Abstract
We evaluated the use of a simple and easy-to-obtain potential marker of tuberculosis (TB) drug efficacy, body weight, and correlated weight loss or gain with the number of CFU of Mycobacterium tuberculosis in lungs and spleens of infected mice. C3H mice were infected intravenously with 106 CFU of virulent M. tuberculosis H37Rv, and body weight was evaluated for several weeks after infection. At day 20, infected untreated mice consistently lost more than 25% of their body weight. Chemotherapy with selected orally active anti-TB drugs was initiated 7 days following infection and continued for 13 days. Drugs that were administered daily by gavage included isoniazid (INH), ethambutol (EMB), rifampin (RIF), and moxifloxacin (MXF). At the most effective doses, each of these drugs inhibited bacterial growth and abolished infection-induced body weight loss. Chemotherapy with 1/10 the standard dose of INH determined in accepted long-term murine models of TB also prevented body weight loss, while chemotherapy with 1/10 the standard dose of RIF did not. With only 2 weeks of chemotherapy, we observed a good reverse correlation between CFU in lung or spleen and body weight of mice. The simple measurement of weight in TB-infected drug-treated mice required only a weight balance, and go/no-go drug efficacy data was available on day 20 without the necessity of prolonged drug treatment and long (3 weeks or more) in vitro culture times to obtain organ CFU values.
A number of experimental formats have been used to evaluate existing drugs, drug combinations, and potential new drugs for activity against Mycobacterium tuberculosis in vivo. Most of the commonly accepted methods include either an intravenous high-dose (106 to 107 CFU) challenge of M. tuberculosis with start of chemotherapy at 0, 1, or 7 days following challenge (3, 4, 16, 17) or a low-dose aerosol TB infection with start of chemotherapy at 20 days following challenge and a duration of 45 days (13). At the end of these experiments, organs (specifically lungs and spleens) of infected control and drug-treated mice are cultured for several weeks and the number of viable CFU in treated mice are compared to those of controls. In addition to these more standard versions of murine TB infection, highly susceptible gamma interferon knock-out (GKO) mice are used (2, 5, 7, 13), and Lenaerts et al. recently proposed a rapid screening model using GKO mice infected with a low-dose aerosol challenge (Erdman strain M. tuberculosis) with a duration of chemotherapy from 18 to 30 days postinfection (10). Again, organs are cultured for several weeks to arrive at the number of CFU/organ, and CFU of control and treated mice are compared. All the models use reduction of mycobacterial load in organs as an index of drug efficacy, and all require 2 months or more of data collection (infection, treatment, organ culture) before data are available for review. Few investigators use survival time of infected and treated mice (too long) or other criteria for assessing drug activity. If these other parameters are used, it is usually in combination with CFU counts in organs (4).
If an investigator is interested in testing one or two drugs in animal models of TB, the complexity and time-consuming process of the test systems described above provide the most definitive data. For the assessment of larger chemical libraries of drugs, where there will certainly be few (if any) drugs with activity against M. tuberculosis in vivo, it is very important to shorten the process of in vivo drug testing and make it cheaper for mass drug screening. Although use of highly susceptible KO mouse strains allows some reduction in time (10), the cost and numbers of mice that are needed for consistent results are prohibitively high for a general screen.
To establish a reasonable equilibrium between the desire for maximal time reduction and the use of immunologically intact mouse strains that better represent human disease, we sought to identify parameters that might reflect antimicrobial activity of a drug in an easily available and less costly susceptible mouse strain, C3H mice. By genetic predisposition, these mice are classified as susceptible to TB. Their TB susceptibility is influenced by a natural allele of polymorphic gene sst1 located in chromosome 1 (8). Unlike GKO mice, which succumb to a host of pathogens, the susceptibility of C3H mice is restricted to a few pathogens, including virulent TB. We also wanted to identify parameters of TB infection that (i) could be consistently obtained with small variation between mice in order to reduce the numbers of mice per group, (ii) was easily quantified through external examination and did not require euthanasia and time-consuming organ culture, and (iii) was rapid in onset.
Development of progressive TB infection in a mouse is accompanied by symptoms and signs of lethal disease: increasing bacterial burden in organs, lung pathology, respiratory failure, progressive body weight loss, and death. In a high-dose challenge model of TB in the extraordinarily susceptible I/St mouse strain, these mice lost up to 25% of their body weight by 20 days after infection (12). Such a rapid and profound body weight loss did not occur in genetically resistant A/Sn mice until after 40 days of infection. In these genetic studies, the A/Sn mouse strain, maintained at the Central Institute for Tuberculosis in Moscow, Russia (but also available from commercial animal vendors in the United States), was the TB-resistant counterpart to the susceptible I/St strain (9, 12, 15). At 20 days, a significant difference in body weight between infected susceptible I/St mice and the resistant A/Sn or (I/St × A/Sn)F1 mice was observed for each sex (P < 0.0001) (9, 12). In fact, there is very good correlation between survival time and body weight loss in general in mice infected with M. tuberculosis. In the genetic studies (12), weight loss was evident in TB-susceptible first-generation backcross (I/St × F1) mice for males (r = 0.78, n = 33, P < 0.0001) and females (r = 0.75, n = 31, P < 0.0001) (9). These data were also confirmed by a lethal TB infection of I/St, C57BL/6, and (I/St × B6)F1 mice (14), which facilitated mapping three genetic loci controlling severity of the TB disease process in mice (15). Although I/St mice could be used in a rapid TB-drug-screening model, this strain is not commercially available, and we chose to test susceptible C3H mice that are available from many animal vendors.
In this study, we evaluated whether weight loss in M. tuberculosis-infected control animals and infected drug-treated mice could predict TB drug activity with sufficient accuracy and within a reasonable timeframe to be functional as a screening tool for identification of new TB drugs.
MATERIALS AND METHODS
M. tuberculosis strains.
M. tuberculosis strain H37Rv was originally obtained from the Institute Pasteur, Paris, France (a kind gift of G. Marchal). Mycobacteria were passaged through C57BL/6 mice (Charles River, Raleigh, N.C.) to maintain virulence. Mice were infected intravenously (i.v.), and 30 days later lungs were removed and homogenized. The lung homogenate was plated on 7H10 agar (Difco) plates, and plates were incubated at 37°C for 3 to 4 weeks. Colonies were collected and suspended in 7H9 broth supplemented with bovine serum albumin (BSA), dextrose, and catalase, and the mycobacterial suspension was cultured two successive times in roller bottles at 37°C for 7 days. The final culture was washed in phosphate-buffered saline (PBS) with 0.05% Tween 80, resuspended in PBS with 0.01% BSA and 0.05% Tween 80, dispensed in aliquots into polypropylene vials, and frozen at −80°C. CFU of the frozen aliquots were determined after thawing by plating serial 10-fold dilutions on 7H10 agar.
Antimicrobial agents.
Isoniazid (INH), ethambutol (EMB), and rifampin (RIF) were purchased from Sigma (St. Louis, Mo.). Moxifloxacin (MXF) was kindly provided by Bayer (West Haven, Conn.).
Animal model.
Specific-pathogen-free female mice of strain C3H/HeNCrlBR (C3H) (average weight, 22 g) were purchased from Charles River Laboratories and were allowed to acclimate for 1 week. At the initiation of the experiment, all mice were weighed, and their weights were recorded. Mice were inoculated i.v. in the tail vein with 0.2 ml of bacterial suspension containing approximately 106 CFU of M. tuberculosis H37Rv in PBS with 0.05% Tween 80. Seven days following infection, six of the infected mice were sacrificed, spleens and lungs were removed and homogenized, and serial 10-fold dilutions of organ homogenates in PBS with 0.05% Tween were plated on agar 7H10 to determine CFU counts in organs. The remaining infected mice were divided into groups (12 animals per group; 6 mice for CFU and body weight measurement and 6 for survival testing). At 7 days, the treatment of infected mice with drugs was initiated. Mice were treated daily with INH (25 mg/kg of body weight), RIF (20 mg/kg), EMB (100 mg/kg), or MXF (100 mg/kg). Two control groups, one of infected mice and one of uninfected mice, received water (placebo control). The dynamics of body weight gain (or loss) of mice was monitored (see below), and drug (or placebo) treatment was continued until day 20. At 20 days, six mice of each group were sacrificed, lungs and spleens were removed and homogenized, and CFU counts per organ were determined. The remaining six mice from each group were used to evaluate body weight changes and mortality. For mouse body weight determination we used portable electronic balance NO4120 for measurement of small-laboratory-animal body weight (Ohaus, Pine Brook, N.J.).
Statistical analyses.
CFU data and body weights were analyzed by analysis of variance, and the significance of differences between groups was analyzed by Student's t test (tSt).
RESULTS
CFU determination in lungs and spleens.
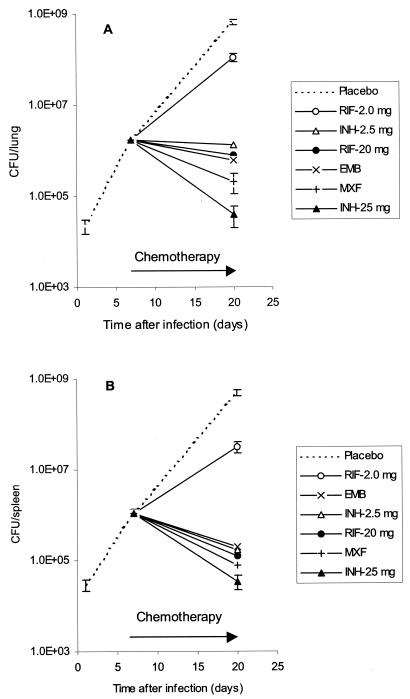
Mice were inoculated i.v. with 106 CFU of virulent M. tuberculosis H37Rv to develop a rapid and progressive TB disease. Chemotherapy was initiated 7 days after inoculation, when bacteria in spleens and lungs achieved significant levels (1.1 × 106 ± 0.2 × 106 and 1.7 × 106 ± 0.3 × 106 CFU, respectively). CFU in organs of untreated control infected mice continued to increase and reached 4.9 × 108 ± 0.8 × 108 in spleen and 6.5 × 108 ± 0.7 × 108 in lungs (Fig. 1) by 20 days.
FIG. 1.
Development of TB infection in lungs (A) and spleens (B) of mice treated with anti-TB drugs. Mice were infected i.v. with 106 CFU of M. tuberculosis H37Rv, and at day 7 chemotherapy was initiated. Differences between CFU for INH (25 mg) and RIF (20 mg) and for EMB (100 mg) are highly significant in lung (P < 0.001) (A) and in spleen (P < 0.05) (B).
Treatment of mice with any drug at its known effective concentration eliminated the increase in CFU in organs and reduced bacterial concentrations to levels below the CFU observed at the start of drug treatment (7 days). In terms of anti-TB activity with this single-drug regimen, the standard anti-TB drugs could be rank ordered as follows (best > worst): INH (3.4× 104 ± 0.6 × 104 CFU in spleen and 4.0 × 104 ± 2.0 × 104 CFU in lung) > MXF (7.8 × 104 ± 5.1 × 104 CFU in spleen and 2.1 × 105 ± 1.0 × 105 CFU in lung) > RIF (1.2 × 105 ± 0.5 × 105 CFU in spleen and 8.0 × 105 ± 2.5 × 105 CFU in lung) ∼ EMB (2.0 × 105 ± 0.6 × 105 CFU in spleen and 6.0 × 105 ± 0.8 × 105 CFU in lung). INH reduced the level of bacteria in spleen and lung more effectively than RIF and EMB, and the difference in activity was almost 1 log (P < 0.05 for spleens and P < 0.001 for lungs for INH and EMB). The activity of MXF was intermediate between INH and EMB or RIF.
Dynamics of body weight during TB infection and treatment.
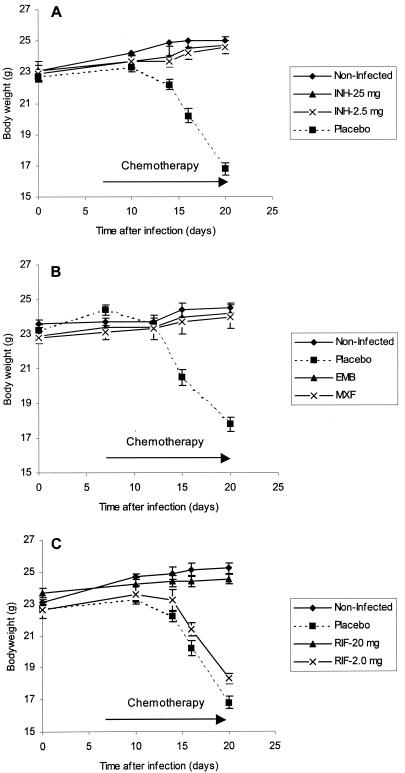
The high-dose TB challenge model was assessed by using weight gain (or loss) as an indicator of time of onset of disease and of drug activity. Chemotherapy with single drugs at their individually effective doses was initiated 7 days after inoculation with M. tuberculosis. Body weights of mice in all groups were monitored starting from time zero. By 10 days, infected placebo control mice started to lose weight; by 20 days these mice lost more than 25% of their body weight (Fig. 2).
FIG. 2.
Dynamics of body weight of uninfected and infected C3H mice treated with INH at 25 and 2.5 mg/kg (A), EMB and MXF at 100 mg/kg each (B), and RIF at 20 and 2.0 mg/kg (C). (A) Difference between untreated mice and all other groups was significant (P < 0.001); there was no statistical difference between uninfected mice and mice treated with either 25 or 2.5 mg of INH/kg. (B) Body weight of mice receiving placebo differs significantly (P < 0.001) from that of all other groups. (C) Difference between body weight of untreated mice (placebo) and mice treated with 20 mg RIF was significant (P < 0.001); the difference between 20 mg of RIF/kg and 2.0 mg of RIF/kg was also significant (P < 0.01); the difference between placebo and 2.0 mg RIF/kg was also significant (P = 0.046).
Uninfected mice and mice treated with INH (25 mg/kg), RIF (20 mg/kg), EMB (100 mg/kg), and MXF (100 mg/kg) did not lose weight at all; they even continued to gain weight. At 20 days, the average body weight of the untreated but infected (placebo) group of mice was between 16.8 and 17.5 g in different experiments, while body weights of the uninfected or infected drug-treated mice were between 23.9 and 25.2 g. The difference between body weight of placebo control mice and any other group was highly significant (P < 0.001). In this model, treatment of mice with standard anti-TB drugs abolished development of severe TB disease and prevented weight loss, one of the signs of TB severity.
Relationship between CFU and body weight in infected mice.
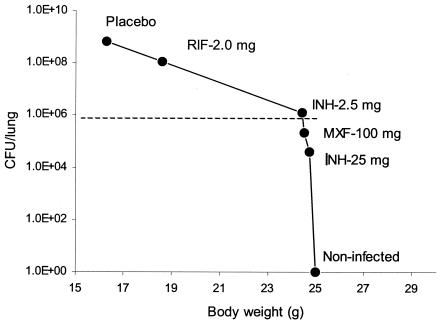
In these studies, drug efficacy and weight loss in mice at the end of the experiment were inversely correlated: drugs that reduced bacteria in lung from ∼106 to 104 CFU also held body weights at the level of uninfected mice. If lung CFU was greater than 106, then mice lost weight. Figure 3 shows a calibration curve for body weight and CFU in the lung.
FIG. 3.
Correlation of CFU M. tuberculosis H37Rv in lung and body weight of mice 20 days after infection.
These data suggest that the rapid in vivo screening assay could distinguish effective drugs (CFU in lung, <106) from drugs with lower efficacy. In addition, the model discriminated between efficacy of drugs in less active groups (106 to 109 CFU/lung, corresponding to different body weights) but did not distinguish between the highly effective drugs (body weights remained roughly the same for lung CFU from 106 to 0). Drug-induced reduction of CFU levels in the lung to ∼106 is a critical point for this model.
Based on these results, we concluded that any drug preventing body weight loss could be considered very effective; if body weight decreased, the drug was less effective and relative efficacy could be determined by the degree of weight loss. In each experiment, mice were numbered. For each body weight, the corresponding CFU in lung and spleen of that mouse at the moment of sacrifice was determined, and we assessed the correlation of body weight and CFU. There was a very good correlation (r = 0.959) between lung and spleen CFU calculated throughout infection and drug treatment. There was a good inverse correlation between CFU in lung and mouse body weight (r = −0.845) as well as between CFU in spleen and body weight (r = −0.844). These data suggest that (i) mouse body weight was good evidence of CFU levels in organs and, therefore, efficacy of drug treatment, and (ii) CFU in any organ—lung or spleen—equally reflected efficacy of the drug treatment.
Efficacy of lower doses of INH and RIF.
To study dose dependency of drug efficacy and its correlation with weight loss, we used 1/10 the standard dose of INH and RIF of 2.5 and 2.0 mg/kg, respectively. Use of a 10-fold-reduced dose of RIF resulted in dramatic increase in CFU in lung and spleens in comparison to the standard dose of the drug, while 1/10 the standard dose of INH continued to control infection in organs (Fig. 1). Figure 2A shows that both 2.5 and 25 mg of INH/kg is sufficient to prevent body weight loss, at least up to 20 days after infection. RIF at standard doses was effective in limiting weight loss, but at 1/10 the standard dose it was not effective (Fig. 2C). Mice treated with 2 mg/kg weighed only 18.3 ± 0.4 g at 20 days after infection. Although weights of mice treated with 2 mg of RIF/kg differed from those of placebo mice (16.8 ± 0.4 g; P = 0.046), the low dose of RIF did not control progression of disease severity. The difference between the standard-dose RIF group (body weight at 20 days was 25.0 ± 2.4 g) and the low-dose group (weight, 18.3 ± 0.4 g) was highly significant (P < 0.01).
Drug efficacy could be evaluated 2 weeks following infection of mice.
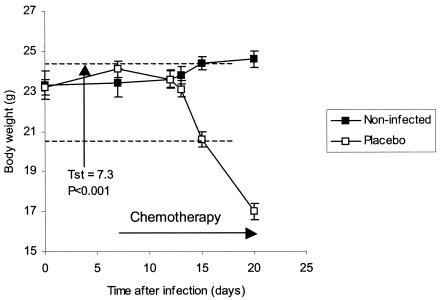
The shorter the screening assay, the more valuable the results, as long as discrimination between effective and ineffective drugs was not compromised. In the in vivo screening assay described above, infected and placebo-treated mice began to lose weight 10 days after infection. At 15 days, these infected mice had body weights of 20.6 ± 0.4 g, while uninfected control mice had body weights of 24.4 ± 0.3 g. This weight difference with six mice per group was very significant (tst = 7.3; P < 0.001) (Fig. 4).
FIG. 4.
Difference in body weights between infected untreated mice and uninfected mice at day 16 following infection (P < 0.001).
These data, collected from a number of similar experiments, suggested that the earliest time to check body weights of control and treated mice for preliminary data on in vivo efficacy of a drug was between 14 and 16 days after infection. If mice did not lose body weight by 14 to 16 days of drug therapy, the drug could be considered potentially active and a candidate for study in the more detailed conventional model(s) of TB. The short 14- to 16-day test allowed us to evaluate drugs prior to the onset of extreme cachexia in infected mice.
In addition, drug efficacy in this screening model can be estimated with a high degree of accuracy by using the slope of body weight loss from 14 to 15 days until that at 20 days (Fig. 2 and 4). The slope of weight loss in infected placebo-treated mice was a straight line. Mice treated with effective concentrations of drugs (INH, RIF, etc.) or uninfected mice both had a horizontal or slightly increasing slope. In Table 1 we present data on individual mouse and group average body weight changes during 24 h between 15 and 16 days after infection. Infected placebo-treated mice lost 1.02 ± 0.06 g; mice treated with an effective (standard) dose of INH (25 mg/kg) gained 0.17 ± 0.05 g; mice treated with an insufficient dose of MXF (10 mg/kg) lost 0.50 ± 0.11 g.
TABLE 1.
Changes in body weight (grams) between days 15 and 16 in mice infected with M. tuberculosis H37Rv and treated with anti-TB drugs (n = 6)a
| Placebo | INH (25 mg/kg) | MXF (10 mg/kg) |
|---|---|---|
| −1.18 | 0.00 | −0.51 |
| −1.00 | 0.10 | −0.28 |
| −1.12 | 0.32 | −0.10 |
| −0.80 | 0.12 | −0.72 |
| −0.92 | 0.08 | −0.80 |
| −1.08 | 0.10 | −0.60 |
Difference between placebo and INH groups, tst = −15.2, P < 0.001. Difference between placebo and MXF, tst = −4.1, P = 0.002. Difference between INH and MXF, tst = 5.29, P < 0.001. Averages for placebo, INH, and MXF were (in grams) −1.02 ± 0.06, 0.17 ± 0.05, and −0.50 ± 0.11, respectively.
Differences between all these groups were statistically significant. Thus, degree (or intensity) of weight loss during a 1-day period beginning approximately 2 weeks after infection can serve as a good estimation of drug efficacy.
Long-term consequences of a short-course drug treatment.
We monitored body weight dynamics and mortality of mice after chemotherapy withdrawal. Of course, such a short course (2 weeks) of drug treatment does not cure mice of their TB infection, but it does reduce the number of bacteria in organs and appears to shift an acute infection into a chronic disease. Development of the signs and symptoms of fulminating TB in drug-treated mice takes place later than untreated controls and depends on the potency of the drug used for treatment. In all cases, however, C3H mice treated with only a short course of effective drugs eventually developed severe disease, suffered weight loss, and died. Mice treated with effective drugs started to lose body weight after 50 days following infection and 30 days following chemotherapy withdrawal. Delay in mortality of these mice depended on drug efficacy during the 2 weeks of treatment (data not shown).
DISCUSSION
In the present study, we analyzed a new approach for rapid in vivo screening of potential anti-TB drug libraries. We showed that weight loss, one of the main signs of disease severity in TB-infected mice, did not occur in mice treated with any of the conventional first-line anti-TB drugs. To shorten the timeframe for discriminating between effective and ineffective drugs, we used a high-dose challenge of M. tuberculosis in genetically susceptible (but immunocompetent) C3H mice. Infected mice that did not receive drugs began to lose weight by 10 days after challenge, and by 20 days after infection these mice lost more than 25% of their body weight. These results correlate with data published by others (9, 12). Chemotherapy with standard doses of INH, EMB, RIF, or MXF starting 7 days after infection absolutely prevented development of TB-related weight loss. In all cases, maintenance of weight in drug-treated mice correlated with reduced CFU in spleen and lung, with 106 CFU being a critical microbial burden in these organs. In all cases, weight loss correlated with increased CFU in lung and spleen (above 106 CFU/organ), whether mice were untreated for the duration of the study or were treated with ineffective doses of a drug.
The rapid screening model of these studies differs in intent from the acute or chronic TB models that are usually used for a detailed analysis of drug activity. The objective here was to find a way to screen, with a high degree of accuracy, a large compound library of many drugs and select only those whose activity in vivo is demonstrable. Drugs that show activity can be moved to the next level of analysis, which takes advantage of the careful and considered details of the established animal models used in TB research laboratories.
Such an in vivo screen needs to be robust enough to reduce numbers of mice required per drug tested, and variance of the parameter between mice should be minimal. It should be easy to measure and be relatively straightforward to perform. The value of measuring weight at a specific time is that this variable was remarkably constant in the two test systems that serve as controls: untreated mice and mice treated with effective doses of known anti-TB drugs. The differences between these two groups on day 16 after infection were consistent and statistically significant. Thus, the positive (infected, drug-treated) and negative (infected, untreated) control groups provided a range in weight sufficient enough that we were able to detect ineffective drug therapy, as demonstrated by weight loss and CFU data with the reduced and ineffective dose of RIF.
As in all screening assays, in vivo or in vitro, seemingly active compounds in the TB-induced weight loss screen must be further tested to determine whether the active compounds exert their activity by appropriate or inappropriate means. It is possible that drugs possessing anabolic properties could give false-positive results in this assay. On the other hand, the assay is not meant to be a definitive judgment of the antitubercular activity of a compound. It is meant to screen large chemical libraries and reduce the number of compounds to a manageable number of actives that can be evaluated in a more informative model of TB disease.
In addition to body weight measurement at 3 weeks of infection, survival of the same mice over 4 to 5 weeks confirmed the results of weight alterations. In our experiments, all mice treated with effective doses of INH, MXF, EMB, and RIF were alive and maintained body weights longer than 30 days after infection, while all nontreated infected mice (placebo) died in the interval of 22 to 28 days. Thus, two criteria could be used for a rapid, relatively inexpensive, and simple evaluation of anti-TB drug activity: body weight by 3 weeks and mortality by 4 weeks.
Interestingly, the TB weight loss model also prioritized the early in vivo efficacy of known anti-TB drugs in the same order that has been demonstrated in the lengthy and complicated long-term TB infection models for mice: INH > MXF > EMB ∼ RIF. In other studies, we tested INH and EMB in a chronic mouse model of TB by using C57BL/6 mice infected with a low dose of M. tuberculosis H37Rv. Chemotherapy was started 20 days following this low-dose challenge, when TB replication stabilized in spleen and lung at the level that does not induce further weight loss; the mice remained alive for months. Determination of CFU in lung and spleen showed that efficacy of INH was significantly higher than that of EMB (unpublished results). In addition, both clinical and experimental studies show that INH is a more active TB drug than EMB (11). RIF has also demonstrated activity in both low- and high-dose challenge models of TB in mice, but in several studies its activity is less effective than that of INH (6). MXF, a fluoroquinolone, was very effective in our rapid model. Its efficacy was nearly comparable to the efficacy of INH and surpassed that of the other anti-TB drugs, EMB and RIF. This is consistent with data of other investigators that used different murine models of TB (1, 10, 17).
It is possible that efficacy of drugs could differ in acute progressive TB, as exemplified by our rapid in vivo screening model and in the stable chronic infection that is more classically used to evaluate individual drugs. However, our data with the first-line TB drugs and MXF suggest that an active drug should be active in both models. We propose to use weight after infection and drug treatment as a rapid and inexpensive tool to select in vivo-active drugs from a subset of compounds in a large chemical library, compounds with in vitro MICs in appropriate ranges. For this in vivo efficacy assay, one needs six mice per group, a balance, and a total duration of 20 days from TB infection of mice until results are obtainable. For further detailed analysis of selected drugs, effective doses, and mechanism(s) of action, one can use the more complicated and time-consuming high-dose challenge or a chronic model of TB disease.
Acknowledgments
This work was supported by National Institutes of Health grant AI-049514-01 (“Second generation antibiotics from ethambutol”).
REFERENCES
- 1.Alvireaz-Freits, E. J., J. L. Carter, and M. H. Cynamon. 2002. In vitro and in vivo activity of gatifloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J. Exp. Med. 178:2243-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cynamon, M. H., S. P. Klemens, C. A. Sharpe, and S. Chase. 1999. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 43:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel, N., N. Lounis, J. I. Baohong, R. L. O'Brien, A. Vernon, L. J. Gaiter, M. Szpytma, C. Truffot-Pernot, G. Hejblum, and J. Grosset. 2000. Antituberculosis activity of once-weekly rifapentine-containing regimens in mice. Am. J. Respir. Crit. Care Med. 161:1572-1577. [DOI] [PubMed] [Google Scholar]
- 5.Flynn, J., L. Chan, J. Triebold, K. J. Dalton, D. K. Stewart, and T. A. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie, S. H., R. D. Gosling, L. O. Uiso, N. E. Sam, E. Bongard, E. G. Kanduma, M. Nyindo, and R. W. Morris. 2003. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 168:1342-1345. [DOI] [PubMed] [Google Scholar]
- 7.Kelly, B. P., K. Furney, M. T. Jessen, and I. M. Orme. 1996. Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:2809-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramnik, I., W. F. Dietrich, P. Demant, and B. R. Bloom. 2000. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:8560-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavebratt, C., A. S. Apt, B. V. Nikonenko, M. Schalling, and E. Schurr. 1999. Severity of tuberculosis in mice is linked to distal chromosome 3 and proximal chromosome 9. J. Infect. Dis. 180:150-155. [DOI] [PubMed] [Google Scholar]
- 10.Lenaerts, A. J. M., V. Gruppo, J. V. Brooks, and I. M. Orme. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47:783-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lounis, N., C. Maslo, C. Truffot-Pernot, J. Grosset, and R. J. Boelaert. 2003. Impact of iron loading on the activity of isoniazid or ethambutol in the treatment of murine tuberculosis. Int. J. Tuberc. Lung Dis. 7:575-579. [PubMed] [Google Scholar]
- 12.Nikonenko, B. V., M. M. Averbakh, Jr., C. Lavebratt, E. Schurr, and A. S. Apt. 2000. Comparative analysis of mycobacterial infection in susceptible I/St and resistant A/Sn inbred mice. Tuberc. Lung Dis. 80:15-25. [DOI] [PubMed] [Google Scholar]
- 13.Orme, I., J. Secrist, S. Anatham, C. Kwong, R. Reynolds, et al. 2001. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 45:1943-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radaeva, T. V., B. V. Nikonenko, and A. S. Apt. 2002. Genetic control of severity of the course of TB infection in mice at complement heredity of resistance. Probl. Tubercl. (Russia) 10:28-30. [Google Scholar]
- 15.Sanchez, F., T. V. Radaeva, B. V. Nikonenko, A.-S. Persson, S. Sengul, M. Schalling, E. Schurr, A. S. Apt, and C. Lavebratt. 2003. Multigenic control of disease severity after virulent Mycobacterium tuberculosis infection in mice. Infect. Immun. 71:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoen, C. M., S. E. Chase, M. S., DeStefano, T. S. Harpster, A. J. Chmielewski, and M. H. Cynamon. 2000. Evaluation of rifalazil in long-term treatment regimens for tuberculosis in mice. Antimicrob. Agents Chemother. 44:1458-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimatsu, T., E. Nuermberger, S. Tyagi, R. Chaisson, W. Bishai, and J. Grosset. 2002. Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob. Agents Chemother. 46:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]