Abstract
The lactoferrin-derived peptide hLF(1-11), but not its control peptide, was highly effective against five multidrug-resistant Acinetobacter baumannii strains in vitro (3 to 4 log reduction) and against four of these strains in an experimental infection in mice (2 to 3 log reduction). Therefore, this peptide is a promising candidate as a novel agent against infections with multidrug-resistant A. baumannii.
The emergence of epidemic Acinetobacter baumannii strains resistant to virtually all antibiotics, including carbapenems, poses a threat to hospitalized patients (6, 7, 14). Therefore, development of novel antibiotics for the treatment of infected patients is urgently required. In this connection, antimicrobial peptides with selective toxicity against bacteria are promising candidates, the more so because resistance to these peptides is as yet limited (4). The synthetic peptide corresponding to the first 11 N-terminal amino acids of human lactoferrin (hLF), hereafter referred to as hLF(1-11), which is highly effective against experimental infections with multidrug-resistant Staphylococcus aureus in rodents (11, 12), could be such an agent. The aim of the present study was to assess whether the hLF(1-11) peptide displays bactericidal activity against multidrug-resistant A. baumannii.
Five well-characterized A. baumannii strains from different locations in Europe were selected for this study (Table 1). The strains had been identified to be A. baumannii by amplified ribosomal DNA restriction analysis (2) and AFLP analysis (9). Strains RUH 134, RUH 875, LUH 7858, and LUH 7312 were from hospital outbreaks. Strain LUH 6034 was from a case of pneumonia, but its association with an outbreak is not known (Sylvain Brisse, personal communication). RUH 875 and RUH 134 are the respective reference strains of European clones I and II (1). Recently, it has been shown that these clones also prevail in the Czech Republic, with LUH 7858 being identified as clone II (10). Strain LUH 6034 from Spain was also identified as clone II (13). The strains were resistant to multiple antibiotics, as derived from MIC determination (http://www.szu.cz/cem/supplement/04/hlfmic.pdf). In particular, strains LUH 7858 and LUH 6034 were resistant or intermediate to 16 and 19 out of 20 antibiotics tested, respectively, including imipenem and meropenem.
TABLE 1.
Acinetobacter baumannii strains used in this study
| Strainc | European clone | Specimen of origin | Year of isolation | City, countrya of origin | Departmentb | Reference or source |
|---|---|---|---|---|---|---|
| RUH 875 | I | Urine | 1984 | Dordrecht, NL | Surgery | 1, 10 |
| RUH 134 | II | Urine | 1982 | Rotterdam, NL | ICU | 1, 10 |
| LUH 6034 (17C003) | II | Sputum | 1997 | Madrid, SP | ICU | 13 |
| LUH 7858 (NIPH 1469) | II | Sputum | 2001 | Prague, CZ | Surgical ICU | 10 |
| LUH 7312 | Sputum | 2001 | Leiden, NL | Surgical ICU | A. T. Bernards |
NL, The Netherlands; SP, Spain; CZ, Czech Republic.
ICU, intensive care unit.
Original strain designations are shown in parentheses.
For assessment of its antimicrobial activity, the synthetic peptide hLF(1-11) (GRRRRSVQWCA; 1,374 Da) and a similar peptide with alanine at positions 2, 3, 6, and 10 (control peptide) were purchased from UCB (Brussels, Belgium). The purity of these synthetic peptides exceeded 95%, as determined by reverse-phase high-performance liquid chromatography. Stocks of these peptides at 1 mM in 5% (vol/vol) acetic acid (pH 3.7) were stored at −20°C and dried in a Speed-Vac (Savant Instruments, Farmingdale, N.Y.) immediately before use.
The in vitro bactericidal activity of the hLF(1-11) peptide against the various strains of A. baumannii was quantified as described previously (11). Briefly, bacterial suspensions corresponding to approximately 2 × 106 CFU/ml were prepared in 10 mM sodium phosphate buffer supplemented with 1% (vol/vol) trypticase soy broth (CM129; Oxoid). Equal volumes of this bacterial suspension and of sodium phosphate buffer-trypticase soy broth containing various concentrations of hLF(1-11) peptide (range, 0 to 144 μM) or control peptide (0 to 72 μM) were mixed and then incubated for 60 min at 37°C. Next, the number of CFU per milliliter was determined. Results are presented as the means and standard deviations from three to four experiments.
The in vivo efficacy of hLF(1-11) against the strains was tested in an experimental mouse thigh model (11, 12) using specific-pathogen-free male Swiss mice weighing 20 to 25 g (Charles River Nederland, Maastricht, The Netherlands). Animal studies were approved by the Animal Experimental Committee of the Leiden University Medical Center (protocol 02029) and complied with relevant laws related to the conduct of animal experiments. Strains were first rendered virulent by two to three passages in mice (12). Strain LUH 6034 did not survive the mouse passages and was therefore not included in the in vivo experiments. In the infection experiments, mice were anesthetized with a single injection of 0.1 ml of saline containing 1 mg of fluanisone and 0.03 mg of fentanyl citrate (Hypnorm; Janssen Pharmaceutics, Tilburg, The Netherlands). Immediately thereafter, approximately 1 × 106 CFU of A. baumannii in 0.1 ml of saline was injected into the right thigh muscle. Approximately 18 h thereafter, 0.1 ml of saline containing hLF(1-11) peptide (range, 0 to 400 μg/kg of body weight) or control peptide (40 μg/kg) was injected intravenously. At 24 h after injection of the peptide, mice were sacrificed by intraperitoneal injection of 0.25 ml of saline containing 12 mg of sodium pentobarbital sodium (60 mg/ml, Nembutal; Sanofi BV, Maassluis, The Netherlands). The infected thigh muscles were removed and, after being weighed, homogenized in 4 ml of phosphate-buffered saline. Appropriate dilutions of the homogenate were plated onto Diagnostic Sensitivity Test agar plates (CM 261; Oxoid), and the number of CFU was determined after overnight incubation at 37°C. Results are expressed as the number of CFU per gram of infected thigh muscle. All negative cultures were assigned the value of 100 CFU/g, which was the minimum detection level. The Mann-Whitney U test was used for data analysis, with the level of significance being set at a P value of <0.05.
Our in vitro results showed that hLF(1-11) killed the five A. baumannii strains in a dose-dependent fashion, with maximum effects (3 to 4 log reduction) seen with 36 to 72 μM peptide, whereas the control peptide was without effect (Table 2). The bactericidal effect of hLF(1-11) was very rapid, i.e., within 5 to 15 min after exposure to the peptide, the maximal reduction in the number of CFU was achieved (data not shown).
TABLE 2.
Bactericidal activity of hLF(1-11) peptide against various A. baumannii strainsa
| Peptide concn (μM) | No. of surviving organisms (CFU/ml)
|
||||
|---|---|---|---|---|---|
| RUH 875 | LUH 7858 | RUH 134 | LUH 7213 | LUH 6034 | |
| hLF(1-11) | |||||
| 0 | 12 × 105 ± 4 × 105 | 9 × 105 ± 2.1 × 105 | 1.2 × 106 ± 0.1 × 106 | 6 × 105 ± 4.4 × 105 | 2 × 106 ± 0.4 × 106 |
| 9 | 7 × 104 ± 8.9 × 104b | 9 × 104 ± 8.8 × 104b | 3 × 104 ± 1 × 104b | 3 × 104 ± 3 × 104b | 8 × 104 ± 4.8 × 104b |
| 18 | 4 × 103 ± 3.2 × 103b | 3 × 104 ± 3.2 × 104b | 5 × 103 ± 2.9 × 103b | 2 × 103 ± 2 × 103b | 15 × 103 ± 3.8 × 103b |
| 36 | 2 × 103 ± 1.4 × 103b | 4 × 103 ± 2.9 × 103b | 3 × 103 ± 1.4 × 103b | 12 × 102 ± 5.7 × 102b | 4 × 103 ± 2.7 × 103b |
| 72 | 12 × 102 ± 7.5 × 102b | 63 × 102 ± 3.8 × 102b | 10 × 102 ± 6.2 × 102b | 670b | 670b |
| Control peptide | |||||
| 72 | 8 × 105 ± 1.4 × 105 | 6 × 105 ± 2.1 × 105 | 2 × 106 ± 1.2 × 106 | 8 × 105 ± 4.8 × 105 | 1 × 106 ± 1.2 × 106 |
Approximately 1 × 106 CFU of A. baumannii was incubated for 1 h at 37°C with a range of concentrations of hLF(1-11) (0 to 72 μM) or with 72 μM control peptide, and then the number of surviving microorganisms (CFU/ml) was determined. Results shown are the means and standard deviations from at least three to six independent experiments.
Values were significantly different (P < 0.05) from those obtained for A. baumannii exposed to control peptide or no peptide.
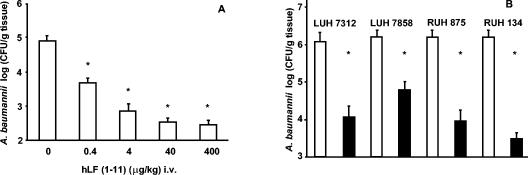
To determine the antibacterial activity of the hLF(1-11) peptide in vivo, mice were first infected with strain LUH 7312 to assess the dose of hLF(1-11) resulting in a maximum reduction in the viable count of bacteria. A dose-dependent bactericidal activity of hLF(1-11) peptide was observed, with a maximal effect (2 to 3 log reduction) after injection of 40 μg of peptide/kg (Fig. 1A). In further experiments, hLF(1-11) peptide, but not the control peptide, reduced (P < 0.01) the number of CFU of the four tested strains at the site of infection (Fig. 1B).
FIG. 1.
Activity of hLF(1-11) peptide against multiresistant A. baumannii strains. (A) Effect of various doses of hLF(1-11) on the number of viable bacteria in mice intramuscularly infected with A. baumannii LUH 7312. Results shown are the means and standard errors of the mean from three to nine animals. i.v., intravenously administered. (B) Bactericidal activity of hLF(1-11) peptide against four different A. baumannii strains in mice with a thigh muscle infection. Results shown are the means and standard errors of the mean from three to six animals. Open bars represent results from animals given control peptide, while filled bars represent results from animals given hLF(1-11). *, P < 0.05.
The main findings of the present study were that hLF(1-11) peptide displayed a dose- and time-dependent bactericidal activity against the various Acinetobacter strains in vitro and, more importantly, that the peptide was highly effective against A. baumannii strains in the experimental thigh muscle infection model. Furthermore, the amount of peptide that killed the bacteria in the mouse thigh experiment (approximately 1 to 1.5% of the injected dose [16]) was considerably lower than that required in our in vitro experiments, as was also observed previously with infections of multidrug-resistant S. aureus (11). This increased in vivo activity of the peptide compared to its in vitro activity may be attributed to effects of the peptide on the immune defense systems, as has been reported for hLF (3, 5, 15). This consideration raises the question of the extent to which in vitro activities of hLF(1-11) can be used to make general assumptions about its efficacy in the infected host.
The recent emergence of panresistant A. baumannii strains emphasizes the need for novel antibiotics to treat infections with these bacteria (8). The present results indicate that hLF(1-11) peptide is an interesting candidate for further exploration, including its ability to eradicate A. baumannii from infected patients, to remove the organisms from body surfaces of colonized patients, or to coat medical devices as a nonaggressive biodisinfectant to prevent adherence.
Acknowledgments
This project was financially supported in part by grant number 310/01/1540 of the Grant Agency of the Czech Republic and AM-Pharma.
Sylvain Brisse and Alexandra Bernards are gratefully acknowledged for the provision of strains LUH 6034 (17C003) and LUH 7312, respectively.
REFERENCES
- 1.Dijkshoorn, L., H. Aucken, P. Gerner-Smidt, P. Janssen, M. E. Kaufmann, J. Garaizar, J. Ursing, and T. L. Pitt. 1996. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 34:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijkshoorn, L., B. van Harsselaar, I. Tjernberg, P. J. Bouvet, and M. Vaneechoutte. 1998. Evaluation of amplified ribosomal DNA restriction analysis for identification of Acinetobacter genomic species. Syst. Appl. Microbiol. 21:33-39. [DOI] [PubMed] [Google Scholar]
- 3.Guillen, C., I. B. McInnes, D. M. Vaughan, S. Kommajosyula, P. H. C. van Berkel, B. P. Leung, A. Aguila, and J. H. Brock. 2002. Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. J. Immunol. 168:3950-3957. [DOI] [PubMed] [Google Scholar]
- 4.Hancock, R. E., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 5.Kai, K., K. Komine, Y. Komine, T. Kuroishi, T. Kozutsumi, J. Kobayashi, M. Ohta, H. Kitamura, and K. Kumagai. 2002. Lactoferrin stimulates a Staphylococcus aureus killing activity of bovine phagocytes in the mammary gland. Microbiol. Immunol. 46:187-194. [DOI] [PubMed] [Google Scholar]
- 6.Landman, D., J. M. Quale, D. Mayorga, A. Adedeji, K. Vangala, J. Ravishankar, C. Flores, and S. Brooks. 2002. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, N.Y.: the preantibiotic era has returned. Arch. Intern. Med. 162:1515-1520. [DOI] [PubMed] [Google Scholar]
- 7.Livermore, D. M. 2002. The impact of carbapenemases on antimicrobial development and therapy. Curr. Opin. Investig. Drugs 3:218-224. [PubMed] [Google Scholar]
- 8.Livermore, D. M. 2003. The treat from the pink corner. Ann. Med. 35:226-234. [DOI] [PubMed] [Google Scholar]
- 9.Nemec, A., T. De Baere, I. Tjernberg, M. Vaneechoutte, T. J. van der Reijden, and L. Dijkshoorn. 2001. Acinetobacter ursingii sp. nov. and Acinetobacter schindleri sp. nov., isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 51:1891-1899. [DOI] [PubMed] [Google Scholar]
- 10.Nemec, A., L. Dijkshoorn, and T. J. van der Reijden. 2004. Long-term predominance of two pan-European clones among multi-resistant Acinetobacter baumannii strains in the Czech Republic. J. Med. Microbiol. 53:147-153. [DOI] [PubMed] [Google Scholar]
- 11.Nibbering, P. H., E. Ravensbergen, M. M. Welling, L. A. van Berkel, P. H. C. van Berkel, E. K. J. Pauwels, and J. H. Nuijens. 2001. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 69:1469-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nibbering, P. H., M. M. Welling, A. Paulusma-Annema, C. P. J. M. Brouwer, A. Lupetti, and E. K. J. Pauwels. 2004. 99mTc-Labeled UBI 29-41 peptide for monitoring the efficacy of antibacterial agents in mice infected with Staphylococcus aureus. J. Nucl. Med. 45:321-326. [PubMed] [Google Scholar]
- 13.Van Dessel, H., L. Dijkshoorn, T. van der Reijden, N. Bakker, A. Paauw, P. van den Broek, J. Verhoef, and S. Brisse. 2004. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 155:105-112. [DOI] [PubMed] [Google Scholar]
- 14.Van Looveren, M., H. Goossens, and the ARPAC Steering Group. 2004. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin. Microbiol. Infect. 10:684-704. [DOI] [PubMed] [Google Scholar]
- 15.Velliyagounder, K., J. B. Kaplan, D. Furgang, D. Lagarda, G. Diamond, R. E. Parkin, and D. H. Fine. 2003. One of two human lactoferrin variants exhibits increased antibacterial and transcriptional activation activities and is associated with localized juvenile periodontitis. Infect. Immun. 71:6141-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welling, M. M., A. Paulusma-Annema, H. S. Balter, E. J. K. Pauwels, and P. H. Nibbering. 2000. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 27:292-301. [DOI] [PubMed] [Google Scholar]