in comparison to that of zanamivir. It has been reported that for small and polar molecules, absorption from the lung is via the tight junctions between cells, and thus, the lung residence time correlates well with the molecular weight (24). Therefore, we anticipated that the dimers would display longer retention in lung tissues than zanamivir, and this has been confirmed by the results of the experiment.
However, the data obtained suggest that it is unlikely that retention is due solely to molecular weight effects, and we believe that the increased residence time for alkyl chain-linked dimers 8 and 9 may be due in part to altered aqueous solubility or a stronger association with lipid membranes, or both. A question may be raised about the safety and tolerability of compounds with long lung residence times. Data from relevant studies are not yet available, but the following points may be made. It is well known that after inhaled administration of a drug, a large percentage is swallowed and does not remain in the lung. For the material that does remain in the lung, our preliminary pharmacokinetic studies (data not included) indicate that a substantial portion is absorbed into the circulation. These factors, together with the likelihood that a therapeutic dose would be small, mean that only very low levels of drug are likely to remain in the lung for extended periods of time (which, nevertheless, from the data presented in this paper, are sufficient for antiviral activity) and may mitigate concerns over safety and tolerability.
In summary, we have identified dimeric NA inhibitors that show remarkably high levels of and prolonged anti-influenza virus activities. We believe that, relative to zanamivir, the superior in vivo prophylactic activities of dimers such as compound 9 can be attributed to a combination of the improved antiviral potency and higher level of compound retention in the lung. These compounds provide a clear example of how suitable multivalent derivatives of a known therapeutic compound can introduce an extra mode of binding and, hence, give dramatically improved activity in vivo. These results raise the prospect for a new type of anti-influenza drug that could be used at very low doses administered once weekly, such that a single dose could be suitable for the treatment of influenza or such that treatment just once a week could be suitable for the prevention of infection.
Acknowledgments
We thank Khim Hoe, senior technical officer, Sequencing & Oligosynthesis Services, Department of Microbiology, Monash University, Clayton, Victoria, Australia, for the specialist electron micrographs.
REFERENCES
- 1.Andrews, D. M., P. C. Cherry, D. C. Humber, P. S. Jones, S. P. Keeling, P. F. Martin, C. D. Shaw, and S. Swanson. 1999. Synthesis and influenza virus sialidase inhibitory activity of analogues of 4-guanidino-Neu5Ac2en (zanamivir) modified in the glycerol side-chain. Eur. J. Med. Chem. 34:563-574. [DOI] [PubMed] [Google Scholar]
- 2.Bertozzi, C. R., and L. L. Kiessling. 2001. Chemical glycobiology. Science 291:2357-2364. [DOI] [PubMed] [Google Scholar]
- 3.Borman, S. 2000. Multivalency: strength in numbers. Chem. Eng. News 78:48-53. [Google Scholar]
- 4.Buxton, R. C., B. Edwards, R. R. Juo, J. C. Voyta, M. Tisdale, and R. C. Bethell. 2000. Development of a sensitive chemiluminescent neuraminidase assay for the determination of influenza virus susceptibility to zanamivir. Anal. Biochem. 280:291-300. [DOI] [PubMed] [Google Scholar]
- 5.Cheer, S. M., and A. J. Wagstaff. 2002. Zanamivir: an update of its use in influenza. Drugs 62:71-106. [DOI] [PubMed] [Google Scholar]
- 6.Demaine, D. A., G. G. A. Inglis, S. J. F. Macdonald, S. E. Shanahan, S. P. Tucker, K. G. Watson, and W.-Y. Wu. 2003. Dimeric compounds and their use as anti-viral agents. WO 03/040138. Chem Abstr. 138:354175. [Google Scholar]
- 7.Fenton, R. J., P. J. Morley, I. J. Owens, D. Gower, S. Parry, L. Crossman, and T. Wong. 1999. Chemoprophylaxis of influenza A virus infections with single doses of zanamivir demonstrates that zanamivir is cleared slowly from the respiratory tract. Antimicrob. Agents Chemother. 43:2642-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gestwicki, J. E., C. W. Cairo, L. E. Strong, K. A. Oetjen, and L. L. Kiessling. 2002. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 124:14922-14933. [DOI] [PubMed] [Google Scholar]
- 9.Green, N. M., L. Konieczny, E. J. Toms, and R. C. Valentine. 1971. Use of bifunctional biotinyl compounds to determine the arrangement of subunits in avidin. Biochem. J. 125:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin, J. H., M. S. Linsell, M. B. Nodwell, Q. Chen, J. L. Pace, K. L. Quast, K. M. Krause, L. Farrington, T. X. Wu, D. L. Higgins, T. E. Jenkins, B. G. Christensen, and J. K. Judice. 2003. Multivalent drug design. Synthesis and in vitro analysis of an array of vancomycin dimers. J. Am. Chem. Soc. 125:6517-6531. [DOI] [PubMed] [Google Scholar]
- 11.Gubareva, L. V., R. Bethell, G. J. Hart, K. G. Murti, C. R. Penn, and R. G. Webster. 1996. Characterization of mutants of influenza A virus selected with the neuraminidase inhibitor 4-guanidino-Neu5Ac2en. J. Virol. 70:1818-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart, G. J., and R. C. Bethell. 1995. 2,3-Didehydro-2,4-dideoxy-4-guanidino-N-acetyl-d-neuraminic acid (4-guanidino-Neu4Ac2en) is a slow-binding inhibitor of sialidase from both influenza A virus and influenza B virus. Biochem. Mol. Biol. Int. 36:695-703. [PubMed] [Google Scholar]
- 13.Honda, T., S. Yoshida, M. Arai, T. Masuda, and M. Yamashita. 2002. Synthesis and anti-influenza evaluation of polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives. Bioorg. Med. Chem. Lett. 12:1929-1932. [DOI] [PubMed] [Google Scholar]
- 14.Judkins, B. D., S. J. F. Macdonald, D. A. Demaine, G. G. A. Inglis, and J. N. Hamblin. 2003. Intermediates for preparing neuraminidase inhibitor conjugates. WO 03/022841. Chem. Abstr. 138:255099. [Google Scholar]
- 15.Kiessling, L. L., L. E. Strong, and J. E. Gestwicki. 2000. Principles for multivalent ligand design. Annu. Rep. Med. Chem. 35:21-330. [Google Scholar]
- 16.Kitov, P. I., H. Shimizu, S. W. Homans, and D. R. Bundle. 2003. Optimization of tether length in nonglycosidically linked bivalent ligands that target sites 2 and 1 of a Shiga-like toxin. J. Am. Chem. Soc. 125:3284-3294. [DOI] [PubMed] [Google Scholar]
- 17.Liu, C., M. C. Eichelberger, R. W. Compans, and G. M. Air. 1995. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J. Virol. 69:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundquist, J. J., and E. J. Toone. 2002. The cluster glycoside effect. Chem. Rev. 102:555-578. [DOI] [PubMed] [Google Scholar]
- 19.Mammen, M., S.-K. Choi, and G. M. Whitesides. 1998. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37:2754-2794. [DOI] [PubMed] [Google Scholar]
- 20.McKimm-Breschkin, J. L., T. J. Blick, A. Sahasrabudhe, T. Tiong, D. Marshall, G. J. Hart, R. C. Bethell, and C. R. Penn. 1996. Generation and characterization of variants of NWS/G70C influenza virus after in vitro passage in 4-amino-Neu5Ac2en and 4-guanidino-Neu5Ac2en. Antimicrob. Agents Chemother. 40:40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKimm-Breschkin, J. L., P. M., Colman, B. Jin, G. Y. Krippner, M. McDonald, P. A. Reece, S. P. Tucker, L. Waddington, K. G. Watson, and W.-Y. Wu. 2003. Tethered neuraminidase inhibitors that bind an influenza virus: a first step towards a diagnostic method for influenza. Angew. Chem. Int. Ed. 42:3117-3121. [DOI] [PubMed] [Google Scholar]
- 22.Murti, K. G., and R. G. Webster. 1986. Distribution of hemagglutinin and neuraminidase on influenza virions as revealed by immunoelectron microscopy. Virology 149:36-43. [DOI] [PubMed] [Google Scholar]
- 23.Palese, P., and R. W. Compans. 1976. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J. Gen. Virol. 33:159-163. [DOI] [PubMed] [Google Scholar]
- 24.Patton, J. S. 1996. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 19:3-36. [Google Scholar]
- 25.Potier, M., L. Mameli, M. Belisle, L. Dallaire, and S. B. Melancon. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287-296. [DOI] [PubMed] [Google Scholar]
- 26.Reece, P. A., K. G. Watson, W.-Y. Wu, B. Jin, and G. Y. Krippner. 1998. Preparation of poly(neuraminic acids) as influenza virus neuraminidase inhibitors. PCT patent application no. WO 98/21243. Chem. Abstr. 129:28172. [Google Scholar]
- 27.Smith, P. W., S. L. Sollis, P. D. Howes, P. C. Cherry, I. D. Starkey, K. N. Cobley, H. Weston, J. Scicinski, A. Merritt, and A. Whittington. 1998. Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 1. Discovery, synthesis, biological activity, and structure-activity relationships of 4-guanidino- and 4-amino-4H-pyran-6-carboxamides. J. Med. Chem. 41:787-797. [DOI] [PubMed] [Google Scholar]
- 28.Varghese, J. N., and P. M. Colman. 1991. Three-dimensional structure of the neuraminidase of influenza virus A/Tokyo/3/67 at 2.2 Å resolution. J. Mol. Biol. 221:473-486. [DOI] [PubMed] [Google Scholar]
- 29.Varghese, J. N., V. C. Epa, and P. M. Colman. 1995. Three-dimensional structure of the complex of 4-guanidino-Neu5Ac2en and influenza virus neuraminidase. Protein Sci. 4:1081-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe, W., K. Konno, K. Ijichi, H. Inoue, T. Yokota, and S. Shigeta. 1994. MTT colorimetric assay system for the screening of anti-orthomyxo- and anti-paramyxoviral agents. J. Virol. Methods 48:257-265. [DOI] [PubMed] [Google Scholar]
- 31.Wright, D., and L. Usher. 2001. Multivalent binding in the design of bioactive compounds. Curr. Org. Chem. 5:1107-1131. [Google Scholar]