Abstract
Background
A lack of neurotrophic support is believed to contribute to the development of diabetic neuropathy. On the other hand, neurotrophins have consistently been shown to increase in the central and peripheral nervous system following exercise, but the effects of exercise intervention on brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in diabetic neuropathy are not understood.
Objectives
This experimental study was designed and carried out at the Tarbiat Modares university (TMU) in Tehran, Iran, to investigate the hypothesis that increased activity as endurance training can help to increase the endogenous expression of neurotrophins in diabetic rats.
Methods
This was an experimental study with 2 × 2 factorial plans performed at TMU in Iran. Sampling was accidental and 28 adult male Wistar rats in the body mass range of 326.3 ± 8.4 g comprised the sample, with each rat randomly assigned to four groups: diabetic control (DC), diabetic training (DT), healthy control (HC), and healthy training (HT). To induce diabetic neuropathy, after 12 hours of food deprivation, an intraperitoneal injection of streptozotocin (STZ) solution (45 mg/Kg) method was used. Two weeks after STZ injection, the endurance training protocol was performed for 6 weeks; 24 hours after the last training session, the rats were sacrificed. Real-time PCR was used for BDNF and NGF expression.
Results
The data indicate that diabetes decreases BDNF and NGF expression in sensory (92%, P = 0.01; 90%, P = 0.038, respectively) and motor (93%, P = 0.05; 60%, P = 0.029, respectively) roots. However, NGF mRNA levels in the DT group were significantly higher than in the HC group ((7.1-fold), P = 0.01; (2.2-fold), P = 0.001, respectively, for sensory and motor roots), but this was not shown for BDNF. In addition, endurance training can increase NGF expression in healthy rats ((7.4-fold), P = 0.01; (3.8-fold), P = 0.001, respectively, for sensory and motor roots).
Conclusions
This study shows that BDNF and NGF expression decreases in diabetic neuropathy. However, this decrease can be reversed through endurance training. These results also indicate that endurance training may have a potential role in compensating for neurotrophin deficiency following diabetic neuropathy.
Keywords: Diabetic Neuropathy, Brain-Derived Neurotrophic Factor (BDNF), Nerve Growth Factor (NGF), Endurance Training
1. Background
Diabetic neuropathy is a descriptive term that includes a spectrum of clinical syndromes and their subsets and also includes peripheral nerve dysfunction in patients with diabetes mellitus after excluding other causes of neuropathy (1). This disease features structural changes in the peripheral nerves, including axonal atrophy, demyelination, the loss of nerve fibers, and the slow regeneration of nerve fibers; it can also lead to symptoms such as pain and the loss of sensation (2).
The etiology of diabetic neuropathy is unclear; however, the accumulation of the final products of glycation, vascular dysfunction, oxidative stress, and changes in neurotrophic support are considered important factors in the genesis of this disease (3). In diabetes, neurotrophin production and its supportive role is reduced; hence, the lack and deficiency of neurotrophic factors and their receptors are involved in the progression of diabetic neuropathy, and it can partially cause the dysfunction of axons and be a factor in the pathogenesis of diabetic neuropathy (4). Atrophy and even neuron death can occur in diabetic neuropathy due to the loss of growth factors (5). In different animal studies, a decreased level of mRNA was seen in diabetic muscle related to nerve growth factor (NGF) and neurotrophin-3 (NT-3) (6, 7). Nevertheless, an increase in brain-derived neurotrophic factor (BDNF) mRNA levels was reported (6, 7). NGF is necessary for the growth and maintenance of neuron phenotypes in the peripheral nervous system and for the functional integrity of the cholinergic neurons in the central nervous system (8). NGF applies its effects through retrograde transport with tyrosine kinase A (TrkA), a high-affinity receptor, and p75, a low affinity receptor (9). Hence, peripheral neuropathies can be associated with a lack of regulation in either the synthesis, transport, or use of NGF in PNS neurons (8). NGF administration in animal models with diabetic neuropathy has protective effects on the neurons of the peripheral nervous system, and it alleviates their neuropathic symptoms, but the use of exogenous NGF (originating outside the body) has side effects such as muscle pain and hyperalgesia (increased pain sensitivity) (8) and also carries some restrictions on its exogenous use (10). BDNF, as another member of the neurotrophin family, is also involved in the neuroplasticity, differentiation, and survival of the central nervous system. It has also been shown that the reduction of BDNF serum levels as a result of diabetes is associated with cognitive disorders (11). Thus, it seems important to consider this neurotrophin. Although many studies have shown that BDNF can promote the regeneration of axons and the reconnection of injured nerve fibers, it also has limitations. The neurotrophin has varying effects on neuronal populations (12), and the fact that there are adverse effects related to the optimal health function of BDNF is a challenge in its medical use (13, 14). It also modifies the expression of important molecules such as synapsin, which is very important for synaptic transmission.
Much of what is currently known about the trophic support has been understood through the exogenous use of neurotrophin proteins. Although they have potential impacts on the characteristics of motor neurons (15) and muscle (16), whether the neurotrophins used as exogenous are placed at the good physiological range of expressing these proteins or not, is unclear. Therefore, a model that raises endogenous neurotrophins may provide a good understanding of their function in the nervous system. As in this way, the possibility to express the neurotrophin in their physiological location is more than their exogenous (17, 18). Exercise training is a model that can affect neurotrophin expression in the proper physiological range. The increased activity of exercise training increases the regeneration of sensory neurons and gene expression that stimulates the proteins required for growth and for the regeneration of axons (19) after injury. Previous studies have shown that increased physical activity can increase the trophic support in the peripheral nerves and their target tissues. For example, a huge increase in BDNF expression throughout the nervous system has frequently been seen in animals, according to the mileage covered, following wheel running (20, 21). Several studies have also shown that NGF in various segments of the nervous and muscular system increases as a result of exercise training (9, 22).
Thus, we expect that the expression of BDNF and NGF will be regulated positively with increased mobility and physical activity and will be regulated negatively with the induction of diabetes. The present study examines the hypothesis that the increased activity of endurance training can help to increase the endogenous expression of neurotrophins in diabetic rats.
2. Objectives
The aim of this study is to investigate the effects of increased activity as endurance training on the gene expression of neurotrophins (BDNF, NGF) at the sensory and motor roots of the sciatic nerve in diabetic rats.
3. Methods
3.1. Experimental Design
This study was designed and carried out at the Tarbiat Modares university (TMU) in Tehran, Iran (2013). In this experimental study, 28 male Wistar rats were randomly divided (simple randomization) into four groups (with seven rats in each group). One of these groups was randomly selected as the control (healthy control (HC)), and one of other groups was randomly selected as healthy training (HT). Type 2 diabetes mellitus was induced in the other two groups with the administration of 45 mg/kg of body weight streptozotocin (STZ) dissolved in fresh citrate buffer. One of the diabetic rat groups was selected randomly as the diabetic control (DC), and the other group’s rats were treated with endurance training (diabetic training (DT)). Throughout the treatment period, the rats were maintained in single cages. The studied groups were as follows:
- Group 1: diabetic control rats (HT): received water and feed.
- Group 2: diabetic training (HT): received water and feed and performed resistance training for six weeks.
- Group 3: diabetic control rats (DC): received water and feed.
- Group 4: diabetic training (DT): received water and feed and performed resistance training for six weeks.
3.2. Animals and Maintenance Conditions
Twenty-eight Wistar rats (200 - 250 g, 10 mo), were purchased from Pasture institute (Tehran, Iran) and maintained in the animal house, School of Medical Sciences of Tarbiat Modares University (TMU). All the animals were maintained in controlled environmental conditions with an average temperature of 22 ± 3°C, a light-dark cycle of 12: 12 hours, and the free access to specific foods and water. After two weeks of familiarization and adjustment of the animals to the new environment and achieving the optimal weight of 326.3 ± 8.4 g (23), the rats were randomly divided into four groups of seven: healthy control (HC), healthy training (HT), diabetes control (DC), and diabetes training (DT). During the familiarization, in order to adapt to the laboratory conditions, treadmills, and manipulation, the animals walked on a treadmill at a speed of 10 meters per minute for 10 - 15 minutes, 5 days per week. All experiments were performed in accordance with the guide for the care and use of laboratory animals (national institutes of health publication No. 85-23, revised 1985) and were approved by the research and ethics committee of Tarbiat Modares University (TMU). The committee’s guidelines for care and use of laboratory animals were also followed.
3.3. Induction of Diabetes by STZ
Diabetes was induced in the appropriate rats after 12 hours of food deprivation, with an intraperitoneal injection of STZ solution (Sigma, St. Louis, MO; 45 mg / Kg dissolved in fresh citrate buffer 0.5 mol/L, PH: 4.5). The nondiabetic rats were also injected with an equivalent volume of citrate buffer. Forty-eight hours after injection, with a small injury by lancet on the tail vein of the rats, a drop of blood was placed on a glucometer strip, and the strip was read by a glucometer (Glucotrend 2, Roche company of Germany). The rats with blood glucose greater than 300 mg/dL entered the present study as the diabetic rats (23). It should be noted that in this study, after the injection of STZ, no symptoms caused by wrong injection, such as abdominal swelling or digestive problems, were observed in the animals. Two weeks after the induction of diabetes, the endurance training protocol was carried out for six weeks. Then all training sessions were held at the end of the sleep cycle of the animals between the hours 4:00 - 6:00 p.m.
3.4. Training Program
The present study used a moderate training intensity (55% - 50% of maximal oxygen consumption), yet one that was efficient in terms of physiology (9). The training groups were exposed to the treadmill training at moderate intensity for six weeks for five days per week. The speed and duration of the treadmill training gradually rose and increased at the following rate:
- 10 meters per minute for 10 minutes in the first week;
- 10 meters per minute for 20 minutes in the second week;
- 14 - 15 meters per minute for 20 minutes in the third week;
- 14 - 15 meters per minute for 30 minutes in the fourth week; and
- 17 - 18 meters per minute for 30 minutes in the fifth week.
In order to achieve a steady state in produced adaptations, all training variables were kept constant at the end of training (9). Training shock was used during the endurance training program, and if necessary, the animals were forced to continue training using the hand or sound stimulus on the cover of the treadmill.
3.5. Behavioral Tests for Mechanical Allodynia and Thermal Hyperalgesia
Thermal hyperalgesia was measured using the method of Hargreaves and et al., with little change (24). In other words, using a radiant heat plantar test (Ugo Bassil, Italy), the animals were placed in a Plexiglas chamber (22 cm length × 22 cm width and 13.3 cm height) on a clean Plexiglas sheet. After 30 minutes of the animal’s adaptation to the new environment, the middle segment of the animal’s paw was exposed to constant thermal radiation with the displacement of the moving source of thermal radiation. After the thermal radiation, the timer was activated, and by foot dragging, the light was cut off and the timer stopped. By recording the paw withdrawal latency (PWL), the damage tolerance to thermal stimuli was measured. Each paw was tested alternately, three times at intervals of 5 to 10 minutes, and the average of the measurements was recorded as the thermal pain threshold. Also, to prevent tissue damage, the test cutoff was considered to be 22 seconds. Finally, the thermal hyperalgesia was calculated as the percent of maximum possible effects, using the following formula:
%MPE = ((base delay -delay after Streptomycin injection) × 100)/ (base delay-time cut off))
In addition, the average of three initial measurements was considered as a base delay.
In order to measure the mechanical allodynia, the animals were also put on a wired network inside a Plexiglas chamber with dimensions of 20 × 20 cm and a height of 30 cm. To allow the animals get used to the new environment, they were put inside the transparent chamber on the mesh 30 minutes before the test. To assess the mechanical allodynia, various Von Frey fibers in the range of 2 g to 60 g (2, 4, 6, 8, 15, 26, 60), made by the Stolting company, USA, were used to measure the sensitivity of the skin to contact stimulation. Each test began with the lowest weight, and in cases where no response was recorded, fibers with a higher weight were used. If two consecutive responses (lifting the leg by the animal) were observed, the same weight was considered as the paw withdrawal threshold (PWT), and the test did not continue. If the animal did not respond to any of the fibers, including the fiber number 60, that fiber was considered as a threshold response. Each experiment was repeated three times at a frequency of at least three minutes, and the mean was calculated as the paw withdrawal threshold (25). In general, the measurement of mechanical allodynia and thermal hyperalgesia was performed both before the STZ injection and 14 days after the injection.
3.6. Fasting Blood Glucose
The initial and final fasting blood glucose (FBG) levels of all groups were recorded after six weeks. Then in a fasting condition, the animals were anesthetized by using ketamine (75 mg/kg bw) and xylazine (10 mg/kg bw) IP. The blood samples were collected by cardiac puncture, and the serum was separated immediately. FBG was measured enzymatically using commercial kits (Pars Azemoon, Tehran, Iran) with the aid of a spectrophotometer (JENWAY 6505 European Union).
3.7. Tissue Extraction
At the end of the six-weeks of training program, 12 hours after the last training session and after the anesthetization, the sensory and motor segment of spinal segments L4, L5, and L6 were isolated under sterile conditions. The desired tissue was immediately frozen in liquid nitrogen, and the sample was kept at 80°C until the molecular analysis was performed.
3.8. RNA Extraction and cDNA Synthesis
Approximately 50 mg of spinal cord tissue was separately homogenized in QIAzol Lysis reagent for a total RNA extraction at a ratio of 1 to 10. To remove the protein components, the obtained product was centrifuged at 4°C, 10 minutes, 12,000 g. Then it was mixed with chloroform at a ratio 1 to 0.5 and was severely shaken for 15 seconds. The product was centrifuged at 4°C, 15 minutes, 12,000 g, and the mineral and water components were separated. After that, the RNA content was removed and mixed with isopropanol at a ratio of 1 to 0.5 and was centrifuged for 10 minutes at room temperature and then at 4°C, 10 minutes, 12,000 g. A pellet containing RNA was rinsed in ethanol and dissolved in 20 μL of RNAS-free water. The RNA concentration was measured (Eppendorff, Germany), and the ratio of 260 to 280 was defined as the optimal purification between 1.8 and 2. CDNA was synthesized by using 1 μg of RNA, random hexamer primer, and the M-Mulv Reverse Transcriptase enzyme (PrimeScript RT Reagent Kit, Takara). All the processes of cDNA synthesis were executed according to kit protocol and company manuals.
3.9. Real-Time PCR
The expression levels of mRNA NGF and BDNF were measured using the quantitative method of Real-Time PCR and Premix SYBR Green II (USA Applied Biosystems). The reaction of the mixture was performed in a final volume of 20 μL, each reaction performed in duplicate. Primers were designed based on information from the NGF, BDNF, and GAPDH genes from NBCI GenBank (Macrogen Inc., Seoul, Korea). The sequences of the primers used are reported in Table 1 (GAPDH was used as a control gene). The temperature used for Real-Time PCR was 95 for 10 minutes, 95 for 15 seconds, 60 for 1 minute (repeat of 40 cycles). The expression of the desired genes was measured by the 2 -ΔΔCT method. All the instruments used were calibrated via standard protocols, and all gene expression processes were performed in the genetics laboratory of Tarbiat Modares university.
Table 1. The Sequences of Primers Used in the Present Study.
| Primer Sequence | GenBank |
|---|---|
| BDNF | NM_012513.3 |
| For: 5′- CGACGTCCCTGGCTGACACTTTT -3′ | |
| Rev: 5′- GTAAGGGCCCGAACATACGATTGG-3′ | |
| NGF | NM_001277055.1 |
| For: 5′- CAC CTC TTC GGA CAC TCT GGA -3′ | |
| Rev: 5′- CGT GGC TGT GGT CTT ATC TCC -3′ | |
| GAPDH | NM_017008 |
| For: 5′- GACATGCCGCCTGGAGAAAC -3′ | |
| Rev: 5′- AGCCCAGGATGCCCTTTAGT -3′ |
3.10. Statistical Analysis
All the data have been described based on the average ± standard deviation. The Kolmogorov-Smirnov test was used to determine the normality of the data distribution, and Levene’s test was used to measure the equality of variances. All data were normal, and given that the present study was a 2 × 2 factorial plan, the two-way ANOVA test and post hoc LSD test were used to determine the significant differences between groups and the interaction of the variables. We also used a repeated measures test to analyze the simultaneous effects of time and groups on blood glucose and body mass, because these variables were measured twice (pre and post). The significance level was considered P ≥ 0.05. All the statistical analyses were performed using SPSS version 20.
4. Results
4.1. Body Mass
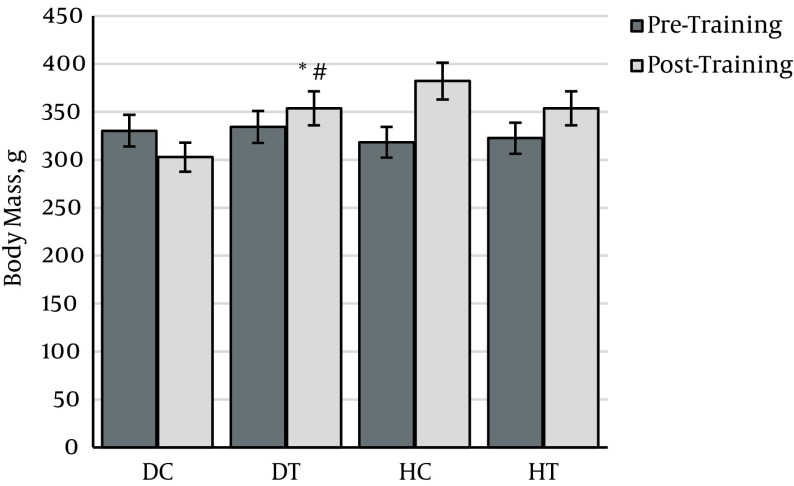
The results related to changes in body mass over six weeks for each group are shown in Figure 1. The results show that six weeks of diabetes caused more significant weight loss in this group than the pre-test (P < 0.01), indicating that atrophy was caused by diabetes. There is also a significant difference between DT and DC (P < 0.01) and between HC and HT (P < 0.01), indicating that although the endurance training could reduce weight loss due to diabetes, it failed to fully compensate for it.
Figure 1. Changes in body mass in different groups.
* Significant difference with healthy untrained group (P < 0.01), # significant difference with normal trained group (P < 0.01), † significant difference with diabetes untrained group (P < 0.01).
4.2. Blood Glucose
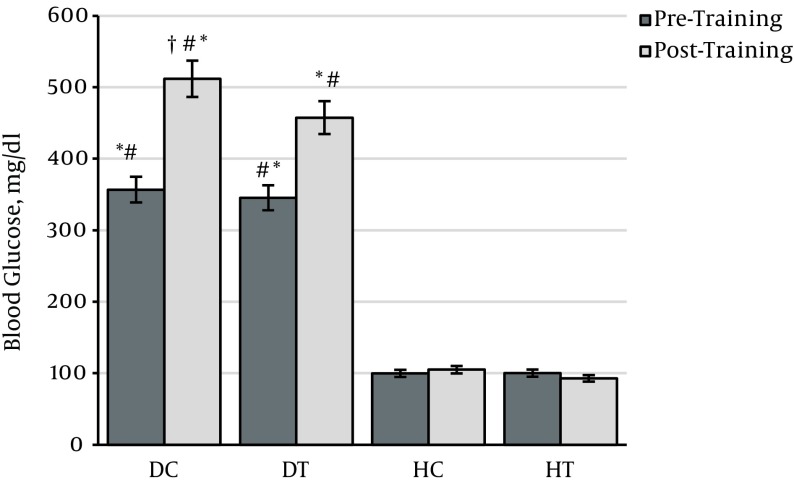
The data on blood glucose before and after the test in the four groups shows that at the beginning of the training program, the DC glucose concentration was significantly higher than that of the healthy groups (P = 0.0001) and that it still showed a significant difference after six weeks of endurance training (P = 0.0001). At the end of the training program, the DT glucose concentration was significantly lower than the DC (P = 0.0001), indicating that six weeks of endurance training could reduce the blood glucose in diabetic rats (Table 2).
Table 2. Changes in Body Mass and Blood Glucose in Different Groupsa.
| Groups | Fasting Blood Glucose, mg/dL | Body Mass, g | ||
|---|---|---|---|---|
| Week 0 | Week 6 | Week 0 | Week 6 | |
| Healthy control (HC) | 99.71 ± 91 | 105.4 ± 12 | 318.23 ± 17 | 382.14 ± 24 |
| Healthy training (HT) | 100.2 ± 13 | 92.85 ± 73 | 353.69 ± 23 | 322.57 ± 28b |
| Diabetic control (DC) | 356.71 ± 35 | 511.85 ± 25b | 330.28 ± 40 | 302.85 ± 25c |
| Diabetic training(DT) | 354.74 ± 38 | 457.42 ± 27b,d | 334.28 ± 33 | 353.69 ± 27d |
aEach value is the mean ± SD of the seven rats in each group.
bBody mass, in comparison with healthy control rats (P < 0.01); FBS, in comparison with healthy control rats (P = 0.0001).
cBody mass, diabetic control group, was compared before and after 6 weeks (P < 0.01); FBS, diabetic control group compared with all other groups after day 42 (P = 0.0001).
dBody mass, in comparison with healthy control rats (P < 0.01); FBS, in comparison with healthy control rats (P = 0.0001).
Figure 2. Changes in plasma glucose levels in different groups.
* Significant difference with healthy untrained group (P < 0.01), # significant difference with healthy trained group (P < 0.01), † significant difference with the diabetes untrained group (P < 0.01).
4.3. Sensitivity to Mechanical and Thermal Stimulation
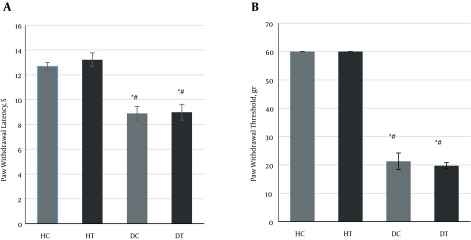
The average latency of the paw withdrawal test in hyperalgesia heat and the changes in the paw withdrawal threshold in the mechanical allodynia test (Table 3) before starting the training program (two weeks after the diabetes induction) in the diabetic groups was significantly lower than that in the healthy groups P ≥ 0.0001).
Table 3. Changes in Latency of Paw Withdrawal in Thermal Hyperalgesia Test (Second) and Paw Withdrawal Threshold in Mechanical Allodynia Test (Gram) in Different Groupsa.
| Groups | Paw Withdrawal Latency (s) | Paw Withdrawal Threshold (g) |
|---|---|---|
| Healthy control (HC) | 12.76 ± 0.22 | 60 ± 0 |
| Healthy training (HT) | 13.2 ± 0.53 | 60 ± 0 |
| Diabetic control (DC) | 8.8 ± 0.55b,c | 22 ± 4b,c |
| Diabetic training(DT) | 9.1 ± 0.73b,c | 20 ± 2b,c |
aEach value is the mean ± SD of the seven rats in each group.
bP < 0.001, in comparison with healthy control rats.
cP < 0.001, in comparison with healthy trained rats.
Figure 3. Changes in A, latency of paw withdrawal in thermal hyperalgesia test (seconds); and B, paw withdrawal threshold in mechanical allodynia test (grams) in different groups. * Significant difference with healthy control group (P < 0.001), # significant difference with healthy trained group (P < 0.001).
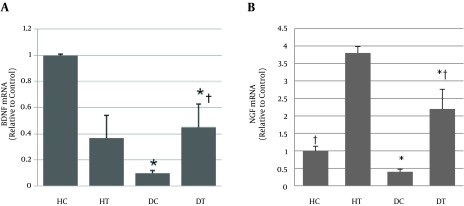
4.4. Gene Expression Levels of BDNF and NGF in Sensory Segment of Spine
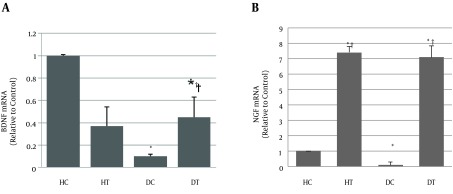
Results of a two-way ANOVA test showed that there was a significant difference between the healthy control group and the diabetes control group for BDNF mRNA (P = 0.01). This means that diabetes significantly decreased the BDNF gene expression in the sensory roots of the sciatic nerve (-0.94-fold). Moreover, no significant difference was seen between the healthy control group and the healthy trained group. In other words, the endurance training did not cause changes in the gene expression of BDNF (P = 0.464) (0.50-fold), although the healthy trained group showed a tendency of a nonsignificant decrease. Nonetheless, the results showed that there was no significant difference between the values of BDNF mRNA between the diabetic trained group and the healthy control group (P = 0.07) (-0.64-fold). In other words, six-week endurance training could compensate for the reduction of BDNF expression caused by diabetes (0.30-fold); however, its absolute values were still lower than those of the healthy control group (Table 4).
Table 4. Values of Gene Expression BDNF and NGF in Sensory and Motor Root of Sciatic Nerve Spinal Segments in Different Groupsa.
| Groups | BDNF mRNA (Relative to Control) | NGF mRNA (Relative to Control) | ||
|---|---|---|---|---|
| Sensory Roots | Motor Roots | Sensory Roots | Motor Roots | |
| Healthy control (HC) | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| Healthy training (HT) | 0.50 ± 0.18 | 0.37 ± 0.17 | 7.4 ± 31b,c | 3.8 ± 0.29b,c |
| Diabetic control (DC) | 0.06 ± 0.03b | 0.1 ± 0.02b | 0.1 ± 0.12b | 0.4 ± 0.09b |
| Diabetic training(DT) | 0.36 ± 0.18c | 0.45 ± 0.18b | 7.1 ± 0.85b,c | 2.2 ± 0.65b,c |
aEach value is the mean ± SD of the seven rats in each group.
bP ≤ 0.05, in comparison with healthy control rats.
cP < 0.05, in comparison with diabetic control rats.
Also, NGF mRNA levels in the DC group were significantly lower than in the HC group (P = 0.038) (-0.90-fold). In contrast to BDNF, the endurance training significantly increased the NGF mRNA in the healthy trained group compared with those of the HC group (P = 0.01) (7.4-fold). The NGF mRNA levels in the DT group were significantly higher than those in the HC group (p = 0.01) (2.2-fold change). Although the NGF mRNA level in HT was higher than in DT, this difference was not significant (P = 0.73), (1.6-fold), (Table 4).
Figure 4. The Values of Gene Expression.
A, BDNF and B, NGF in the sensory root of sciatic nerve spinal segments in research groups. * Significant difference with healthy control group (P < 0/05), # significant difference with diabetic group (P < 0/01).
4.5. Gene Expression Levels of BDNF and NGF in Spinal Motor Segment
The results showed a significant difference for BDNF mRNA between the healthy control group and the diabetic control group (P = 0.05) (-0.90-fold). In other words, diabetes significantly decreased the BDNF gene expression in the motor roots of the sciatic nerve. Nor did the endurance training alone cause changes in the gene expression of BDNF (P = 0.299) (-0.63-fold). Nevertheless, these results suggest that the BDNF mRNA levels in the diabetic trained group were higher than those in the diabetic control group (0.35-fold) but that they were significantly lower compared to the healthy control group (P = 0.01) (-0.65-fold). In other words, six weeks of training could compensate for the reduction of BDNF expression caused by diabetes but could not return it to its original level (Table 4).
In this regard, the NGF mRNA levels in the DC group were significantly lower than in the HC group (P = 0.029) (-0.60-fold). Additionally, the HT group showed higher levels of NGF mRNA compared to the HC group (P = 0.001) (3.8-fold). Similar to the sensory segment, the endurance training significantly increased the NGF mRNA levels in the DT group compared to the healthy rats (P = 0.001) (2.2-fold) (Table 4).
Figure 5. The Values of Gene Expression.
A, BDNF and B, NGF in the motor root of sciatic nerve spinal segments in research groups. * Significant difference with healthy control group (P < 0/001), # significant difference with diabetic group (P < 0.001).
5. Discussion
The aim of the present study was to investigate the effects of six weeks of endurance training on NGF and BDNF gene expression in the sensory and motor segments of spinal cord of rats with diabetes induced by STZ. With respect to the first part, namely, the effects of diabetes on body mass, the results showed that there was a significant difference between the diabetic group in pre- and post-test, while on the other hand, there was a significant difference between the healthy control group and diabetes in the body mass (Table 2). These results suggest that diabetes alone could reduce the weight of the animals that could be a sign of atrophy caused by diabetes. Nonetheless, when the values of the pre- and post-tests of the diabetic group were compared with each other, we did not see a significant difference between them. Thus we can conclude that endurance training may compensate for a part of the muscular atrophy caused by diabetes.
In addition, at the end of the training program, the blood glucose concentration of the DT group was significantly lower than that of the diabetic control group, indicating that six weeks of endurance training could reduce the plasma glucose in diabetic rats (Table 2). These results are consistent with the results of several other studies (26, 27). There are some possible mechanisms for this reduction, such as the increase of insulin sensitivity, the increase of glucose transporters (Glut4), and the increase of blood flow to skeletal muscles (26, 27).
In this study, to ensure the occurrence of diabetic neuropathy, the behavioral tests for mechanical allodynia and thermal hyperalgesia were used. The results showed that two weeks after the induction of diabetes, the sensitivity to Von Frey tactile and thermal stimuli significantly increased; these results support the occurrence of diabetic neuropathic pain. Pain is one of the clearest signs of neuropathy characterized by features such as hyperalgesia (increased response to a stimulus that is normally painful) and allodynia (response to a stimulus that normally does not cause pain) (28, 29), and the result is a hyperglycemia in diabetes status (30). The results showed that BDNF and NGF gene expression was reduced in diabetic rats compared to the healthy rats in the sensory and motor roots of the sciatic nerve; this confirms the hypothesis of reduced trophic support as one of the causes of diabetic neuropathy. BDNF does play an important role in the regeneration of injured peripheral axons (31), and there is some evidence that exercise increases BDNF expression in motor neurons but not in sensory neurons following peripheral nerve injury (32). Neurotrophins play an important role in the development, survival, differentiation, and function of neurons. Neurotrophins regulate the axonal and dendritic growth of neurons and nerve regeneration in response to the injury of undamaged nerve bases (33). In this regard, several studies have revealed the role of NGF neurotrophin in diabetic neuropathy. Findings from previous studies have shown that diabetes is associated with the reduced retrograde transport of NGF, less gene expression of NGF, and the receptors p75, and TrkA (9) in target tissues. In line with these findings, our results showed that NGF levels were significantly decreased in the DC group compared with the HC group. The role of NGF in the etiology of diabetic sensory neuropathy has been shown to be due to a defect in the retrograde transport of NGF in the sciatic nerve (34) and the intestines of diabetic rats. These studies have proven clear relationships between incomplete NGF expression in target tissues of the lower extremities and the retrograde transport of NGF decreased the dorsal root ganglion (6) and the expression of neuropeptide, calcitonin gene-related peptide and substance P which are the neuronal targets for NGF in type C fibers (35). In previous studies, it has been reported that NGF is the primary neurotrophin in the sensory nervous system, but this neurotrophin is probably produced through BDNF incremental adjustment (36), stimulating the production of VEGF (37). And since diabetes creates disorders in the nervous system myelin (38), it indirectly has major effects on the motor neurons.
In this study, the endurance training considerably increased NGF and BDNF gene expression in both parts of the spinal cord in the training group compared with the DC group. Nonetheless, in relation to BDNF, six weeks of endurance training could not bring the values to their initial levels; however, they could compensate for the reduced expression of BDNF caused by diabetes (Table 4). In contrast, one of the most interesting findings of this study is the incremental adjustment of NGF gene expression after training in both the sensory and motor parts of diabetic rats’ spinal cords compared with those of healthy rats (Table 4). The increased activity of endurance training can deal with the decreased NGF caused by diabetes and can even increase it to higher levels than those in healthy rats.
Researchers have reported that in a diabetic state, a defect in the synthesis of NGF can occur due to hyperglycemia or hypoinsulinemia or accumulated polyol. It changes in corticosterone concentration and 1, 25-dihydroxyvitamin D3 and antioxidant system failure. Corticosterone reduces NGF synthesis, whereas 1, 25-dihydroxyvitamin D3 increases it (5). In relation to the mechanisms of the effects of physical activity on the synthesis of NGF, training can increase the expression of NGF by affecting the above issues. For example, regular training can reduce the secretion of glucocorticoids (9) and can also strengthen the antioxidant capacity (39).
Results from previous studies have shown that BDNF expression is rapidly affected by physical activity; its levels significantly rise even after six hours of training in rats, and this increase is associated with an increase in the propagation of nerve cells and neurogenesis (40). In contrast, in rats that did high-intensity training, an inverse relationship was seen with the training intensity. The increase of neurotrophic factors indicate that there are limitations in neurogenesis arising from the training programs and from moderate-intensity training. Consequently, they lead to an increase in BDNF (41). Different studies have also shown a direct relationship between changes in the levels of NGF and training. For example, Chae et al. showed that six weeks of endurance training stopped the apoptosis of muscle cells through increased levels of NGF in the soleus muscle of rats with diabetes induced by STZ (9). Running on a treadmill and swimming also kept the levels of NGF in the hippocampus significantly higher than in the control group after four weeks of training, and it also stimulated neurogenesis (28). It has also been shown that voluntary training increased the TrkC and NT-3 gene and protein expression in the spinal cord and soleus muscle of healthy adult rats (42), and the reports suggest that it also increases the neural–muscular activity of BDNF. (43). Voluntary running on a treadmill also increases the expression of growth factors such as NT-3, BDNF, and GAP-43 in the spinal cord of injured rats. (44). However, Cobianchi et al. demonstrated that aerobic exercise training reduced both the levels of BDNF in the dorsal root ganglion and the neuropathic pain in rats following peripheral nerve injury (45). Similarly, Detloff et al. very recently showed that aerobic exercise can normalize spinal levels of glial cell-derived neurotrophic factor (GDNF), prevent excessive sprouting of pain afferents, and reduce tactile allodynia in rats following spinal cord injury (46).
Interestingly, the results showed that BDNF mRNA in the healthy trained groups decreased compared with the healthy control group. To interpret this contradiction with the reports presented above on the increased regulation of BDNF with training, the study of Gomez-Pinilla et al. should be mentioned. (47). They illustrated that there was an inequality between mRNA and BDNF protein in both the spinal cord and the soleus muscle after five days of running. Moreover, BDNF protein levels in the spinal cord were greater than mRNA levels, but this was the opposite in the muscle (47). Therefore, we assume that as a result of endurance training: 1, BDNF translation capacity increased; or 2, the retrograde transport of BDNF of skeletal muscles increased and supplied the spinal cord needs for BDNF; or 3, there was a combination of both. In addition, in relation to the possible mechanisms of increased BDNF, we can mention the increase of sensory input during the training (47), the increased activity of glucocorticoids and cholinergic systems (48), the increase of NGF (34), and the intracellular increase of Ca+ (49). However, nonmeasurement of BDNF protein levels can be considered a limitation and something to be measured in later studies.
In conclusion, this study showed that diabetes reduces the expression of BDNF and NGF in the sensory and motor roots of the sciatic nerve. Six weeks of training was able to compensate for the reduction of BDNF expression caused by diabetes, but it could not return the BDNF expression to its original level; in relation to NGF, however, endurance training could increase its gene expression to levels higher than the normal state. The results of this study clearly prove the hypothesis of reduced neurotrophic support in diabetic neuropathy. It would appear that the neurons are biologically compatible with the increase and decrease of activity and that such changes could help the survival of neurons.
5.1. Strong Points of Study
Given the considerable prevalence of diabetic neuropathy and the lack of effective treatment from a neurological point of view, considering neurotrophin activation via physical activity may lead to more suitable treatments. To the best of our knowledge, this study is the first to evaluate the relationship between physical activity and neurotrophin activation.
5.2. Weak Points of Study
Only gene expressions cannot demonstrate any physiological alterations. In the present study, the protein changes were not evaluated; therefore, protein measurement must also be evaluated, so that it leads to more valid conclusions.
Acknowledgments
The article was based on part of two MSc theses in exercise physiology (Ghazaleh Sorkhkamanzadeh and Amir-Bahador Dakhili), granted by the Physical education department, Tarbiat Modares university.
Footnotes
Authors’ Contribution:Proposal design, Rasoul Eslami; material preparation; Reza Gharakhanlou, Abdol Reza Kazemi; carrying out the Experiment, Amir Bahador Dakhili; data analysis: All authors; manuscript Preparation, Rasoul Eslami, Ghazaleh Sorkhkamanzadeh.
Financial Disclosure:The authors declare that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding/Support:Neuroscience Research Center, Institute of Neuropharmacology, and Kerman University of Medical Sciences, Kerman, IR Iran and Physical Education Department, Tarbiat Modares University.
References
- 1.Bril V. Treatments for diabetic neuropathy. J Peripher Nerv Syst. 2012;17 Suppl 2:22–7. doi: 10.1111/j.1529-8027.2012.00391.x. [DOI] [PubMed] [Google Scholar]
- 2.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–9. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9(1):36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 4.Boucek P. Advanced Diabetic Neuropathy: A Point of no Return? Rev Diabet Stud. 2006;3(3):143–50. doi: 10.1900/RDS.2006.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, et al. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69(4):229–85. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 6.Fernyhough P, Diemel LT, Tomlinson DR. Target tissue production and axonal transport of neurotrophin-3 are reduced in streptozotocin-diabetic rats. Diabetologia. 1998;41(3):300–6. doi: 10.1007/s001250050907. [DOI] [PubMed] [Google Scholar]
- 7.Fernyhough P, Diemel LT, Hardy J, Brewster WJ, Mohiuddin L, Tomlinson DR. Human recombinant nerve growth factor replaces deficient neurotrophic support in the diabetic rat. Eur J Neurosci. 1995;7(5):1107–10. doi: 10.1111/j.1460-9568.1995.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 8.Aloe L, Rocco ML, Bianchi P, Manni L. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chae CH, Jung SL, An SH, Jung CK, Nam SN, Kim HT. Treadmill exercise suppresses muscle cell apoptosis by increasing nerve growth factor levels and stimulating p-phosphatidylinositol 3-kinase activation in the soleus of diabetic rats. J Physiol Biochem. 2011;67(2):235–41. doi: 10.1007/s13105-010-0068-9. [DOI] [PubMed] [Google Scholar]
- 10.De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, et al. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci U S A. 2005;102(10):3811–6. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhen YF, Zhang J, Liu XY, Fang H, Tian LB, Zhou DH, et al. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology (Berl). 2013;227(1):93–100. doi: 10.1007/s00213-012-2942-3. [DOI] [PubMed] [Google Scholar]
- 12.Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148(2):475–94. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- 13.Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci. 2012;35(2):221–32. doi: 10.1111/j.1460-9568.2011.07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constandil L, Aguilera R, Goich M, Hernandez A, Alvarez P, Infante C, et al. Involvement of spinal cord BDNF in the generation and maintenance of chronic neuropathic pain in rats. Brain Res Bull. 2011;86(5-6):454–9. doi: 10.1016/j.brainresbull.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez M, Collins WF. Modulation of motoneuron excitability by brain-derived neurotrophic factor. J Neurophysiol. 1997;77(1):502–6. doi: 10.1152/jn.1997.77.1.502. [DOI] [PubMed] [Google Scholar]
- 16.Carrasco DI, English AW. Neurotrophin 4/5 is required for the normal development of the slow muscle fiber phenotype in the rat soleus. J Exp Biol. 2003;206(Pt 13):2191–200. doi: 10.1242/jeb.00412. [DOI] [PubMed] [Google Scholar]
- 17.Ogborn DI, Gardiner PF. Effects of exercise and muscle type on BDNF, NT-4/5, and TrKB expression in skeletal muscle. Muscle Nerve. 2010;41(3):385–91. doi: 10.1002/mus.21503. [DOI] [PubMed] [Google Scholar]
- 18.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–19. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 19.Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101(22):8473–8. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschenes MR, Tenny KA, Wilson MH. Increased and decreased activity elicits specific morphological adaptations of the neuromuscular junction. Neuroscience. 2006;137(4):1277–83. doi: 10.1016/j.neuroscience.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12(12):4171–80. [PubMed] [Google Scholar]
- 22.Chae CH, Lee HC, Jung SL, Kim TW, Kim JH, Kim NJ, et al. Swimming exercise increases the level of nerve growth factor and stimulates neurogenesis in adult rat hippocampus. Neuroscience. 2012;212:30–7. doi: 10.1016/j.neuroscience.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Calcutt NA. Modeling diabetic sensory neuropathy in rats. In: Luo ZD, editor. Pain Research. Vol. 99. USA: Humana Press; 2004. pp. 55–65. [DOI] [PubMed] [Google Scholar]
- 24.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 25.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57(3):375–82. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 26.Mostarda C, Rogow A, Silva IC, De La Fuente RN, Jorge L, Rodrigues B, et al. Benefits of exercise training in diabetic rats persist after three weeks of detraining. Auton Neurosci. 2009;145(1-2):11–6. doi: 10.1016/j.autneu.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56(3):836–48. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunha JM, Funez MI, Cunha FQ, Parada CA, Ferreira SH. Streptozotocin-induced mechanical hypernociception is not dependent on hyperglycemia. Braz J Med Biol Res. 2009;42(2):197–206. doi: 10.1590/s0100-879x2009000200008. [DOI] [PubMed] [Google Scholar]
- 29.Chen YW, Hsieh PL, Chen YC, Hung CH, Cheng JT. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth Analg. 2013;116(2):482–90. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]
- 30.Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68(11):1229–43. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, et al. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32(14):5002–9. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houle JD. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;1438:8–21. doi: 10.1016/j.brainres.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karamoysoyli E, Burnand RC, Tomlinson DR, Gardiner NJ. Neuritin mediates nerve growth factor-induced axonal regeneration and is deficient in experimental diabetic neuropathy. Diabetes. 2008;57(1):181–9. doi: 10.2337/db07-0895. [DOI] [PubMed] [Google Scholar]
- 34.Gold SM, Schulz KH, Hartmann S, Mladek M, Lang UE, Hellweg R, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. 2003;138(1-2):99–105. doi: 10.1016/s0165-5728(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 35.Apfel SC, Arezzo JC, Brownlee M, Federoff H, Kessler JA. Nerve growth factor administration protects against experimental diabetic sensory neuropathy. Brain Res. 1994;634(1):7–12. doi: 10.1016/0006-8993(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 36.Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, et al. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17(21):8476–90. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98(7):4160–5. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cermenati G, Abbiati F, Cermenati S, Brioschi E, Volonterio A, Cavaletti G, et al. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J Lipid Res. 2012;53(2):300–10. doi: 10.1194/jlr.M021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011;10:12. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987(1):93–9. doi: 10.1016/s0006-8993(03)03258-x. [DOI] [PubMed] [Google Scholar]
- 42.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16(6):1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 43.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193(2):411–9. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13(6):1078–84. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 45.Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013;240:157–67. doi: 10.1016/j.expneurol.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houle JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Pinilla F, Ying Z, Roy RR, Hodgson J, Edgerton VR. Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J Neurophysiol. 2004;92(6):3423–32. doi: 10.1152/jn.00432.2004. [DOI] [PubMed] [Google Scholar]
- 48.da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, et al. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci. 1993;13(9):3818–26. doi: 10.1523/JNEUROSCI.13-09-03818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saarelainen T, Vaittinen S, Castren E. TrkB-receptor activation contributes to the kainate-induced increase in BDNF mRNA synthesis. Cell Mol Neurobiol. 2001;21(4):429–35. doi: 10.1023/A:1012775808253. [DOI] [PMC free article] [PubMed] [Google Scholar]