Abstract
Neuropsychiatry disorders are common health problems affecting approximately 1% of the population. Twin, adoption, and family studies have displayed a strong genetic component for many of these disorders; however, the underlying pathophysiological mechanisms and neural substrates remain largely unknown. Given the critical need for new diagnostic markers and disease-modifying treatments, expanding the focus of genomic studies of neuropsychiatric disorders to include the role of non-coding RNAs (ncRNAs) is of growing interest. Of known types of ncRNAs, microRNAs (miRNAs) are 20–25-nucleotide, single-stranded, molecules that regulate gene expression through post-transcriptional mechanisms and have the potential to coordinately regulate complex regulatory networks. In this review, we summarize the current knowledge on miRNA alteration/dysregulation in neuropsychiatric disorders, with a special emphasis on schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD). With an eye toward the future, we also discuss the diagnostic and prognostic potential of miRNAs for neuropsychiatric disorders in the context of personalized treatments and network medicine.
Keywords: Non-coding RNA, MiRNA, Schizophrenia, Bipolar disorder, Neuroplasticiry
1. Introduction
Neuropsychiatric disorders are chronic and severe medical conditions characterized by a complex range of symptoms including psychosis, depression, mania, and cognitive deficits. The underlying pathogenic mechanisms of these disorders remain largely elusive; however it is considered that both genetic factors and environmental exposures play important roles. In particular, as discussed below in more detail, recent genome-wide association studies (GWAS) and next-generation sequencing of common neuropsychiatric disorders such as schizophrenia (SCZ), bipolar disorder (BD),and major depressive disorder (MDD), have identified multiple genetic variants associated with risk, indicating that variation in a single gene is insufficient to cause the underlying disorder (McCarroll and Hyman, 2013). This etiology contrasts to rare neuropsychiatric disorders, such as Fragile X syndrome (FMR1) (Fu et al., 1991), Rett syndrome (MECP2) (Amir et al., 1999), and Pitt-Hopkins syndrome (TCF4) (Amiel et al., 2007), where highly penetrant, most often de novo mutations in a single gene are sufficient to cause characteristic neurodevelopmental, cognitive, and behavioral symptoms of each disorder.
MiRNAs play a role in almost all biological processes, such as cell proliferation, development, differentiation, and apoptosis (Krol et al., 2010). Compared to other organs, the brain has a particularly high percentage of tissue-specific and tissue-enriched miRNAs, implying possible roles in neuronal differentiation, synaptic plasticity, and neurite outgrowth (Sun and Shi, 2015). On the other hand, deregulation of miRNA expression and function is associated with the pathogenesis of neuropsychiatric disorders.
Considering emerging evidence for the involvement and important roles of miRNAs in the pathogenesis of neuropsychiatric disorder, as well as accumulating evidence for single nucleotide polymorphisms (SNPs) and copy number variations (CNVs) in miRNAs and their target genes, we aim here to summarize recent studies and discuss the possible biomarker value of miRNAs for diagnostic assessment improvement and personalized medicine.
2. Pathogenesis of neuropsychiatric disorders
2.1. Alterations in neurodevelopment
Neural development starts during early stages of embryogenesis, and involves proliferation and differentiation of neurons, migration of immature neurons to their ultimate brain region, outgrowth of axons and dendrites, and generation, as well as proper maintenance, of synapses (Stiles and Jernigan, 2010). The neurodevelopmental hypothesis of SCZ has been linked to adverse conditions, such as genetic or environmental factors leading to abnormal or altered brain development and inappropriate connections of neurons during the perinatal period (Rapoport et al., 2012). The damage of the brain from the altered early development causes reduced cortical volume, altered gray matter loss, and ventricular enlargement at the onset of SCZ (Kochunov and Hong, 2014). In addition to SCZ, altered brain development has been implicated in BD. Neuroimaging studies showed that BD patients lose gray matter volume of emotion related brain areas including the insula and orbitofrontal, rostral, and dorsolateral prefrontal cortical (DLPFC) areas (Najt et al., 2015).
Recent genetic and molecular findings further support a role for altered neurodevelopment in neuropsychiatric disorders. For example, GWAS have shown that the ANK3 gene encoding the protein Ankyrin-G, which has many roles in cellular processes including neural development, is associated with risk for BD (Chen et al., 2013; Ferreira et al., 2008; Muhleisen et al., 2014). As evidence of this, Ankyrin-G has been shown to play a key role in organizing the axon initial segment in polarized neurons, to mediate AMPA-receptor mediated synaptic transmission, and to maintain proper dendritic spine morphology in glutamatergic neurons (Smith et al., 2014), and Durak et al. have recently reported that loss of ANK3 elevated the number of newborn neurons in the embryonic “ mouse brain (Durak et al., 2015). Furthermore, recent studies using BD-patient derived induced pluripotent stem cell (iPSC) models have provided a new line of evidence for altered neurodevelopmental processes in BD (Chen et al., 2014; Madison et al., 2015), including alterations of miRNAs such as miR-34a that targets ANK3 as discussed below in more detail (Bavamian et al., 2015).
2.2. Disrupted synaptic plasticity
Synaptic plasticity is a term that describes the changes in the synapse strength that occur over time. Synaptic plasticity plays a major role in establishing and maintaining the correct connections between neurons. All brain activities, including higher cortical functions involved in cognition, depend on the presence of the correct synaptic connections (Cowan et al., 1984; Tau and Peterson, 2010).
Historically, SCZ is associated with abnormal connections or disconnections between brain regions. Electroencephalography (EEG) and magnetoencephalography/electroencephalogram (MEG/EEG) studies supported the disconnection hypothesis (Uhlhaas and Singer, 2010). Additionally, functional neuroimaging studies have found reduced frontotemporal connectivity by functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) (Stephan et al., 2009). These anatomical changes may be mediated by abnormal synaptic plasticity that leads to changes in size and numbers of dendritic spines and postsynaptic receptor density and thereby affects connectivity patterns in the brain.
Post-mortem studies have also revealed several abnormalities in synaptic formation and plasticity in BD. These abnormalities include lower number of neurons in specific brain regions, and decreased glial cell density in frontal cortical regions (Harrison, 2002; Vawter et al., 2000). Apart from these morphological changes, protein marker of synaptic numbers (such as Growth Associated Protein 43) is lower in depression, and increases with antidepressant treatment (Schloesser et al., 2008). Region-specific alterations in the expression of synaptic vesicular proteins, including synaptosomal-associated protein 25 (SNAP-25), syntaxin, synaptobrevin and synaptophysin, have been reported in brain samples of patients with BD (Gray et al., 2010).
2.3. Large-scale human genetic studies
Adoption and twin studies have confirmed that heritable genetic risk factors in psychiatric disorders (including SCZ and BD) play a major role in disease etiology. In two Swedish population studies, heritability for SCZ and BD was reported as 64% and 58%, respectively (Lichtenstein et al., 2009, J. Song et al., 2015). Linkage and association studies have identified several chromosomal regions that are linked to psychiatric disorders. For SCZ, these studies have recently led to the association of over 108 loci, including multiple components of glutamatergic synapses and chromatin modifications (Network & Pathway Analysis Subgroup of Psychiatric Genomics, 2015; Schizophrenia Working Group of the Psychiatric Genomics, 2014), although further studies are required to unambiguously elucidate causal genetic factors in each locus [reviewed in (Sullivan et al., 2012; Kotlar et al., 2015)]. Additional genetic findings on rare variants, as identified by CNV and exome sequencing studies in SCZ, have also pointed to a key role for synaptic proteins as well as for the actin cytoskeleton (Fromer et al., 2014; Kirov et al., 2012; Need et al., 2012; Purcell et al., 2014; Timms et al., 2013). Furthermore, emerging functional studies on these rare variants in SCZ provide support for dysregulation of synaptic signaling pathways including G1T1-PAK3 involved in actin cytoskeleton dynamics (Kim et al., manuscript under review).
The results of GWAS point out to several chromosomal regions, which are associated with BD risk (Chen et al., 2013; Ferreira et al., 2008; Muhleisen et al., 2014), although the total sample size analyzed to date is smaller than that of SCZ. This includes genetic variation within the loci encoding the axonal initial segment protein Ankyrin-G encoded by the ANK3 gene (Chen et al., 2013; Ferreira et al., 2008; Hughes et al., 2015; Muhleisen et al., 2014; Rueckert et al., 2013) as well as the CACNA1C gene encoding a subunit of the L-type calcium channel, both of which play key roles in diverse aspects of neuronal development and function (Berger and Bartsch, 2014; Sinnegger-Brauns et al., 2009).
Several CNVs are associated with increased risk for psychiatric disorders. More than 15 genomic regions are associated with increased risk for SCZ; however, these variants are less common in BD (Kotlar et al., 2015). A deletion in 2p16.3 region, which contains synaptic protein gene neurexin-1 (NRXN1), increases the risk of SCZ 8.9 fold (Vinas-Jornet et al., 2014). Another locus (3q29 region) that contains 22 genes has also been implicated as a risk for SCZ: a recent meta-analysis of 25,904 SCZ patients and 62,871 healthy controls indicate that the 3q29 deletion confers a 41.1-fold increased risk for SCZ (Mulle, 2015). Various candidate genes are located in this region, including Disks large homolog 1 (DLG1), p21 activated kinase (PAK2), and F-box only protein 5 (FBXO45) (Mulle, 2015). Further studies for understanding of biological results of these CNVs will help us clarify the mechanism of psychiatric diseases.
In sum, on-going large-scale, genetic studies on SCZ, BD, and MDD populations are likely to yield more accurate and replicable findings that will facilitate the discovery of the true causal variants within the multiple loci implicated. Gaining insight into how these genetic risk factors collectively impact the biological mechanisms underlying the pathophysiology of any one individual will require a deeper understanding of the molecular mechanisms that coordinately regulate neurodevelopment and neuroplasticity. For this, consideration of how non-genetic mechanisms, i.e. epigenetic mechanisms and post-transcriptional forms of gene regulation, involving non-coding RNAs and other regulatory molecules that operate at the level of specific cells within defined neurocircuits is likely to be informative.
2.4. Alterations in epigenetic mechanisms
While genetic factors play important roles in the etiology of neuropsychiatric disorders, the results of twin studies indicate that additional factors, such as epigenetic mechanisms, are essential for disease onset. Epigenetic mechanisms include histone and DNA modifications such as acetylation, ubiquitination, SUMOylation, methylation, phosphorylation of noncoding RNAs (Nestler et al., 2015). Epigenetic mechanisms contribute to brain development and physiological function of the brain. Disruption of epigenetic regulatory mechanisms could be associated with psychiatric disorders (Nestler et al., 2015).
Postmortem human brain and blood studies in patients with SCZ have shown that promoter regions of several genes including REELIN, SRY-box 10 (SOX10), major histocompatibility complex (HL4), gluta-mate decarboxylase 1 (GAD1), catechol-O-methyltransferase (COMT) and BDNF (Abdolmaleky et al., 2005; Carrard et al., 2011; Ikegame et al., 2013; Kundakovic et al., 2015; Walton et al., 2014) are methylated. Target genes of abnormal DNA methylation are common SCZ and BD. HLA9 and GAD1 gene methylation changes were observed in the brain tissue of patients with BD (Kaminsky et al., 2012; Ruzicka et al., 2015). Dynamic changes in histone acetylation and methylation have also been shown to contribute to rodent stress models and antidepressants drugs affect these same histone-modifying mechanisms (Nestler et al., 2015), although the full relevance of these observations to human disease is unknown given challenges inherent to modeling complex behaviors in rodent models. Understanding if changes in miRNA expression occur in neuropsychiatric disorders due to epigenetic regulatory mechanism, such as DNA methylation in miRNA genes, represents an important avenue for future studies.
3. MiRNAs
MiRNAs are short, single stranded, endogenous, and noncoding RNAs that regulate gene expression by binding to complementary sequences principally in their target mRNAs′ 3′-untranslated region (UTR), and also to a lesser extent to the 5′-UTR and coding regions (Hamzeiy et al., 2014). A region of about 2–8 to nucleotides within the structure of the miRNA is called the “seed sequence/region”. Target recognition is primarily determined by this seed sequence, leading to the regulation of gene expression (Lewis et al., 2003). Here the definition of a miRNA target refers to a specific pairing of a miRNA and a mRNA sequence, with the recognition that formal proof of a target of a miRNA requires extensive experimental validation beyond what can be simply predicted based upon sequence homology. In many cases the manipulation of a miRNA may lead to alterations of the expression of a gene through secondary indirect effects of a miRNA on other regulatory mechanisms or genes, which is an important caveat when considering list of reported target genes in many studies.
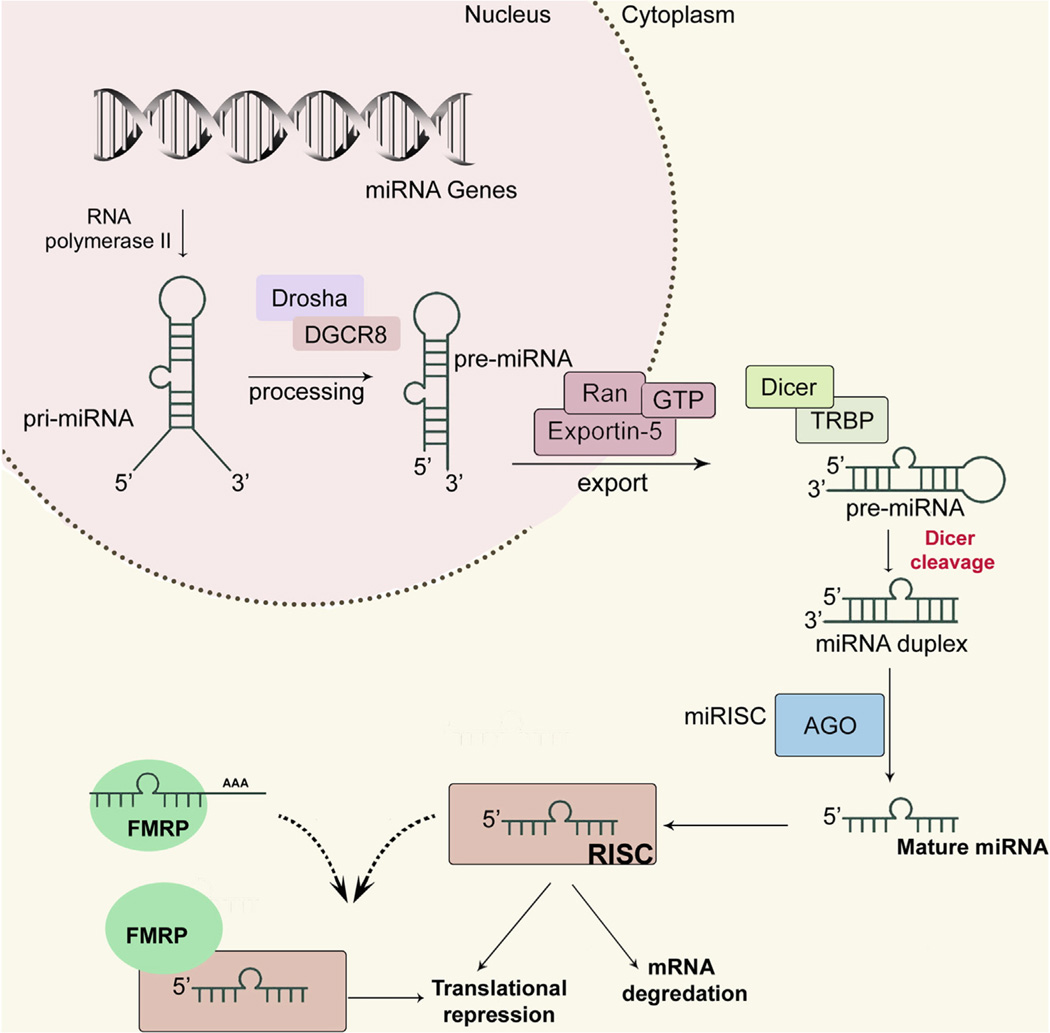
The canonical miRNA biogenesis applies to the maturation of most miRNA families, except unique miRNA families that have been described before (Hammond, 2015). In the canonical pathway, a primary transcript (pri-miRNA) is transcribed in the nucleus by RNA polymerase II; next, Drosha and ‘DiGeorge syndrome critical region gene 8’ (DGCR8) process the pri-miRNA to generate precursor miRNA (pre-miRNA). Pre-miRNAs are then exported to the cytoplasm via exportin 5, a double-stranded RNA-binding protein, where Dicer cleaves the pre-miRNA hairpin loop to form ~22 base pair mature miRNAs. Finally, mature miRNAs are loaded into the RNA-induced silencing complex (RISC) to regulate translation process (Fig. 1) (Ha and Kim, 2014). Depending on the complementarity between the miRNA and its target(s), miRNAs either repress translation, or cause degradation of target mRNAs (Dalmay, 2013).
Fig. 1.
Overview of miRNA biogenesis and its interaction with FMRP. RNA polymerase transcribes the miRNA genes, and Drosha processes the resulting pri-miRNAs. Then, pre-miRNAs are exported into the cytoplasm and are further processed by Dicer. Mature miRNAs are loaded into the RISC, which in turns binds to complementary sequences on 3′UTRs of target mRNAs. According to an alternative hypothesis, FMRP binds to some mRNAs, and miRNA-RISC can interact with FMRP-mRNA complex, thereby leading to translational inhibition.
Conventional methods used to measure miRNA expression include microarrays, real-time PCR, in situ hybridization. In addition, various methods are currently being developed, such as nanoparticle-derived probes, isothermal amplification, electrochemical methods, and next-generation sequencing (Tian et al., 2014). Considering that i) single base differences can significantly affect miRNA binding to target genes, and ii) different miRNA family members share closely-related sequences, sequencing is a highly valuable approach to detect and quantify specific miRNAs.
Approximately 75% of annotated miRNAs are expressed in the brain and contribute both physiological brain development and neural function including neurogenesis, neuronal differentiation, and synaptogenesis (Ji et al., 2013). Moreover, miRNAs play critical roles in cell fate determination of neurons and glia, by suppressing specific target genes. For example, miR-124 is one of the best-studied miRNAs, which is richly expressed in the brain and is related to brain development and neuronal differentiation (Sun et al., 2015). Another brain-specific miRNA is miR-9, which is expressed in both neurons and glia. miR-9 plays an essential role in various aspects of neurogenesis (Smirnova et al., 2005).
MiRNAs appear to be differentially distributed between distinct brain areas (Hommers et al., 2015). In addition to brain region or neuron type specific expression differences, neurons display differential intraneuronal miRNA compartmentalization. The contributor mechanisms of specific enrichment of miRNAs within synapses, dendrites, and axons remain unknown, but involvement of some RNA-binding proteins for delivering or anchoring miRNAs to particular neuron areas is possible (O’Carroll and Schaefer, 2013). For example, Fragile X mental retardation protein (FMRP), a component of the RISC that interacts with Ago1 (Jin et al., 2004), is expressed in many tissues. FMRP expression is especially high in dendrites and spines of neurons (Li and Jin, 2009). Indeed, several brain-enriched miRNAs, including miR-125b, miR-128, and miR-132, are associated with FMRP (Edbauer et al., 2010). Mutations in protein activator of interferon induced protein kinase (PRKRA), which encodes PACT one of the RISC loading complex (RLC) proteins, cause young-onset dystonia-parkinsonism disorder (Camargos et al., 2008). TRIM32 positively regulates miRNA activity, and shows strong expression in differentiating neurons in the ventricular zone (VZ) (Kawahara et al., 2012). Collectively, these reports suggest that miRNA biogenesis is closely related to the pathogenesis of nervous system disorders.
4. Genetic variants in miRNA-related genes
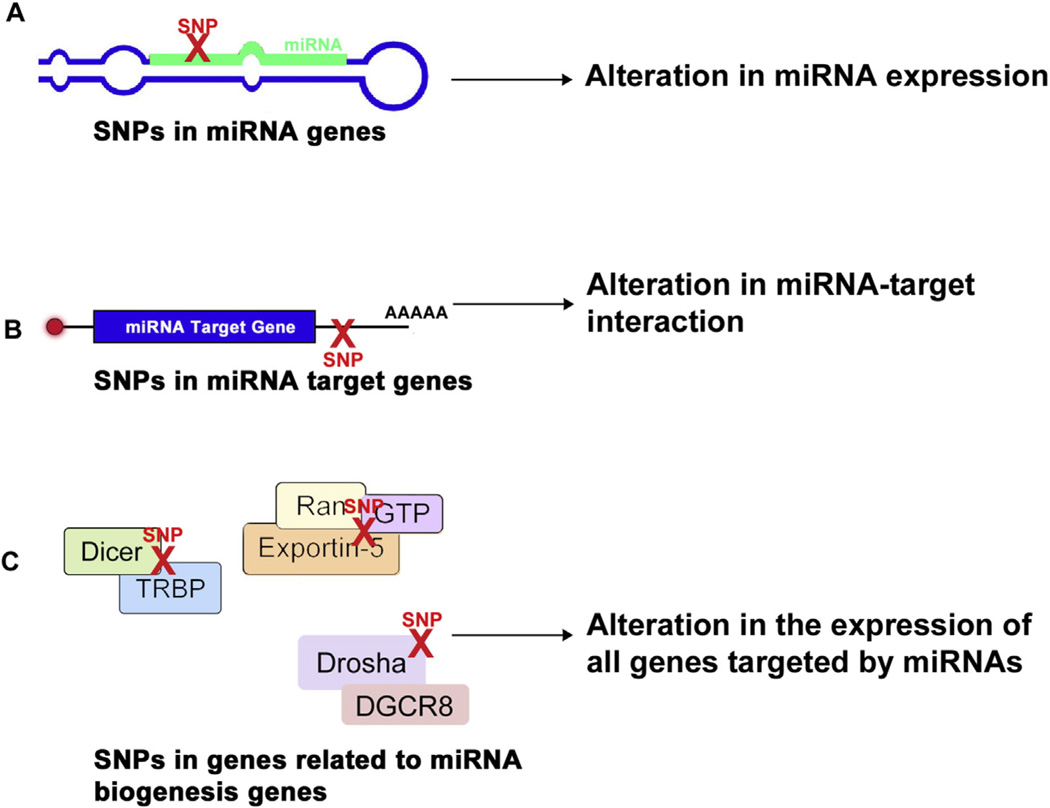
In the past few years accumulating evidence have supported that changes in miRNA regulation or function are associated with psychiatric disorders, including major depressive disorder (MDD), BD and SCZ. Alterations in miRNA activity can occur as a result of different variations (Fig. 2): (1) in the machinery genes related to miRNA biogenesis; (2) in the miRNA promoter sequences; (3) in the sequences responsible for pre-miRNA cleavage; (4) the miRNA binding sites in target genes; or (5) in the seed sequences (Hogg and Harries, 2014).
Fig. 2.
The effect of SNPs on miRNA-mediated regulation of gene expression. SNPs in different steps of miRNA biogenesis exert diverse effects on the regulation of gene expression. (A) SNPs in miRNA genes can alter miRNA expression. (B) SNPs in target mRNAs also affect miRNA function by altering miRNA-target interaction. (C) SNPs in miRNA machinery genes affect the entire transcriptome that is subject to miRNA-mediated regulation.
In this review, we focus on three categories of polymorphisms in the miRNA regulatory pathway: (1) SNPs or CNVs in miRNA genes; (2) SNPs or CNVs in miRNA binding sites in target genes and (3) SNPs or CNVs in genes involved in miRNA biogenesis and processing with the major findings summarized in Table 1.
Table 1.
Variants in miRNA genes in neuropsychiatric disorders.
| MiRNA | Population | Number of samples | Functional result | |
|---|---|---|---|---|
| Hansen et al. (2007) | SNP in miR-198 (rs1700) and miR-206 (rs17578796) |
Scandinavian | Danish subjects: 420 SCZ patients, 1006 control subjects Norwegian subjects: 257 SCZ patients, 293 control subjects Swedish subjects: 163 SCZ patients, 177 control subjects |
One pathway was identified with 8 common targets to both miRNAs (PTPN, CCND2, CREB5, MET, N-PAC, MEIS1, PUM2.AP1G1) |
| Burmistrova et al. (2007) | SNP in miR-130b (rs861843) | Russian | 300 SCZ patients, 316 control subjects | Not assessed |
| Feng et al. (2009) | SNP in miR-188, miR-18b/18b*, miR-502, miR-505, miR-510, miR-660, miR-325, miR-509-3, let-7f-2 |
Caucasian | 193 SCZ patients, 191 control subjects | CLCN5, HMGA2, NRXN3, DISCI, NRG1, MECP2, RGS4, GRM3, ERBB4, MAGI2, DLG2 |
|
Xu et al. (2010) Schizophrenia Psychiatric Genome-Wide Association Study (2011) |
SNP in pre-miR-30e (ss178077483) SNP in miR-137 (rs1625579) |
Chinese Genome-Wide Association Study |
456 SCZ patients, 453 control subjects 17,836 SCZ patients, 33,859 control subjects |
Ubc9 Implicated in regulation of adult neurogenesis and neuronal maturation. Four predicted targets (TCF4, CACNA1C, CSMD and C10orf26) were found to have SNPs associated with schizophrenia |
|
Whalley et al. (2012) Begemann et al. (2010) |
SNP in miR-137 (rs1625579) SNP in miR-498 (rs3822674) |
Scottish Caucasian, 95.3%; other, 1.6%; unknown, 3.1%) |
44 high genetic risk of SCZ, 81 controls 792 SCZ patients, 159 patients with schizoaffective disorder, 120 suspected schizophrenic psychosis cases, 1079 control subjects |
Not assessed 3′UTR of candidate schizophrenia genes, a SNP in the complexin 2 (CPLX2) 3′UTR in a predicted binding site of miR-498. |
| Green et al. (2013) | SNP in miR-137 (rs1625579) | Australian | 526 SCZ patients, 91 patients with schizoaffective disorder, 764 control subjects |
Not assessed |
| Cummings et al. (2013) | SNP in mir-137 (rs1625579) | Irish | 821 SCZ patients, BD patients and schizoaffective disorder, 171 control subjects |
Associated with a specific psychosis phenotype. |
| Ripke et al. (2013) | SNP in miR-137 (rs1198588) | Swedish | 5001 SCZ patients, 6243 control subjects | The SNP with the strongest association to schizophrenia (rs1198588) is 39 kb upstream of MIR137, and might regulate the transcription of MIR137. |
| Psychosis Endophenotypes International et al. (2014) | SNP in miR-137 (rs1702294) and miR-548aj2(rs215411) |
Genome-Wide Association Study |
36,989 SCZ patients, 113,075 control subjects | Not assessed |
| Warnica et al. (2015) | CNVs at eight loci: Iq21.1, 2q13, 12q21.31, 14q32.33, 15q11-15q13, 16p11.2, 16p13.11, and 19q13.42 |
Canadian | 420 SCZ patients, 2357 control subjects | Predicted gene targets: CAPRIN1, NEDD4, NTRK2, PAK2, RHOA, and SYNGAP1 |
| Whalley et al. (2012) | SNP in miR-137 (rs1625579) | Scottish | 90 high genetic risk of BD, 81 control subjects | Not assessed |
| Forstner et al. (2015) | SNPs in miR-199, miR-640, miR-708, miR-581, miR-644, miR-135a-1, miR-1908, let-7g |
Genome-Wide Association Study |
9747 BD patients, 14,278 controls | Target gene and pathway analyses revealed 18 significant canonical pathways, including brain development and neuron projection. |
| Saus et al. (2010a,b) | SNP in pre-mir-182 (rs76481776) | Spanish | 359 MDD patients, 341 control subjects | Associated with late insomnia in MDD. |
| Xu et al. (2010) | SNP in pre-miR-30e (ss178077483) | Chinese | 1088 MDD patients, 1102 control subjects | Not assessed |
|
Variants in miRNA target genes in neuropsychiatric disorders | ||||
| SNP/CNV | Population | Number of samples | Functional result | |
| Begemann et al. (2010) | SNP in CPLX2 gene (affecting miR-498 binding) (rs3822674) |
Caucasian | 1071 SCZ patients, 1079 control subjects | CPLX2 gene is associated with altered cognition in SCZ patients. |
| Gong et al. (2013) | SNPs in GABRA6 (rs3219151), COMT (rs165599) and RCS4 (rs10759) (affecting miR-124 binding) |
Chinese | 598 SCZ patients, 500 control subjects | In vitro luciferase assays demonstrated that regulator of G-protein signaling 4 (RGS4) downregulation was mediated by miR-124, and that miR-124 binding can be modified by SNP rs10759. |
| Liu et al. (2012) | SNPs in TBCW15 gene (rs17110432, rs11178988, rs11178989) possibly affecting miRNA binding |
Chinese | 746 SCZ patients, 1599 control subjects | Not assessed |
| Kandaswamy et al. (2014) | SNP in CRM7 gene (rs56173829) predicted to differential miRNA binding. |
British | 553 BD patients and 547 control subjects | Bioinformatic analyses predicted a change in the centroid secondary structure of RNA and alterations in the miRNA binding sites for the mutated base of rs56173829. |
| Rahman et al. (2010) | SNP in predicted binding site of miR-625 and miR-1302 in P2RX7 gene (rs1653625l |
Unspecified | 171 MDD or BD patients, 178 control subjects | P2RX7 |
| Jensen et al. (2014) |
SNPs: Predicted binding site of miR-330-3p in MAP2K5 gene; only significant in African Americans (rs41305272) |
European Americans: 465 cases, 2010 controls African Americans: 427 cases, 2584 controls |
314 MDD patients, 252 control subjects | MAP2K5. Enriched pathways include TGF, WNT and cytoskeletal remodeling, neurotrophin family signaling, roles of HDAC and CaMK in control of skeletal myogenesis, nervous system development, system development, neurogenesis, axonal guidance, FSH-beta signaling pathway, FGF-ErbB signaling. Genes involved in mental disorders, psychiatry and psychology, and schizophrenia. |
|
Variants in miRNA biogenesis genes in neuropsychiatric disorders | ||||
| Gene | Population | Number of samples | Functional result | |
| Beveridge et al. (2010) | Upregulation: DGCR8 (miRNA biogenesis gene) |
Caucasian | 21 SCZ patients, 21 control subjects |
Upregulation of the microprocessor component DGCR8 mRNA; related to an increase of both mature miRNA and precursor forms of miR-181b and miR-26b |
| Santarelli et al. (2011) | Upregulation: Confirmed by qPCR: Dicer (miRNA biogenesis gene) |
Caucasian | 37 cases (SCZ or schizoaffective disorder), 37 control subjects |
|
| Zhou et al. (2013) | Two polymorphisms in the DGCR8 and DICER genes (rs3757 and rs3742330) |
Chinese | 255 SCZ patients, 252 control subjects | Polymorphisms in two miRNA machinery genes, functional significance of these variants is, as yet, undetermined. |
| Smalheiser et al. (2012) | No significant differences were found in Dicer, Drosha and DGCR8 mRNAs (miRNA biogenesis gene). |
Unspecified | 18 MDD, 17 controls | Not assessed |
| He et al. (2012) | SNPs: DGCR8 (miRNA biogenesis gene) (rs3757), AGOl (RISC component) (rs636832) |
Chinese | 314 MDD, 252 controls | Not assessed |
4.1. Variants in miRNA genes in neuropsychiatric disorders
Variations in pri- and pre-miRNAs can affect miRNA processing, and variations in mature miRNAs can influence miRNA-binding to its target transcripts. In addition, SNPs in promoters of miRNA genes or miRNA host genes may have impact on miRNA expression.
SNPs in miRNA genes have been most extensively studied in SCZ among various neuropsychiatric disorders. Hansen et al. have carried out the first study in this field, and showed that SNPs in miR-198 (rs1700) and miR-206 (rs17578796) are associated with SCZ (Hansen et al., 2007). SNPs in other miRNAs, including miR-498, pre-miR-30e, and miR-130b, have also associated with SCZ in different ethnic groups (Begemann et al., 2010; Green et al., 2013; Whalley et al., 2012; Xu et al., 2010). Additionally, different SNPs (rs1625579; rs1198588, rs1702294) in the upstream region of the host gene for miR-137 have been strongly associated with SCZ (Cummings et al., 2013; Green et al., 2013; Psychosis Endophenotypes International et al., 2014; Ripke et al., 2013; Schizophrenia Psychiatric Genome-Wide Association Study, 2011; Whalley et al., 2012). Besides SNPs, variable number tandem repeats (VNTRs) and rare variants have been also reported within the same MIR137 locus (Duan et al., 2014; Strazisar et al., 2015; Warburton et al., 2015). Recently, Warnica et al. reported CNVs at eight loci (1q21.1, 2q13, 12q21.31, 14q32.33, 15q11-15q13, 16p11.2, 16p13.11, and 19q13.42), which overlap with a total of 25 miRNAs (Warnica et al., 2015), providing abundant evidence for the potential contribution of disrupted miRNA levels to the underlying pathophysiology.
To date, less evidence is available for a role of genetic variation in miRNAs in BD and MDD. Whalley et al. performed the first study in individuals with higher genetic risk of developing BD, and found a SNP in miR-137 (rs1625579) gene (Whalley et al., 2012). A recent comprehensive analysis of 609 autosomal miRNAs was performed in the context of a large bipolar disorder genome-wide association study (9747 patients and 14,278 controls). This study provided evidence for the nominally significant association of 98 unique miRNA loci that was reduced to 9 loci after correction for multiple testing, including miR-499, miR-708 and miR-1908 (Forstner et al., 2015).
Finally, 2 SNPs in pre-mir-182 (rs76481776) and pre-miR-30e (ss178077483) are associated with MDD (Saus et al., 2010a,b; Xu et al., 2010). With larger GWAS of MDD underway, it is likely that additional miRNAs will be implicated by genetic variation associated with MDD.
42. Variants in miRNA target genes in neuropsychiatric disorders
Since there is a strict recognition requirement between the miRNA seed region and its target, a naturally occurring SNP in a gene may have significant functional implications for miRNA binding and post-transcriptional regulation of gene expression.
One of the earliest studies on psychiatric diseases was carried out on SCZ patients. Begemann et al. found a SNP in rs3822674 in the complexin 2 gene (CPLX2) that affects binding of miR-498 to its target site (Begemann et al., 2010). Previous studies found common variants in the CPLX2 gene to be highly associated with cognitive dysfunction in SCZ patients (Eastwood and Harrison, 2005; Ripke et al., 2013). Other SNP findings associated with SCZ are located in predicted binding site of miR-625 and miR-1302 in purinergic receptor P2X (P2RX7) gene, in the miR-124 binding site of regulator of G protein signaling 4 (RGS4) gene (rs10759); and 3 other SNPs (rs17110432, rs11178988 and rs11178989) in the 3′-UTR of TBC1 domain family member 15 (TBC1D15) gene (Gong et al., 2013; Liu et al., 2012; Rahman et al., 2010). P2RX is a ligand gated ion channel that is activated by adenosine triphosphate (ATP), and mediates GABA release (Hansen et al., 2008). Altered GABA neurotransmission has been seen in prefrontal cortex (PFC) of schizophrenic patients (Lewis et al., 1999). Deletion of 22q11.2 is a well-known SCZ-associated CNV (Bassett and Chow, 1999; Chow et al., 1999). Notably, miR-185 is located on this locus, and the role of this miRNA and its downstream pathways has also been implicated in SCZ (Brzustowicz and Bassett, 2012; Forstner et al., 2013; Gardiner et al., 2012).
So far, only three studies have investigated the role of SNPs in miRNA target genes in mood disorders. The first study was conducted on patients with BD or MDD, which showed a significant association between the SNP in P2RX7 gene (rs1653625) and disease conditions (Rahman et al., 2010). Purinergic signaling in the peripheral and central nervous systems was discovered in the 1970s (Burnstock, 2006) and suggesting as a susceptibility gene for both MDD and BD (Halmai et al., 2013). The over activity of P2 receptors has been seen in MDD and P2X receptor antagonism leads anti-depressant and anti-anxiety effects in mice (Ortiz et al., 2015). Additionally, a SNP was detected in the metabotropic glutamate receptor 7 (GRM7) gene, which was predicted to cause alterations in the miRNA binding sites for the mutated base of rs56173829 (Kandaswamy et al., 2014). In another study, a SNP in the mitogen-activated protein kinase kinase 5 (MAP2K5) gene (which contains a predicted binding site of miR-330-3p), is associated with MDD in African American subjects, but not in European American subjects (Jensen et al., 2014).
A full understanding of the functional consequence of these SNPs in miRNA target genes awaits further biological investigations.
4.3. Variants in miRNA biogenesis genes in neuropsychiatric disorders
SNPs, chromosomal deletions, insertions, duplications or CNVs in regions related to miRNA biogenesis are associated with susceptibility to neuropsychiatric disorders (Table 1). For instance, there is a strong and specific connection between 22q11.2 microdeletion and SCZ. One gene disrupted by the 22q11.2 microdeletion is DGCR8 (Fenelon et al., 2011; Merico et al., 2014; Zhao et al., 2015). In addition, DGCR8 expression is upregulated in the DLPFC (BA9) of SCZ patients (Beveridge et al., 2010). In a subsequent study on SCZ, Santarelli et al. found an increase in the levels of miR-15 family miRNAs, such as miR-15a, miR-15b, miR-195 and DGCR8 mRNA expression in the superior temporal gyrus and DLPFC (Santarelli et al., 2011). Despite the lack of studies on deregulated miRNA biogenesis in BD, 2 SNPs located in DGCR8 (rs3757) and AGO1 (RISC component) (rs636832) are associated with MDD (He et al., 2012). In contrast, no association was observed between the mRNA levels of Dicer, Drosha and DGCR8; but this finding is based on data from small subset of MDD patients and control subjects (Smalheiser et al., 2012).
5. MiRNA expression studies in neuropsychiatric disorders
Besides mutations in miRNA-related genes, altered miRNA expression levels have been identified in multiple studies. Expression studies have generally used postmortem brain tissue samples, cerebral spinal fluid (CSF) or peripheral blood; thus, they can demonstrate the correlation between miRNAs and disease. Here, we summarized the studies that demonstrate altered miRNA expression in SCZ, BD, and MDD (Tables 2–4). It is important to note that despite the high number of disease-associated miRNAs, only a small number of miRNAs are common between different studies. This is not completely surprising, given the polygenic and complex nature of these disorders. However, the limitations of these studies (including limited sample size, cellular heterogeneity of brain regions, and cell-specific miRNA expression) must be considered when interpreting the results. Thus, more detailed, large-scale, association studies are needed to further clarify the involvement of miRNAs in these diseases.
Table 2.
Studies of miRNAs in schizophrenia.
| MiRNA | Sample type | Methods | Number of samples |
Affected function/pathway | |
|---|---|---|---|---|---|
| Burmistrova et al. (2007) | No significant difference in miR-130b | Postmortem tissue — parietal cortex (BA7) |
Microarray | 12 SCZ patients, 12 control subjects |
Not assessed |
| Perkins et al. (2007) | Down-regulation: miR-26b, miR-30b, miR-29b, miR-195, miR-92, miR-30a-5p, miR-30d, miR-20b, miR-29c, miR-29a, miR-212, miR-7, miR-24, miR-30e, miR-9-3p Up-regulation: miR-106b |
Postmortem tissue — PFC (BA9) |
Custom microarray and real-time PCR |
15 SCZ patients, 21 control subjects |
Authors’ pathway analysis revealed predicted enrichment of pathways involved in synaptic plasticity, including MAPK and phosphatidylinositol signaling and focal adhesion. |
| Beveridge et al. (2008) | Up-regulation: miR-181b | Postmortem tissue — superior temporal gyrus (BA22) |
Microarray | 21 SCZ patients, 21 control subjects |
Authors validated two of the putative target genes of miR-181b, VSNL1 and GRIA2, as miR-181b target genes by using target gene reporter assay and in vitro cell culture silencing studies. |
| Zhu et al. (2009) | Down-regulation: miR-346 | Postmortem tissue — DLPFC (BA46) |
Real-time PCR |
193 SCZ patients, 191 control subjects |
Authors compared the targets of miR-346 with previous SCZ-related genetic association studies. With the exception of CSF2RA, all the genes predicted to be targeted by miR-346 do not have undisputed positive results. |
| Beveridge et al. (2010) |
STG and DLPFC. Up-regulation: DGCR8 (miRNA biogenesis gene), miR-107, miR-15 family members (miR-15a/b, miR-16 and miR-195), miR-181b, let-7e STG: Up-regulation: miR-20a, miR-26b DLPFC: Up-regulation: miR-128a, miR-16, miR-181a, miR-20a, miR-219, miR-27a, miR-29c, miR-7, miR-19a, miR-26b, let-7d |
Postmortem tissue — superior temporal svrus (BA22) and DLPFC (BA9) |
Microarray real-time PCR |
21 SCZ patients, 21 control subjects |
Author’s pathway analysis showed enrichment of neuronal pathways including axonal guidance, LTP, Wnt, ErbB and MAPK signaling; miR-15 family predicted to target neuronal genes |
|
S. H. Kim et al. (2010), A. H. Kim et al. (2010) |
Up-regulation: miR-34a, miR-132/132*, miR-212, miR-544, miR-7, miR-154* |
Postmortem tissue — DLPFC (BA46) |
TLDA array | 35 SCZ patients, 35 control subjects |
Author’s pathway analysis revealed some predicted pathways that are involved in nervous system function and disease, including schizophrenia. miR-132 and miR-212 both downregulate TH and PGD. |
| Moreau et al. (2011) | Down-regulation: miR-33, miR-138, miR-151, miR-210, miR-324-3p, miR-22, miR-425, miR-106b, miR-338, miR-15a, miR-339 Up-regulation: miR-193b, miR-545, miR-301, miR-27b, miR-148b, miR-639, miR-186, miR-99a, miR-190 |
Postmortem tissue — PFC (BA9) |
Real-time PCR |
35 SCZ patients, 35 control subjects |
Not assessed |
| Santarelli et al. (2011) | Up-regulation: Confirmed by qPCR: Dicer (miRNA biogenesis gene), miR-17, miR-107, miR-134, miR-150, miR-199a*, miR-25, miR-328, miR-382, miR-187a, miR-652 |
Postmortem tissue — DLPFC (BA46) |
Microarray real-time PCR |
37 cases (SCZ or schizoaffective disorder), 37 control subjects |
Authors’ pathway analysis revealed several neurologically important and schizophrenia-related pathways including nervous system development, neurogenesis, neuron differentiation and axonogenesis |
| Banigan et al. (2013) | Up-regulation: miR-497 | Postmortem tissue — PFC (BA9) — exosomes |
Luminex miRNA expression assay, real-time PCR |
8 SCZ patients, 13 control subjects |
Not assessed |
| Wong et al. (2013) | Up-regulation: miR-17 | Postmortem tissue — DLPFC | Microarray real-time PCR |
37 SCZ patients, 37 control subjects |
Authors validated NPAS3 as miR-17 target gene by using luciferase reporter assay. NPAS3 is expressed by GABAergic interneurons and developmentally important transcription factor that has been associated with psychiatric illness. |
| Miller et al. (2012) | Down-regulation, corrected p-value: miR-132, miR-132* Down-regulation, uncorrected p-value: miR-150, miR-133a Up-regulation, uncorrected p-value: miR-320, miR-320c, miR-628-3p, miR-874, miR-105, miR-17*, let-7b |
Human and mouse Postmortem tissue — DLPFC (BA46) |
Microarray | 35 SCZ, 37 control subjects |
Authors’ pathway analysis revealed some predicted enrichments of nervous system pathways for miR-132, including protein kinase A signaling, LTP and LDP, neuronal CREB signaling and DNA methylation. |
| Mellios et al. 2012) | Down-regulation: (In females, not males): miR-30b |
Human and mouse Postmortem tissue — PFC (BA9 and 10) and parietal cortex (BA7) |
Real-time PCR |
PFC: 30 SCZ, 30 control subjects Parietal cortex: 11 SCZ, 12 control subjects |
According to authors’ pathway analysis, miR-30b is predicted to target several schizophrenia-linked genes including metabotropic glutamate receptors GRM3 and GRM5. |
| Pietersen et al. (2014a,b) | Down-regulation: miR-1243, miR-150, miR-378, miR-520d-3p, miR-875-5p Up-regulation: miR-126, miR-30b, miR-328, miR-628-5p, miR-99b |
Postmortem tissue — laser-captured pyramidal neurons from layer 3 of STG (Brodmann’s area 42) |
Megaplex miRNA TaqMan arrays |
9 SCZ patients, 9 control subjects |
Authors’ pathway analysis revealed revealed some predicted pathways including TGFβ signaling, extracellular matrix-receptor interaction, DNA damage, apoptosis and actin cytoskeleton regulation pathways. |
| Pietersen et al. 2014b) | Down-regulation: miR-106a, miR-218, miR-342 Up-regulation: miR-151, miR-338-5p, miR-197, miR-342, miR-518f, miR-1274b, miR-151-3p, miR-197, miR-34a, miR-520c-3p |
Postmortem tissue — laser-captured parvalbumin-immunoreactive neurons from layer 3 of STG (Brodmann’s area 42) |
Megaplex miRNA TaqMan arrays |
8 SCZ patients, 8 control subjects |
Authors’ pathway analysis revealed some predicted pathways including WNT and NOTCH signaling, DNA damage, apoptosis, cell cycle and actin cytoskeleton regulation pathways. |
| Yu et al. (2015) | Down-regulation: miR-132, miR-134, miR-1271, miR-664*, miR-200c and miR-432. |
Blood — PBMCs | Microarray real-time PCR |
105 SCZ patients and 130 control subjects |
Not assessed |
| Gardiner et al. (2012) | Down-regulation: miR-31, miR-99b, miR 107, miR-134, miR-431, miR-433, miR-487b |
Blood — PBMCs | Microarray real-time PCR |
112 SCZ patients and 76 control subjects |
Authors’ pathway analysis revealed significantly enriched pathways including axon guidance, regulation of the actin cytoskeleton, long-term potentiation, long-term depression, neuroactive ligand-receptor interaction, focal adhesion, neurotrophin, mammalian target of rapamycin, calcium, mitogen-activated protein kinase and ErbB signaling pathways. |
| Lai et al. (2011) | Down-regulation: miR-432 Up-regulation: miR-34a, miR-449a, miR-548d, miR-564, miR-572, miR-652 |
Blood — WBCs | TaqMan low density array v.1.0 |
30 SCZ patients, 30 control Subjects |
Not assessed |
| Shi et al. (2012) |
Down-regulation: miR-195 Up-regulation: let-7g, miR-181b, miR-219-2-3p, miR-1308 |
Blood — serum | Real-time PCR |
115 SCZ patients, 40 control subjects |
Authors selected 9 miRNA to investigate based on the data obtained from the literature, SCZ Gene database, NCBI database and tried to find the most promising miRNAs that reflect SCZ illnesses status, 7 of them found altered expression. |
Table 4.
Studies of miRNAs in major depressive disorder.
| MiRNA | Sample type | Methods | Number of samples | Affected function/pathway | |
|---|---|---|---|---|---|
| Smalheiser et al. (2012) | Downregulation: miR-142-5p, miR-137, miR-489, miR-148b, miR-101, miR-324-5p, miR-301a, miR-146a, miR-335, miR-494, miR-20a/b, miR-376a, miR-190, miR-155, miR-660, miR-130a, miR-27a, miR-497, miR-10a, miR-142-3p |
Postmortem tissue — PFC (BA9) |
Real-time PCR |
18 MDD patients and 17 control subjects |
Authors’ target analysis revealed several validated (VEGFA, BCL2, DNMBb; targeted by miR-20a/b, 34a and 34b*) and predicted (targets of 3 or more downregulated miRNAs: ESR1, AJBE2D1, UBE2W, CAMK2G, AKAP1, NOVA1, GABRA4, CACNA1C, SMAD5, MITF, BACH2, MYCN, ARID4A) targets. |
| Smalheiser et al. (2014) | Downregulation: miR-508-3p, miR-152-3p | Postmortem tissue — PFC (BA10) |
TLDA array | 15 MDD patients, 15 control subjects 4 |
Not assessed |
| Maussion et al. (2012) | Upregulation: miR-491-3P and miR 185* | Postmortem tissue — PFC (BA10) |
Microarray | 38 suicide completers (23 MDD patients; 4 SCZ patients; 1 BD patient) 17 control subjects |
Authors demonstrated a significant correlation between miR-185* and TrkB-T1, a BDNF receptor lacking a tyrosine kinase domain that is highly expressed in astrocytes and regulates BDNF-evoked calcium transient. They also validated TrkB-T1 as a target for miR-185* by luciferase assay. |
| Lopez et al. (2014a) | Upregulation: miR-139-5p, miR-320c and miR-34c-5p |
Postmortem tissue — PFC (BA44) |
Real-time PCR |
16 suicide completers (all MDD patients) 15 control subjects |
Authors demonstrated a significant correlation between altered miRNAs and the expression levels of both SAT1 and SMOX. |
| Lopez et al. (2014b) | Downregulation: miR-1202 | Postmortem tissue — PFC (BA44) |
Real-time PCR |
16 suicide completers (all MDD patients) 15 control subjects |
Authors quantified the expression levels of the predicted gene targets of miR-1202 and upregulation in the level of metabotropic glutamate receptor 4 (GRM4) were found. |
| Fan et al. (2014) | Upregulation: miR-26b miR-1972, miR-4485, miR-4498, and miR-1743 Downregulation: |
Blood - PBMCs |
Microarray real-time PCR |
81 MDD patients, 46 control subjects |
Authors’ target analysis revealed several pathways associated with nervous system and brain functions. |
|
J. Song et al. (2015), M. F. Song et al. (2015) |
Downregulation: miR-16 | Cerebrospinal fluid (CSF) and blood |
Real-time PCR |
36 MDD patients, 30 control subjects |
Authors emphasized the relationship between miR-16 and serotonin neurotransmitter system via targeting SERT gene (SLC6A4). |
| Li et al. (2015) | Upregulation: miR-644, miR-450b, miR-328, miR-182 Downregulation: miR-335, miR-583, miR-650, miR-708 and miR-654 |
Blood | Real-time PCR |
18 MDD patients, 18 control subjects |
Authors confirmed that miR-335 can directly target glutamate receptor, metabotropic 4 (GRM4), a member of the metabotropic glutamate receptor III family. |
| Wan et al. (2015) | Downregulation: miR-451a Upregulation: miR-221-3p, miR-34a-5p and let-7d-3p |
CSF and blood |
Real-time PCR |
CSF: 6 MDD patients, 6 control subjects. Blood: 32 MDD patients, 21 control subjects |
Authors’ pathway analysis revealed several pathways related to PI3K-Akt signaling, axon guidance, Wnt signaling, neurotrophin signaling, Hippo signaling, mTOR signaling, ErbB signaling, cell cycle, apoptosis, long-term depression. |
| Belzeaux et al. (2012) | Downregulation: miR-517b, miR-636, miR-1243, miR-381, miR-200c Upregulation: miR-589, miR-579, miR-941, miR-133a, miR-194, miR-107, miR-148a, miR-652, miR-425-3p |
Blood - PBMCs |
Microarray | 16 MDD patients and 13 control subjects |
Unspecified |
| Li et al. (2013) | Upregulation: miR-182, miR-132 | Blood - serum |
Real-time PCR |
40 MDD patients and 40 control subjects |
Authors emphasized the relationship between the serum BDNF levels and the miR-132/miR-l 82 levels. They provided evidence supporting that miR-182 is a putative BDNF-regulatory miRNA. |
| Camkurt et al. (2015) | Downregulation: miR-320a. Upregulation: miR-451a miR-17-5p and miR-223-3p. |
Blood - plasma |
Real-time PCR |
50 MDD patients and 41 control subjects |
Authors emphasized on GRIN2A and DISCI, two of the predicted target genes of miR-320a, and also SLC17A7, one of the predicted targets of miR-451. |
5.1. Schizophrenia
The changes in miRNA expression profiles in SCZ have been investigated in several studies. In most of the miRNA profiling studies, postmortem brain tissues from SCZ patients are used, and a vast number of differentially expressed miRNAs have been identified (Banigan et al., 2013; Beveridge et al., 2008, 2010; A. H. Kim et al., 2010; Levinson et al., 2014; Perkins et al., 2007; Santarelli et al., 2013; Wong et al., 2013; Zhu et al., 2009).
Although the studies vary in their findings, some of these miRNA alterations are consistent. For example, upregulation of miR-181a and miR-181b is detected in the superior temporal gyrus (BA22) (Beveridge et al., 2008), and DLPFC from SCZ patients (Beveridge et al., 2008, 2010), and in blood (Shi et al., 2012). miR-181a is strongly enriched in the synaptodendritic compartment of the nucleus accumbens. It can regulate the glutamate receptor 2 subunit (GluA2) of AMPA receptors, which are vitally involved in synaptic plasticity, at the post-transcriptional level (Saba et al., 2012). On the other hand, alteration of some miRNAs, like miR-132 (A. H. Kim et al., 2010; Miller et al., 2012; Yu et al., 2015) and miR-134 (Gardiner et al., 2012; Santarelli et al., 2011; Yu et al., 2015) were inconsistent between different studies.
Studies of miRNA expression in peripheral tissues have also revealed associations with SCZ. Lai et al. found 6 upregulated miRNAs and 1 downregulated miRNA in the white blood cells of SCZ patients compared to healthy controls (Lai et al., 2011). Gardiner et al. identified 7 downregulated miRNAs in peripheral blood mononuclear cells of SCZ patients (Gardiner et al., 2012). Shi et al. also analyzed miRNA expression in the serum of SCZ patients, and observed 4 upregulated miRNAs whereas one miRNA was downregulated (Shi et al., 2012). In a recent study, global plasma miRNAs were profiled in an initial discovery cohort of 164 SCZ patients and 187 controls followed by replication in a cohort of 400 SCZ patients, 213 controls, and 162 non-SCZ psychiatric patients, and miR-130b and miR-193a-3p levels are upregulated in SCZ but not controls or other psychiatric disorders (Wei et al., 2015). The functional targets of these miRNAs include a number of genes, such as BDNF, the dopamine receptor (DRD1), the synaptic protein neuregulin 1 (NRG1) (Stefansson et al., 2002), neurotrophic tyrosine kinase receptor (TrkB-T1) and early growth response gene 3 (EGR3) (S. H. Kim et al., 2010; Yamada et al., 2007), that have been linked with schizophrenia in previous studies.
Whole transcriptome sequencing is an important analytical technique that uses massively parallel RNA-seq to carry out transcriptome analyses at a far higher resolution (Nagalakshmi et al., 2010). Pietersen et al. used laser capture microdissection (LCM)-isolated neurons from the superior temporal gyrus of postmortem schizophrenia and normal control brain. Using whole transcriptome sequencing, the authors identified 15 miRNAs that were differentially expressed in SCZ (Pietersen et al., 2014b). The same group also profiled the miRNA expression of LCM-isolated pyramidal neurons from the superior temporal gyrus of postmortem brains from SCZ and normal control subjects, and identified 10 miRNAs that were differentially expressed in neurons using the same methods (Pietersen et al., 2014a). A number of the differentially expressed miRNAs are in concordance with previous studies on postmortem SCZ samples. These miRNAs include miR-150 (Miller et al., 2009; Santarelli et al., 2011), miR-328 (Santarelli et al., 2011), and miR-30b (Mellios et al., 2012; Perkins et al., 2007). Additionally, Kohen et al. used RNA-seq of dentate gyrus granule cells LCM-isolated from hippocampus of postmortem SCZ, BD MDD patients and control brain. Their results showed evidence of disrupted miR-182 signaling in subjects with SCZ and MDD (Kohen et al., 2014).
5.2. Bipolar disorder
Studies of miRNA expression in BD have also showed significant alterations in postmortem brain tissue from affected subjects. These studies have usually focused on two brain areas; PFC (BA9/BA10) and DLPFC (BA46) and detected several upregulated and downregulated miRNAs (Banigan et al., 2013; A. H. Kim et al., 2010; Miller et al., 2012; Moreau et al., 2011; Smalheiser et al., 2012). On the other hand, Zhu et al. analyzed miR-346 levels in DLPFC (BA 46) tissues from SCZ and BD, but did not found significant alteration in BD patients (Zhu et al., 2009). Despite substantial heterogeneity of miRNA identified in association with BD, a few miRNAs have appeared in multiple studies (see Table 3 for complete and detailed list of studies).
Table 3.
Studies of miRNAs in bipolar disorder.
| MiRNA | Methods | Sample type | Number of samples | Affected function/pathway | |
|---|---|---|---|---|---|
| S. H. Kim et al. (2010), A. H. Kim et al. (2010) | Downregulation: miR-154*, miR-29a, miR-520c-3p, miR-140-3p, miR-767-5p, miR-874, miR-32, miR-573 Upregulation: miR-504, miR-145, miR-145*, miR-22*, miR-133b, miR-154*, miR-889 |
TLDA array | Postmortem tissue - DLPFC (BA46) |
35 BD patients and 35 control subjects |
Using in silico target gene prediction programs, authors reported some predicted genes like tyrosine hydroxylase (TH), phosphogluconate dehydrogenase (PGD) and metabotropic glutamate receptor 3 (GRM3) that are involved in networks overrepresented for neurodevelopment, behavior pathways, and SZ and BP disease development. |
| Moreau et al. (2011) | Downregulation: miR-330, miR-33, miR-193b, miR-545, miR-138, miR-151, miR-210, miR-324-3p, miR-22, miR-425, miR-181a, miR-106b, miR-193a, miR-192, miR-301, miR-27b, miR-148b, miR-338, miR-639, miR-15a, miR-186, miR-99a, miR-190, miR-339 |
Real-time PCR |
Postmortem tissue -PFC |
35 BD patients and 35 control subjects |
Not assessed |
| Miller et al. (2012) | Downregulation, uncorrected p-value: miR-132, miR-132*, miR-150 Upregulation, uncorrected p-value: miR-320, miR-320c, miR-628-3p, miR-874, miR-105, miR-17*, let-7b, let-7f–l* Upregulation, corrected p-value: miR-383, miR-32*, miR-490-5p, miR-196b, miR-513-5p, miR-876-3p, miR-449b, miR-297, miR-188-5p, miR-187 |
Microarray | Postmortem tissue - DLPFC |
31 BD patients and 34 control subjects |
Not assessed |
| Smalheiser et al. (2014) | Downregulation: miR-145-5p, miR-485-5p, miR-370, miR-500a-5p, miR-34a-5p Upregulation: miR-17-5p, miR-579, miR-106b-5p, miR-29c-3p |
TLDA array | Postmortem tissue - PFC (BA10) |
15 BD patients and 15 control subjects |
Not assessed |
| Banigan et al. (2013) | Upregulation: miR-29c | Luminex miRNA expression assay, real-time PCR |
Postmortem tissue - PFC (BA9) - exosomal miRNA |
9 BD patients, 13 control subjects |
Authors emphasized the possible relationship between miR-29c and lithium. miR-29c is induced by canonical Wnt signaling, which is antagonized by GSK-3, a known substrate of inhibition by lithium. |
| Zhu et al. (2009) | No significant alteration in miR-346 | Real-time PCR |
Postmortem tissue - DLPFC (BA 46) |
35 BD patients, 34 controls, 35 SCZ patients |
Not assessed |
| Bavamian et al. (2015) | Upregulation: miR-34a | Real-time PCR |
Postmortem tissue - cerebellar tissue and BD patient-derived neuronal cultures |
29 BD patients, 34 control subjects |
Authors validated two of the putative target genes of miR-34a, Ankyrin-3 (ANK3) and voltage-dependent L-type calcium channel subunit beta-3 (CACNB3), as direct miR-34a targets. |
| Strazisar et al. (2015) | Downregulation: miR-137 | Sanger-based sequencing |
Blood | 345 BD patients, 426 SCZ patients, 1376 control subjects |
Authors’ pathway analysis revealed some pathways that involved in nervous system development and proper synaptic function. |
| Walker et al. (2015) | Upregulation: miR-15b, miR-132 and miR-652 |
TaqMan microRNA assay, real-time PCR |
Blood | 34 unaffected individuals with higher genetic risk of developing a mood disorder, 46 control subjects |
miR-132 is transcribed from a cluster of miRNAs that are known to play an important role in neuronal development and function |
| Rong et al. (2011) | Downregulation: miR-134 | Real-time PCR |
Plasma | 21 BD patients, 21 control subjects |
Authors emphasized the connection between BD and one of the validated miR-134 targets Limk1, that controls synaptic development, maturation and/or plasticity. |
In addition to studies in peripheral blood, CSF, and post-mortem tissues, Bavamian et al. demonstrated a new avenue of investigation to connect dysregulation of miRNAs to BD. In this study, the authors analyzed induced neurons (iNs), neurons induced pluripotent stem cells (iPSCs) from a family with BD and post-mortem brain tissue from BD subjects and controls and demonstrated the elevation of miR-34a expression in the context of BD (Bavamian et al., 2015; Madison et al., 2015). Moreover, miR-34a was shown to directly target the expression of the BD risk factors ANK3 and the voltage-dependent L-type calcium channel subunit beta-3 (CACNB3), with an extended network of BD risk genes and dendritic morphology affected upon its overexpression. This leads to the conclusion that through the regulation of a molecular network essential for neurodevelopment and synaptogenesis, miR-34a may provide a critical link between multiple genetic risk factors for BD and its underlying pathogenesis. Given the opportunity to investigate early stages of neurodevelopment and the response to diverse pharmacological agents, such studies with iN and iPSC models of BD and other neuropsychiatric disorders hold tremendous promise for dissecting the regulation and function of miRNAs in the future (Haggarty et al., 2016).
5.3. Major depressive disorder
To date, five studies have focused on miRNA expression profiles in MDD by using postmortem brain tissue samples. Similar to BD, these studies used PFC (BA9 or BA10) as the starting material. Additional studies have focused on changes of miRNA expression in peripheral blood or CSF (Belzeaux et al., 2012; Camkurt et al., 2015; Li et al., 2015; Li et al., 2013; Wan et al., 2015). Interestingly, targets of altered miRNAs in MDD play significant roles in several pathways associated with nervous system and brain functions, such as serotonin transporter (SERT) and metabotropic glutamate receptor 4 (GRM4).
Further investigation of these more robust miRNAs and their target genes should provide insight into the development these disorders, and these miRNAs could potentially serve as biomarkers (please refer to Table 4 for complete and detailed list of studies).
6. Effect of drugs on miRNA expression
6.1. Antipsychotics
The effects of different antipsychotic drugs (haloperidol, clozapine, risperidone, etc.), on miRNA expression are summarized in Table 5. In the first study, upregulation in the levels of miR-199a, miR-128a, and miR-128b were observed in rats treated with haloperidol (Perkins et al., 2007). In another animal study, Kocerha et al. found that haloperidol and clozapine decrease miR-219 level in C57BL/6 mice, and this miRNA negatively regulates the function of NMDA receptor (Kocerha et al., 2009). Santarelli et al. investigated the changes in miRNA expression associated with haloperidol, clozapine and olanzapine treatment in the mouse brain. The results of this study showed that olanzapine treatment leads to a decrease in miR-193 level, whereas haloperidol treatment leads to a decrease in miR-434-5p and miR-22 levels. Yet, the results of clozapine treatment were excluded due to incompatible results of real time-PCR validation (Santarelli et al., 2013). According to previous observations, these miRNAs are elevated in the DLPFC brain tissue of SCZ patients (Moreau et al., 2011).
Table 5.
Pharmacological modulation of miRNAs in neuropsychiatric disorders.
| Drug | Sample | MiRNA | Main findings | Targets | Reference |
|---|---|---|---|---|---|
| Antipsychotics | |||||
| Haloperidol | Rat | Up-regulation: miR-199a, miR-128a, miR-128b |
Three miRNAs were up-regulated in response to haloperidol treatment in rats as compared to untreated controls but None of these miRNAs was differentially expressed in their SCZ patient group |
Not assessed | Perkins et al. (2007) |
| Haloperidol Clozapine |
C57BL/6 mice | Down-regulation: miR-219 | Dizocilpine is a NMDA-R antagonist that can rapidly produce schizophrenia-like behavioral Pretreatment with haloperidol and clozapine prevented dizocilpine-induced effects on miR-219. |
It has been proposed that miR-219 negatively regulates the function of NMDA receptors |
Kocerha et al. (2009) |
| Risperidone | SCZ patients receiving drug treatment = 40 |
Down-regulation: miR-365 and miR-520c-3p |
Among the seven miRNAs screened, the expression levels of miR-365 and miR-520c-3p were significantly down-regulated after 1 year of risperidone treatment |
Not assessed | Liu et al. (2013) |
| Haloperidol Olanzapine |
Mouse | Confirmed with real-time PCR: Haloperidol: Down-regulation: miR-434-5p, miR-22 Olanzapine: miR-193 |
For the haloperidol treatment group, miR-434-5p and miR-22, and for the olanzapine group, miR-193 was down-regulated, But validation of the clozapine treatment group was conflicting (miR-329 and miR-342-5p were significant down-regulation by qPCR and up-regulated on the microarray). |
Metabolic pathways were enriched in olanzapine and clozapine treatments, possibly associated with their weight gain side effects. Neurologically and metabolically relevant miRNA-gene interaction networks were identified in the olanzapine treatment group. |
Santarelli et al. (2013) |
| Olanzapine Quetiapine Ziprasidone Risperidone |
Plasma control subjects = 20 SCZ patients receiving drug treatment = 20 |
Down-regulation: miR-181b | Among 20 patients, each drug group has 5 patients. 9 miRNA that were reported to be associated with SCZ were selected and after six weeks of antipsychotic treatment, only the expression level of miR-181b had significantly decreased. |
miR-181b might implicate several target genes associated with synaptic transmission, nervous system development disorders. |
Song et al. (2014) |
| Aripiprazole Risperidone |
Plasma SCZ patients receiving drug treatment and remitted = 79, SCZ patients receiving drug treatment but unremitted = 28 |
Down-regulation: miR-130b and miR-193a-3p |
Among 107 SCZ patients who completed the 1-year follow-up, 79 achieved the remission criteria. The baseline levels of plasma miR-130b and miR-193a-3p between patients who remitted and those without remission were compared. |
The validated downstream target genes for miR-13 0b include PDGFRA, RUNX3, ITGB1, PPARG, FMRl, and STAT3, and for miR-193a-3p they include ErbB4, S6K2, and MCL1. Classification of these genes: -SCZ susceptibility genes (PDGFRA, PPARG, ErbB4), -neurodevelopment-related genes (RUNX3, ITGB1, FMR1, STAT3), and -neuroprotective genes (S6 K2 and MCL1). |
Wei et al. (2015) |
| Mood stabilizers | |||||
| Lithium | Human whole blood BD patients = 5 Control subjects = 21 |
miR-134 | miR-134 down-regulation in patients Increase in miR-134 levels after lithium treatment |
Targeted genes/gene pathways: Limk-1, dendritic spine size regulation |
Rong et al. (2011) |
| Lithium/VPA combination |
Rat cerebellar granule cells |
Down-regulation: miR-34a and miR-495 Up-regulation: miR-182, miR-147, and miR-222 |
The pathways associated with mood stabilizer-regulated miRNAs this study, are strongly associated with pathways implicated in neuropsychiatric diseases such as SCZ. |
Hunsberger et al. (2013) | |
| Lithium or VPA |
Rat hippocampus | Down-regulation: let-7b, let-7c, miR-128a, miR-24a, miR-30c, miR-34a, and miR-221 Up-regulation: miR-144 |
Alteration in hippocampal miRNA levels following chronic treatment with lithium or valproate (VPA), and the predicted effectors of these miRNAs are also genetic risk candidates for bipolar disorder. |
These miRNAs are involved in neurite outgrowth, neurogenesis, and signaling of PTEN, ERK, and Wnt/β-catenin pathways. The effectors of miRNAs targeted by both lithium and VPA treatments were CAPN6, DPP10, GRM7, ESRRG, FAM126A and THRB. |
Zhou et al. (2009) |
| Lithium | Lymphoblastoid cell lines (LCLs) BD patients = 10 Unaffected siblings = 10 |
miR-34a, miR-152, miR-155, and miR-221 |
After derivation of LCLs from patients and their unaffected siblings, miR-221, miR-152, miR-155 and miR-34a up-regulated at treatment time-point days 4 and 16. |
Chen et al. (2009) | |
| Lithium/VPA combination |
SH-SY5Y | Down-regulation: miR-30a-5p | Croce et al. (2014) | ||
| Lithium | SH-SY5Y | Down-regulation: miR-34a | Neuroprotective and anti-oxidant effects of lithium is found to related miR-34a expression. |
Alural et al. (2015) | |
| Antidepressants | |||||
| Fluoxetine | Mouse brain | miR-16 | After infusion of fluoxetine in mouse brain, a 2.5-fold increase in the level of miR-16 has been observed. |
miR-16 was identified as a complementarity to the 3′ untranslated region of the SERT mRNA by Using in silico computational target prediction. |
Baudry et al. (2010) |
| Fluoxetine | Human CSF MDD = 9 and Mouse hippocampus tissue |
miR-16 | After fluoxetine treatment, miR-16 levels decreased in mouse hippocampus. Also, after fluoxetine treatment, miR-16 targeting molecules BDNF, Wnt2, 15d–PGJ2 levels increased in human CSF samples. |
They proposed miR-16 as regulator between SRI treatment and hippocampal neurogenesis. BDNF, Wnt2 and 15-deoxy-delta12, 14-prostaglandin J2 (15d–PGJ2) act synergistically on the hippocampus by decreasing miR-16 and increasing serotonin transporter (SERT) and bcl-2 levels. |
Launay et al. (2011) |
| Escitalopram | Human whole blood MDD = 10 |
Up-regulation: miR-130b*, miR-505*, miR-29-b-2*, miR-26a/b, miR-22*, miR-664, miR-494, let7d/e/f/g, miR-629, miR-106b*, miR-103, miR-191, miR-128, miR-502-3p, miR-374b, miR-132, miR-30d, miR-500, miR-589, miR-183, miR-574-3p, miR-140-3p, miR-335, miR-361-5p Down-regulation: miR-34c-5p, miR-770-5p |
28 miRNAs were up-regulated, and 2 miRNAs were strongly down-regulated after 12-week escitalopram treatment |
miRNA target gene prediction and functional annotation analysis showed that there was a significant enrichment in several pathways associated with neuronal brain function (such as neuroactive ligand-receptor interaction, axon guidance, long-term potentiation and depression). |
Bocchio-Chiavetto et al. (2013) |
| Citalopram | Human whole blood | miR-1202 | A decrease in miR-1202 levels in “depressed patients and increase in miR-1202 levels after 8 weeks of treatment |
miR-1202 regulates the expression of the metabotropic glutamate receptor 4 (GRM4) gene and predicts antidepressant response at baseline. |
Lopez et al. (2014a,b) |
| Ketamine | Rat hippocampus tissue | miR-206 | 18 miRNAs were significantly reduced, while 22 miRNAs were significantly increased. But researchers focused on miR-206 expression due to its modulator effect on BDNF expression. |
miR-206 strongly modulated the expression of BDNF. miRNA target gene analysis referred the enrichments in several pathways associated with neuronal brain function, such as the neuroactive ligand-receptor interaction (miR-132-3p, miR-206, miR-181a-5p, miR-150-5p), amphetamine addiction (miR-497-5p, miR-29a-3p, miR-132-3p, miR-181 a-5p, miR-29c-3p), Wnt signaling pathway (miR-29a-3p, miR-98-5p), dopaminergic synapse (miR-132-3p, miR-181a-5p), ErbB signaling pathway (miR-221-3p), mTOR and TNF signaling pathway (miR-206, miR-132-3p) |
Yang et al. (2014) |
| Citalopram | Human whole blood MDD = 18 Control = 18 |
miR-335 | Down-regulation of miR-335 levels in MDD patients and up-regulated after citalopram treatment. |
Regulatory loop between GRM4 and miR-335 has been observed. The expression of miR-335 was increased and GRM4 was decreased in the blood samples of MDD patients after citalopram treatment. |
Li et al. (2015) |
In another study, peripheral samples obtained from SCZ patients (before and after treatment) were used and the expression levels of 9 SCZ-associated miRNAs were investigated. Before treatment, the expression levels of miRNA-181b, miRNA-30e, miRNA-34a and miRNA-7 were significantly upregulated in the SCZ-group. After 6-week antipsychotic treatment, expression level of miR-181b was significantly down-regulated (H. T. Song et al., 2014). Finally, a recent study showed that increased levels of miR-130b and miR-193a-3p in the plasma of SCZ patients could be suppressed after 1 year of treatment with 2 antipsychotic drugs (aripiprazole and risperidone) (Wei et al., 2015).
6.2. Mood stabilizers
Changes in miRNA expression in response to mood stabilizers, such as lithium and valproic acid (VPA), have been investigated in several studies (Table 5). One study in rats showed an alteration in hippocampal miRNA levels following chronic treatment with lithium or VPA (Zhou et al., 2009). In vitro studies on cell lines derived from BD patients have shown that 4 miRNAs (hsa-miR-34a-5p, hsa-miR-152-3p, miR-155-5p, hsa-miR-221-3p) were dysregulated after 16 days of treatment (Chen et al., 2009). Expression changes after drug treatment have also . been investigated in cell lines. For example, rat cerebellar granule cells were treated with lithium/VPA combination and 7 miRNAs (let-7b, let-7c, miR-128a, miR-24a, miR-30c, miR-34a, and miR-221) were downregulated, whereas only miR-144 was upregulated after treatment (Hunsberger et al., 2013). Combination of lithium and VPA has been also tested in vitro, which leads to a decrease in miR-30a-5p in SH-SY5Y human neuron-like cells (Croce et al., 2014). Recently, our group investigated the role of miR-34a in neuroprotective effects of lithium, and found that lithium decreases miR-34a expression and increases expression of its target genes (BCL2, NRF2 and BDNF) in SH-SY5Y cells (Alural et al., 2015). Given that i) miR-34a levels are elevated in patients with BD, miR-34a represents a potentially important link between pharmacological treatment options for BD and molecular mechanisms underlying disease pathophysiology (Bavamian et al., 2015).
6.3. Antidepressants
Regarding a possible involvement of miRNAs in the action of antidepressant drugs, effects of different drugs on miRNA expression have been investigated (Table 5). Baundry et al. found that infusion of fluoxetine in mouse brain increases miR-16 levels in serotonergic raphe nuclei, and the authors proposed that miR-16 functions as a regulator between serotonin reuptake inhibitor (SRI) treatment and hippocampal neurogenesis (Baudry et al., 2010). The same laboratory used mouse hippocampus tissue and patient-derived CSF to analyze miR-16 expression. Stereotaxic injection of fluoxetine into raphe nuclei resulted in a decrease in the endogenous level of miR-16 in the hippocampus and corresponding increase in serotonin transporter (SERT), the target of SRls (Launay et al., 2011). Fluoxetine treatment was also found to increase secretion of in BDNF, Wnt2 and 15-deoxy-delta12,14-prosta-glandin J2 from serotonergic neurons that coordinately regulated miR-16 levels in the hippocampus (Launay et al., 2011). Elevated levels of these same factors were observed in the CSF of depressed patients upon fluoxetine treatment suggesting an important role for miR-16 in SRI response (Launay et al., 2011). Another anti-depressant drug, escitalopram, also alters peripheral miRNA expression in MDD patients. Bocchio-Chiavetto et al. reported that 28 miRNAs are upregulated, and 2 miRNAs are downregulated after 12 weeks of escitalopram treatment (Bocchio-Chiavetto et al., 2013). Taken together, these results suggest that miRNA regulation may play a key role in shaping neuroplasticity involved in depressive behavior and the response to clinically effective therapeutics.
7. Biomarker discovery studies on miRNAs
7.1. Diagnostic potential of miRNA alteration in blood and CSF
Biomarkers are valuable tools to diagnose individuals at the early stages of disease, to develop therapeutic strategies, and to provide prognostic information. Currently, diagnosis of neuropsychiatric disorders relies on behavioral and clinical symptoms, which appear after several years of disease progression. Therefore, establishment of biomarkers that allows early detection is of utmost importance for the management of these disorders. In this regard, miRNAs can also be isolated from circulating blood cells or CSF, and they are promising non-invasive biomarkers for a wide variety of diseases (Stoicea et al., 2016).
Lai et al. reported the first study on miRNAs as possible biomarkers for SCZ. In this study, the authors identified a 7-miRNA signature in peripheral blood mononuclear cells (PBMCs), which is able to distinguish SCZ patients from control subjects with 93% accuracy (Lai et al., 2011). In another study, 10 SCZ-linked miRNAs were analyzed, and 5 of these miRNAs (miR-30e, miR-181b, miR-34a, miR-346 and miR-7) were shown to discriminate SCZ patients from healthy controls with 71% accuracy, a specificity of 95% and a sensitivity of 92% (Sun and Shi, 2015). Fan et al. investigated miRNA changes in PBMC samples obtained from young SCZ patients and control subjects and receiver operating characteristics (ROC) analysis of 9 miRNAs showed very high sensitivity and specificity for diagnosis of SCZ (AUC = 0.973, 95% confidence interval ‘ (CI): 0.945–1.000) (Fan et al., 2015). Fan et al. suggested that alterations in peripheral levels of miRNA-26b, miRNA-1972, miRNA-4485, miRNA-4498, and miRNA-4743 are associated with MDD (AUC = 0.636, 95% confidence interval (CI): 0.58–0.90) (Fan et al., 2014). Recently, Wan et al. analyzed differentially expressed miRNAs in both CSF and serum samples from MDD patients. The authors observed three upregulated miRNAs and one downregulated miRNA, and ROC analysis of these miRNAs showed very high sensitivity and specificity for diagnosis of MDD (AUC for each miRNA > 0.9; sensitivity > 90%; specificity > 84%) (Wan et al., 2015). Concerning plasma-based miRNA expression profiling studies in MDD, the combined use of miR-101-3p and miR-93-5p has been suggested as an optimal normalization method to contribute to more reliable and accurate results for biomarker discovery for MDD (Liu et al., 2014).
An important caveat of these studies seeking to use miRNAs as diagnostics (and below for predicting drug response) is the fact that patients are almost always on various medications, sometimes combinations, making it difficult to control for a suite of factors. Future studies will benefit from more explicit and careful study of this confounding factor.
7.2. Altered miRNAs as biomarkers to predict drug response
MiRNAs not only can be utilized for monitoring treatment but also promising predictive biomarkers for predicting drug response. The discovery of the role of miRNAs in drug resistance and drug response can potentially improve diagnosis, treatment and prognosis in patients. Furthermore, real-time analysis of miRNA expression can be used to adjust drug treatment to achieve optimal response in patients.
7.2.1. Schizophrenia
In the context of SCZ treatment, Wei et al. identified two upregulated miRNAs (miR-130b and miR-193a-3p) in the plasma of SCZ patients, which were suppressed in remitted patients after one year of treatment with antipsychotic drugs (aripiprazole and risperidone). The baseline levels of these two miRNAs were lower in patients in remission, compared to patients who were not in remission. Thus, their findings suggest that miR-130b and miR-193a-3p levels can be used as potential biomarkers to predict drug response in SCZ patients (Wei et al., 2015).
7.2.2. Bipolar disorder
Lithium and valproic acid are commonly used mood stabilizers for treatment of BD. Different studies have suggested that changes in miRNA expression can be used to monitor response to mood stabilizers. For instance, Hunsberger et al. showed that lithium treatment down-regulates let-7 miRNA family expression in lymphoblastoid cells in BD lithium responders (Hunsberger et al., 2015). In another example, Rong et al. reported that plasma miRNA-134 levels in drug-free patients with BD mania are significantly lower compared to control subjects. The authors also reported that plasma miRNA-134 levels increase significantly after four weeks of treatment (Rong et al., 2011). Overall, these findings suggest that monitoring let-7 family and miRNA-134 expression in BD may serve as a potential marker to predict efficacy of treatment.
7.2.3. Major depressive disorder
Alternation in miRNA expression in patients with MDD has been evaluated as a SSR1 response biomarker. For example, citalopram-responding patients were found to have reduced serum miR-1202 levels at baseline, compared to non-responders, and expression of miR-1202 increased during the course of treatment in antidepressant-responsive MDD patients (Lopez et al., 2014a,b). Additionally, miR-151-3p expression in LCLs has been shown to be a marker of paroxetine sensitivity (Oved et al., 2012). Overall, while miR-1202 and miR-151-3p have potential for use as bio-markers to predict response to antidepressants, further work is required to more comprehensively elucidate the potential roles of miRNAs in the variability of drug response and to provide mechanistic insight into the basis for such differential response.
8. Conclusion & perspective
Although still in its infancy, the studies summarized above reflect the growing recognition of the importance of ncRNAs in the form of miRNAs in the potential etiological mechanisms of multiple neuropsychiatric disorder, diagnostics, and the response to pharmacological treatments. In terms of the significance of many of the findings reported to date, it will be critical for the field to rigorously test the reproducibility and generalizability of the observations made in independent samples and larger cohorts. Here, the ability to systematically study the expression of miRNAs in patient-derived samples from peripheral blood, CSF, patient-derived iNs and iPSCs, and post-mortem tissue holds promise for the elucidation of the role specific miRNAs in different cell types, stages of neurodevelopment, and in response to pharmacological agents (Haggarty et al., 2016).
With growing recognition of the polygenic nature of neuropsychiatric disorders like SCZ, BD and MDD, the ability of a single miRNA to simultaneously control the expression of multiple genetic factors linked to the etiology of each disease or to a pathway of interest makes understanding the impact of disease-associated genetic variation on miRNA expression and function of paramount importance. In this context, through the systematic investigation of the network of genes affected by dysregulated miRNAs in different neuropsychiatric disorders, either due to genetic variation itself in the miRNAs or their target genes, it may be possible to provide insight into the unique, as well as overlapping, complex mechanisms underlying disease pathophysiology (Barabasi et al., 2011). As the ability of ncRNAs to be used as potential therapeutic agents themselves improves through advances in delivery of nucleic acids (Kumar et al., 2015), along with the creation of RNA-targeted therapeutics (Bernat and Disney, 2015), this network-based approach may in turn help facilitate the discovery of novel therapeutics for treating and ideally preventing neuropsychiatric disorders.
Acknowledgments
We apologize for the inability to cite a number of other important papers in the field due to space limitations. We want to thank Dr. Erdogan Pekcan Erkan for his kind help in the manuscript review. Research in the Haggarty laboratory on ncRNAs and neuropsychiatric disease has been supported in part by the National Institute of Mental Health (R33MH087896; R01MH091115; R01MH095088), and the Pitt-Hopkins Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
Dr. Haggarty has served on scientific advisory boards for Rodin Therapeutics and PsyBrain; and has received speaker honoraria and/or consulting fees from Sunovion Pharmaceuticals, Biogen-Idec, and AstraZeneca. None of these entities was involved in the writing of this manuscript.
Contributors
All authors (B.A., S.G., and SJ.H.) contributed to the article preparation, editing and approved the final version.
References
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Tsuang MX. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;134B(1):60–66. doi: 10.1002/ajmg.b.30140. http://dx.doi.org/10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Alural B, Ozerdem A, Allmer J, Gene K, Gene S. Lithium protects against paraquat neurotoxicity by NRF2 activation and miR-34a inhibition in SH-SY5Y cells. Front Cell. Neurosci. 2015;9:209. doi: 10.3389/fncel.2015.00209. http://dx.doi.org/10.3389/fncel.2015.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, Colleaux L. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am. J. Hum. Genet. 2007;80(5):988–993. doi: 10.1086/515582. http://dx.doi.org/10.1086/515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver LB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. http://dx.doi.org/10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Banigan MG, Kao RE, Kozubek JA, Winslow AR, Medina J, Costa J, Delalle I. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS One. 2013;8(1):e48814. doi: 10.1371/journal.pone.0048814. http://dx.doi.org/10.1371/journal.pone.0048814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat. Rev. Genet. 2011;12(1):56–68. doi: 10.1038/nrg2918. http://dx.doi.org/10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol. Psychiatry. 1999;46(7):882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329(5998):1537–1541. doi: 10.1126/science.1193692. http://dx.doi.org/10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, Haggarty SJ. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol. Psychiatry. 2015;20(5):573–584. doi: 10.1038/mp.2014.176. http://dx.doi.org/10.1038/mp2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann M, Grube S, Papiol S, Malzahn D, Krampe H, Ribbe K, Ehrenreich H. Modification of cognitive performance in schizophrenia by complexin 2 gene polymorphisms. Arch. Gen. Psychiatry. 2010;67(9):879–888. doi: 10.1001/archgenpsychiatry.2010.107. http://dx.doi.org/10.1001/archgenpsychiatry.2010.107. [DOI] [PubMed] [Google Scholar]
- Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Treziny C, Verrier L, Ibrahim EC. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl. Psychiatry. 2012;2:e185. doi: 10.1038/tp.2012.112. http://dx.doi.org/10.1038/tp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SM, Bartsch D. The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res. 2014;357(2):463–476. doi: 10.1007/s00441-014-1936-3. http://dx.doi.org/10.1007/S00441-014-1936-3. [DOI] [PubMed] [Google Scholar]
- Bernat V, Disney MD. RNA structures as mediators of neurological diseases and as drug targets. Neuron. 2015;87(1):28–46. doi: 10.1016/j.neuron.2015.06.012. http://dx.doi.org/10.1016/j.neuron.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, Cairns MJ. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum. Mol. Genet. 2008;17(8):1156–1168. doi: 10.1093/hmg/ddn005. http://dx.doi.org/10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll AP, Tooney RA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010;15(12):1176–1189. doi: 10.1038/mp.2009.84. http://dx.doi.org/10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur. Neuropsychopharmacol. 2013;23(7):602–611. doi: 10.1016/j.euroneuro.2012.06.013. http://dx.doi.org/10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Brzustowicz LM, Bassett AS. miRNA-mediated risk for schizophrenia in 22q11.2 deletion syndrome. Front Genet. 2012;3:291. doi: 10.3389/fgene.2012.00291. http://dx.doi.org/10.3389/fgene.2012.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmistrova OA, Goltsov AY, Abramova LI, Kaleda VG, Orlova VA, Rogaev EI. MicroRNA in schizophrenia: genetic and expression analysis of miR-130b (22q11) Biochemistry (Mosc) 2007;72(5):578–582. doi: 10.1134/s0006297907050161. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. http://dx.doi.org/10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Camargos S, Scholz S, Simon-Sanchez J, Paisan-Ruiz C, Lewis P, Hernandez D, Singleton AB. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7(3):207–215. doi: 10.1016/S1474-4422(08)70022-X. http://dx.doi.org/10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- Camkurt MA, Acar S, Coskun S, Gunes M, Gunes S, Yilmaz MF, Tamer L. Comparison of plasma microRNA levels in drug naive, first episode depressed patients and healthy controls. J. Psychiatr. Res. 2015;69:67–71. doi: 10.1016/j.jpsychires.2015.07.023. http://dx.doi.org/10.1016/j.jpsychires.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Carrard A, Salzmann A, Perroud N, Gafner J, Malafosse A, Karege F. Genetic association of the phosphoinositide-3 kinase in schizophrenia and bipolar disorder and interaction with a BDNF gene polymorphism. Brain Behav. 2011;1(2):119–124. doi: 10.1002/brb3.23. http://dx.doi.org/10.1002/brb3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang N, Burmeister M, McInnis MG. MicroRNA expression changes in lymphoblastoid cell lines in response to lithium treatment. Int. J. Neuropsychopharmacol. 2009;12(7):975–981. doi: 10.1017/S1461145709000029. http://dx.doi.org/10.1017/S1461145709000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DT, Jiang X, Akula N, Shugart YY, Wendland JR, Steele CJ, Strauss J. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol. Psychiatry. 2013;18(2):195–205. doi: 10.1038/mp.2011.157. http://dx.doi.org/10.1038/mp.2011.157. [DOI] [PubMed] [Google Scholar]
- Chen HM, DeLong CJ, Bame M, Rajapakse I, Herron TJ, McInnis MG, O’Shea KS. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl. Psychiatry. 2014;4:e375. doi: 10.1038/tp.2014.12. http://dx.doi.org/10.1038/tp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS. Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol. Psychiatry. 1999;46(10):1436–1442. doi: 10.1016/s0006-3223(99)00150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Croce N, Bernardini S, Caltagirone C, Angelucci F. Lithium/valproic acid combination and L-glutamate induce similar pattern of changes in the expression of miR-30a-5p in SH-SY5Y neuroblastoma cells. Neruomol. Med. 2014;16(4):872–877. doi: 10.1007/s12017-014-8325-7. http://dx.doi.org/10.1007/s12017-014-8325-7. [DOI] [PubMed] [Google Scholar]
- Cummings E, Donohoe G, Hargreaves A, Moore S, Fahey C, Dinan TG, Corvin A. Mood congruent psychotic symptoms and specific cognitive deficits in carriers of the novel schizophrenia risk variant at MIR-137. Neurosci. Lett. 2013;532:33–38. doi: 10.1016/j.neulet.2012.08.065. http://dx.doi.org/10.1016/j.neulet.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Dalmay T. Mechanism of miRNA-mediated repression of mRNA translation. Essays Biochem. 2013;54:29–38. doi: 10.1042/bse0540029. http://dx.doi.org/10.1042/bse0540029. [DOI] [PubMed] [Google Scholar]
- Duan J, Shi J, Fiorentino A, Leites C, Chen X, Moy W, Gejman PV. A rare functional noncoding variant at the GWAS-implicated MIR137/MIR2682 locus might confer risk to schizophrenia and bipolar disorder. Am. J. Hum. Genet. 2014;95(6):744–753. doi: 10.1016/j.ajhg.2014.11.001. http://dx.doi.org/10.1016/j.ajhg.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durak O, de Anda FC, Singh KK, Leussis MP, Petryshen TL, Sklar P, Tsai LH. Ankyrin-G regulates neurogenesis and Wnt signaling by altering the subcellular localization of beta-catenin. Mol. Psychiatry. 2015;20(3):388–397. doi: 10.1038/mp.2014.42. http://dx.doi.org/10.1038/mp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr. Res. 2005;73(2–3):159–172. doi: 10.1016/j.schres.2004.05.010. http://dx.doi.org/10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65(3):373–384. doi: 10.1016/j.neuron.2010.01.005. http://dx.doi.org/10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HM, Sun XY, Guo W, Zhong AF, Niu W, Zhao L, Lu J. Differential expression of microRNA in peripheral blood mononuclear cells as specific biomarker for major depressive disorder patients. J. Psychiatr. Res. 2014;59:45–52. doi: 10.1016/j.jpsychires.2014.08.007. http://dx.doi.org/10.1016/j.jpsychires.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Fan HM, Sun XY, Niu W, Zhao L, Zhang QL, Li WS, Lu J. Altered microRNA expression in peripheral blood mononuclear cells from young patients with schizophrenia. J. Mol. Neurosci. 2015;56(3):562–571. doi: 10.1007/s12031-015-0503-z. http://dx.doi.org/10.1007/s12031-015-0503-z. [DOI] [PubMed] [Google Scholar]
- Fenelon K, Mukai J, Xu B, Hsu PK, Drew LJ, Karayiorgou M, Gogos JA. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108(11):4447–4452. doi: 10.1073/pnas.1101219108. http://dx.doi.org/10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Sun G, Yan J, Noltner K, Li W, Buzin CH, Sommer SS. Evidence for X-chromosomal schizophrenia associated with microRNA alterations. PLoS One. 2009;4(7):e6121. doi: 10.1371/journal.pone.0006121. http://dx.doi.org/10.1371/journal.pone.0006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L Wellcome Trust Case Control, C. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008;40(9):1056–1058. doi: 10.1038/ng.209. http://dx.doi.org/10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner AJ, Degenhardt F, Schratt G, Nothen MM. MicroRNAs as the cause of schizophrenia in 22q11.2 deletion carriers, and possible implications for idiopathic disease: a mini-review. Front. Mol. Neurosci. 2013;6:47. doi: 10.3389/fnmol.2013.00047. http://dx.doi.org/10.3389/fnmol.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner AJ, Hofmann A, Maaser A, Sumer S, Khudayberdiev S, Muhleisen TW, Nothen MM. Genome-wide analysis implicates microRNAs and their target genes in the development of bipolar disorder. Transl. Psychiatry. 2015;5:e678. doi: 10.1038/tp.2015.159. http://dx.doi.org/10.1038/tp.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, O’Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–184. doi: 10.1038/nature12929. http://dx.doi.org/10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gardiner E, Beveridge NJ, Wu JQ, Carr V, Scott RJ, Tooney PA, Cairns MJ. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol. Psychiatry. 2012;17(8):827–840. doi: 10.1038/mp.2011.78. http://dx.doi.org/10.1038/mp.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Wu CN, Xu J, Feng G, Xing QH, Fu W, Zhao XZ. Polymorphisms in microRNA target sites influence susceptibility to schizophrenia by altering the binding of miRNAs to their targets. Eur. Neuropsychopharmacol. 2013;23(10):1182–1189. doi: 10.1016/j.euroneuro.2012.12.002. http://dx.doi.org/10.1016/j.euroneuro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Gray LJ, Dean B, Kronsbein HC, Robinson PJ, Scarr E. Region and diagnosis-specific changes in synaptic proteins in schizophrenia and bipolar I disorder. Psychiatry Res. 2010;178(2):374–380. doi: 10.1016/j.psychres.2008.07.012. http://dx.doi.org/10.1016/j.psychres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Green MJ, Cairns MJ, Wu J, Dragovic M, Jablensky A, Tooney PA Australian Schizophrenia Research, B. Genome-wide supported variant MIR137 and severe negative symptoms predict membership of an impaired cognitive subtype of schizophrenia. Mol. Psychiatry. 2013;18(7):774–780. doi: 10.1038/mp.2012.84. http://dx.doi.org/10.1038/mp.2012.84. [DOI] [PubMed] [Google Scholar]
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. http://dx.doi.org/10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Haggarty SJ, Silva MC, Cross A, Brandon NJ, Perlis RH. Advancing drug discovery for neuropsychiatric disorders using patient-specific stem cell models. Mol. Cell. Neurosci. 2016 doi: 10.1016/j.mcn.2016.01.011. http://dx.doi.org/10.1016/j.mcn.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmai Z, Dome P, Vereczkei A, Abdul-Rahman O, Szekely A, Gonda X, Nemoda Z. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J. Affect. Disord. 2013;150(1):104–109. doi: 10.1016/j.jad.2013.02.033. http://dx.doi.org/10.1016/j.jad.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Hammond SM. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. http://dx.doi.org/10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzeiy H, Allmer J, Yousef M. Computational methods for microRNA target prediction. Methods Mol. Biol. 2014;1107:207–221. doi: 10.1007/978-1-62703-748-8_12. http://dx.doi.org/10.1007/978-1-62703-748-8_12. [DOI] [PubMed] [Google Scholar]
- Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, Werge T. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS One. 2007;2(9):e873. doi: 10.1371/journal.pone.0000873. http://dx.doi.org/10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T, Jakobsen KD, Fenger M, Nielsen J, Krane K, Fink-Jensen A, Werge T. Variation in the purinergic P2RX(7) receptor gene and schizophrenia. Schizophr. Res. 2008;104(1-3):146–152. doi: 10.1016/j.schres.2008.05.026. http://dx.doi.org/10.1016/j.schres.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of primary mood disorder. Brain. 2002;125(Pt 7):1428–1449. doi: 10.1093/brain/awf149. [DOI] [PubMed] [Google Scholar]
- He Y, Zhou Y, Xi Q, Cui H, Luo T, Song H, Ying B. Genetic variations in microRNA processing genes are associated with susceptibility in depression. DNA Cell Biol. 2012;31(9):1499–1506. doi: 10.1089/dna.2012.1660. http://dx.doi.org/10.1089/dna.2012.1660. [DOI] [PubMed] [Google Scholar]
- Hogg DR, Harries LW. Human genetic variation and its effect on miRNA biogenesis, activity and function. Biochem. Soc. Trans. 2014;42(4):1184–1189. doi: 10.1042/BST20140055. http://dx.doi.org/10.1042/BST20140055. [DOI] [PubMed] [Google Scholar]
- Hommers LG, Domschke K, Deckert J. Heterogeneity and individuality: microRNAs in mental disorders. J. Neural Transm. 2015;122(1):79–97. doi: 10.1007/s00702-014-1338-4. http://dx.doi.org/10.1007/s00702-014-1338-4. [DOI] [PubMed] [Google Scholar]
- Hughes T, Hansson L, Sonderby IE, Athanasiu L, Zuber V, Tesli M, Djurovic S. A loss-of-function variant in a minor isoform of ANK3 protects against bipolar disorder and schizophrenia. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.021. http://dx.doi.org/10.1016/j.biopsych.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Fessler EB, Chibane FL, Leng Y, Maric D, Elkahloun AG, Chuang DM. Mood stabilizer-regulated miRNAs in neuropsychiatric and neurodegenerative diseases: identifying associations and functions. Am. J. Transl. Res. 2013;5(4):450–464. [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Chibane FL, Elkahloun AG, Henderson R, Singh R, Lawson J, Chuang DM. Novel integrative genomic tool for interrogating lithium response in bipolar disorder. Transl. Psychiatry. 2015;5:e504. doi: 10.1038/tp.2014.139. http://dx.doi.org/10.1038/tp.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame T, Bundo M, Sunaga F, Asai T, Nishimura F, Yoshikawa A, Iwamoto K. DNA methylation analysis of BDNF gene promoters in peripheral blood cells of schizophrenia patients. Neurosci. Res. 2013;77(4):208–214. doi: 10.1016/j.neures.2013.08.004. http://dx.doi.org/10.1016/j.neures.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Jensen KP, Kranzler HR, Stein MB, Gelernter J. The effects of a MAP2K5 microRNA target site SNP on risk for anxiety and depressive disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014;165B(2):175–183. doi: 10.1002/ajmg.b.32219. http://dx.doi.org/10.1002/ajmg.b.32219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Lv X, Jiao J. The role of microRNAs in neural stem cells and neurogenesis. J. Genet. Genomics. 2013;40(2):61–66. doi: 10.1016/j.jgg.2012.12.008. http://dx.doi.org/10.1016/j.jgg.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7(2):113–117. doi: 10.1038/nn1174. http://dx.doi.org/10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Kaminsky Z, Tochigi M, Jia P, Pal M, Mill J, Kwan A, Petronis A. A multi-tissue analysis identifies HLA complex group 9 gene methylation differences in bipolar disorder. Mol. Psychiatry. 2012;17(7):728–740. doi: 10.1038/mp.2011.64. http://dx.doi.org/10.1038/mp.2011.64. [DOI] [PubMed] [Google Scholar]
- Kandaswamy R, McQuillin A, Curtis D, Gurling H. Allelic association, DNA resequencing and copy number variation at the metabotropic glutamate receptor GRM7 gene locus in bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014;165B(4):365–372. doi: 10.1002/ajmg.b.32239. http://dx.doi.org/10.1002/ajmg.b.32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Imai T, Okano H. MicroRNAs in neural stem cells and neurogenesis. Front. Neurosci. 2012;6:30. doi: 10.3389/fnins.2012.00030. http://dx.doi.org/10.3389/fnins.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Song JY, Joo EJ, Lee KY, Ahn YM, Kim YS. EGR3 as a potential susceptibility gene for schizophrenia in Korea. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B(7):1355–1360. doi: 10.1002/ajmg.b.31115. http://dx.doi.org/10.1002/ajmg.b.31115. [DOI] [PubMed] [Google Scholar]
- Kim AH, Reimers M, Maher B, Williamson V, McMichael O, McClay JL, Vladimirov VI. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 2010;124(1-3):183–191. doi: 10.1016/j.schres.2010.07.002. http://dx.doi.org/10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry. 2012;17(2):142–153. doi: 10.1038/mp.2011.154. http://dx.doi.org/10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Wahlestedt C. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2009;106(9):3507–3512. doi: 10.1073/pnas.0805854106. http://dx.doi.org/10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr. Bull. 2014;40(4):721–728. doi: 10.1093/schbul/sbu070. http://dx.doi.org/10.1093/schbul/sbu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R, Dobra A, Tracy JH, Haugen E. Transcriptome profiling of human hippocampus dentate gyrus granule cells in mental illness. Transl. Psychiatry. 2014;4:e366. doi: 10.1038/tp.2014.9. http://dx.doi.org/10.1038/tp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlar AV, Mercer KB, Zwick ME, Mulle JG. New discoveries in schizophrenia genetics reveal neurobiological pathways: a review of recent findings. Eur. J. Med. Genet. 2015;58(12):704–714. doi: 10.1016/j.ejmg.2015.10.008. http://dx.doi.org/10.1016/j.ejmg.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. http://dx.doi.org/10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Kumar L, Verma S, Vaidya B, Gupta V. Exosomes: natural carriers for siRNA delivery. Curr. Pharm. Des. 2015;21(31):4556–4565. doi: 10.2174/138161282131151013190112. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. U. S. A. 2015;112(22):6807–6813. doi: 10.1073/pnas.1408355111. http://dx.doi.org/10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CY, Yu SL, Hsieh MH, Chen CH, Chen HY, Wen CC, Chen WJ. MicroRNA expression aberration as potential peripheral blood biomarkers for schizophrenia. PLoS One. 2011;6(6):e21635. doi: 10.1371/journal.pone.0021635. http://dx.doi.org/10.1371/journal.pone.0021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay JM, Mouillet-Richard S, Baudry A, Pietri M, Kellermann O. Raphe-mediated signals control the hippocampal response to SRI antidepressants via miR-16. Transl. Psychiatry. 2011;1:e56. doi: 10.1038/tp.2011.54. http://dx.doi.org/10.1038/tp.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray NR, Sullivan PF. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol. Psychiatry. 2014;76(7):510–512. doi: 10.1016/j.biopsych.2014.07.029. http://dx.doi.org/10.1016/j.biopsych.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neuro-transmission and prefrontal cortical dysfunction in schizophrenia. Biol. Psychiatry. 1999;46(5):616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Li X, Jin P. Macro role(s) of microRNAs in fragile X syndrome? Neruomol. Med. 2009;11(3):200–207. doi: 10.1007/s12017-009-8081-2. http://dx.doi.org/10.1007/s12017-009-8081-2. [DOI] [PubMed] [Google Scholar]
- Li YJ, Xu M, Gao ZH, Wang YQ, Yue Z, Zhang YX, Wang PY. Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS One. 2013;8(5):e63648. doi: 10.1371/journal.pone.0063648. http://dx.doi.org/10.1371/journal.pone.0063648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Meng H, Cao W, Qiu T. MiR-335 is involved in major depression disorder and antidepressant treatment through targeting GRM4. Neurosci. Lett. 2015;606:167–172. doi: 10.1016/j.neulet.2015.08.038. http://dx.doi.org/10.1016/j.neulet.2015.08.038. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. http://dx.doi.org/10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang F, Li T, Lu M, Wang L, Yue W, Zhang D. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics. 2012;13:661. doi: 10.1186/1471-2164-13-661. http://dx.doi.org/10.1186/1471-2164-13-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yuan YB, Guan LL, Wei H, Cheng Z, Han X, Yu X. MiRNA-365 and miRNA-520c-3p respond to risperidone treatment in first-episode schizophrenia after a 1 year remission. Chin. Med. J. 2013;126(14):2676–2680. [PubMed] [Google Scholar]
- Liu X, Zhang L, Cheng K, Wang X, Ren G, Xie P. Identification of suitable plasma-based reference genes for miRNAome analysis of major depressive disorder. J. Affect. Disord. 2014;163:133–139. doi: 10.1016/j.jad.2013.12.035. http://dx.doi.org/10.1016/j.jad.2013.12.035. [DOI] [PubMed] [Google Scholar]
- Lopez JP, Fiori LM, Gross JA, Labonte B, Yerko V, Mechawar N, Turecki G. Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int. J. Neuropsychopharmacol. 2014a;17(1):23–32. doi: 10.1017/S1461145713000941. http://dx.doi.org/10.1017/S1461145713000941. [DOI] [PubMed] [Google Scholar]
- Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, Turecki G. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat. Med. 2014b;20(7):764–768. doi: 10.1038/nm.3582. http://dx.doi.org/10.1038/nm.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, Haggarty SJ. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol. Psychiatry. 2015;20(6):703–717. doi: 10.1038/mp.2015.7. http://dx.doi.org/10.1038/mp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussion G, Yang J, Yerko V, Barker P, Mechawar N, Ernst C, Turecki G. Regulation of a truncated form of tropomyosin-related kinase B (TrkB) by Hsa-miR-185* in frontal cortex of suicide completers. PLoS One. 2012;7(6):e39301. doi: 10.1371/journal.pone.0039301. http://dx.doi.org/10.1371/journal.pone.0039301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Hyman SE. Progress in the genetics of polygenic brain disorders: significant new challenges for neurobiology. Neuron. 2013;80(3):578–587. doi: 10.1016/j.neuron.2013.10.046. http://dx.doi.org/10.1016/j.neuron.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Galdzicka M, Ginns E, Baker SP, Rogaev E, Xu J, Akbarian S. Gender-specific reduction of estrogen-sensitive small RNA, miR-30b, in subjects with schizophrenia. Schizophr. Bull. 2012;38(3):433–443. doi: 10.1093/schbul/sbq091. http://dx.doi.org/10.1093/schbul/sbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico D, Costain G, Butcher NJ, Warnica W, Ogura L, Alfred SE, Bassett AS. MicroRNA dysregulation, gene networks, and risk for schizophrenia in 22q11.2 deletion syndrome. Front. Neurol. 2014;5:238. doi: 10.3389/fneur.2014.00238. http://dx.doi.org/10.3389/fneur.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, Bridgemohan C, Wu BL. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J. Med. Genet. 2009;46(4):242–248. doi: 10.1136/jmg.2008.059907. http://dx.doi.org/10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, Wahlestedt C. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. U. S. A. 2012;109(8):3125–3130. doi: 10.1073/pnas.1113793109. http://dx.doi.org/10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol. Psychiatry. 2011;69(2):188–193. doi: 10.1016/j.biopsych.2010.09.039. http://dx.doi.org/10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, Cichon S. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat. Commun. 2014;5:3339. doi: 10.1038/ncomms4339. http://dx.doi.org/10.1038/ncomms4339. [DOI] [PubMed] [Google Scholar]
- Mulle JG. The 3q29 deletion confers N40-fold increase in risk for schizophrenia. Mol. Psychiatry. 2015;20(9):1028–1029. doi: 10.1038/mp.2015.76. http://dx.doi.org/10.1038/mp.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalakshmi U, Waern K, Snyder M. RNA-Seq: a method for comprehensive transcriptome analysis. Curr. Protoc. Mol. Biol. 2010:11–13. doi: 10.1002/0471142727.mb0411s89. http://dx.doi.org/10.1002/0471142727.mb0411s89 (Chapter 4, Unit 4 11) [DOI] [PubMed] [Google Scholar]
- Najt P, Wang F, Spencer L, Johnston JA, Cox Lippard ET, Pittman BP, Blumberg HP. Anterior cortical development during adolescence in bipolar disorder. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.026. http://dx.doi.org/10.1016/j.biopsych.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AC, McEvoy JP, Gennarelli M, Heinzen EL, Ge D, Maia JM, Goldstein DB. Exome sequencing followed by large-scale genotyping suggests a limited role for moderately rare risk factors of strong effect in schizophrenia. Am. J. Hum. Genet. 2012;91(2):303–312. doi: 10.1016/j.ajhg.2012.06.018. http://dx.doi.org/10.1016/j.ajhg.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Pena CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic basis of mental illness. Neuroscientist. 2015 doi: 10.1177/1073858415608147. http://dx.doi.org/10.1177/1073858415608147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network & Pathway Analysis Subgroup of Psychiatric Genomics, C. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat. Neurosci. 2015c;18(2):199–209. doi: 10.1038/nn.3922. http://dx.doi.org/10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll D, Schaefer A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology. 2013;38(1):39–54. doi: 10.1038/npp.2012.87. http://dx.doi.org/10.1038/npp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz R, Ulrich H, Zarate CA, Jr, Machado-Vieira R. Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;57:117–131. doi: 10.1016/j.pnpbp.2014.10.016. http://dx.doi.org/10.1016/j.pnpbp.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oved K, Morag A, Pasmanik-Chor M, Oron-Karni V, Shomron N, Rehavi M, Gurwitz D. Genome-wide miRNA expression profiling of human lymphoblastoid cell lines identifies tentative SSRI antidepressant response biomarkers. Pharmacogenomics. 2012;13(10):1129–1139. doi: 10.2217/pgs.12.93. http://dx.doi.org/10.2217/pgs.12.93. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Hammond SM. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. http://dx.doi.org/10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Mauney SA, Kim SS, Lim MP, Rooney RJ, Goldstein JM, Woo TU. Molecular profiles of pyramidal neurons in the superior temporal cortex in schizophrenia. J. Neurogenet. 2014a;28(1–2):53–69. doi: 10.3109/01677063.2014.882918. http://dx.doi.org/10.3109/01677063.2014.882918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Mauney SA, Kim SS, Passeri E, Lim MP, Rooney RJ, Woo TU. Molecular profiles of parvalbumin-immunoreactive neurons in the superior temporal cortex in schizophrenia. J. Neurogenet. 2014b;28(1–2):70–85. doi: 10.3109/01677063.2013.878339. http://dx.doi.org/10.3109/01677063.2013.878339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramon M, Pirinen M, Strange A, Lin K, Spencer CC Psychosis Endophenotypes International, C, Wellcome Trust Case–control, C. A genome-wide association analysis of a broad psychosis phenotype identifies three loci for further investigation. Biol. Psychiatry. 2014;75(5):386–397. doi: 10.1016/j.biopsych.2013.03.033. http://dx.doi.org/10.1016/j.biopsych.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, Sklar P. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185–190. doi: 10.1038/nature12975. http://dx.doi.org/10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman OA, Sasvari-Szekely M, Szekely A, Faludi G, Guttman A, Nemoda Z. Analysis of a polymorphic microRNA target site in the purinergic receptor P2RX7 gene. Electrophoresis. 2010;31(11):1790–1795. doi: 10.1002/elps.200900664. http://dx.doi.org/10.1002/elps.200900664. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry. 2012;17(12):1228–1238. doi: 10.1038/mp.2012.23. http://dx.doi.org/10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, Sullivan PF. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 2013;45(10):1150–1159. doi: 10.1038/ng.2742. http://dx.doi.org/10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong H, Liu TB, Yang KJ, Yang HC, Wu DH, Liao CP, Shen QJ. MicroRNA-134 plasma levels before and after treatment for bipolar mania. J. Psychiatr. Res. 2011;45(1):92–95. doi: 10.1016/j.jpsychires.2010.04.028. http://dx.doi.org/10.1016/j.jpsychires.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Rueckert EH, Barker D, Ruderfer D, Bergen SE, O’Dushlaine C, Luce CJ, Sklar P. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol. Psychiatry. 2013;18(8):922–929. doi: 10.1038/mp.2012.104. http://dx.doi.org/10.1038/mp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka WB, Subburaju S, Benes FM. Circuit- and diagnosis-specific DNA methylation changes at gamma-aminobutyric acid-related genes in postmortem human hippocampus in schizophrenia and bipolar disorder. JAMA Psychiatry. 2015;72(6):541–551. doi: 10.1001/jamapsychiatry.2015.49. http://dx.doi.org/10.1001/jamapsychiatry.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R, Storchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, Schratt GM. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol. Cell. Biol. 2012;32(3):619–632. doi: 10.1128/MCB.05896-11. http://dx.doi.org/10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol. Psychiatry. 2011;69(2):180–187. doi: 10.1016/j.biopsych.2010.09.030. http://dx.doi.org/10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Santarelli DM, Liu B, Duncan CE, Beveridge NJ, Tooney PA, Schofield PR, Cairns MJ. Gene-microRNA interactions associated with antipsychotic mechanisms and the metabolic side effects of olanzapine. Psychopharmacology. 2013;227(1):67–78. doi: 10.1007/s00213-012-2939-y. http://dx.doi.org/10.1007/s00213-012-2939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saus E, Soria V, Escaramis G, Crespo JM, Valero J, Gutierrez-Zotes A, Urretavizcaya M. A haplotype of glycogen synthase kinase 3beta is associated with early onset of unipolar major depression. Genes Brain Behav. 2010a;9(7):799–807. doi: 10.1111/j.1601-183X.2010.00617.x. http://dx.doi.org/10.1111/j.1601-183X.2010.00617.x. [DOI] [PubMed] [Google Scholar]
- Saus E, Soria V, Escaramis G, Vivarelli F, Crespo JM, Kagerbauer B, Estivill X. Genetic variants and abnormal processing of pre-miR-182, a circadian clock modulator, in major depression patients with late insomnia. Hum. Mol. Genet. 2010b;19(20):4017–4025. doi: 10.1093/hmg/ddq316. http://dx.doi.org/10.1093/hmg/ddq316. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study, C. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011C;43(10):969–976. doi: 10.1038/ng.940. http://dx.doi.org/10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014C;511(7510):421–427. doi: 10.1038/nature13595. http://dx.doi.org/10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33(1):110–133. doi: 10.1038/sj.npp.1301575. http://dx.doi.org/10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- Shi W, Du J, Qi Y, Liang G, Wang T, Li S, Xiao Z. Aberrant expression of serum miRNAs in schizophrenia. J. Psychiatr. Res. 2012;46(2):198–204. doi: 10.1016/j.jpsychires.2011.09.010. http://dx.doi.org/10.1016/j.jpsychires.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Striessnig J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol. 2009;75(2):407–414. doi: 10.1124/mol.108.049981. http://dx.doi.org/10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS One. 2012;7(3):e33201. doi: 10.1371/journal.pone.0033201. http://dx.doi.org/10.1371/journal.pone.0033201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS One. 2014;9(1):e86469. doi: 10.1371/journal.pone.0086469. http://dx.doi.org/10.1371/journal.pone.0086469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005;21(6):1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. http://dx.doi.org/10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schurmann B, Penzes P. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron. 2014;84(2):399–415. doi: 10.1016/j.neuron.2014.10.010. http://dx.doi.org/10.1016/j.neuron.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HT, Sun XY, Zhang L, Zhao L, Guo ZM, Fan HM, Lu J. A preliminary analysis of association between the down-regulation of microRNA-181b expression and symptomatology improvement in schizophrenia patients before and after anti-psychotic treatment. J. Psychiatr. Res. 2014;54:134–140. doi: 10.1016/j.jpsychires.2014.03.008. http://dx.doi.org/10.1016/j.jpsychires.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landen M, Lichtenstein P. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;17(2):184–193. doi: 10.1111/bdi.12242. http://dx.doi.org/10.1111/bdi.12242. [DOI] [PubMed] [Google Scholar]
- Song MF, Dong JZ, Wang YW, He J, Ju X, Zhang L, Lv YY. CSF miR-16 is decreased in major depression patients and its neutralization in rats induces depression-like behaviors via a serotonin transmitter system. J. Affect. Disord. 2015;178:25–31. doi: 10.1016/j.jad.2015.02.022. http://dx.doi.org/10.1016/j.jad.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 2002;71(4):877–892. doi: 10.1086/342734. http://dx.doi.org/10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. http://dx.doi.org/10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol. Rev. 2010;20(4):327–348. doi: 10.1007/s11065-010-9148-4. http://dx.doi.org/10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoicea N, Du A, Lakis DC, Tipton C, Arias-Morales CE, Bergese SD. The MiRNA journey from theory to practice as a CNS biomarker. Front. Genet. 2016;7:11. doi: 10.3389/fgene.2016.00011. http://dx.doi.org/10.3389/fgene.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazisar M, van der Ven K, Cammaerts S, Forero DA, Lenaerts AS, Nordin A, Del-Favero J. MIR137 variants identified in psychiatric patients affect synaptogenesis and neuronal transmission gene sets. Mol. Psychiatry. 2015;20(4):472–481. doi: 10.1038/mp.2014.53. http://dx.doi.org/10.1038/mp.2014.53. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat. Rev. Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. http://dx.doi.org/10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E, Shi Y. MicroRNAs: small molecules with big roles in neurodevelopment and diseases. Exp. Neurol. 2015;268:46–53. doi: 10.1016/j.expneurol.2014.08.005. http://dx.doi.org/10.1016/j.expneurol.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Sun Y, Luo ZM, Guo XM, Su DF, Liu X. An updated role of microRNA-124 in central nervous system disorders: a review. Front. Cell. Neurosci. 2015;9:193. doi: 10.3389/fncel.2015.00193. http://dx.doi.org/10.3389/fncel.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147–168. doi: 10.1038/npp.2009.115. http://dx.doi.org/10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms AE, Dorschner MO, Wechsler J, Choi KY, Kirkwood R, Girirajan S, Tsuang DW. Support for the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiatry. 2013;70(6):582–590. doi: 10.1001/jamapsychiatry.2013.1195. http://dx.doi.org/10.1001/jamapsychiatry.2013.1195. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. http://dx.doi.org/10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol. Psychiatry. 2000;48(6):486–504. doi: 10.1016/s0006-3223(00)00978-1. [DOI] [PubMed] [Google Scholar]
- Vinas-Jornet M, Esteba-Castillo S, Gabau E, Ribas-Vidal N, Baena N, San J, Guitart M. A common cognitive, psychiatric, and dysmorphic phenotype in carriers of NRXN1 deletion. Mol. Genet. Genomic Med. 2014;2(6):512–521. doi: 10.1002/mgg3.105. http://dx.doi.org/10.1002/mgg3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RM, Rybka J, Anderson SM, Torrance HS, Boxall R, Sussmann JE, Evans KL. Preliminary investigation of miRNA expression in individuals at high familial risk of bipolar disorder. J. Psychiatr. Res. 2015;62:48–55. doi: 10.1016/j.jpsychires.2015.01.006. http://dx.doi.org/10.1016/j.jpsychires.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Liu J, Hass J, White T, Scholz M, Roessner V, Ehrlich S. MB-COMT promoter DNA methylation is associated with working-memory processing in schizophrenia patients and healthy controls. Epigenetics. 2014;9(8):1101–1107. doi: 10.4161/epi.29223. http://dx.doi.org/10.4161/epi.29223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Liu Y, Wang X, Wu J, Liu K, Zhou J, Zhang C. Identification of differential microRNAs in cerebrospinal fluid and serum of patients with major depressive disorder. PLoS One. 2015;10(3):e0121975. doi: 10.1371/journal.pone.0121975. http://dx.doi.org/10.1371/journal.pone.0121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton A, Breen G, Bubb VJ, Quinn JP. A GWAS SNP for schizophrenia is linked to the internal MIR137 promoter and supports differential allele-specific expression. Schizophr. Bull. 2015 doi: 10.1093/schbul/sbv144. http://dx.doi.org/10.1093/schbul/sbv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnica W, Merico D, Costain G, Alfred SE, Wei J, Marshall CR, Bassett AS. Copy number variable microRNAs in schizophrenia and their neurodevelopmental gene targets. Biol. Psychiatry. 2015;77(2):158–166. doi: 10.1016/j.biopsych.2014.05.011. http://dx.doi.org/10.1016/j.biopsych.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Yuan Y, Liu S, Wang C, Yang F, Lu Z, Xu Q. Detection of circulating miRNA levels in schizophrenia. Am. J. Psychiatry. 2015;172(11):1141–1147. doi: 10.1176/appi.ajp.2015.14030273. http://dx.doi.org/10.1176/appi.ajp.2015.14030273. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Papmeyer M, Romaniuk L, Sprooten E, Johnstone EC, Hall J, McIntosh AM. Impact of a microRNA MIR137 susceptibility variant on brain function in people at high genetic risk of schizophrenia or bipolar disorder. Neuropsychopharmacology. 2012;37(12):2720–2729. doi: 10.1038/npp.2012.137. http://dx.doi.org/10.1038/npp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Duncan CE, Beveridge NJ, Webster MJ, Cairns MJ, Weickert CS. Expression of NPAS3 in the human cortex and evidence of its posttranscriptional regulation by miR-17 during development, with implications for schizophrenia. Schizophr. Bull. 2013;39(2):396–406. doi: 10.1093/schbul/sbr177. http://dx.doi.org/10.1093/schbul/sbr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Li F, Zhang B, Zhang K, Zhang F, Huang X, Liu P. MicroRNAs and target site screening reveals a pre-microRNA-30e variant associated with schizophrenia. Schizophr. Res. 2010;119(1–3):219–227. doi: 10.1016/j.schres.2010.02.1070. http://dx.doi.org/10.1016/j.schres.2010.02.1070. [DOI] [PubMed] [Google Scholar]
- Yamada K, Gerber DJ, Iwayama Y, Ohnishi T, Ohba H, Toyota T, Yoshikawa T. Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2007;104(8):2815–2820. doi: 10.1073/pnas.0610765104. http://dx.doi.org/10.1073/pnas.0610765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang Q, Wang X, Luo C, Wan Y, Li J, Zhang C. MicroRNA expression profile and functional analysis reveal that miR-206 is a critical novel gene for the expression of BDNF induced by ketamine. Neruomol. Med. 2014;16(3):594–605. doi: 10.1007/s12017-014-8312-z. http://dx.doi.org/10.1007/s12017-014-8312-z. [DOI] [PubMed] [Google Scholar]
- Yu HC, Wu J, Zhang HX, Zhang GL, Sui J, Tong WW, Lv LX. Alterations of miR-132 are novel diagnostic biomarkers in peripheral blood of schizophrenia patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;63:23–29. doi: 10.1016/j.pnpbp.2015.05.007. http://dx.doi.org/10.1016/j.pnpbp.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Zhao D, Lin M, Chen J, Pedrosa E, Hrabovsky A, Fourcade HM, Lachman HM. MicroRNA profiling of neurons generated using induced pluripotent stem cells derived from patients with schizophrenia and schizoaffective disorder, and 22q11.2 del. PLoS One. 2015;10(7):e0132387. doi: 10.1371/journal.pone.0132387. http://dx.doi.org/10.1371/journal.pone.0132387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Manji HK. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34(6):1395–1405. doi: 10.1038/npp.2008.131. http://dx.doi.org/10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang J, Lu X, Song X, Ye Y, Zhou J, Wang L. Evaluation of six SNPs of microRNA machinery genes and risk of schizophrenia. J. Mol. Neurosci. 2013;49(3):594–599. doi: 10.1007/s12031-012-9887-1. http://dx.doi.org/10.1007/s12031-012-9887-1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kalbfleisch T, Brennan MD, Li Y. A microRNA gene is hosted in an intron of a schizophrenia-susceptibility gene. Schizophr. Res. 2009;109(1–3):86–89. doi: 10.1016/j.schres.2009.01.022. http://dx.doi.org/10.1016/j.schres.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]