Abstract
The enhanced expression of glucosylceramide based glycosphingolipids is a hallmark of many forms of renal disease including diabetic nephropathy, polycystic kidney disease, and renal cell carcinoma. A common feature of each of these renal disorders is the preference metabolism of via aerobic glycolysis. While aerobic glycolysis is an inefficient way to generate ATP, aerobic glycolysis promotes the formation of substrates important for the production of biomass, including lipids, amino acids, and nucleotides through the pentose phosphate pathway. Two products that are essential for the synthesis of glucosylceramide and more complex glycosphingolipids are generated through the pentose phosphate pathway. These products are reducing equivalents in the form of NADPH and UDP-glucose. In experimental models of each of these disorders, inhibition of glucosylceramide synthase with eliglustat or related analogues reverses the disease phenotype suggesting that blocking glycosphingolipid synthesis should be explored as a potential treatment strategy.
Keywords: diabetes mellitus, glucosylceramide, eliglustat, polycystic kidney disease, sphingolipids
Introduction
Historically, interest in the metabolism of sphingolipids arose with the discovery of the lysosome by Christian de Duve and the recognition that several lysosomal storage diseases arise from deficiencies in hydrolytic enzymes involved in the degradation of sphingolipids and more specifically, glycosphingolipids (GSLs) [1]. Fabry disease, a lysosomal storage disorder resulting from a loss of α-galactosidase A activity, the accumulation of globotriaosylceramide, ultimately resulting in progressive renal failure, is one such disorder [2]. These discoveries were followed by significant efforts in defining the biochemical pathways, the anabolic and catabolic enzymes associated with these pathways, and the cellular biology associated with these novel lipid molecules. With these efforts several studies have been reported suggesting that sphingolipids may play a role in the pathophysiology of common clinical disorders, including those associated with aberrant renal growth. This review discusses recent work in this area and proposes a unifying hypothesis linking enhanced glycosphingolipid metabolism with aberrant renal growth. This review was written on the occasion of a symposium honoring David Warnock for his many accomplishments in academic nephrology and in particular for his studies of Fabry disease.
Sphingolipid metabolism
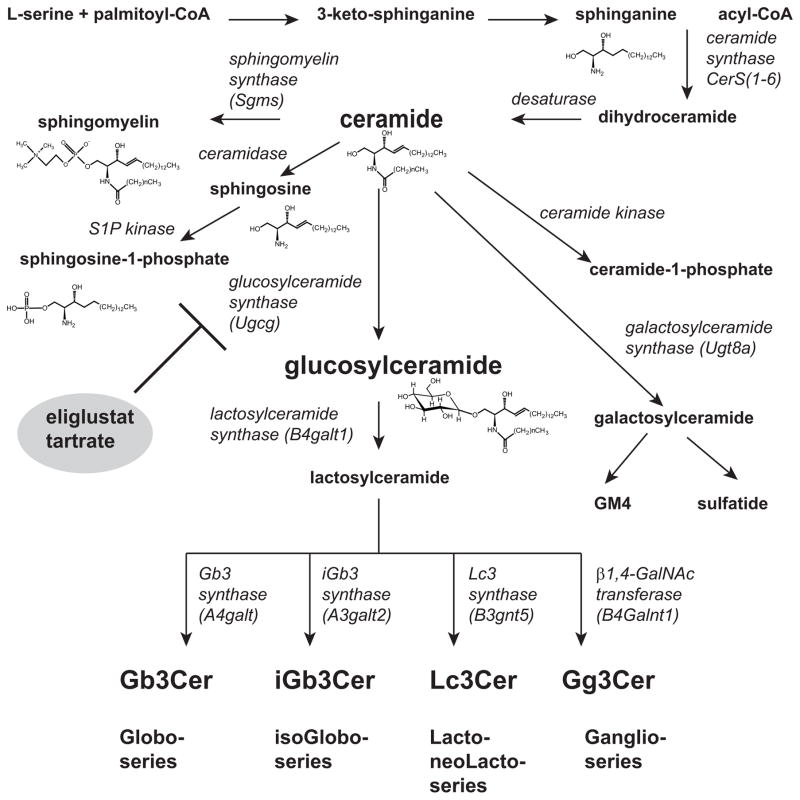
By definition, sphingolipids are any compound containing a long chain sphingoid amine [3]. The precursor long chain base, dihydrosphingosine, is initially formed by a condensation reaction that utilizes serine and palmitoyl-CoA as substrates. The subsequent acylation and oxidation of dihydrosphingosine results in ceramide formation, the primary lipid moiety found in most glycosphingolipids. Ceramide refers to a lipid containing a sphingoid base, typically sphingosine, and a fatty acyl group in amide linkage. The acyl groups of ceramides may differ in their carbon chain lengths, degree of saturation, and numbers of hydroxyl groups. Ceramide is an intermediate in the formation of multiple types of sphingolipids. Free sphingosine results from the deacylation of ceramide by various ceramidases. Sphingosine can be phosphorylated to form sphingosine-1-phosphate, the ligand for a family of G protein coupled receptors, termed sphingosine 1-phosphate receptors [4]. Alternatively, the 1-carbon hydroxyl on ceramide can be modified through several alternative pathways. Phosphorylcholine exchange with phosphatidylcholine results in the formation of sphingomyelin, one of the most abundant phospholipids found in cells. Phosphorylation of ceramide leads to ceramide 1-phosphate, a metabolite with its own biological activities that include membrane fusion and regulation of cytosolic phospholipase A2 [5].
Glycosphingolipids are formed by the addition of sugars to ceramide [6]. The number of sugars varies from only one to greater than twenty. The term cerebroside denotes the presence of a single sugar. Glucosylceramide and galactosylceramide are the most commonly found cerebrosides. These glycans may be neutral or negatively charged, the negative charge arising from the presence of one or more sialic acids or from the addition of a sulfate group. Mammalian tissues contain over 300 distinct glycosphingolipids based on the present of glycosyltransferases in the Golgi apparatus. Most GSLs are characterized by the presence of glucose as the first sugar, other GSLs have galactose and less commonly fucose as their base cerebrosides. The glucosylceramide based GSLs are built on a lactosylceramide backbone, in which lactosylceramide synthase (B4galt1) has added a galactose. Based on the next sugar to be added to the lactosylceramide, GSLs comprising one of four series are formed. These series and the responsible enzymes include the globo-series (Gb3 synthase, A4galt); the isoGlobo-series (iGb3 synthase, A3galt3); the lacto-/neoLacto-series (Lc synthase, B3gnt5); and Ganglio-series (β1,4-GalNAc transferase, B4Galnt1) (figure 1A). Over twenty glucosylceramide based ganglio-series GSLs are found, each with their own distinct biological activities.
Figure 1.
Primary pathways for sphingolipid synthesis. Long chain base synthesis begins with the condensation of serine and palmitoyl-CoA eventually resulting in the formation of ceramide, a key intermediate in sphingolipids synthesis. The glycosylation of ceramide primarily results in the formation of the single sugar containing cerebroside, glucosylceramide. Further glycosylation pathways result in the formation of over 300 mammalian glycosphingolipids through the primary series, globo-, isoglobo, ganglio-, and lacto-, indicated at the lower portion of the figure.
A role for GSLs in common disorders of the kidney
Fabry disease, a deficiency in the lysosomal enzyme α-galactosidase A, is a classic form of chronic renal disease that results from impaired GSL metabolism. In this setting, globotriaosylceramide and globotriaosylsphingosine, the deacylated counterpart, accumulate within the lysosome and other membrane compartments resulting in the progressive loss of renal function [7]. While the mechanisms responsible for the loss of renal function are not well delineated, recent work has implicated both limited nitric oxide bioavailability and endothelial nitric oxide synthase uncoupling as potential causes for the vasculopathy [8].
Less well appreciated is the possibility that aberrant GSL metabolism may underlie some of the most common diseases of the kidney. In particular, diseases associated with aberrant renal growth including diabetic nephropathy [9], polycystic kidney disease [10], and renal cell carcinoma [11] have been associated with changes in GSL levels and associated metabolism. Glucosylceramide, lactosylceramide, and ganglioside GM3 are elevated in the kidneys and renal tissues from patients with these diseases and in experimental models of these disorders. Importantly, inhibition of glucosylceramide formation with the use of small molecule inhibitors of glucosylceramide synthase reverses or prevents the abnormal renal phenotypes associated with these experimental models. These latter findings suggest that not only might glucosylceramide and glucosylceramide based GSLs serve as biomarkers for these disorders, but that blockade of glucosylceramide synthesis may be a pharmacological strategy for their treatment.
Linking GSL synthesis to aberrant renal growth
What might be the metabolic link between GSL metabolism and enhanced renal growth? A clue to this nexus may lie in understanding the relative contributions of mitochondrial oxidative phosphorylation and aerobic glycolysis to GSL synthesis. In the presence of oxygen, terminally differentiated tissues, including the kidney, will metabolize glucose through the process of oxidative phosphorylation. Glucose is first metabolized to pyruvate via glycolysis and then is oxidized in the mitochondria to form CO2. Oxygen is required as the first electron acceptor to completely oxidize glucose and is therefore essential to this pathway. As a result, approximately 36 mol of ATP are produced per mole of glucose. When oxygen is limited, cells redirect pyruvate by generating lactate resulting in anerobic glycolysis and the production of only 2 mol of ATP per mol glucose. The Warburg effect is the observation that cancer cells convert most of their glucose to lactate regardless of whether oxygen is limiting or not. However, it is now appreciated that this pathway, so called aerobic glycolysis, is characteristic of proliferating tissues in general and not simply those that have undergone malignant transformation [12].
Two explanations have been proposed for the preference for proliferating cells to utilize aerobic glycolysis [13]. The first is that ATP is not rate limiting under such conditions. Proliferating cells are exposed to a continuous supply of glucose and other substrates. Indeed, proliferating cells are observed to maintain high ratios of ATP to ADP and of NADH to NAD+. The second explanation is that proliferating cells have metabolic needs that extend beyond that of ATP. Because a proliferating cell must reproduce all of its cellular components, there is a requirement to produce nucleotides, lipid, and amino acids in addition to ATP. For the production of each of these components there is a requirement to consume more equivalents of carbon, and NADPH than of ATP. For example, the synthesis of a single molecule of palmitate requires 7 molecules of ATP, 8 molecules of acetyl-CoA, and 28 electrons from 14 molecules of NADPH. Thus metabolism of glucose exclusively through oxidative phosphorylation runs counter to the metabolic needs of proliferating cells.
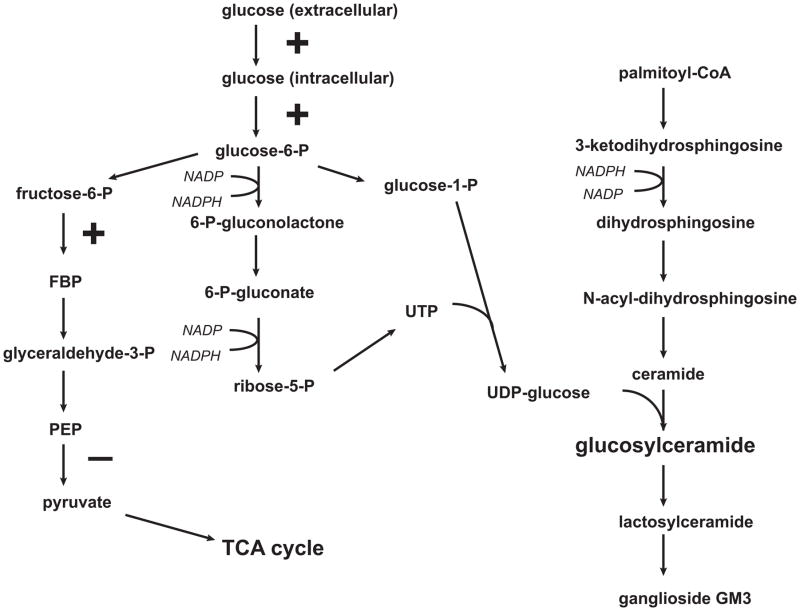
Proliferating cells appear to be “hard wired” for aerobic glycolysis. Growth factors stimulate glucose availability through stimulation of glucose transport and hexokinase and the inhibition of flux through mitochondrial metabolism by inhibition of pyruvate kinase or in cancer cells pyruvate kinase M2 [14]. The net result is increased flux of glucose through the pentose phosphate pathway. Two important substrates for glycolipid synthesis result from this flux. First, more reducing equivalents are available in the form of NADPH. Second, nucleotide sugars, including UDP-glucose and UDP-galactose, are formed which are critical in the Leloir pathways for glycolipid synthesis (figure 2).
Figure 2.
Intersection of aerobic glycolysis and glycosphingolipid synthesis. Glucose metabolism is shunted from oxidative phosphorylation and the TCA cycle to aerobic glycolysis in the presence of growth factors. Enzymes stimulated by growth factors are indicated by a “+” sign and include hexokinase and phosphofructokinase. Glucose transport is also growth factor stimulated. Conversion of phosphoenolpyruvate to pyruvate via pyruvate kinase or in malignant cells pyruvate kinase M2 is inhibited by growth factors favoring glucose metabolism through the pentose phosphate shunt. Two metabolites that are critical for glucosylceramide formation are produced as a result. These include reducing equivalents in the form of NADPH and sugar nucleotides, notably UDP-glucose.
While GSLs represent a only a small fraction of the total chemical mass of a cell, the increase in glycolipid content in kidneys that have undergone hyperplasia or hypertrophy has been well documented as was mentioned above. In these examples aerobic glycolysis is favored due to the presence of increased growth factors such as EGF in autosomal dominant polycystic kidney disease, IGF1 in diabetic nephropathy, or the acquisition of mutations that alter growth factor dependent signaling pathways. In some disorders these metabolic changes may be irreversible to the extent that they arise from a mutation in a gene resulting in the persistent expression of a growth factor or the loss or gain of function of a signaling protein. In other cases, the metabolic changes may be reversible as is observed in the suppression of respiration and oxidative phosphorylation that is characteristic of hyperglycemia. This reversible shift in energy metabolism is referred to as the Crabtree effect[15].
The secondary effects of increased GSL formation may result in additional mechanisms that underlie the specific pathology of these disorders. In each of these examples, renal cell carcinoma [11], polycystic kidney disease [16], and diabetic hypertrophy [17], the experimental use of inhibitors of glucosylceramide synthase has been shown to reverse the pathological phenotype. The actual basis for this reversal, however, is unknown. Two possibilities may be considered. First, there may be specific GSLs that are critical mediators of these particular diseases. In one well studied example, ganglioside GM3 may result in insulin resistance through sequestration of the insulin receptor outside of its normal caveolar locale and associated signalsome [18]. Second, the inhibition of glucosylceramide synthesis may result in the accumulation of a precursor or loss of a product that may specifically signal the cell to turn off aerobic glycolysis. While the former question has been actively pursued through studies linking GSLs to a variety of signaling pathways, particularly those involving growth factors, the latter question does not appear to have been addressed but is certainly worthy or future studies.
Understanding the connection between inhibition of glucosylceramide synthase and the reversal of either renal hypertrophy or cyst growth is more than a scientific exercise. Eliglustat tartrate was recently approved as the first “stand alone” substrate reduction agent for the treatment of Gaucher disease type 1 [19,20]. Eliglustat was very well tolerated by the majority of patients that participated in the phase 2 and 3 trials leading to its approval, and the toxicity profile was very favorable including those subjects who remained on the drug for over five years. Thus the use of compounds that potently and selectively inhibit glucosylceramide synthesis should be seriously considered as the basis for clinical study.
Acknowledgments
This work was supported by NIH grants UH2NS092981 and 2 RO1 DK055823.
Abbreviations
- GSL
glycosphingolipid
Footnotes
Contribution from the Special Symposium to celebrate the contributions of David G. Warnock, MD to Academic Nephrology, the University of Alabama at Birmingham, Division of Nephrology, November 9, 2015.
References
- 1.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 2.Kint JA. Fabry’s disease: alpha-galactosidase deficiency. Science. 1970;167:1268–1269. doi: 10.1126/science.167.3922.1268. [DOI] [PubMed] [Google Scholar]
- 3.Merrill AH, Jr, Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA, Wang E. Sphingolipids--the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 4.Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem Rev. 2011;111:6299–6320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamour NF, Chalfant CE. Ceramide-1-phosphate: the “missing” link in eicosanoid biosynthesis and inflammation. Mol Interv. 2005;5:358–367. doi: 10.1124/mi.5.6.8. [DOI] [PubMed] [Google Scholar]
- 6.Kolter T, Proia RL, Sandhoff K. Combinatorial ganglioside biosynthesis. J Biol Chem. 2002;277:25859–25862. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- 7.Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105:2812–2817. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu L, Vivekanandan-Giri A, Pennathur S, Smid BE, Aerts JM, Hollak CE, Shayman JA. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int. 2014;86:58–66. doi: 10.1038/ki.2013.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA. A Role for Glycosphingolipid Accumulation in the Renal Hypertrophy of Streptozotocin-Induced Diabetes- Mellitus. J Clin Invest. 1993;91:797–803. doi: 10.1172/JCI116299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshmukh GD, Radin NS, Gattone VH, Shayman JA. Abnormalities of Glycosphingolipid, Sulfatide, and Ceramide in the Polycystic (Cpk/Cpk) Mouse. J Lipid Res. 1994;35:1611–1618. [PubMed] [Google Scholar]
- 11.Chatterjee S, Alsaeedi N, Hou J, Bandaru VV, Wu L, Halushka MK, Pili R, Ndikuyeze G, Haughey NJ. Use of a glycolipid inhibitor to ameliorate renal cancer in a mouse model. Plos One. 2013;8:e63726. doi: 10.1371/journal.pone.0063726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 13.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Ruiz R, Rigoulet M, Devin A. The Warburg and Crabtree effects: On the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys Acta. 2011;1807:568–576. doi: 10.1016/j.bbabio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Natoli TA, Smith LA, Rogers KA, Wang B, Komarnitsky S, Budman Y, Belenky A, Bukanov NO, Dackowski WR, Husson H, Russo RJ, Shayman JA, Ledbetter SR, Leonard JP, Ibraghimov-Beskrovnaya O. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16:788–792. doi: 10.1038/nm.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subathra M, Korrapati M, Howell LA, Arthur JM, Shayman JA, Schnellmann RG, Siskind LJ. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol. 2015;309:F204–215. doi: 10.1152/ajprenal.00150.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A. 2007;104:13678–13683. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mistry PK, Lukina E, Ben Turkia H, Amato D, Baris H, Dasouki M, Ghosn M, Mehta A, Packman S, Pastores G, Petakov M, Assouline S, Balwani M, Danda S, Hadjiev E, Ortega A, Shankar S, Solano MH, Ross L, Angell J, Peterschmitt MJ. Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. JAMA. 2015;313:695–706. doi: 10.1001/jama.2015.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox TM, Drelichman G, Cravo R, Balwani M, Burrow TA, Martins AM, Lukina E, Rosenbloom B, Ross L, Angell J, Puga AC. Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385:2355–2362. doi: 10.1016/S0140-6736(14)61841-9. [DOI] [PubMed] [Google Scholar]