Abstract
Background
Valproic acid (VPA) and carbamazepine (CBZ), two widely used antiepileptic drugs, have recently been found to inhibit histone deacetylases (HDAC). HDAC inhibitors (HDACIs) have various effects on cancer cells.
Objectives
The aim of this study was to compare the anticancer activity of these drugs on SW480 colon cancer cell lines.
Methods
In the present experimental study, implemented during 2014 - 2015 in Iran, after incubation of cells into 96-well plates with 5,500 cells/well, the tested drugs were added, and cytotoxic effects were assessed by MTT. Moreover, after incubation of 8×106 cells in 75 cm² flasks to obtain β-catenin levels and 106 cells in a six-well plate to obtain vascular endothelial growth factor (VEGF) levels , these levels were estimated using enzyme-linked immunosorbent assay (ELISA) analysis.
Results
Through MTT assay, we found that the inhibitory concentration of 50% (IC50) values for VPA and CBZ were 2.5 mM and 5 μM, respectively in comparison to controls in terms of total concentration and times evaluated (P < 0.0001). We also found that treatments with these drugs decreased levels of β-catenin (P < 0.0001) and VEGF (P < 0.0001) significantly more than controls.
Conclusions
VPA and CBZ treatments caused a decrease in β-Catenin and VEGF levels in SW480 colon cancer cell lines. These results suggest that CBZ can be considered a potential antitumor drug with potencies different from VPA.
Keywords: Histone Deacetylase Inhibitor, β-Catenin, VEGF
1. Background
Valproic acid (VPA) is a broad spectrum antiepileptic drug that has also been used in the treatment of bipolar disorders, neuropathic pain, and migraine prophylaxis. However, the mechanisms of action for VPA are currently unknown. Its antiepileptic effects primarily depend on the increased gamma aminobutyric acid function and its interactions with sodium and calcium channels. The anticonvulsant drug carbamazepine (CBZ) is known to have antimanic and prophylactic effects in the treatment of manic depressive disorders. CBZ blocks Na+ channels (1).
In the past few years, histone deacetylases inhibitors (HDACIs) have proven to be powerful inducers of cancer cell growth arrest (including in drug resistant subtypes), differentiation, and apoptotic cell death of transformed cells. They also inhibit angiogenesis and sensitize cancer cells to overcome drug resistance when used in combination with other anticancer agents. In addition, histone hyperacetylation has proven to be important in the carcinoma process, and HDACIs firmly bind to histones and prevent the transcription and expression of tumor suppressor genes. Several HDACIs are currently in Phase I and Phase II clinical trials as cancer therapeutics (2, 3). Furthermore, multiple proteins are targets of histone deacetylases (HDAC). Preclinical evidence suggests their synergy and additive activity with many other anticancer agents. However, use of some of them is demonstrated to be limited by their toxicity (4, 5).
Among HDACIs, VPA has some agreeable features from a clinical point of view. It is a very well-known drug that has been in use for a long time. VPA has a longer in vivo half-life compared than other HDACIs (6). Finally, we found evidence that HDAC is a target of CBZ in the differentiation of HepG2 liver carcinoma cell lines. These findings suggest a role for CBZ in the treatment of liver cancer (7). These recent findings that CBZ, a clinically well characterized and tolerated drug, is also an HDACI suggest that it can be considered as a valuable alternative separation agent. Moreover, the inhibitory concentration of 50% (IC50) for HDAC inhibition is well within its therapeutic range and has no adverse drug reactions, such as those induced by VPA in terms of hepatotoxicity, mitochondrial toxicity, and hyperammonemic encephalopathy (8).
Colon cancer is a major global health problem. Colorectal cancer is the second most common reason for cancer mortality (9). It is the third most common cancer worldwide, with over one million new cancer cases and over half a million deaths per year (10). It is critically important to aggressively explore pharmacological treatment strategies that can effectively overcome cancer drug resistance and its adverse effects.
To the best of our knowledge, there are no reports on in vitro or in vivo biological activities of CBZ or its effects on colon cancer cells. Here, for the first time, we investigated the antitumor and cytotoxic activity of CBZ against human colon cancer cell lines. This study was performed to determine the biological and therapeutic effects of HDACIs in treating colon cancer. We focused particularly on CBZ and VPA, which are recognized as the least toxic HDACIs. Up to this date, no studies have been conducted on the biological anticancer effectiveness of CBZ; measurements of β-catenin and vascular endothelial growth factor (VEGF) protein levels are conducted for this reason. Due to the fact that 90% of colon cancers are caused by gene mutations that induce β-catenin production, its measurement is vital. VPA has clinical anticancer applications, and in our study it is used as a positive control to compare with CBZ, so that if CBZ proves effective, it can also be used clinically. The side effects of these two drugs are far less severe than those of the chemotherapy drugs presently available. Therefore, the use of these drugs may reduce the necessary dose of chemotherapy drugs in patients and consequently their side effects. Hence, these results are very valuable. Moreover, resistance to chemotherapy drugs can be reduced by using these drugs, and in cases in which patients do not respond to chemotherapy, their use is vital.
2. Objectives
The aim of this study was to compare the cytotoxic effects of VPA and CBZ and determine their effects on β-catenin and VEGF levels, two key proteins involved in carcinogenesis and tumor metastasis.
3. Methods
3.1. Materials
Reagents were purchased from the following sources: Quantikin human VEGF enzyme-linked immunosorbent assay (ELISA) and Surveyor Ic human total β-catein ELISA from R&D Systems (USA). All other chemicals used in this study were obtained from Sigma (USA). The human colon adenocarcinoma cell lines (SW480) were obtained from the Avicenna Institute.
3.2. Cell Culture
Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 in RPMI1640 supplemented with 10% (v/v) fetal calf serum. The present experimental study was conducted during the period 2014 - 2015 in Tehran, Iran.
3.3. Cytotoxicity assay
3.3.1. Sampling
In order to determine the IC50, cells were seeded into 96-well plates with 5,500 cells/well in 0.19 mL of a complete medium.
3.3.2. Protocols
Starting the next day, cells were treated for three consecutive days in a complete medium with different concentrations: 0, 1.25, 2.5, and 5 mM of VPA and 0, 2.5, 5, and 10 μM of CBZ. Over these days, cell viability was measured by the conventional MTT dye reduction assay. In brief, 100 μL of 25 mg/5 mL MTT reagent were added to each viable cell well with active mitochondria to reduce the MTT to an insoluble purple formazan precipitate, which was solubilized by the subsequent addition of 100 μL of DMSO.
3.3.3. Measurements
The formazan dye was measured with the aid of an ELISA reader: Bio Tek E1800TM (USA). All assays were performed in triplicate and repeated three times (kappa coefficient > 95%). The cytotoxic effect of each treatment is given by the following: cell viability = (treated cells / untreated control cells) × 100; which is in accordance with the optical density read from the ELISA reader.
3.4. β -Catenin Level
Experiments to determine the effects of VPA and CBZ on β-catenin levels were conducted using the Surveyor IC human total β-catenin immunoassay kit following the manufacturer’s instructions.
3.4.1. Sampling
In brief, 8 × 106 cells were seeded into 75 cm² flasks and allowed to attach by overnight incubation.
3.4.2. Protocols
The cells were treated with 0, 5, and 10 μM CBZ for 48 hours and with 0, 2.5, and 5 mM of VPA for 72 hours. The medium was removed, washed, and lysed by a specific lysis buffer provided by the kit. The cells were then scraped off the dish and collected by centrifugation. The lysate was used to determine the β-catenin levels.
3.5. VEGF Level
3.5.1. Sampling
VEGF protein released by SW480 cells was measured by a human VEGF ELISA kit based on the manufacturer’s instructions. In brief, 106 cells were seeded into a six-well plate and allowed to attach by overnight incubation.
3.5.2. Protocols
The cells were treated with 0, 5, and 10 μM of CBZ for 48 hours and with 0, 2.5, and 5 mM of VPA for 72 hours. We removed particulates by centrifugation. The cell culture supernatants were used to determine the VEGF levels.
3.5.3. Measurements
The total protein concentration level was measured by using the BCA protein assay reagent. The absorbance was quantified using an ELISA reader. All assays were performed in duplicate and repeated three times, yielding similar results (kappa coefficient > 96%).
3.6. Ethical Considerations
This experimental study was approved by the ethics committee of Medical Sciences of Shahid Baheshti University in Tehran, Iran (Code: IR.SBMU.SM.REC.1394.4). Date of approval: 2015/8/18.
3.7. Statistical Analysis
Statistical analysis was performed using the SPSS software program, version 16 (SPSS Inc., Chicago). Moreover, one-way ANOVA analysis was applied to determine the differences between the results of the studied groups using the graph pad prism5 software. Values of P < 0.05 were considered statistically significant.
4. Results
4.1. Cytotoxicity Results
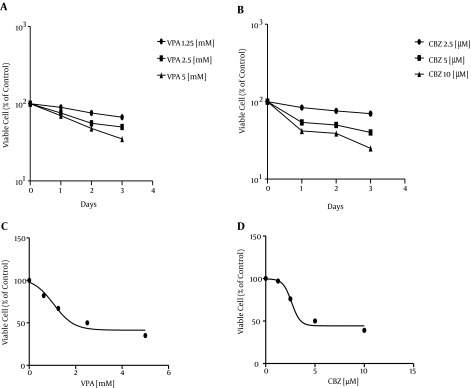
A panel of SW480 cell lines was treated with a single dose of 1.25, 2.5, and 5 mM of VPA and 2.5, 5, and 10 μM of CBZ for 24, 48, and 72 hours, and the cell culture growth was assessed by MTT assay (Figure 1A, B). Cell growth > 50% was inhibited relative to untreated controls in SW480 cell lines. A concentration response curve with SW480 cells indicated an IC50 value of 2.5 mM for VPA and 5 μM for CBZ (Figure 1 C, D). Time-course experiments showed that after three days of exposure to 2.5 mM VPA and two days of exposure to 5 μM CBZ, 50% viable cells were present compared to vehicle-treated controls. We considered levels with P < 0.0001 as significant (Table 1).
Figure 1. This Diagram Shows That cell Viability After Treatment With VPA and CBZ for Days 1, 2, and 3 Significantly Differs From Cell Viability in Controls (P < 0.0001).
Table 1. Comparing Experimental Results of Treatments With VPA and CBZ.
| Concentration of Drug | Drug | |||
|---|---|---|---|---|
| Variable | VPA | CBZ | ||
| (%) | P Values Compared to Control | (%) | P Values Compared to Control | |
| Day 1 | ||||
| Control | 100 | 100 | ||
| C1 | 90 | 0.0001 > | 84 | 0.0001 > |
| C2 | 76 | 0.0001 > | 54 | 0.0001 > |
| C3 | 70 | 0.0001 > | 42 | 0.0001 > |
| Day2 | ||||
| Control | 100 | 100 | ||
| C1 | 76 | 0.0001 > | 76 | 0.0001 > |
| C2 | 56 | 0.0001 > | 50 | 0.0001 > |
| C3 | 48 | 0.0001 > | 39 | 0.0001 > |
| Day 3 | ||||
| Control | 100 | 100 | ||
| C1 | 67 | 0.0001 > | 70 | 0.0001 > |
| C2 | 50 | 0.0001 > | 40 | 0.0001 > |
| C3 | 35 | 0.0001 > | 25 | 0.0001 > |
4.2. β-Catenin Level Results
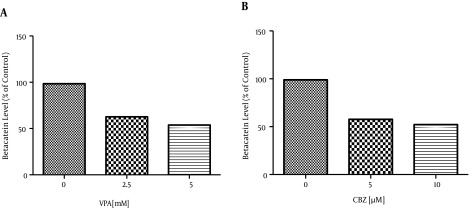
The Wnt/β-catenin signaling pathway plays an important role in carcinogenesis and tumor metastasis, which is involved in many cancer models (11, 12). Thus, targeting the Wnt/β-catenin inhibitors is of great significance for cancer chemotherapy. Treatments with VPA at 2.5 and 5 mM and with CBZ at 5 and 10 μM resulted in a dose dependent decrease of the β-catenin levels in the human colon cancer SW480 cells compared to water-treated cells for VPA and DMSO treated cells for CBZ. For example, the β-catenin levels in SW480 cells treated for 72 hours with 2.5 and 5 mM of VPA decreased by about 35.76% and 44.6%, respectively. Those treated for 48 hours with 5 and 10 μM of CBZ decreased by about 41.26% and 46.81%, respectively compared to levels in the control group (Figure 2). According to one-way ANOVA, the reductions were significant (P < 0.0001) (Table 2).
Figure 2. This Diagram Shows That β-Catenin Levels After Treatment With VPA and CBZ Significantly Differ From Levels in Controls (P < 0.0001).
Table 2. Comparing Experimental Results of Treatments With VPA and CBZ.
| Variable | Drug | |||
|---|---|---|---|---|
| VPA | CBZ | |||
| (%) | P Values | (%) | P Values | |
| β-catenin | ||||
| Control | 98.46 | 0.0001 > | 98.99 | 0.0001 > |
| IC50 | 62.7 | 0.0001 > | 57.73 | 0.0001 > |
| 2IC50 | 53.86 | 0.0001 > | 52.18 | 0.0001 > |
| VEGF | ||||
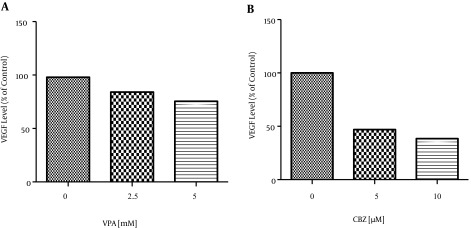
| Control | 97.97 | 0.0001 > | 100 | 0.0001 > |
| IC50 | 84.09 | 0.0001 > | 46.93 | 0.0001 > |
| 2IC50 | 75.38 | 0.0001 > | 38.40 | 0.0001 > |
4.3. VEGF Level Results
Certain HDACIs mediate their antitumor activity by acting on cell cycle development and survival, affecting tumor angiogenesis via the reduction of hypoxia inducible factor (HIF)-1 and VEGF expression (13, 14). The VEGF levels in SW480 cells treated for 72 hours with 2.5 and 5 mM of VPA decreased by about 13.88% and 22.59%, respectively, and those treated for 48 hours with 5 and 10 μM of CBZ decreased by about 53.07% and 61.6%, respectively compared to levels in the control group (Figure 3). According to one-way ANOVA, the reductions were significant (P < 0.0001) (Table 2).
Figure 3. This Diagram Shows that VEGF Levels After Treatment by VPA and CBZ Significantly Differ from Those of Controls (P < 0.0001).
5. Discussion
HDACIs are a new class of drugs known to have anticancer activity in hematological and solid malignancies (4, 15). Published data suggest a wide range in the number of genes disordered in response to HDACI treatment, namely between 1 and 22%. This depends on factors such as class of compound, dosage, incubation time, and choice of cell lines (16-18). There are now so many HDACIs available, with such different chemical structures and biological and biochemical properties, that we can be hopeful that at least a few of them will succeed, probably in combination with other therapies. A well-known anticonvulsant recently discovered to have impressive HDACI activity is VPA, which is commonly used in the treatment of seizures and bipolar disorders (19, 20). It is well tolerated in patients that have an accepted safety characterization (21, 22). Some antiepileptic drugs, such as VPA and CBZ, are recognized as having HDAC inhibition properties, which can modify aberrantly-silenced gene expression by an epigenetic mechanism (23). CBZ-induced acetylation of histone H4 in the HepG2 liver carcinoma cell lines and inhibited HDAC 3 and HDAC 7 (7). This is the first study to explain the low micromolar potency of CBZ in human colon cancer cells. We believe that an indirect comparison to other HDACIs is important. Our results indicate that in comparison with other HDACIs, such as VPA, CBZ had greater potency and required only 5 μM to achieve an IC50 in the SW480 cells. Other HDACIs, such as VPA, required millimolar concentrations in order to achieve an IC50 in the SW480 cell lines. This high concentration of VPA resulted in the dose-limiting neurotoxicity studied in the clinical trials (24). VPA had a similar effect on the cell cycle distribution compared with other HDACIs, such as trichostatin A, sodium butyrate, and SAHA (25-27). All these agents have been reported to decrease S-phase and G2-M phase cells and increase the collection of G0-G1 phase cells after treatment. We found that 72-hour VPA and 48-hour CBZ acute treatments induce a dose-dependent effect, with maximal induction for a 5.0 mM dose at 72 hours for VPA and a 10 μM dose at 48 hours for CBZ. SW480 cells culture treated with VPA required a 24 hour longer drug response time than those treated with CBZ.
We then detected the effects of VPA on colon cancer cell viability in vitro. We found that 72- hour treatment with VPA and 48-hour treatment with CBZ resulted in a dose-dependent decrease in the cell viability of the SW480 cell lines (Figure 1). Hence, we examined the effects of VPA on β-catenin protein levels using the ELISA in the SW480 cells. Both 5 μM of CBZ and 2.5 mM of VPA decreased β-catenin protein levels. However, the rate of β-catenin decrease differed for each drug. CBZ caused β-catenin to decline within 48 hours of treatment, whereas the effect of VPA was not evident until 72 hours after treatment (Figure 2). Therefore, identification of the relative levels of the Wnt signaling elements in human tumor tissue may help to determine whether up-regulation or down-regulation of Wnt activity is an efficient therapeutic strategy for use with HDACIs. VPA has been shown to inhibit proliferation and induce differentiation of cell lines, and it may well find broader clinical use in treatment of other types of cancer. Whether this occurs through hyperacetylation and activation of P53 or through regulation of other HDAC targets is a subject for future study.
In the present study, for the first time, we showed that CBZ treatment significantly inhibited human SW480 colon cancer cell growth. Wnt/β-catenin signaling is involved in cancer and in many other diseases. Deregulation of Wnt/β-catenin signaling has been associated with the pathogenesis of many kinds of human cancers. The obstruction of Wnt/β-catenin signaling represents a new direction in developing novel drugs for cancer therapy (28).
Treatments of SW480 cells with 5 μM of CBZ and 2.5 mM of VPA decreased the β-catenin protein levels by 41.26% and 35.76%, respectively. The HDACIs’ antitumor and antiangiogenic effects were correlated with the down-regulation of angiogenesis-related genes, such as HIF and VEGF. HDACIs regulate the expression of multiple genes that play an important role in tumor progression and angiogenesis (29, 30). VEGF induces endothelial cell proliferation and migration. We have shown here that VPA and CBZ decreased the hypoxia-mediated induction of VEGF secretion in the SW480 cell lines. Treatment of SW480 cells with 5 μM of CBZ and 2.5 mM of VPA decreased the VEGF protein levels by 53.07% and 13.88%, respectively. For the first time, we showed that CBZ is a potent inhibitor of SW480 colon cancer cell growth. It induced cell death in the SW480 human colon cancer cells, which are regulated by the β-catenin signaling pathway. Our study also showed a possible antiangiogenic action of HDACIs in SW480 cell lines. The effects of HDACIs in decreasing VEGF levels thus supports their anticancer activity. Prior studies have shown that VPA can be used in the treatment of cancer patients. Later clinical trials with VPA reported neurotoxicity in some patients, which may limit its use. Thus, research to find medicines that do not have such side effects and can replace VPA clinically is very important. We observed that CBZ is a good alternative and can replace VPA. The only remaining task is to determine its effectiveness in cancer patients through future studies.
Acknowledgments
We would like to thank the Pharmacology Laboratory of Shahid Baheshti University.
Footnotes
Authors’ Contribution:All authors contributed to design of the study, analysis, interpretation of the data, and drafting of the article.
Funding/Support:This study was funded and supported in part by the Shahid Baheshti University of Medical Sciences, Grant No.177-93/4/16. It was also part of a Ph.D. thesis supported by the Shahid Baheshti University of Medical Sciences..
References
- 1.McNamara JO. Drugs effective in the therapy of the epilepsies. 12 ed. New York: McGraw-Hill; 2011. pp. 583–607. [Google Scholar]
- 2.Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13(6):477–83. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Kramer OH, Gottlicher M, Heinzel T. Histone deacetylase as a therapeutic target. Trends Endocrinol Metab. 2001;12(7):294–300. doi: 10.1016/s1043-2760(01)00438-6. [DOI] [PubMed] [Google Scholar]
- 4.Sandor V. Phase I trial of the histone deacetylase inhibitor, depsipeptide in refractory neoplasms. Cancer Res. 2002;8(3):718–28. [PubMed] [Google Scholar]
- 5.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 6.Blaheta RA, Michaelis M, Driever PH, Cinatl J. Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev. 2005;25(4):383–97. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- 7.Beutler AS, Li S, Nicol R, Walsh MJ. Carbamazepine is an inhibitor of histone deacetylases. Life Sci. 2005;76(26):3107–15. doi: 10.1016/j.lfs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323–38. doi: 10.1016/j.clinbiochem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Xu R, Zhou B, Fung PC, Li X. Recent advances in the treatment of colon cancer. Histol Histopathol. 2006;21(8):867–72. doi: 10.14670/HH-21.867. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Khalil S, Tan GA, Giri DD, Zhou XK, Howe LR. Activation status of Wnt/ss-catenin signaling in normal and neoplastic breast tissues: relationship to HER2/neu expression in human and mouse. PLoS One. 2012;7(3):33421. doi: 10.1371/journal.pone.0033421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26(37):5420–32. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 14.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 15.Takai N, Desmond JC, Kumagai T, Gui D, Said JW, Whittaker S, et al. Histone deacetylase inhibitors have a profound antigrowth activity in endometrial cancer cells. Clin Cancer Res. 2004;10(3):1141–9. doi: 10.1158/1078-0432.ccr-03-0100. [DOI] [PubMed] [Google Scholar]
- 16.Glaser KB. Gene expression histone deacetylase inhibitors in T24 and MDA cell lines. Cancer Ther. 2003;2(2):151–63. [PubMed] [Google Scholar]
- 17.Gray SG, Qian CN, Furge K, Guo X, Teh BT. Microarray profiling of the effects of histone deacetylase inhibitors on gene expression in cancer cell lines. Int J Oncol. 2004;24(4):773–95. doi: 10.3892/ijo.24.4.773. [DOI] [PubMed] [Google Scholar]
- 18.de Ruijter AJ, Meinsma RJ, Bosma P, Kemp S, Caron HN, van Kuilenburg AB. Gene expression profiling in response to the histone deacetylase inhibitor BL1521 in neuroblastoma. Exp Cell Res. 2005;309(2):451–67. doi: 10.1016/j.yexcr.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276(39):36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669–94. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 21.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20(24):6969–78. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaheta RA, Cinatl J. Anti-tumor mechanisms of valproate: a novel role for an old drug. Med Res Rev. 2002;22(5):492–511. doi: 10.1002/med.10017. [DOI] [PubMed] [Google Scholar]
- 23.Stettner M, Kramer G, Strauss A, Kvitkina T, Ohle S, Kieseier BC, et al. Long-term antiepileptic treatment with histone deacetylase inhibitors may reduce the risk of prostate cancer. Eur J Cancer Prev. 2012;21(1):55–64. doi: 10.1097/CEJ.0b013e32834a7e6f. [DOI] [PubMed] [Google Scholar]
- 24.Hoti N, Chowdhury W, Hsieh JT, Sachs MD, Lupold SE, Rodriguez R. Valproic acid, a histone deacetylase inhibitor, is an antagonist for oncolytic adenoviral gene therapy. Mol Ther. 2006;14(6):768–78. doi: 10.1016/j.ymthe.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Kutko MC. Histone deacetylase 8 inhibitors induce growth suppression in human rhabdomyosarcoma in vitro. Cancer Res. 2003;9(15):5749–55. [PubMed] [Google Scholar]
- 26.Sawa H, Murakami H, Ohshima Y, Sugino T, Nakajyo T, Kisanuki T, et al. Histone deacetylase inhibitors such as sodium butyrate and trichostatin A induce apoptosis through an increase of the bcl-2-related protein Bad. Brain Tumor Pathol. 2001;18(2):109–14. doi: 10.1007/BF02479423. [DOI] [PubMed] [Google Scholar]
- 27.Li GC, Zhang X, Pan TJ, Chen Z, Ye ZQ. Histone deacetylase inhibitor trichostatin A inhibits the growth of bladder cancer cells through induction of p21WAF1 and G1 cell cycle arrest. Int J Urol. 2006;13(5):581–6. doi: 10.1111/j.1442-2042.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 28.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287(5458):1606–9. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 29.Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21(3):427–36. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 30.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7(4):437–43. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]