Abstract
WCK 771, the arginine salt of S-(−)-nadifloxacin, was evaluated in animal models of staphylococcal infection and in vitro. For 302 methicillin-susceptible strains the MIC at which 50% of isolates are inhibited (MIC50) and the MIC90 of WCK 771 were 0.03 and 0.03 μg/ml, respectively, and for 198 methicillin-resistant strains the MIC50 and the MIC90 were 0.5 and 1.0 μg/ml, respectively. All methicillin-susceptible staphylococci were quinolone susceptible, and almost all methicillin-resistant staphylococci were quinolone resistant. WCK 771 was more potent than moxifloxacin, trovafloxacin, levofloxacin, and ciprofloxacin and had potency comparable to that of clinafloxacin. Only WCK 771 and clinafloxacin demonstrated strong potencies against vancomycin-intermediate Staphylococcus aureus strains (MICs = 1 μg/ml). WCK 771 is not a substrate of the NorA pump, as evident from the lack of an effect of reserpine on the MICs and similar protective doses against infections caused by efflux-positive and -negative staphylococci. WCK 771 was effective by both the oral and the subcutaneous routes in mice infected intraperitoneally with quinolone-susceptible methicillin-susceptible S. aureus (MSSA) strains. For infections caused by quinolone-resistant methicillin-resistant S. aureus (MRSA) strains, the activity of WCK 771 administered subcutaneously was superior to those of trovafloxacin and sparfloxacin, with a 50% effective dose range of 27.8 to 46.8 mg/kg of body weight. The activity of WCK 771 was superior to those of moxifloxacin, vancomycin, and linezolid in a mouse cellulitis model of infection caused by one MSSA and two MRSA strains, with effective doses of 2.5 and 5 mg/kg for the MSSA strain and 10-fold higher effective doses for MRSA strains. WCK 771, like vancomycin and linezolid, eradicated MRSA from mouse liver, spleen, kidney, and lung when it was administered subcutaneously at a dose of 50 mg/kg for four doses. These studies have demonstrated the effectiveness of WCK 771, administered orally and parenterally, for the treatment of diverse staphylococcal infections in mice, including those caused by quinolone-resistant strains.
Staphylococcus aureus is a major cause of disease and health care expenditures worldwide. Among the staphylococci, methicillin resistance is widespread in hospitals and is also emerging in community settings (24). Increased prevalences of methicillin-resistant S. aureus (MRSA) have been shown in the United States, Europe, and Japan (4, 27, 34). Of even greater concern is the emergence of vancomycin-resistant S. aureus (VRSA) strains (3, 5, 8), which compromise the therapeutic value of vancomycin. Among the three approved anti-MRSA agents, linezolid is bacteriostatic (6) and vancomycin is weakly bactericidal (12). Daptomycin, the sole drug rapidly bactericidal for MRSA, is thus far approved by the U.S. Food and Drug Administration only for the treatment of skin and soft tissue infections. Clinicians need access to multiple therapeutic options, preferably to agents with potent bactericidal actions.
Although newer quinolones, such as moxifloxacin and gatifloxacin, have potent bactericidal activities against many gram-positive bacteria, they lack adequate activities against quinolone-resistant staphylococci (13). In the present study, the antistaphylococcal activity of WCK 771 (Fig. 1), a novel arginine salt of the tricyclic fluoroquinolone S-(−)-nadifloxacin, was examined by using in vitro models and several animal infection models. The fluoroquinolones trovafloxacin, sparfloxacin, moxifloxacin, and clinafloxacin, which have optimized antistaphylococcal activities, were used as comparators (30).
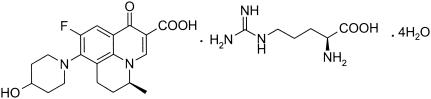
FIG. 1.
Structure of WCK 771.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study included recent clinical isolates of staphylococci from the King Edward Memorial Hospital, Mumbai, India. These strains were collected in 2002 from outpatients and included isolates from a variety of sources from patients in all age groups. Duplicate isolates from the same patient were excluded. Additionally, three reference strains were used: S. aureus Smith (ATCC 13709), obtained from the American Type Culture Collection, and S. aureus 1199B (NorA+) and S. aureus 1199 (NorA−), provided by G. W. Kaatz (Wayne State University, Detroit, Mich.) (1). S. aureus 1199B is a laboratory-derived construct derived from wild-type strain 1199; it contains a grlA mutation and hyperexpresses a norfloxacin efflux pump. Reference strains used for quality control for MIC testing included S. aureus ATCC 25923.
Antimicrobial agents.
WCK 771, ciprofloxacin, sparfloxacin, and clinafloxacin were synthesized at Wockhardt Research Centre (Aurangabad, India). Trovafloxacin, levofloxacin, and moxifloxacin were recovered from their commercial preparations in tablet form, and teicoplanin was recovered from its commercial parenteral preparation. Linezolid was obtained from Symed Laboratories (Hyderabad, India), and vancomycin was obtained from Sigma-Aldrich (St. Louis, Mo.). The purities and potencies of the agents recovered from commercial preparations were documented by showing purity of >98.5% by high-pressure liquid chromatographic analysis and by showing that the MICs of the standard antibacterials were within acceptable limits against quality control strains (19).
MIC determinations in vitro.
MICs were determined by the agar dilution method with Mueller-Hinton agar (Difco, Detroit, Mich.), according to the recommendations of NCCLS (19). The inocula were adjusted to deliver 104 CFU/spot with a multipoint inoculator (Applied Quality Services, Horshan, West Sussex, United Kingdom). The plates were incubated at 35°C for 18 h, and the MICs were read as the lowest concentration of drug that completely inhibited bacterial growth. The comparators included were clinafloxacin, moxifloxacin, trovafloxacin, levofloxacin, ciprofloxacin, vancomycin, and teicoplanin. Quinolones were also tested in the presence of 20 μg of reserpine per ml, as described previously (16).
In vivo murine infection models.
The protocols for the in vivo efficacy studies with WCK 771 and the other agents described here were reviewed and approved by Wockhardt Animal Ethics Committee (WAEC), which functions under the Indian Government's regulations for animal experimentation. Fifty percent of the WAEC members are external to the Wockhardt Research Center. The statistical significance of differences was determined with SYSTAT software, version 10.2. Data from all experiments were analyzed by analysis of variance (ANOVA) with the post hoc test (Scheffe test).
(i) Effect of staphylococcal NorA efflux pump on quinolone efficacy in intraperitoneal infection model.
To determine the influence of the NorA efflux pump on the in vivo efficacies of the fluoroquinolones, S. aureus 1199B (NorA+) and S. aureus 1199 (NorA−) systemic infections were established in mice. S. aureus 1199B is a NorA-hyperexpressing strain with a grlA mutation, and S. aureus 1199 is a wild-type strain. These strains were grown overnight on Trypticase soy agar plates at 37°C and suspended in 5% hog gastric mucin (Difco). Swiss mice (six mice per dose; age, 4 weeks; weight, 20 g) were inoculated intraperitoneally with 0.5 ml of bacterial suspension so that each mouse received 2 × 108 to 3 × 108 CFU. All untreated control animals died within 24 h of infection. All agents were administered orally. For S. aureus 1199, WCK 771, moxifloxacin, and sparfloxacin were administered at doses of 2.5, 5, and 10 mg/kg of body weight; ciprofloxacin was administered at doses of 10, 20, and 30 mg/kg; and levofloxacin was administered at doses of 5, 10, and 20 mg/kg. For S. aureus 1199B, the WCK 771 and sparfloxacin doses were 2.5, 5, and 10 mg/kg; the moxifloxacin doses were 5, 10, and 20 mg/kg; the levofloxacin doses were 10, 20, and 50 mg/kg; and the ciprofloxacin doses were 100, 200, and 300 mg/kg. These doses were administered twice, at 1 and 4 h postinfection. Mice were observed for survival for up to 7 days. The 50% effective doses (ED50s) and the 95% confidence intervals were calculated by probit analysis (18).
(ii) MSSA and MRSA intraperitoneal infection model.
The efficacies of WCK 771, moxifloxacin, trovafloxacin, and sparfloxacin, administered subcutaneously (s.c.) and orally, against methicillin-susceptible S. aureus (MSSA) strains, S. aureus ATCC 25923 and S. aureus Smith (ATCC 13709), were evaluated. These strains were grown overnight on agar plates at 37°C and suspended in 5% hog gastric mucin (Difco). Swiss mice (six mice per dose group; age, 4 weeks; weight, 20 g) were inoculated intraperitoneally with 0.5 ml of bacterial suspension so that each mouse received 2 × 108 to 3 × 108 CFU. The treatment was given at doses of 0.6, 1.2, 2.5, and 5.0 mg/kg twice at 1 and 4 h postinfection by the s.c. and the oral routes. All untreated control animals died within 24 h of infection. The efficacies of WCK 771, trovafloxacin, sparfloxacin, and vancomycin were also tested against six clinical MRSA strains (ciprofloxacin MICs > 8 μg/ml) causing infections in animals. Swiss mice (six mice per dose group; age, 4 weeks; weight, 20 g) were inoculated intraperitoneally with 0.5 ml of a bacterial suspension so that each mouse received 2 × 108 to 5 × 108 CFU. Treatment was by s.c. administration of WCK 771, trovafloxacin, and sparfloxacin at 30, 50, 75, and 100 mg/kg and vancomycin at 2.5, 5, 10, and 15 mg/kg; two doses were administered, at 1 and 4 h postinfection. Mice were observed for survival for up to 7 days. ED50s and 95% confidence intervals were calculated by probit analysis (18).
(iii) Staphylococcal cellulitis model.
A group of 18 Swiss mice (age, 4 weeks; weight, 23 to 26 g; six animals per dose per experiment in three replicate studies), as well as a control group (18 animals), were infected with S. aureus Smith (MSSA) and two MRSA strains, MRSA 5027 and MRSA 32, by subcutaneous injection of 106 to 107 CFU/animal. Infecting strains were suspended in 5% hog mucin (Difco), and 0.5 ml was injected into the right groin region of the mice (33). For S. aureus Smith infection, four doses of WCK 771 and the comparator drugs (moxifloxacin, vancomycin, and linezolid) were given s.c. (at 1, 4, 24, and 27 h after infection). For MRSA 32 and MRSA 5027 infections, three doses of WCK 771, moxifloxacin, vancomycin, and linezolid were given s.c. (at 1, 3, and 5 h after infection). The lesions that formed at the site of infection were excised 24 h after administration of the last dose and were homogenized in 2 ml of ice-chilled saline to determine the bacterial load, and the results are expressed in relation to the initial bacterial load determined 1 h after infection in control animals.
(iv) Efficacy in disseminated systemic infection.
Three groups each with 10 Swiss mice (age, 4 weeks; weight, 23 to 26 g), comprising five males and five females per group, were infected by intraperitoneal injection of 0.5 ml of 106 to 107 CFU of MRSA 32 per ml suspended in 5% hog mucin (Difco). One group served as an untreated control, while the remaining two groups were treated at 1, 4, 24, and 27 h after infection with vancomycin and WCK 771 s.c. at doses of 20 and 50 mg/kg, respectively. Since WCK 771 was expected to have superior bactericidal action compared to that of vancomycin, the efficacy of WCK 771 at the ED90 was compared with that of vancomycin at four times the ED90. The lungs, livers, spleens, and kidneys of the mice in the treatment groups were removed 48 h after administration of the last dose. Organ tissue homogenates were prepared in 3 ml of chilled sterile saline with a tissue homogenizer (IKA-WERKE, Staufen, Germany). Homogenates were suitably diluted in sterile saline and plated on agar plates to enumerate the bacterial load of each organ. The organs of the control untreated animals were excised at the time of onset of treatment, and the bacterial loads were determined. Bacterial eradication was assessed by comparing the reduction of bacterial counts in each organ in the infected groups with the bacterial loads of the control animals at the time of onset of treatment.
Pharmacokinetics in mice.
The pharmacokinetic profiles of WCK 771 and comparators, each at a dose of 50 mg/kg, were studied. The 50-mg/kg dose was selected on the basis of the fact that this dose of WCK 771 achieves the therapeutically relevant end points of bacterial eradication and survival of mice infected with MRSA strains. Groups of 21 fed Swiss mice (age, 4 weeks; weight, 23 to 26 g), with three mice used for each time point, were given single s.c. doses of 50 mg of WCK 771, moxifloxacin, trovafloxacin, sparfloxacin, vancomycin, or linezolid per kg. Blood samples (1.5 ml) were collected by retroorbital puncture at 0, 0.25, 0.5, 1, 2, 4, and 6 h postdosing and were placed in 2-ml Eppendorf tubes. The blood samples were allowed to coagulate for 1 h at 37°C, and serum was separated by centrifugation. Drugs were extracted from 1 ml of serum with solid-phase extraction cartridges (OASIS HLB; Waters) and eluted from the cartridges with 2 ml of methanol. The concentrations of drugs in the methanolic eluates were estimated by a validated high-pressure liquid chromatography assay (Agilent-1100). A liquid chromatography-mass spectrometry-mass spectrometry method was used for the analysis of vancomycin in mouse serum samples. Pharmacokinetic parameters, including the area under the concentration-time curve (AUC), the maximum concentration of drug in serum (Cmax), and half-life (t1/2), were determined by the WinNonlin method (10). Protein-bound and non-protein-bound serum fractions were determined on the basis of the methods published in the literature (2, 7, 15, 17, 26, 28, 29, 32).
RESULTS
MIC determinations.
On the basis of their susceptibilities to ciprofloxacin, all MSSA isolates and coagulase-negative staphylococci were quinolone susceptible, and almost all (>99%) MRSA isolates were quinolone resistant (Table 1). The ciprofloxacin MICs for 161 of the 196 ciprofloxacin-resistant isolates (82.1%) were fourfold or more lower in the presence of 20 μg of reserpine per ml. The potency of WCK 771 against Indian staphylococcal isolates was comparable to that of clinafloxacin. The gradation of potency in terms of the MIC at which 90% of isolates are inhibited (MIC90) for MSSA and methicillin-susceptible Staphylococcus epidermidis (MSSE) was WCK 771 = clinafloxacin < moxifloxacin = trovafloxacin < levofloxacin < ciprofloxacin = vancomycin < teicoplanin, and that for MRSA and methicillin-resistant S. epidermidis (MRSE) was WCK 771 = clinafloxacin < vancomycin = teicoplanin < moxifloxacin = trovafloxacin < levofloxacin < ciprofloxacin. The MIC90s of WCK 771 and clinafloxacin for MRSA and MRSE strains were identical; however, the MIC90s of the other fluoroquinolones for MRSA strains were two- to fourfold higher than those for MRSE strains. Such differential susceptibilities between MSSA and MSSE strains were not observed for any of the fluoroquinolones studied. Of 198 quinolone-resistant isolates (ciprofloxacin MICs ≥ 4.0 μg/ml), 78 (39.4%) had a reserpine-inhibited quinolone efflux pump, with the ciprofloxacin MICs for these isolates being fourfold or more lower in the presence of reserpine.
TABLE 1.
MICs of WCK 771 and other fluoroquinolones for 500 isolates of staphylococci from India
| Organism (no. of strains) and agent | MIC (μg/ml)
|
% Resistant | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| MSSA (244) | ||||
| WCK 771 | 0.015-0.25 | 0.03 | 0.03 | NAa |
| Clinafloxacin | 0.015-0.25 | 0.03 | 0.06 | NA |
| Moxifloxacin | 0.03-0.5 | 0.03 | 0.12 | 0 |
| Trovafloxacin | 0.015-0.5 | 0.06 | 0.12 | 0 |
| Levofloxacin | 0.12-1.0 | 0.25 | 0.5 | 0 |
| Ciprofloxacin | 0.12-2.0 | 0.5 | 1.0 | 0 |
| Vancomycin | 0.5-2.0 | 1.0 | 2.0 | 0 |
| Teicoplanin | 0.5-4.0 | 1.0 | 2.0 | 0 |
| MSSE (58) | ||||
| WCK 771 | 0.015-0.25 | 0.03 | 0.03 | NA |
| Clinafloxacin | 0.25-0.25 | 0.03 | 0.06 | NA |
| Moxifloxacin | 0.03-0.5 | 0.03 | 0.06 | 0 |
| Trovafloxacin | 0.03-0.5 | 0.06 | 0.12 | 0 |
| Levofloxacin | 0.125-1.0 | 0.25 | 0.5 | 0 |
| Ciprofloxacin | 0.125-2.0 | 0.5 | 1.0 | 0 |
| Vancomycin | 1.0-4.0 | 1.0 | 2.0 | 0 |
| Teicoplanin | 0.5-16.0 | 1.0 | 2.0 | 0 |
| MRSA (176) | ||||
| WCK 771 | 0.015-4.0 | 0.5 | 1.0 | NA |
| Clinafloxacin | 0.015-4.0 | 0.25 | 1.0 | NA |
| Moxifloxacin | 0.03-8.0 | 1.0 | 4.0 | 13.85 |
| Trovafloxacin | 0.015-16 | 1.0 | 4.0 | 12.65 |
| Levofloxacin | 0.125-64 | 4.0 | 16.0 | 99.0 |
| Ciprofloxacin | 0.25-128 | 4.0 | 64 | 99.0 |
| Vancomycin | 0.5-2.0 | 1.0 | 2.0 | 0 |
| Teicoplanin | 0.5-2.0 | 1.0 | 2.0 | 0 |
| MRSE (22) | ||||
| WCK 771 | 0.03-1.0 | 0.5 | 1.0 | NA |
| Clinafloxacin | 0.03-2 | 0.5 | 1.0 | NA |
| Moxifloxacin | 0.03-4 | 2.0 | 2.0 | 20.0 |
| Trovafloxacin | 0.03-8 | 1.0 | 2.0 | 40.0 |
| Levofloxacin | 0.125-16 | 4.0 | 8.0 | 92.0 |
| Ciprofloxacin | 0.5-128 | 4.0 | 16.0 | 92.0 |
| Vancomycin | 1.0-2.0 | 1.0 | 2.0 | 0 |
| Teicoplanin | 0.5-8.0 | 1.0 | 1.0 | 0 |
NA, not applicable.
Mouse systemic infections.
The results of studies of the impact of efflux-mediated resistance on in vivo efficacy in terms of the ED50s and ED90s for infections caused by S. aureus 1199 (NorA−) and S. aureus 1199B (Nor A+) are shown in Table 2. There was a onefold difference in the ED50s and ED90s of WCK 771 for the two strains. The maximum adverse impact of efflux was observed for ciprofloxacin, with the ED50s of ciprofloxacin for the infections caused by the NorA+ strains elevated >20 times above those for the infections caused by the NorA− strain. The ED50s of levofloxacin and moxifloxacin were elevated five and three times, respectively. The activity of WCK 771 was significantly (P < 0.05) superior to those of the comparator quinolones against both S. aureus 1199B NorA+ and S. aureus 1199 NorA−.
TABLE 2.
Effect of efflux-mediated resistance on in vivo efficacy of WCK 771 administered orally
| Infecting strain and agent | MIC (μg/ml) | ED (mg/kg [95% confidence limit])a
|
|
|---|---|---|---|
| 50% | 90% | ||
| S. aureus 1199 NorA− | |||
| WCK 771 | 0.015 | 2.2 (1.4-3.5) | 3.1 (1.61-6.0) |
| Ciprofloxacin | 0.25 | 19.4* (15.5-24.4) | 36.1* (23.8-55.0) |
| Levofloxacin | 0.25 | 5.6* (4.5-7.0) | 10.6* (7.3-15.4) |
| Moxifloxacin | 0.06 | 3.1 (1.8-5.2) | 9.4 (4.9-16) |
| Sparfloxacin | 0.06 | 4.4 (3.3-5.9) | 7.4 (4.9-11.3) |
| S. aureus 1199B NorA+ | |||
| WCK 771 | 0.03 | 3.04 (2.5-3.6) | 6.6 (3.8-11.6) |
| Ciprofloxacin | 8 | 443* (80-1,084) | >500* |
| Levofloxacin | 1 | 27.4* (23.5-31.9) | 44.8* (34.7-57.9) |
| Moxifloxacin | 0.25 | 10.4* (8.4-12.9) | 23.6* (15.7-35.3) |
| Sparfloxacin | 0.25 | 5.8* (4.8-7.0) | 13* (9.1-18.7) |
ED50s and ED90s represent the means, and the 95% confidence limits are based on the results of three or four experiments. The results for ciprofloxacin, levofloxacin, moxifloxacin, and sparfloxacin were compared with those for WCK 771 by a one-way ANOVA, and results showing P values <0.05 are indicated with an asterisk.
The comparative ED50s and ED90s for in vivo efficacy against systemic MSSA infections are shown in Table 3. The ED90 of WCK 771 administered orally ranged from 4.37 to 5.0 mg/kg. The efficacy of WCK 771 administered orally was significantly (P < 0.05) superior to those of sparfloxacin and moxifloxacin for both strains tested. The efficacy of WCK 771 administered by the s.c. route was comparable to those of moxifloxacin and sparfloxacin. However, trovafloxacin had the highest efficacy by both routes.
TABLE 3.
In vivo efficacy of WCK 771 by oral and s.c. administration for systemic MSSA infections caused by two strains of S. aureus
| Infecting strain and quinolone | MIC (μg/ml) | ED (mg/kg [95% confidence limit])a
|
|||
|---|---|---|---|---|---|
| s.c. route
|
Oral route
|
||||
| 50% | 90% | 50% | 90% | ||
| S. aureus ATCC 25923 | |||||
| WCK 771 | 0.03 | 0.59 (0.37-0.95) | 1.08 (0.67-1.71) | 2.8 (1.9-4.1) | 4.37 (3.0-6.3) |
| Moxifloxacin | 0.06 | 0.6 (0.3-1.2) | 1.7 (1.0-2.9) | 3.2* (1.9-5.2) | 9.6* (4.9-18) |
| Trovafloxacin | 0.015 | 0.26* (0.17-0.41) | 0.37 (0.2-0.5) | 1.0* (0.6-1.7) | 1.51* (0.9-2.4) |
| Sparfloxacin | 0.06 | 0.96* (0.66-1.39) | 1.7 (1.2-2.5) | 5.4* (4,3-6.3) | 7.0* (5.6-8.7) |
| S. aureus Smith (ATCC 13709) | |||||
| WCK 771 | 0.015 | 0.86 (0.5-1.3) | 2.4 (1.2-4.9) | 1.7 (0.93-0.06) | 5.0 (2.3-10.9) |
| Moxifloxacin | 0.06 | 1.0 (0.6-1.5) | 2.3 (1.3-4.2) | 3.2* (1.9-5.2) | 9.6* (4.9-18) |
| Trovafloxacin | 0.015 | 0.2* (0.04-0.9) | 0.9 (0.3-0.3) | 0.9* (0.6-1.4) | 2.18* (1.2-3.7) |
| Sparfloxacin | 0.06 | 0.6 (0.4-0.9) | 1.2 (0.7-2.1) | 3.85* (2.5-5.8) | 8.7* (5.1-14.9) |
ED50s and ED90s represent the means, and the 95% confidence limits are based on the results of three or four experiments. The results for ciprofloxacin, levofloxacin, moxifloxacin, and sparfloxacin were compared with those for WCK 771 by a one-way ANOVA, and results showing P values <0.05 are indicated with an asterisk.
The efficacy of WCK 771 against six clinical isolates of MRSA is shown in Table 4. The ED50s of WCK 771 ranged from 27.8 to 46.8 mg/kg, and the ED90 range was 48.6 to 70.4 mg/kg. The efficacy of WCK 771 was significantly (P < 0.05) superior to those of trovafloxacin and sparfloxacin for five of the six strains studied. Against MRSA 32 infection, the efficacy of WCK 771 was superior to that of sparfloxacin but was comparable to that of trovafloxacin. The protective doses of vancomycin were significantly lower for all six MRSA strains.
TABLE 4.
In vivo efficacies of WCK 771 and comparators, administered by s.c. injection, for systemic MRSA infections caused by six clinical isolates
| Infecting strain and agent | MIC (μg/ml) | ED (mg/kg [95% confidence limit])a
|
|
|---|---|---|---|
| 50% | 90% | ||
| MRSA 5023 | |||
| WCK 771 | 1 | 33.9 (26.8-42.8) | 48.6 (36.9-63.9) |
| Trovafloxacin | 2 | 66.5* (52.7-84.0) | >75* |
| Sparfloxacin | 8 | 67.3* (50.4-77.7) | 89.4* (75-106.5) |
| Vancomycin | 1 | 4.8* (2.6-9.0) | 10.1* (5.6-18) |
| MRSA 33 | |||
| WCK 771 | 1 | 38.3 (27.7-53.0) | 70.4 (47.6-104) |
| Trovafloxacin | 2 | >50* | >50* |
| Sparfloxacin | 8 | >100* | >100* |
| Vancomycin | 1 | 3.7* (1.18-11.7) | 5.69* (1.72-17.6) |
| MRSA 34 | |||
| WCK 771 | 1 | 44.6 (30.3-65.3) | 62.3 (42.3-91.7) |
| Trovafloxacin | 2 | >100* | >100* |
| Sparfloxacin | 8 | >100* | >100* |
| Vancomycin | 1 | 9.9* (8.7-11.4) | 11.8* (10.3-13.4) |
| MRSA 5002 | |||
| WCK 771 | 1 | 46.8 (36-60.8) | 56.8 (43.7-73.8) |
| Trovafloxacin | 2 | >100* | >100* |
| Sparfloxacin | 2 | >100* | >100* |
| Vancomycin | 1 | 9.36* (7.2-12.1) | 11.39* (8.7-14.7) |
| MRSA 32 | |||
| WCK 771 | 1 | 27.8 (18.7-41.3) | 62.1 (41.8-92.3) |
| Trovafloxacin | 2 | 46.8 (36-60) | 56.8 (43.7-73.6) |
| Sparfloxacin | 8 | >100* | >100* |
| Vancomycin | 1 | 2.18* (0.6-7.0) | 7.0* (2.2-22.7) |
| MRSA 97 | |||
| WCK 771 | 1 | 46.8 (36-60.8) | 56.8 (43.7-73.8) |
| Trovafloxacin | 4 | >100* | >100* |
| Sparfloxacin | 8 | >100* | >100* |
| Vancomycin | 1 | 8.3* (4.0-17.3) | 10.6* (5.1-22.1) |
ED50s and ED90s represent the means, and the 95% confidence limits are based on the results of three or four experiments. The results for ciprofloxacin, levofloxacin, moxifloxacin, and sparfloxacin were compared with those for WCK 771 by a one-way ANOVA, and results showing P values <0.05 are indicated with an asterisk.
Staphylococcal cellulitis model.
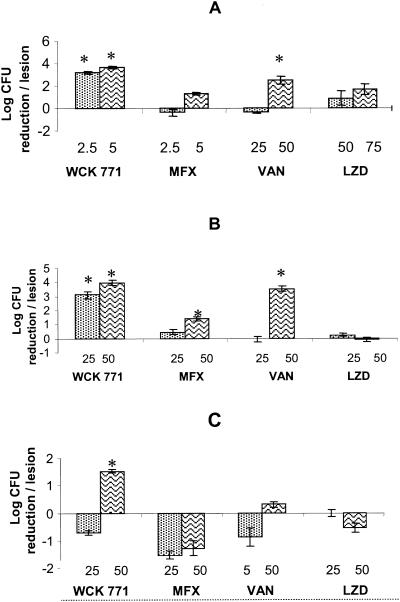
The MICs of the test drugs for the staphylococcal strains used in the cellulitis model are shown in Table 5, and histograms of the log CFU reduction per lesion for one MSSA strain and two MRSA strains are shown in Fig. 2. For infections with S. aureus Smith, WCK 771 at a dose of 2.5 mg/kg brought about a 3-log10 reduction in the bacterial load per lesion, whereas moxifloxacin showed a bacteriostatic effect (Fig. 2A). Vancomycin was bacteriostatic at 25 mg/kg (P > 0.05), but at the 50-mg/kg dose it resulted in about a 2.5-log10 reduction in the organism load per lesion (P < 0.05). Linezolid at 50 mg/kg reduced the organism load by only 0.89 log10 per lesion and linezolid at 75 mg/kg reduced the organism load by 1.68 log10 per lesion, but these changes were not statistically significant (P > 0.05). WCK 771, linezolid, and vancomycin reduced the bacterial loads in a dose-dependent manner.
TABLE 5.
MICs of fluoroquinolones for staphylococcal strains used in mouse cellulitis model
| Compound | MIC (μg/ml)
|
||
|---|---|---|---|
| S. aureus Smith ATCC 13709 | MRSA 5027 | MRSA 32 | |
| WCK 771 | <0.03 | 0.25 | 1 |
| Moxifloxacin | 0.03 | 1 | 4 |
| Vancomycin | 2 | 2 | 2 |
| Linezolid | 4 | 4 | 2 |
FIG. 2.
Efficacy of WCK 771 in mouse cellulitis model. The results are shown as histograms of the reductions in viable counts per lesion at the end of therapy compared to the viable counts at the onset of treatment in the controls. Bars indicate standard deviations. MXF, moxifloxacin; VAN, vancomycin; LZD, linezolid. Values above the drug names indicate the dose (in milligrams per kilogram). Results are shown as the organism load reduction at the end of the study period compared with the load in the untreated controls at the start of treatment; results that are significantly different (P < 0.05) from those for the controls are indicated with an asterisk. (A) S. aureus Smith (ATCC 13709; MSSA) infection; treatment schedule, 1, 4, 24, and 27 h postinfection; (B) MRSA 5027 infection; treatment schedule, 1, 3, and 5 h postinfection; (C) MRSA 32 infection; treatment schedule, 1, 3, and 5 h postinfection.
WCK 771 was the most efficacious compound against MRSA 5027 infections, and at 25 mg/kg it significantly (P < 0.05) reduced the bacterial load by 3 log10 per lesion, whereas vancomycin showed a bacteriostatic effect (Fig. 2B). Moxifloxacin at the same dose reduced the count marginally. At 50 mg/kg, WCK 771 and vancomycin showed comparable efficacies (3.97- and 3.53-log10 reductions per lesion, respectively), with the efficacies of both compounds being superior to that of moxifloxacin at this dose (1.43-log10 reduction per lesion); however, the reductions were statistically significant (P < 0.05) for all three agents. Linezolid was bacteriostatic, even at a higher dose of 50 mg/kg.
For MRSA 32 infection, none of the agents at a dose of 25 mg/kg reduced the bacterial loads. On the contrary, treatment with this dose increased the bacterial loads by 0.5 to 1.5 log10 per lesion (Fig. 2C). However, at the 50-mg/kg doses of these agents, only WCK 771 reduced the bacterial count significantly (by 1.5 log10 per lesion [P < 0.05]), whereas vancomycin had a bacteriostatic effect; and the lesions of moxifloxacin- and linezolid-treated animals showed increased bacterial counts.
Disseminated systemic infection.
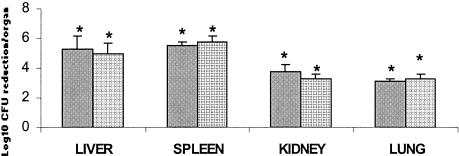
The effect of WCK 771 treatment on multiorgan eradication of MRSA 32 48 h after administration of the last dose is shown in Fig. 3. Treatment with WCK 771 at 50 mg/kg resulted in a >3-log10 reduction in organism loads in the liver, kidney, spleen, and lung (P < 0.05 for all organs). The bacterial loads in the liver and spleen showed about a 5-log10 reduction per organ, whereas the bacterial loads in the kidney and lung were reduced by 3 to 4 log10 per organ. Treatment with vancomycin at 20 mg/kg for 2 days gave a response comparable to that of WCK 771.
FIG. 3.
Efficacy of eradication of MRSA 32 from multiple organs. The histograms show the reductions of the organism loads per organ 48 h after the end of therapy compared with the organism loads in the untreated controls at the start of treatment. Bars indicate standard deviations. Results that are significantly different (P < 0.05) from those for the controls are indicated with an asterisk. Bars on the left for each organ, vancomycin at 20 mg/kg twice a day for 2 days; bars on the right for each organ, WCK 771 at 50 mg/kg twice a day for 2 days.
Mouse pharmacokinetics.
The pharmacokinetics of the antibacterial agents in mice in terms of the AUC for serum, Cmax, and t1/2 are shown in Table 6, as are the plasma protein binding properties of these agents. Among the fluoroquinolones administered s.c. at a dose of 50 mg/kg, trovafloxacin showed the highest AUCs from 0 to 6 h (AUC0-6s; 34.50 μg · h/ml), followed by WCK 771 (23.02 μg · h/ml), sparfloxacin (20.18 μg · h/ml), and moxifloxacin (12.05 μg · h/ml). When ciprofloxacin was administered by the oral route it attained a lower AUC0-6 of 7.36 μg · h/ml. Vancomycin and linezolid showed two to four times higher AUCs compared to that of WCK 771. The highest Cmax and the longest t1/2 were attained by vancomycin and trovafloxacin, respectively. Among the fluoroquinolones, WCK 771 showed the highest Cmax (14.5 μg/ml). On the basis of these values, the AUC0-24s achieved for total and unbound drug in serum with 50-mg/kg doses and the dosing regimen used for the MRSA mouse cellulitis studies (three doses over 5 h, with the bacterial load determined 24 h after administration of the last dose) are as follows: WCK 771, 69.1 and 13.8 μg · h/ml, respectively; moxifloxacin, 36.2 and 18.1 μg · h/ml, respectively; vancomycin, 173.5 and 104.1 μg · h/ml, respectively; and linezolid, 265.5 and 185.9 μg · h/ml, respectively. The AUC/MIC ratios for unbound drug in serum achieved with this dosing regimen would therefore exceed 25, the threshold usually associated with the efficacies of concentration-dependent agents (15), for isolates for which the MICs were as follows (adjusted to doubling dilution values): WCK 771, ≤0.5 μg/ml; moxifloxacin, ≤0.5 μg/ml; vancomycin, ≤4 μg/ml; and linezolid, ≤4 μg/ml.
TABLE 6.
Values of pharmacokinetic parameters for WCK 771 and other agents in mice
| Agenta | Route | AUC0-6 (μg · h/ml [SD]) | Cmax (μg/ml [SD]) | t1/2 (h [SD]) | Unbound fraction (%) in serumb |
|---|---|---|---|---|---|
| WCK 771 | s.c. | 23.02 (1.49) | 14.51 (3.47) | 0.47 (0.2) | 20 |
| Moxifloxacin | s.c. | 12.05 (0.89) | 9.49 (0.69) | 1.10 (0.15) | 50 |
| Trovafloxacin | s.c. | 34.50 (3.06) | 8.72 (1.81) | 1.85 (0.63) | 25 |
| Sparfloxacin | s.c. | 20.18 (2.44) | 6.65 (0.91) | 1.72 (0.41) | 55 |
| Ciprofloxacin | Oral | 7.36 (1.34) | 3.92 (0.18) | 0.90 (0.86) | 70 |
| Vancomycin | s.c. | 57.83 (5.26) | 65.84 (2.56) | 0.71 (0.09) | 60c |
| Linezolid | s.c. | 88.51 (14.57) | 48.16 (6.75) | 1.19 (0.20) | 70 |
Each agent was administered at a dose of 50 mg/kg.
Determined from published data (see Materials and Methods).
Very variable.
DISCUSSION
WCK 771 is an arginine salt of the S-(−) isomer of nadifloxacin. Because the S-(−) isomer is primarily responsible for antibacterial activity, the potency of WCK 771 is two to four times higher than that of racemic nadifloxacin (22). The comparative assessment of WCK 771 with other fluoroquinolones demonstrates that this new agent is a highly potent antistaphylococcal fluoroquinolone with improved potency against even fluoroquinolone-resistant strains of S. aureus and coagulase-negative staphylococci. In the present study the MIC90 of WCK 771 was low (1.0 μg/ml) for 198 MRSA and MRSE isolates, which included a significant proportion of quinolone-resistant strains, as evident from the elevated MIC50s of ciprofloxacin and levofloxacin.
The potency of WCK 771 (MIC90 = 1 μg/ml) against MRSA and MRSE strains is considerably higher than that of levofloxacin (MIC90 = 16 μg/ml) or ciprofloxacin (MIC90 = 128 μg/ml). It is two- to fourfold more potent than moxifloxacin and trovafloxacin. Additionally, WCK 771 has potency comparable to that of clinafloxacin, one of the most potent anti-MRSA quinolones developed to date (9, 25). WCK 771 was also found to be the most potent agent tested against 234 clinical isolates of MSSA and MSSE. Thus, on the basis of its antistaphylococcal potency, WCK 771 represents a significant advance over the quinolones available at present.
A large proportion (53.2%) of fluoroquinolone-resistant strains was detected among the clinical isolates of S. aureus used in this study. Among the oxacillin-resistant MRSA isolates, almost all were resistant to ciprofloxacin and levofloxacin, while the rates of resistance to other fluoroquinolones varied from 13.9% for moxifloxacin to 12.7% for trovafloxacin. However, although the MICs of WCK 771 were higher for quinolone-resistant isolates than quinolone-susceptible ones, the WCK 771 MICs ranged from 0.12 to 4 μg/ml for ciprofloxacin-resistant staphylococci. Racemic nadifloxacin has been reported to have a similar potency against a large number of staphylococcal isolates collected from 1994 to 2000 (20-22). WCK 771 also resists NorA-mediated efflux (S. V. Gupte, M. V. Patel, S. K. Agarwal, D. J. Upadhyay, S. C. Nair, S. S. Bhagwat, N. Shetty, S. B. Bhavsar, N. J. De Souza, and H. F. Khorakiwala, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother, abstr. F- 537, 2001) and is bactericidal against fluoroquinolone-resistant staphylococci (16). WCK 771 has also previously been reported to have good potency against vancomycin-intermediate S. aureus strains MU-3 and MU-50 (P. K. Deshpande, V. N. Desai, S. V. Bhavsar, N. C. Chaturvedi, S. A. Ghalsasi, S. Aher, R. D. Yeole, D. Pawar, M. C. Shukla, M. V. Patel, S. V. Gupte, N. J. De Souza, and H. F. Khorakiwala, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother, abstr. F 430, 2003) and, with an MIC of 0.5 μg/ml, was fourfold more potent than clinafloxacin against a VRSA isolate (3). Significantly, WCK 771 was bactericidal against this VRSA strain, which bears mutations in the quinolone resistance-determining regions of gyrA and grlB. The potencies of all other fluoroquinolones tested against vancomycin-intermediate S. aureus and VRSA strains were four- to eightfold less than that of WCK 771. These findings indicate that WCK 771 may be able to overcome currently prevailing molecular mechanisms of fluoroquinolone and vancomycin resistance, provided that its pharmacokinetic properties are adequate.
In assessing the impact of the NorA efflux pump on WCK 771 activity, it was observed that the activity of the drug against a NorA-hyperexpressing S. aureus strain was not affected by the presence of reserpine and that there was little difference between the MICs for NorA− strains and those for NorA+ strains (Table 2). This is in agreement with a report that the activity of racemic nadifloxacin is not influenced by the expression of the norA gene (23). The comparable EDs of WCK 771 for the treatment of infections due to NorA+ and NorA− strains further substantiates the ability of this agent to overcome NorA-mediated efflux. In contrast, there was a significant elevation of the protective dose of ciprofloxacin in animals infected with the NorA+ strain compared to that in animals infected with the NorA− strain. The protective dose of sparfloxacin, which is reported to be a poor substrate for NorA-mediated efflux (35), did not show a significant rise against the NorA+ strain. These observations underscore the therapeutic significance of efflux-mediated resistance. The in vivo efficacy data obtained in the systemic MSSA infection model demonstrates that the overall efficacy of WCK 771 administered by either the oral or the s.c. route is comparable to those of moxifloxacin and trovafloxacin and is superior to that of sparfloxacin (Table 3).
The efficacy of WCK 771 against systemic MRSA infections is superior to those of trovafloxacin and sparfloxacin, with ED90s (48.6 to 70.4 mg/kg) considerably lower than those of the comparator fluoroquinolones (>50 to >100 mg/kg) (Table 4). The ED90s of WCK 771 were about fivefold higher than those of vancomycin. When the comparable MICs of WCK 771 and vancomycin are considered, the doses of the former agent that afford protection in the mouse model are somewhat higher. This could be due to the relatively higher level of serum protein binding (∼80%) of WCK 771 compared to that of vancomycin (∼40%). The free Cmax of WCK 771, when it was dosed at the ED90, obtained in the systemic MRSA infection model (50 mg/kg) was about 3 μg/ml, which is less than half the free Cmax of a 10-mg/kg dose of vancomycin (∼8 μg/ml, as derived from the pharmacokinetic values obtained with a 50-mg/kg dose).
The organ load reduction shown in the disseminated systemic infection model (Fig. 3) indicates the promising potential of WCK 771 for the treatment of systemic MRSA infections, as this new agent demonstrated activity comparable to that of vancomycin in eradicating MRSA from organs. The dose of WCK 771 needed to eradicate infection was 2.5 times higher than that of vancomycin. However, in terms of multiples of the ED90 required for systemic infection, WCK 771 and vancomycin were dosed at one and four times their ED90s, respectively. The effectiveness of WCK 771 may be attributable to its powerful bactericidal action.
An excellent correlation between the bactericidal properties of WCK 771 in vitro and pathogen eradication in vivo was observed for mouse skin infection caused by MSSA strain S. aureus Smith and two MRSA strains (Fig. 2). The comparative assessment of WCK 771 with other potent antistaphylococcal drugs, such as moxifloxacin, vancomycin, and linezolid, by the s.c. route showed the advantage associated with WCK 771 therapy for both MSSA and MRSA infections. WCK 771 at a dose of 2.5 mg/kg achieved a superior bactericidal response against MSSA in vivo compared to those of the other drugs tested when all drugs were administered at 5 mg/kg. Pharmacokinetic data for WCK 771 at a dose of 50 mg/kg in mice showed that although the calculated unbound serum AUC and Cmax values for WCK 771 are lower than the values for any of the comparator agents studied (Table 6), the AUC/MIC ratios for unbound drug in serum were the highest for WCK 771, and the significant reduction in the bacterial load correlated well with AUC/MIC ratios that exceeded 25. These results reflect the activity that can be achieved in humans, as the AUC values achieved with the highest doses used in our model are similar to the values that can be achieved in humans. Specifically, the AUC0-24 of unbound moxifloxacin in human serum when moxifloxacin is dosed at 400 mg as a single dose per day is 25 μg · h/ml (11), which is similar to the value of 18.1 μg · h/ml achieved in our model. The AUC0-24 values for total and unbound WCK 771 achieved in human phase I studies when the drug was dosed at 200, 400, and 600 mg are 46.6 and 9.3, 93.5 and 18.7, and 154.5 and 30.9 μg · h/ml, respectively (N. Maharaj, R. Jha, Y. Chugh, R. Yeole, M. Patel, N. De Souza, H. Khorakiwala, B. More, N. Gotay, S. Dalvi, and N. Kshirsagar, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-21, 2004); and it is envisioned that a dosing regimen of 600 mg twice a day will be effective against isolates for which MICs are as high as 2 μg/ml. Our findings with this animal model support the clinical development of WCK 771 for the treatment of almost all infections due to quinolone-resistant staphylococci.
Among the comparator drugs studied, vancomycin and linezolid administered s.c. showed excellent pharmacokinetic values in mice in terms of both AUC and Cmax, as well as in terms of AUC/MIC ratios. However, the relatively poor pharmacodynamic effects of both these drugs compared to those of the fluoroquinolones might have resulted in a lower eradication efficacy due to the slower bactericidal action of vancomycin and the bacteriostatic effect of linezolid (6, 12). The superior pharmacokinetic advantages of vancomycin and linezolid are reflected by the lower protective doses that we found in the mouse protection studies (survival with linezolid treatment; data not shown). Trovafloxacin showed superior AUC and t1/2 values compared to those of WCK 771 and the comparator quinolones, which correlated with trovafloxacin having the highest efficacy among the fluoroquinolones studied in the MSSA systemic infection model. However, its lower efficacy against infections caused by quinolone-resistant MRSA could be attributed to its higher MICs coupled with its relatively higher level of protein binding. In the MSSA survival model, sparfloxacin was the next best agent after trovafloxacin, in line with its good pharmacokinetic profile.
The strong potency of WCK 771 against MSSA and MRSA strains is possibly due to its unique bactericidal mechanism of action and its resistance to efflux-mediated resistance, resulting in the combination of high-affinity targeting of staphylococcal DNA gyrase, coupled with efflux resistance, creating high intracellular drug concentrations. The use of various older fluoroquinolones (e.g., norfloxacin, ciprofloxacin, ofloxacin, and levofloxacin) that primarily target staphylococcal topoisomerase IV has led to widespread cross-resistance among members of this class due to the relative mutational vulnerability of topoisomerase IV (J. Campion, P. J. McNamara, and M. E. Evans, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1324, 2003). The reported 50% inhibitory concentration of racemic nadifloxacin for DNA gyrase is sixfold lower than that of topoisomerase IV (31). Since the R isomer of nadifloxacin is devoid of significant antibacterial activity, the S-(−) isomer would have an even higher affinity for DNA gyrase. Recent publications suggest that DNA gyrase tolerates a smaller range of mutations than topoisomerase IV does (14). The superior anti-MRSA potency of WCK 771 compared with those of the other agents tested is a possible reflection of a further improvement in affinity for DNA gyrase.
In summary, on the basis of the data presented in this study, WCK 771 has the potential to provide an attractive option for the treatment of infections due to MRSA, fluoroquinolone-resistant staphylococci, and staphylococci with lowered susceptibilities to glycopeptides, provided that ongoing clinical studies demonstrate its favorable pharmacokinetic parameters and favorable clinical outcomes with treatment with this agent.
Acknowledgments
All studies were part of Wockhardt's anti-infective research program, which is supported by Wockhardt Ltd., an Indian pharmaceutical company.
We thank S. Takalkar and R. Yeole for technical assistance.
REFERENCES
- 1.Aeschlimann, J. R., L. D. Dresser, G. W. Kaatz, and M. J. Rybak. 1999. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boswell, F. J., J. P. Ashby, J. M. Andrews, and R. Wise. 2002. Effect of protein binding on the in vitro activity and pharmacodynamics of faropenem. J. Antimicrob. Chemother. 50:525-532. [DOI] [PubMed] [Google Scholar]
- 3.Bozdogan, B., D. Esel, C. Whitener, F. A. Browne, and P. C. Appelbaum. 2003. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the Hershey Medical Center. J. Antimicrob. Chemother. 52:864-868. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Reduced susceptibility of Staphylococcus aureus to vancomycin—Japan, 1996. Morb. Mortal. Wkly. Rep. 46:624-626. [PubMed] [Google Scholar]
- 5.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 6.Chiang, F. Y., and M. Climo. 2003. Efficacy of linezolid alone or in combination with vancomycin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3002-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykhuizen, R. S., G. Harvey, N. Stephenson, D. Nathwani, and I. M. Gould. 1995. Protein binding and serum bactericidal activities of vancomycin and teicoplanin. Antimicrob. Agents Chemother. 39:1842-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldrick, B. 2002. First reported case of VRSA in the United States. Am. J. Nurs. 102:17. [DOI] [PubMed] [Google Scholar]
- 9.Harrington, G. D., L. T. Zarins, M. A. Ramsey, S. F. Bradley, and C. A. Kauffman. 1995. Susceptibility of ciprofloxacin-resistant staphylococci and enterococci to clinafloxacin. Diagn. Microbiol. Infect. Dis. 21:27-31. [DOI] [PubMed] [Google Scholar]
- 10.Heatherington, A. C., P. Vicini, and H. Golde. 1998. A pharmacokinetic/pharmacodynamic comparison of SAAM II and PC/WinNonlin modeling software. J. Pharm. Sci. 87:1255-1263. [DOI] [PubMed] [Google Scholar]
- 11.Herington, J. A., J. A. Fedirici, and J. M. Remy. 2000. Factors affecting the in vitro activity of moxifloxacin, p. 73-79. In D. Adam and R. Finch (ed.), Moxifloxacin in practice. Maxim Medical, Oxford, United Kingdom.
- 12.Hermsen, E. D., L. B. Hovde, J. R. Hotchkiss, and J. C. Rotschafer. 2003. Increased killing of staphylococci and streptococci by daptomycin compared with cefazolin and vancomycin in an in vitro peritoneal dialysate model. Antimicrob. Agents Chemother. 47:3764-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper, D. C. 2002. Fluoroquinolone resistance among gram-positive cocci. Lancet Infect. Dis. 2:530-538. [DOI] [PubMed] [Google Scholar]
- 14.Ince, D., X. Zhang, L. C. Silver, and D. C. Hooper. 2003. Topoisomerase targeting with and resistance to gemifloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, M. R. 2001. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 7:589-596. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, M. R., S. Bajaksouzian, A. Windau, P. C. Appelbaum, M. V. Patel, S. V. Gupta, S. S. Bagwat, N. J. De Souza, and H. Khorakiwala. 2004. In vitro activity of the new quinolone WCK 771 against staphylococci. Antimicrob. Agents Chemother. 48:3338-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, L., M. V. Miles, H. Lakkis, and A. L. Zaritsky. 1996. Vancomycin-binding characteristics in patients with serious infections. Pharmacotherapy 16:1024-1029. [PubMed] [Google Scholar]
- 18.Miller, L. C., and M. L. Tainter. 1994. Estimation of the ED50 and its error by means of logarithmic-probit graph paper. Proc. Soc. Exp. Biol. Med. 57:261-264. [Google Scholar]
- 19.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A6. NCCLS, Wayne, Pa.
- 20.Nishijima, S., I. Kurokawa, and H. Nakaya. 2002. Susceptibility change to antibiotics of Staphylococcus aureus strains isolated from skin infections between July 1994 and November 2000. J. Infect Chemother. 8:187-189. [DOI] [PubMed] [Google Scholar]
- 21.Nishijima, S., M. Nakagawa, N. Tsuboi, H. Akamatsu, T. Horio, M. Fujita, and S. Kawabata. 1996. Activity of nadifloxacin against methicillin-resistant Staphylococcus aureus isolated from skin infections: comparative study with seven other fluoroquinolones. J. Int. Med. Res. 24:12-16. [DOI] [PubMed] [Google Scholar]
- 22.Nishijima, S., S. Namura, H. Akamatsu, S. Kawai, Y. Asada, S. Kawabata, and M. Fujita. 1995. In vitro activity of nadifloxacin against both methicillin-susceptible and -resistant clinical isolates of Staphylococcus aureus from patients with skin infections. Drugs 49(Suppl. 2):230-232. [DOI] [PubMed] [Google Scholar]
- 23.Oizumi, N., S. Kawabata, M. Hirao, K. Watanabe, S. Okuno, T. Fujiwara, and M. Kikuchi. 2001. Relationship between mutations in the DNA gyrase and topoisomerase IV genes and nadifloxacin resistance in clinically isolated quinolone-resistant Staphylococcus aureus. J. Infect. Chemother. 7:191-194. [DOI] [PubMed] [Google Scholar]
- 24.Rubin, R. J., C. A. Harrington, A. Poon, K. Dietrich, J. A. Greene, and A. Moiduddin. 1999. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg. Infect. Dis. 5:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheel, O., D. J. Lyon, V. T. Rosdahl, F. A. Adeyemi-Doro, T. K. Ling, and A. F. Cheng. 1996. In-vitro susceptibility of isolates of methicillin-resistant Staphylococcus aureus 1988-1993. J. Antimicrob. Chemother. 37:243-251. [DOI] [PubMed] [Google Scholar]
- 26.Slatter, J. G., L. A. Adams, E. C. Bush, K. Chiba, P. T. Daley-Yates, K. L. Feenstra, S. Koike, N. Ozawa, G. W. Peng, J. P. Sams, M. R. Schuette, and S. Yamazaki. 2002. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 32:907-924. [DOI] [PubMed] [Google Scholar]
- 27.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, W. R. Jarvis, et al. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 28.Stalker, D. J., and G. L. Jungbluth. 2003. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin. Pharmacokinet. 42:1129-1140. [DOI] [PubMed] [Google Scholar]
- 29.Sun, H., E. G. Maderazo, and A. R. Krusell. 1993. Serum protein-binding characteristics of vancomycin. Antimicrob. Agents Chemother. 37:1132-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahata, M., M. Yonezawa, S. Kurose, N. Futakuchi, N. Matsubara, Y. Watanabe, and H. Narita. 1996. Mutations in the gyrA and grlA genes of quinolone-resistant clinical isolates of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 38:543-546. [DOI] [PubMed] [Google Scholar]
- 31.Takei, M., H. Fukuda, R. Kishii, and M. Hosaka. 2001. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob. Agents Chemother. 45:3544-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnidge, J. 1999. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs 58(Suppl. 2):29-36. [DOI] [PubMed] [Google Scholar]
- 33.Ueda, Y., and M. Sunagawa. 2003. In vitro and in vivo activities of novel 2-(thiazol-2-ylthio)-1β-methylcarbapenems with potent activities against multiresistant gram-positive bacteria. Antimicrob. Agents Chemother. 47:2471-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss, A., D. Milatovic, C. Wallrauch-Schwarz, V. T. Rosdahl, and I. Braveny. 1994. Methicillin-resistant Staphylococcus aureus in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 13:50-55. [DOI] [PubMed] [Google Scholar]
- 35.Yu, J. L., L. Grinius, and D. C. Hooper. 2002. NorA functions as a multidrug efflux protein in both cytoplasmic membrane vesicles and reconstituted proteoliposomes. J. Bacteriol. 184:1370-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]