Abstract
Background
Hyperoxia is common early in the course of resuscitation of critically ill patients. It has been associated with mortality in some, but not all, studies of cardiac arrest patients and other critically ill cohorts. Reasons for the inconsistency are unclear and may depend on unmeasured patient confounders, the timing and duration of hyperoxia, population characteristics, or the way that hyperoxia is defined and measured. We sought to determine whether, in a prospectively collected cohort of mechanically ventilated patients with traumatic injuries with and without head trauma, higher maximum partial pressure of arterial oxygen (PaO2) within 24 hours of admission would be associated with increased risk of in-hospital mortality.
Methods
Critically ill patients with traumatic injuries undergoing invasive mechanical ventilation enrolled in the Validating Acute Lung Injury biomarkers for Diagnosis (VALID) study were included in this study. All arterial blood gases (ABGs) from the first 24 hours of admission were recorded. Primary analysis was comparison of the highest PaO2 between hospital survivors and non-survivors.
Results
A total of 653 patients were evaluated for inclusion. Of these, 182 were not mechanically ventilated or did not have an ABG measured in the first 24 hours, leaving 471 patients in the primary analysis. In survivors, the maximum PaO2 was 141 mmHg (median, interquartile range 103 - 212) compared to 148 mmHg (IQR 105 - 209) in non-survivors (p = 0.82). In the subgroup with head trauma (n = 266), the maximum PaO2 was 133 mmHg (IQR 97 - 187) among survivors and 152 mmHg (108 - 229) among nonsurvivors (p = 0.19). After controlling for age, injury severity score, number of arterial blood gases, and fraction of inspired oxygen, maximum PaO2 was not associated with increased mortality (OR 1.27 for every fold increase of PaO2 (95% CI 0.72 - 2.25).
Conclusions
In mechanically ventilated patients with severe traumatic injuries, hyperoxia in the first 24 hours of admission was not associated with increased risk of death or worsened neurological outcomes in a setting without brain tissue oxygenation monitoring.
Electronic supplementary material
The online version of this article (doi:10.1186/s12890-017-0370-1) contains supplementary material, which is available to authorized users.
Background
Trauma accounts for approximately 10% of deaths worldwide and causes a disproportionately high amount of disability and lost years of life [1]. The magnitude of this problem may be even greater than recognized, as a significant number of patients survive hospitalization but ultimately succumb to complications of their injuries [2]. Therefore, improvements in management of patients with severe traumatic injury are needed.
Exposure to supraphysiologic levels of partial pressure of arterial oxygen (hereafter “hyperoxia”) is common in mechanically ventilated, critically ill patients [3–9]. The effect of hyperoxia on outcomes in a non-cardiac arrest population of critically ill patients remains unknown. Patients with severe traumatic injury may be especially susceptible to detrimental neurological effects of hyperoxia due to the prevalence of traumatic brain injury (TBI) in this population, but the biological and clinical effects of hyperoxia are complex and incompletely understood [3–5, 10–12]. The observed prevalence and degree of hyperoxia vary widely among centers [3–9] and have been associated with both better [13, 14] and worse [3, 6–9] outcomes in critically ill adults.
The association of hyperoxia with outcome has been previously studied in patients with critical traumatic injury. One large retrospective cohort analysis of TBI patients showed a U-shaped relationship between in-hospital and 6 month mortality and arterial oxygen tension in the first 48 h of admission, but there was no significant relationship between hyperoxia and mortality on multivariate analysis [15]. In this study, analysis was limited to arterial blood gases recorded according to APACHE II methodology (i.e., the highest alveolar-arterial gradient for patients on FiO2 0.5 or greater, or the lowest PaO2 for patients on FiO2 < 0.5). This approach would tend to select for the blood gas associated with lowest oxygen tension and thus may not be ideal for measurement of any potential effects of early hyperoxia on outcomes. In another analysis focusing on PaO2 in the first 24 h, there was an independent association between mortality and hyperoxia (defined as PaO2 > 300 mmHg) in a TBI cohort [9]. Thus, data is mixed on this question perhaps due to differences in the time point analyzed and approach to assessing arterial oxygen tension.
Because the peri-contusional brain tissue in TBI is at risk for hypoxic-ischemic injury, strategies have been developed to directly measure the partial pressure of oxygen in brain tissue (PBrO2) using brain probe monitors to guide oxygen management via adjustments in FiO2 and/or mean airway pressure. A small phase II RCT suggests improved outcomes with such an approach [16] and results of a larger RCT studying an aggressive PBrO2-guided oxygen management are expected soon [17]. However, these techniques are not yet universally adopted and may not be possible in resource-limited settings. Furthermore, even at institutions using this modality, there is an early period of resuscitation that occurs prior to implantation of PBrO2 monitors. Consequently, the safe range of PaO2 in critically ill adults without brain tissue oxygenation monitoring early after traumatic injury is unclear.
We performed a retrospective cohort study in mechanically ventilated patients with severe traumatic injuries to test the hypothesis that the maximum measured partial pressure of arterial oxygen (PaO2) within 24 h of admission would be associated with an increased risk of in-hospital mortality and that this association would be stronger in patients with head injury.
Methods
Patients
The study population consisted of 653 consecutive patients who were prospectively enrolled in the Validating Acute Lung Injury Markers for Diagnosis (VALID) study on the morning after admission to the Trauma ICU. Methods for the VALID study have been previously published [18–20], and the Vanderbilt Institutional Review Board approved the study. The VALID study included critically ill patients who were ≥18 years old and who were admitted to Vanderbilt ICUs for at least 2 days. Of the 653 patients, 182 were excluded from the analysis as they were not receiving invasive mechanical ventilation or did not have a confirmed arterial blood gas (ABG) measured in the first 24 h of admission (Fig. 1).
Fig. 1.
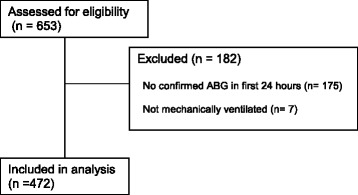
CONSORT Diagram. Of 653 patients admitted to the Trauma ICU during the enrollment period and assessed for eligibility, 471 had an ABG in the first 24 h from admission and were mechanically ventilated; these patients were included in the analysis
Measurements
All arterial blood gas analysis measurements obtained through arteriopuncture or arterial catheters for clinical purposes during the first 24 h of admission were recorded. The maximum PaO2 in the 24 h after admission, the corresponding fraction of inspired oxygen (FiO2), and the number of ABGs measured during the 24 h after admission were also recorded. Other data collection included the APACHE II score [21], the injury severity score (ISS) [22] and the presence or absence of head injury (i.e., any traumatic insult to the face, neck, cranium, or intracranial contents). Head injury was noted to be present or absent based on the report or electronic medical record documentation of the bedside attending physician. The Glasgow Coma Scale (GCS) score [23] at time of hospital discharge was also included for analysis, as this has been shown to act as a reasonable surrogate for long-term neurologic outcomes in patients with traumatic brain injury [24].
Statistical analysis
Patients’ baseline characteristics were summarized by median with interquartile range for continuous variables and frequencies with percentages for categorical variables. To compare patient’s characteristics between in-hospital mortality groups (survivors vs. non-survivors), Wilcoxon rank-sum test (continuous variables) and Pearson χ 2 test (categorical variables) were used. In the primary analysis, association between the highest PaO2 measured in the 24 h after admission and in-hospital mortality was evaluated using a logistic regression model controlling for age, injury severity score, number of measured ABGs, and fraction of inspired oxygen. The ISS and age were included in the model because they are known to have associations with outcome in this population. The number of measured ABGs was included because the timing and number of ABG assessments was not standardized in this study and is an inherent confounder of the likelihood of having a high PaO2 measurement. As clinicians may be more likely to check an ABG (and therefore more likely to document hyperoxia) in patients who are receiving a high FiO2, this was also adjusted for in the analysis. GCS was not included in the adjustment model because this measure is heavily affected by both sedation practices and TBI and thus may not be internally consistent in its interaction with outcomes of interest in this population. As a secondary investigation, the association between the highest PaO2 and the Glasgow Coma Scale (GCS) at time of discharge was evaluated using a proportional odds model adjusting for the same set of factors as in the primary analysis. A planned subgroup analysis of those with head injury was also conducted and reported.
In all models, the maximum PaO2 values were transformed using logarithmic function. Missing baseline values were imputed using a multiple imputation method (30-iteration) [25]. Statistical analysis was conducted with R version 3 (R Core Team) [26]; a two-sided significance level of 0.05 was used for statistical inference.
Results
Clinical characteristics
The patient population consisted exclusively of critically ill patients with severe traumatic injuries admitted to the Vanderbilt Trauma ICU consecutively from February 2006 to February 2011 (Table 1). summarizes characteristics of survivors and non-survivors. Survivors were significantly younger, had higher Glasgow Coma Scale (GCS) scores and lower APACHE II scores on admission. Gender, ISS, number of ABGs measured, FiO2, and maximum PaO2 were not significantly different between the two groups.
Table 1.
Baseline characteristics of 471 critically-Ill trauma patients included in the analysis
| Characteristic | N | Overall | Survivors | Non-survivors | p-value |
|---|---|---|---|---|---|
| n = 471 | n = 422 (89.6%) | n = 49 (10.4%) | |||
| Age (Years) | 471 | 42 (27, 55) | 41 (27, 54) | 51 (43, 67) | <0.001 |
| Men (n, %) | 471 | 342 (73%) | 308 (73%) | 34 (69%) | 0.59 |
| Head Trauma (Yes) | 471 | 266 (56%) | 231 (55%) | 35 (71%) | 0.03 |
| APACHE II score | 471 | 25 (20, 28) | 24 (20, 28) | 28 (25, 31) | <0.001 |
| Injury Severity Score | 470 | 29 (18, 36) | 29 (18, 36) | 29 (20, 39) | 0.37 |
| Number of ABGs measured | 471 | 3 (2, 5) | 3 (2, 5) | 3 (2, 6) | 0.44 |
| FiO2 Associated with Maximum PaO2 | 352 | 0.40 (0.40, 0.60) | 0.40 (0.40, 0.60) | 0.40 (0.40, 0.60) | 0.56 |
| Maximum PaO2 (mmHg) | 471 | 142 (103, 212) | 141 (103, 212) | 148 (105, 209) | 0.82 |
| GCS | 471 | 11 (8, 15) | 11 (9, 15) | 3 (3, 9) | <0.001 |
In-hospital mortality and maximum PaO2
Among the 471 patients in the primary analysis, 49 (10.4%) died during hospitalization. There was no difference between the maximum PaO2 of survivors [median 141 mmHg (IQR 103–212)] and nonsurvivors [148 (105–209), p = 0.82] (Fig. 2). A logistic regression model adjusting for potential confounders showed no association of maximum PaO2 with mortality, with an adjusted odds ratio for every fold increase of PaO2 of 1.27 (95% CI 0.72–2.25) (Table 2).
Fig. 2.
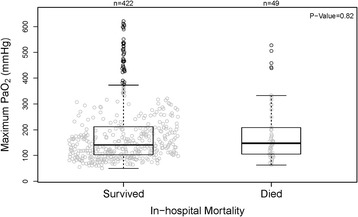
No association was seen between maximum PaO2 in the first 24 h after admission and in hospital mortality in an unadjusted analysis
Table 2.
Logistic regression model for in-hospital mortality
| Characteristic | Odds ratio | 95% Confidence interval | p-value |
|---|---|---|---|
| Age (Increment of 5 years) | 1.20 | 1.10–1.31 | <0.001 |
| Injury Severity Score (Increment of 5) | 1.41 | 1.03–1.94 | 0.03 |
| Number of ABGs Measured | 1.05 | 0.91–1.22 | 0.49 |
| FiO2 at time of ABG (Increment of 10%) | 0.94 | 0.77–1.15 | 0.54 |
| Maximum PaO2 (Increment of 1 fold) | 1.27 | 0.72–2.25 | 0.41 |
Head injury subgroup
As the effect of hyperoxia might differ between patients with head injury and those without, we conducted a subgroup analysis to determine if the adjusted association between mortality and maximum PaO2 was different for those with head injury. Of the entire cohort of 471 patients analyzed for this study, 266 (56.5%) carried a diagnosis of head injury. In this subgroup, the maximum PaO2 among survivors was 133 mmHg (97–187) compared to 152 mmHg (108–229) among nonsurvivors (p = 0.19). After controlling for age, ISS, number of ABGs measured, and the FiO2 at the time of PaO2measurement, there was no significant association between maximum PaO2 and mortality in patients with or without head trauma (OR 1.55, 95% CI 0.79–3.02, p = 0.20, and OR 1.01, 95% CI 0.31–3.28 p = 0.98, respectively) (Table 3). Head injury did not modify the effect of PaO2 on mortality (p = 0.21).
Table 3.
Logistic regression model for in-hospital mortality in head trauma patients
| Characteristic | Odds ratio | 95% Confidence interval | p-value |
|---|---|---|---|
| Age (Increment of 5 years) | 1.22 | 1.09–1.36 | <0.001 |
| Injury Severity Score (Increment of 5) | 1.28 | 0.86–1.91 | 0.23 |
| Number of ABGs Measured | 1.15 | 0.98–1.37 | 0.10 |
| FiO2 at time of ABG (Increment of 10%) | 0.88 | 0.69–1.12 | 0.29 |
| Maximum PaO2 (Increment of 1 fold) | 1.55 | 0.79–3.02 | 0.20 |
To study the potential association between PaO2 and neurologic outcomes, Glasgow Coma Scale (GCS) scores were collected on the day of hospital discharge for all patients. After adjusting for potential confounders, there was no association between the maximum PaO2 measured within 24 h of admission and the GCS measured on the day of hospital discharge (OR for maximum PaO2 0.96, 95% CI 0.71–1.31, p = 0.82). When this analysis was restricted to patients with and without head injury, there was still no association between maximum PaO2 and lower GCS at discharge (OR 1.10, 95% CI 0.76–1.60 p = 0.62 in the head injury group and OR 0.66, 95% CI 0.38–1.15 p = 0.14 in the non head injury group [Additional file 1: Tables S1, Additional file 2: Tables S2 and Additional file 3: Tables S3]. The presence of head injury did not modify the effect of PaO2 on GCS at hospital discharge (p = 0.11) (Table 4).
Table 4.
Proportional odds regression model for GCS
| Characteristic | Odds ratioa | 95% Confidence interval | p-value |
|---|---|---|---|
| Age (Increment of 5 years) | 1.05 | 1.00–1.10 | 0.04 |
| Injury Severity Score (Increment of 5) | 1.24 | 1.05–1.48 | 0.01 |
| Number of ABGs Measured | 1.11 | 1.02–1.21 | 0.01 |
| FiO2 at time of ABG (Increment of 10%) | 0.93 | 0.83–1.05 | 0.25 |
| Maximum PaO2 (Increment of 1 fold) | 0.96 | 0.71–1.31 | 0.82 |
aodds ratio for lower GCS
APACHE II score was not included in the reported logistic regression models because PaO2 is a component of APACHE II. However, since APACHE II score was associated with mortality on univariate analysis, a sensitivity analysis that included APACHE II as a variable was done for all models. Inclusion of APACHE II did not alter the reported conclusions; these analyses are reported in [Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6].
Discussion
Contrary to our hypothesis, we did not find an association between increased PaO2 within 24 h of ICU admission and in-hospital mortality in mechanically ventilated patients with severe traumatic injuries. Additionally, we did not detect such an association in the subgroup of patients with head injury. These findings provide reassuring evidence that hyperoxia early in the course of severe traumatic injury does not have major adverse effects.
It is worth noting that arterial hyperoxia as measured in this study is distinct from cerebral hyperoxia (i.e., elevated oxygen tension in brain tissue). PBrO2 monitoring in individuals with TBI allows for an individualized approach to oxygen targets in TBI patients [27–29] and its use may obviate concern for brain tissue oxygen toxicity in this population. However, this technology is not available at all centers. Furthermore, in the early period preceding insertion of the PBrO2 monitor, the management of oxygenation is still empiric. It is tempting to assume that a liberal oxygen target is best in these patients because of the high incidence of cerebral hypo-oxygenation, as is recommended in pre-hospital trauma care guidelines [29]. However, there may be some patients who would not benefit or would be harmed by such an approach; therefore, this question required further study. Our analysis affirms a prior report that such an approach is indeed safe [15]. However, because the literature is mixed as to the effects of hyperoxia in TBI and our study was not powered to exclude a small effect in this secondary subgroup analysis, further investigation remains warranted.
The significance of these findings is most apparent when considered in light of the available literature on this topic. Considerable evidence has accrued that hyperoxia can have harmful biochemical and physiological effects. Elevated PaO2 may increase the formation of reactive oxygen species (ROS) in the neuronal tissue bed, favoring the induction of neuronal cell death, and potentially contributing to poor neurological outcomes [10, 11]. Supraphysiologic levels of PaO2 can also cause cerebral vasoconstriction and heterogeneous tissue bed perfusion patterns, potentially resulting in paradoxically lower delivery of oxygen and other important substrates to cerebral tissue [11, 30, 31].
Conversely, a beneficial effect of increased PaO2 has been postulated for certain patient groups [14, 32]. Human studies have associated hyperoxia with improvements in intracranial pressure, tissue bed oxygenation in both peri-contusional and remote neuronal tissue, and more aerobic neural metabolic profiles in patients with TBI [14]. Early reductions in neurologic deficits and radiographic patterns of injury in patients with stroke who were exposed to hyperoxia have also been reported, supporting a potentially neuroprotective role for hyperoxia in the injured brain [32].
In previous cohort studies, hyperoxia has been associated with increased mortality in a variety of clinical settings [3, 5–9, 33] but these associations have not been seen in all studies [4, 15, 34–37]. Data in the general mechanically ventilated population have been mixed with the largest retrospective cohort study to date finding no effect [4, 5]. A recent meta-analysis found arterial hyperoxia to correlate with mortality but noted substantial heterogeneity of effect that could be due to differences in study design [38].
In light of this heterogeneity, several aspects of the current study enhance the validity of its findings. One strength of this analysis is that all ABGs obtained in the first 24 h after admission were analyzed rather than just those that contributed to APACHE scoring. Furthermore, PaO2 was analyzed as a continuous variable with the odds ratio of mortality for every fold increase of PaO2 above 50 mmHg reported. This measurement of oxygen exposure differs from that used in other studies, and may address some of the limitations of prior reports. Past studies of hyperoxia have used an arbitrary PaO2 cutoff of 200 or 300 mmHg, quintiles of hyperoxia exposure, or in the minority, PaO2 as a continuous variable [3–7, 9]. We chose to analyze PaO2 as a continuous variable because hyperoxia has no consensus definition, arbitrary partial pressure cut-off values may not accurately reflect biology, and because it increases statistical power. Analysis of the data using an arbitrary cutoff that has been used in some prior studies (300 mmHg) did not change our results (data not shown). The highest PaO2 during the first 24 h was chosen as a marker of exposure to hyperoxia for this trial. This early time point was chosen because hyperoxia is more common in our center during the early period. Also, vulnerable tissues may be most susceptible to any ill effects of hyperoxia early after trauma. Other publications have examined a varied range of time points and, in some cases, only analyzed the ABG with the lowest alveolar-arterial gradient, lowest PaO2, or average PaO2, thus potentially missing many cases of hyperoxia exposure [8, 15]. This early time point also increases the relevance of our results to the empiric management of oxygen prior to brain tissue monitor placement in centers with PbrO2 monitoring capability.
Our study is subject to some limitations. The retrospective design limits interpretation of the results. Although about half of our patients were classified as having head injury, this diagnosis was defined as the bedside physician noting head injury in the medical record and likely includes patients with varying degrees of trauma to the neck, face, and cranium. Sufficient data were not collected as part of the VALID study to know whether patients had true TBI. Other explanations for not finding a significant difference in PaO2 between survivors and non-survivors include a relatively low median PaO2 observed (142 mmHg) in our entire cohort compared with past studies of oxygen exposure (major studies ranging from 99 to 247 mmHg) [3–9]. Additionally, it is not known if risk accumulates over time with continuing exposure to hyperoxia, and our study was not designed to assess for such an effect. Furthermore, our study may have been underpowered to detect a small effect of hyperoxia. With our sample size of 471, we would have had 80% power to detect an association if the odds ratio corresponding to one-standard-deviation increase in maximum PaO2 were 1.53. To achieve 80% power to detect our observed OR of 1.27 would require N = 1973. Finally, there may be outcomes associated with increased PaO2 other than mortality that the current study did not analyze, including neurologic outcomes, which have previously been shown to worsen in association with exposure to hyperoxia [6]. However, GCS at time of discharge has been shown to be a reasonable surrogate for longer-term neurologic outcomes [24] and a lower GCS did not associate with maximum PaO2 in our cohort.
Conclusions
The optimal oxygenation strategy in critically ill patients with traumatic injuries who do not have brain tissue monitoring remains unknown. This study finds no association of hyperoxia with mortality or lower GCS at discharge in a population of mechanically ventilated patients who are critically ill with traumatic injuries. Our findings are reassuring that liberal oxygen supplementation can be safely applied in the management of the general trauma ICU population as well as in patients with head injury. However, these findings bear confirmation in a larger cohort of TBI patients, because our study was underpowered to exclude a small effect in this subgroup. When viewed in light of previous research in this area showing harm from hyperoxia in some populations and not others, this study supports the hypothesis that the association of higher PaO2 with mortality depends on the nature of the patient population studied.
Acknowledgments
Not applicable.
Funding
This study was funded by the NIH through the following grants: HL103836 and T32 HL087738.
Availability of data and materials
The data used for this manuscript is derived from the Validating Acute Lung Injury Markers for Diagnosis (VALID) study database. The authors decline to publish the database online as it is stored in a non-electronic format.
Authors’ contributions
DWR: literature search, data collection, data interpretation, writing, and critical revision; WLE: study design, data collection; DRJ: study design, data analysis, data interpretation, writing, and critical revision; GRB: study design and critical revision; ZZ: data analysis, data interpretation, writing, and critical revision, TK: data analysis, data interpretation, and critical revision; AKM: study design, data interpretation; LBW: study design, data analysis, data interpretation, and critical revision. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Vanderbilt University Institutional Review Board approved this study. Details of the consent process for this study have been previously published [39].
Additional files
Logistic regression model for in-hospital mortality in patients without head injury. (DOCX 14 kb)
Proportional odds regression model for GCS at discharge in patients with head injury. (DOCX 14 kb)
Proportional odds regression model for GCS at discharge in patients without head injury. (DOCX 14 kb)
Logistic regression model for in-hospital mortality (including APACHE). (DOCX 14 kb)
Logistic regression model for in-hospital mortality in patients with head injury (including APACHE). (DOCX 14 kb)
Proportional odds regression model for GCS at discharge (including APACHE). (DOCX 14 kb)
Contributor Information
Derek W. Russell, Phone: 903 530 2570, Email: dwrussell@uabmc.edu
David R. Janz, Email: djanz@lsuhsc.edu
William L. Emerson, Email: willemerson@gmail.com
Addison K. May, Email: Addison.may@vanderbilt.ed
Gordon R. Bernard, Email: Gordon.bernard@vanderbilt.edu
Zhiguo Zhao, Email: alex.zhao@vanderbilt.edu.
Tatsuki Koyama, Email: tatsuki.koyama@vanderbilt.edu.
Lorraine B. Ware, Email: Lorraine.ware@vanderbilt.edu
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Callcut RA, Wakam G, Conroy AS, et al. Discovering the truth about life after discharge: Long-term trauma related mortality. J Trauma Acute Care Surg. 2016;80:210-17. [DOI] [PMC free article] [PubMed]
- 3.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 4.Eastwood G, Bellomo R, Bailey M, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012;38:91–98. doi: 10.1007/s00134-011-2419-6. [DOI] [PubMed] [Google Scholar]
- 5.de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janz DR, Hollenbeck RD, Pollock JS, et al. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012;40:3135–3139. doi: 10.1097/CCM.0b013e3182656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42:387–396. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 8.Brenner M, Stein D, Hu P, et al. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147:1042–1046. doi: 10.1001/archsurg.2012.1560. [DOI] [PubMed] [Google Scholar]
- 9.Rincon F, Kang J, Vibbert M, et al. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014;85:799–805. doi: 10.1136/jnnp-2013-305505. [DOI] [PubMed] [Google Scholar]
- 10.Nyquist PA. Toxicity with hyperoxia in brain injury? Retrospection identifies unforeseen obstacles. Crit Care Med. 2014;42:469–470. doi: 10.1097/CCM.0b013e3182a525a4. [DOI] [PubMed] [Google Scholar]
- 11.Floyd TF, Clark JM, Gelfand R, et al. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol. 2003;95:2453–2461. doi: 10.1152/japplphysiol.00303.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ainslie PN, Shaw AD, Smith KJ, et al. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin Sci (Lond) 2014;126:661–670. doi: 10.1042/CS20130343. [DOI] [PubMed] [Google Scholar]
- 13.Rockswold SB, Rockswold GL, Zaun DA, et al. A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg. 2013;118:1317–1328. doi: 10.3171/2013.2.JNS121468. [DOI] [PubMed] [Google Scholar]
- 14.Tolias CM, Reinert M, Seiler R, et al. Normobaric hyperoxia--induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurosurg. 2004;101:435–444. doi: 10.3171/jns.2004.101.3.0435. [DOI] [PubMed] [Google Scholar]
- 15.Raj R, Bendel S, Reinikainen M, et al. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013;17:R177. doi: 10.1186/cc12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narotam PK, Morrison JF, Nathoo N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg. 2009;111:672–682. doi: 10.3171/2009.4.JNS081150. [DOI] [PubMed] [Google Scholar]
- 17.University of Texas Southwestern Medical Center. Brain Tissue Oxygen Monitoring in Traumatic Brain Injury (TBI) (BOOST2). In: ClinicalTrials.gov [Internet]. Bethesda: National Library of Medicine (US). 2000–[cited 2016 Oct-08]. Available from: https://clinicaltrials.gov/ct2/show/NCT00974259 NLM Identifier: NCT00974259.
- 18.O’Neal HR, Jr, Koyama T, Koehler EA, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware LB, Koyama T, Zhao Z, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17:R253. doi: 10.1186/cc13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Baker SP, O’Neill B, Haddon W, Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. doi: 10.1097/00005373-197403000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Jennett B, Teasdale G, Braakman R, et al. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–1034. doi: 10.1016/S0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- 24.Leitgeb J, Mauritz W, Brazinova A, et al. Glasgow Coma Scale score at intensive care unit discharge predicts the 1-year outcome of patients with severe traumatic brain injury. Eur J Trauma Emerg Surg. 2013;39:285–292. doi: 10.1007/s00068-013-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell FE., Jr . Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 26.RCT (2014) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 27.Brain Trauma F. American Association of Neurological S. Congress of Neurological S et al. Guidelines for the management of severe traumatic brain injury. X. Brain oxygen monitoring and thresholds. J Neurotrauma. 2007;24(Suppl 1):S65–S70. doi: 10.1089/neu.2007.9986. [DOI] [PubMed] [Google Scholar]
- 28.Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805–811. doi: 10.3171/jns.2005.103.5.0805. [DOI] [PubMed] [Google Scholar]
- 29.Branson RD, Johannigman JA. Pre-hospital oxygen therapy. Respir Care. 2013;58:86–97. doi: 10.4187/respcare.02251. [DOI] [PubMed] [Google Scholar]
- 30.Diringer MN, Aiyagari V, Zazulia AR, et al. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J Neurosurg. 2007;106:526–529. doi: 10.3171/jns.2007.106.4.526. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto O, Auer RN. Hypoxia, hyperoxia, ischemia, and brain necrosis. Neurology. 2000;54:362–371. doi: 10.1212/WNL.54.2.362. [DOI] [PubMed] [Google Scholar]
- 32.Singhal AB, Benner T, Roccatagliata L, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- 33.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care. 2015;19:348. doi: 10.1186/s13054-015-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaahersalo J, Bendel S, Reinikainen M, et al. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurologic outcome. Crit Care Med. 2014;42:1463–1470. doi: 10.1097/CCM.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 35.Bellomo R, Bailey M, Eastwood GM, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15:R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelskyla A, Parr MJ, Skrifvars MB. Prevalence and factors correlating with hyperoxia exposure following cardiac arrest--an observational single centre study. Scand J Trauma Resusc Emerg Med. 2013;21:35. doi: 10.1186/1757-7241-21-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ihle JF, Bernard S, Bailey MJ, et al. Hyperoxia in the intensive care unit and outcome after out-of-hospital ventricular fibrillation cardiac arrest. Crit Care Resusc. 2013;15:186–190. [PubMed] [Google Scholar]
- 38.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association Between Arterial Hyperoxia and Outcome in Subsets of Critical Illness: A Systematic Review, Meta-Analysis, and Meta-Regression of Cohort Studies. Crit Care Med. 2015;43:1508–1519. doi: 10.1097/CCM.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 39.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20:1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this manuscript is derived from the Validating Acute Lung Injury Markers for Diagnosis (VALID) study database. The authors decline to publish the database online as it is stored in a non-electronic format.


