Abstract
This study investigated whether adolescent nicotine exposure in one generation of rats would impair the cognitive capacity of a subsequent generation. Male and female rats in the parental F0 generation were given twice-daily i.p. injections of either 1.0 mg/kg nicotine or an equivalent volume of saline for 35 days during adolescence on postnatal days 25-59 (P25-59). After reaching adulthood, male and female nicotine-exposed rats were paired for breeding as were male and female saline control rats. Only female offspring were used in this experiment. Half of the offspring of F0 nicotine-exposed breeders and half of the offspring of F0 saline control rats received twice-daily i.p. injections of 1.0 mg/kg nicotine during adolescence on P25-59. The remainder of the rats received twice-daily saline injections for the same period. To evaluate transgenerational effects of nicotine exposure on complex cognitive learning abilities, F1 generation rats were trained to perform a highly structured serial pattern in a serial multiple choice (SMC) task. Beginning on P95, rats in the F1 generation were given either 4 days of massed training (20 patterns/day) followed by spaced training (10 patterns/day) or only spaced training. Transgenerational effects of adolescent nicotine exposure were observed as greater difficulty in learning a “violation element” of the pattern, which indicated that rats were impaired in the ability to encode and remember multiple sequential elements as compound or configural cues. The results indicated that for rats that received massed training, F1 generation rats with adolescent nicotine exposure whose F0 generation parents also experienced adolescent nicotine exposure showed poorer learning of the violation element than rats that experienced adolescent nicotine exposure only in the F1 generation. Thus, adolescent nicotine exposure in one generation of rats produced a cognitive impairment in the next generation.
1. Introduction
Recent research and theory on epigenetic and transgenerational effects of drugs and toxic chemicals suggest that experiences with these agents in one generation may ultimately produce neurobehavioral effects in subsequent generations (e.g., Bohacek, Gapp, Saab, & Mansuy, 2013; Jensen, 2013; Szyf, 2007). This raises the question of whether nicotine exposure in adolescence, which has been shown to cause long lasting changes in cognitive learning capacity (Pickens, Rowan, Bevins, & Fountain, 2013; Renaud, Pickens, & Fountain, 2015), would also cause neurobehavioral changes that would be passed down to future generations. The current study was aimed at determining if adolescent exposure to nicotine in one generation could affect the adult cognitive abilities of a subsequent generation.
Recent research has documented the effects of exposure to nicotine during critical development periods on brain physiology and morphology (for reviews, see Dwyer, McQuown, & Leslie, 2009; Smith, McDonald, Bergstrom, Ehlinger, & Brielmaier, 2015). Several studies have also demonstrated that adolescent nicotine exposure causes adult cognitive deficits including impairments of visuospatial attention and impulse control (Counotte et al., 2009), context fear learning (Spaeth, Barnet, Hunt, & Burk, 2010), and serial pattern learning (Fountain, Rowan, Kelley, Willey, & Nolley, 2008; Pickens et al., 2013).
Serial pattern learning in the serial multiple choice (SMC) task (Fountain, 1990; Fountain & Rowan, 1995a; 1995b) is a paradigm modeled after a nonverbal task for studying cognitive function in humans (Restle & Brown, 1970a, 1970b). In the SMC task, rats are placed inside an octagonal operant chamber equipped with a nose poke receptacle on each wall and they are trained to perform serial patterns by choosing the receptacles in the proper sequential order. One may refer to the receptacles by numbering them 1 through 8 in a clockwise manner. A serial pattern that is often used in the SMC task is made up of 24 elements composed of eight 3-element chunks:
123-234-345-456-567-678-781-818-
The digits in the sequence refer to the order in which the rat is supposed to choose the receptacles. The first element of each 3-element chunk (bolded) is termed the chunk-boundary element, the two elements that follow it are known as within-chunk elements, and the last element of the last chunk of this pattern (underlined) is termed the violation element because it violates the rules that describe the rest of the pattern. Trials within chunks are separated by 1 sec, and the dashes between chunks represent 3-sec pauses that serve as a phrasing cues (Fountain et al., 2008; Stempowski, Carman, & Fountain, 1999). Previous research has shown that this task recruits multiple cognitive systems that interact together to allow the rat to learn complex serial patterns (Chenoweth & Fountain, 2015; Fountain et al., 2012; Muller & Fountain, 2010; 2016). For example, learning to anticipate chunk-boundary elements has been shown to depend on stimulus-response (S-R) learning and serial position learning (Muller & Fountain, 2010; 2016; Stempowski et al., 1999). Violation element anticipation is believed to rely on multiple-item memory (Kundey & Fountain, 2010; Muller & Fountain, 2010; 2016). Within-chunk elements, on the other hand, are encoded via rule learning (Muller & Fountain, 2010).
Pickens et al. (2013) used an SMC task to assess the effects of adolescent nicotine on adult rat serial pattern learning. Pickens et al. (2013) exposed male and female adolescent rats to either 1.0 mg/kg/day of nicotine or the vehicle control via once-daily intraperitoneal (i.p.) injections for 35 days beginning on postnatal day 25 (P25). The rats were then given a drug-free period of 35 days until they reached the adult age of P95. At that time, they were trained in the SMC task where they had to perform the pattern presented above 10 times per day for 49 days. Pickens et al. (2013) found that adolescent nicotine exposure caused adult learning deficits in the SMC task. Adolescent nicotine caused differential impairments for males and females. Specifically, males exposed to nicotine in adolescence had impaired learning for chunk-boundary elements compared to vehicle controls whereas females did not. Females exposed to nicotine in adolescence, on the other hand, had impaired learning for the violation element whereas males did not. No differences in performance were found for either sex on within-chunk elements. The results are strong evidence that adolescent nicotine exposure caused specific learning deficits for some elements of the pattern and that the deficits were sex-specific (Pickens et al., 2013).
To date, there has been no research assessing the transgenerational effects of adolescent nicotine exposure on cognitive systems. This study examined the extent to which adolescent exposure to nicotine in a parental F0 generation of rats would affect the cognitive abilities of the subsequent F1 generation. To this end, the SMC task was used to assess learning and memory abilities in adulthood in the F1 generation only. Half the male-female mating pairs of rats in the parental F0 generation received adolescent nicotine exposure, whereas the other half received vehicle injections. Only females of the subsequent F1 generation were used in the behavioral experiment, half receiving adolescent nicotine and the other half receiving vehicle injections. Rats in the nicotine exposure groups received twice-daily i.p. injections of 1.0 mg/kg injections for a total exposure of 2.0 mg/kg/day, whereas injection controls received twice-daily injections of vehicle. All F1 rats were trained in the SMC task. The goal of the study was to determine whether or not adolescent exposure to nicotine in the F0 generation would cause transgenerational effects that would impair the learning abilities of the F1 generation with or without their direct exposure to adolescent nicotine.
2. Material and methods
2.1. Animal care and nicotine treatment
The subjects were 84 Long Evans rats (Rattus norvegicus), 12 male and 72 female, bred in-house. This study utilized two sets of subjects, a parental F0 generation (12 males and 12 females) and progeny F1 generation (60 females). The F1 progeny were the only rats to undergo behavioral testing. All rats were housed in plastic shoe-box cages with free access to food and water and on a 15:9-h light-dark cycle with testing occurring during the light portion of the cycle.
Injections of either nicotine or saline vehicle occurred from P25-59 for both the F0 and F1 generation rats. Rats received twice-daily intraperitoneal (i.p.) injections of 1.0 mg/kg nicotine bitartrate (Sigma Chemical, Saint Louis, MO; expressed as the weight of the free base) or saline vehicle as 1.0 ml/kg body weight. Rats were weighed and injected daily for 35 consecutive days and were then given 35 drug free days prior to testing as adults. No adverse physical effects were observed after nicotine administration.
2.1.1. F0 parental generation treatment and breeding
Twelve naïve male and 12 naïve female rats served as the parental F0 generation subjects. Six males and 6 females were randomly assigned to the experimental group that received nicotine injections; the remaining 6 males and 6 females were assigned to the control group that received saline vehicle injections. Beginning on postnatal day 21 (P21), rats were housed in groups of 3 in plastic shoe-box cages. Rats were housed so that each individual in a box was from a different litter but part of the same experimental condition. Rats in grouped housing were differentiated from one another by colored tail markings. At P60 rats were separated and housed individually in shoe-box cages. At P90 males and females of the F0 generation were paired. Control females were paired with control males, and nicotine-exposed females were paired with nicotine-exposed males.
2.1.2. F1 generation treatment
Female rats from the F1 generation served as subjects in the behavioral studies reported below. Sixty female pups from 12 litters, one litter from each F0 pair, were randomly assigned to nicotine and control conditions. Fifteen female pups from the nicotine-exposed breeding pairs and 15 pups from the control breeding pairs were randomly assigned to the experimental groups that received adolescent nicotine injections. Similarly, 15 pups from nicotine-exposed breeding pairs and 15 pups from control breeding pairs were assigned to control groups that received vehicle injections. Thus the 6 litters from the F0 Nicotine rats were equally divided amongst the F1 Nicotine and F1 Control conditions, resulting in F0 Nicotine F1 Nicotine and F0 Nicotine or F0 Control groups. The 6 litters from the F0 Control rats were also equally divided amongst the F1 Nicotine and F1 Control conditions, resulting in F0 Control F1 Nicotine and F0 Control F1 Control groups (see Fig. 1). For the F1 generation, all rats were housed in groups of 3 in plastic shoe-box cages so that each individual in a box was from a different litter but part of the same experimental condition. Rats in grouped housing were differentiated from one another by colored tail markings. At P60, rats were separated and housed individually in shoe-box cages. After the period of injections, rats were given free access to food and water until P90.
Fig. 1.
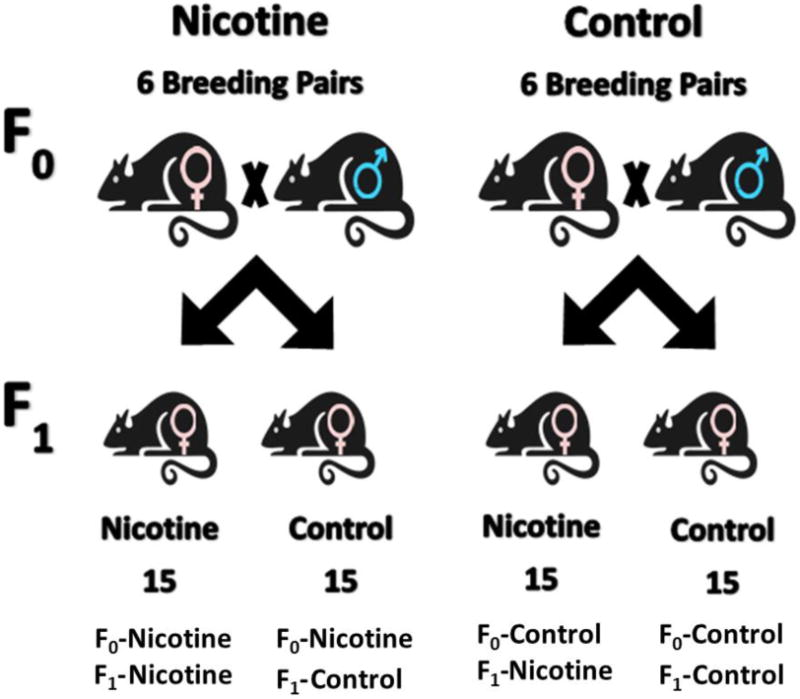
Diagram of the paring of F0 generation to acquire the F1 generation. 6 nicotine-exposed females were paired with 6 nicotine-exposed males. 30 of their female offspring were divided into 2 equal groups where 1 group would be exposed to nicotine and the other group would not be. The same procedure was conducted with 6 control females and 6 control males.
As detailed in Supplemental Information, analyses of body weights showed that at the beginning of injections on P25 for the F1 generation, there was no adverse effect of adolescent nicotine exposure in the F0 generation on the weight of F1 generation offspring. In fact, a small but significant increase in body weights for F1 rats born of nicotine exposed parents was observed between groups, although body weight differences did change over the period of injections as expected between groups by the end of the period of injections at P59. However, by P90 when the behavioral experiment began, there were no significant group differences in body weights.
On P90, water intake was restricted for shaping and behavioral testing. Rats received no water for approximately 36 hours prior to shaping. Starting on the first day of shaping and for the remainder of behavioral testing, rats received 5 minutes of access to water daily. Animals were monitored daily for signs of dehydration such as loss of skin elasticity, listlessness, and yellowing of the fur. Furthermore, animals were examined regularly by the attending veterinarian. Additional access to water was provided to keep rats at or above 80% of their free-feeding weight (which was recorded prior to water deprivation). Rats received free access to food in their home cage throughout the experiment. These water deprivation procedures have been used successfully in a number of prior experiments (e.g., Pickens et al., 2013; Renaud et al., 2015; Rowan et al., 2015) and have produced no adverse effects on the health of the rats or their ability to perform the SMC task.
2.2. Apparatus
Three clear ¼-inch Plexiglas® shaping chambers (15 × 30 × 30 cm) with wire mesh flooring and a single nose poke receptacle (2.5-cm diameter PVC pipe end caps painted flat black) were used in this study. The receptacle was centered 5.0 cm above the floor on the end wall of the chamber. It contained an infra-red emitter and detector which were located on the left and right side as well as a white LED cue light positioned on the back of the receptacle. Six clear ¼-inch Plexiglas® octagonal test chambers with wire mesh floors were used as the experimental apparatus. Each wall measured 15 cm wide × 30 cm tall with parallel walls 40 cm apart. One nose poke receptacle, as described above, was centered on each chamber wall 5.0 cm above the floor.
An opening located at the bottom of each nose-poke receptacle, connected to a solenoid and syringe by plastic tubing, served to deliver water to the chamber. All chambers were enclosed within a sound attenuating chamber made of wood. 10-ml syringes served as water reservoirs and were attached to an internal wall of this enclosure. Syringes were connected by Tygon tubing (VWR Scientific, Performance Plastics 1/32-inch, #R-3603) to solenoids (General Valve Corp. Vac. 20 psig. 24 volts) and then to the receptacles. The solenoid controlled the delivery of water drops to the nose-poke receptacles.
White noise was used in order to reduce audible distractions during testing. All chambers were controlled by a computer running a MedPC interface (Med Associates interface; Grayson Stadler power supply Model E 783 DA) which was located in a separate room of the laboratory. Animals were monitored from the computer room via closed circuit cameras mounted inside the enclosures.
2.3. Procedure
2.3.1. Shaping procedure
Rats received two days of nose poke shaping beginning on P93. On the first day of shaping, the rats were placed in the chamber and the program was initialized. At that point, the light in the nose poke receptacle illuminated and each nose poke resulted in the light turning off and was rewarded with a 0.025 ml drop of water. On the second day of training, a 2-s intertrial interval was added following each nose poke where the light would be turned off and nose poke responses would not be rewarded. Criterion for being included in the study was set at 240 responses within one hour on each of these two consecutive days. All rats met criterion on both shaping days.
2.3.2. Testing procedure
On P95, rats were placed in the octagonal test chamber and the program was initialized. At the beginning of each trial, all eight lights in the nose poke receptacle illuminated and the rat was allowed to make a response at any one of the receptacles. If a correct choice was made, all lights turned off and the response was rewarded with a 0.025 ml drop of water delivered to the receptacle. If a wrong choice was made, the correction procedure was initiated in which all lights would turn off except for the light of the correct receptacle. When the rat made a nose poke response for the correct receptacle, the rat was given a drop of water. After the correction procedure resulted in the rat's response to the indicated correct receptacle, the sequence continued as if a correct response had been produced on the trial. The computer recorded where responses occurred as well as how many correct and incorrect choices were made.
Rats were required to make responses in the receptacles in a specific 24-element pattern: 123-234-345-456-567-678-781-818- with each digit representing a clockwise position in the octagonal chamber. Dashes (-) represent a 3-s intertrial interval during which all the lights were extinguished and responses were not reinforced. This served as a phrasing cue to the animals, arranging the pattern in such a way as to make it easier to learn. Other intertrial intervals between pattern elements were 1-s. The first digit of each chunk (the bolded digit) is called the chunk boundary, the two digits following the chunk boundary are called the within-chunk elements, and the last digit in the pattern (the underlined digit) is called the violation element since it violates the structure of the pattern.
Rats were started in the experiment in squads according to their birth dates. Starting dates for each squad were staggered according to which date the animals reached P95. Each squad was composed of approximately equal number of rats from each group. On the first 4 days of the experiment, 9 rats from the F0-Control F1-Nicotine group, 5 rats from the F0-Nicotine F1-Nicotine group, 6 rats from the F0-Control F1-Control group, and 5 rats from the F0-Nicotine F1-Control group were required to perform 20 patterns per day. To more easily accommodate behavioral testing each day, daily testing was reduced to 10 patterns per day for the remainder of the study. As a result, rats in squads started beyond the 4th day of testing received only 10 patterns per day throughout training. Thus, 2 cohorts of rats were created, namely, groups that received Massed training versus Spaced training, each of which included all groups of the study. All rats had 90 minutes to complete their patterns each day and training was continued until animals had performed 490 total patterns. Rats performed an average of approximately 10 patterns per day (M = 10.23, SD = .85). For this reason, data were analyzed and presented by 10-pattern blocks for all rats.
2.4. Statistical analysis
An ANOVA was used to examine the effects of massed versus spaced training and, more importantly, the effects of F0-exposure and F1-exposure to adolescent nicotine on rats' acquisition for each element type, namely, within-chunk, chunk-boundary, and violation elements. Main effects and interactions were considered significant if p < .05. To assess differences in acquisition of pattern elements, a 2 (spacing of training) × 2 (F0 exposure) × 2 (F1 exposure) × 49 (block) repeated measures ANOVA was conducted on rats' daily total correct responses for each element type. When significant effects were observed, planned comparisons based on the appropriate error term of the ANOVA (Fisher's Least Significant Difference tests) were conducted to determine the direction of the effect as well as specific days within acquisition when groups differed. Then a curve parallelism F-test, conducted in SigmaPlot was used to determine whether the slope of acquisition for the violation element differed between groups. Lastly, a 2 (spacing of training) × 2 (F0 exposure) × 2 (F1 exposure) × 24 (element) repeated measures ANOVA was used to compare the number of errors made on each element of the pattern pooled across the 49 blocks of training.
3. Results
Analyses were conducted to assess the effects of massed versus spaced training and, more importantly, to determine whether F0-exposure and F1-exposure to nicotine in adolescence affected various aspects of pattern acquisition. We directed particular attention to determining whether adolescent nicotine exposure in the F0 generation alone or in both the F0 and F1 generations produced greater cognitive impairment than exposure in the F1 generation alone. Such a result would be evidence of transgenerational effects of adolescent nicotine exposure on adult cognitive capacity.
3.1. Spacing Effects but No Transgenerational Adolescent Nicotine Effects on Within-Chunk Element Acquisition
To assess the effects of F0 or F1 adolescent nicotine exposure and spacing on acquisition of within-chunk elements, we conducted a 2 (spacing of training) × 2 (F0 exposure) × 2 (F1 exposure) × 49 (block) repeated measures ANOVA. The ANOVA found significant main effects of block, F(48,2496) = 116.41, p < .001, η2 = 0.65, and spacing, F(1,52) = 26.84, p < .001, η2 = 0.31. The analysis also found significant interactions for spacing × block, F(48,2496) = 4.69, p < .001, η2 = 0.03, and spacing × F1 exposure × block, F(48,2496) = 1.59, p = .006, η2 = 0.01. Effects involving F0 exposure as a factor were not significant (p > .05) and accounted for very little of the variance1. Thus, spacing of training and offspring F1 generation drug exposure significantly affected acquisition for the within-chunk elements, but there was no evidence of transgenerational effects of adolescent F0 exposure. Fig. 2 shows acquisition of within-chunk elements by spacing of training and F1 exposure (collapsing across F0 exposure) for the 49 blocks of training. Planned comparisons based on the appropriate error term from the ANOVA identified the significant differences (ps < 0.05) that follow.
Fig. 2.
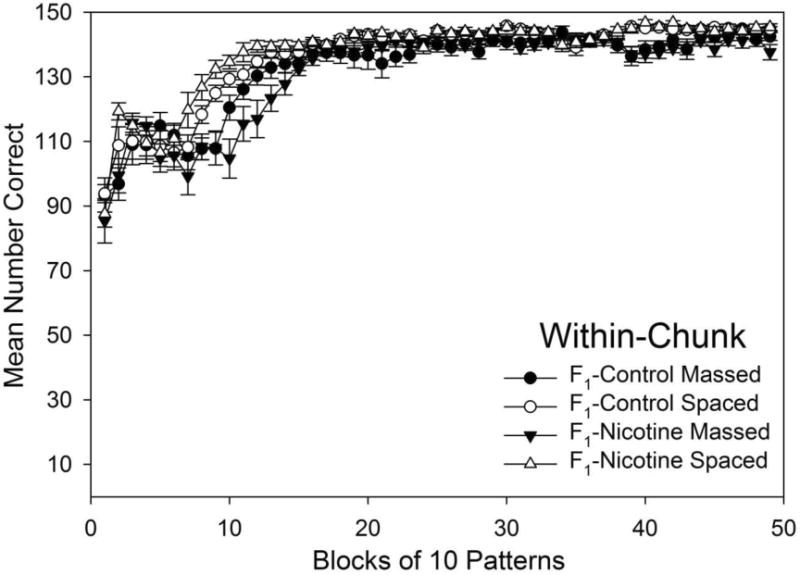
Acquisition of within-chunk elements grouped by F1 exposure and spacing of training for all 49 blocks training. Mean number of correct responses were averaged for each block of 10 patterns across all days of training. Error bars: ± SEM.
3.1.1. F1 Adolescent Nicotine Effects on Within-Chunk Element Acquisition
Although there was no evidence of transgenerational effects of adolescent F0-Nicotine on F1 generation learning for within-chunk elements, same-generation F1-adolescent nicotine effects were observed. F1-Nicotine groups had significantly slower acquisition (i.e., fewer correct responses) compared to F1-Control groups' performance when spacing was held constant. This effect was observed as follows: F1-Nicotine Massed made fewer correct responses than F1-Control Massed on blocks 1, 5, and 10-13; and F1-Nicotine Massed groups made fewer correct responses than F1-Control Spaced groups on blocks 1, 2, 7-14, 39-41, 45, and 49. Thus, rats that received F1-Nicotine made fewer correct choices when learning within-chunk elements than rats that did not.
3.1.2. Spacing Effects on Within-Chunk Element Acquisition
Spacing effects on within-chunk element acquisition were observed as Massed groups' significantly slower acquisition (i.e., fewer correct responses) compared to Spaced groups' performance with F1-nicotine exposure held constant. This effect was observed as follows: F1-Nicotine Massed groups made fewer correct responses than F1-Nicotine Spaced groups on blocks 2, 7-15, 39-42, and 49; F1-Control Massed groups made fewer correct responses than F1-Control Spaced groups on blocks 2, 8-10, 21, and 39. Thus, rats that received a few sessions of massed training at the beginning of serial pattern learning made fewer correct choices when learning within-chunk elements than rats that received spaced training.
3.2. Spacing Effects but No Transgenerational Adolescent Nicotine Effects on Chunk-Boundary Element Acquisition
To assess the effects of F0 or F1 adolescent nicotine exposure and spacing on acquisition of chunk-boundary elements, we conducted a 2 (spacing of training) × 2 (F0 exposure) × 2 (F1 exposure) × 49 (block) repeated measures ANOVA. The ANOVA found significant main effects of block, F(48,2496) = 475.35, p < .001, η2 = 0.88, and spacing, F(1,52) = 16.59, p < .001, η2 = 0.22. The analysis also found significant interactions for spacing × block, F(48,2496) = 5.29, p < .001, η2 = 0.01, and spacing × F1 exposure × block, F(48,2496) = 1.77, p = .001, η2 = 0.003. Effects involving F0 exposure were not significant (p > .05) and accounted for very little of the variance2. Thus, spacing of training and offspring F1 generation drug exposure significantly affected acquisition for chunk-boundary elements, but there was no evidence of transgenerational effects of adolescent F0 exposure.
Fig. 3 shows acquisition of chunk-boundary elements by spacing of training and F1 exposure (collapsing across F0 exposure) for the 49 blocks of training. Planned comparisons based on the appropriate error term from the ANOVA identified the significant differences (ps < 0.05) that follow.
Fig. 3.
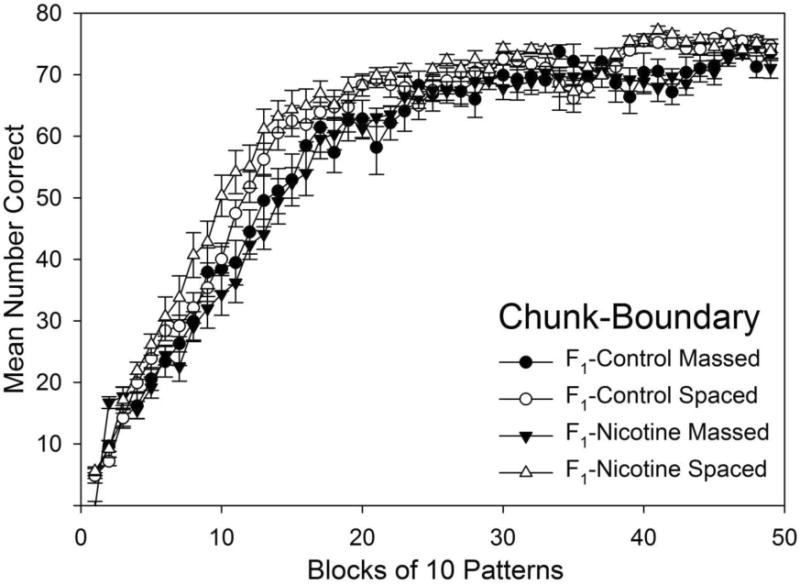
Acquisition of chunk-boundary element grouped by F1 exposure and spacing of training for all 49 blocks of training. Mean number of correct responses were averaged for each block of 10 patterns across all days of training. Error bars: ± SEM.
3.2.1. F1 Adolescent Nicotine Effects on Chunk-Boundary Element Acquisition
Although there was no evidence of transgenerational effects of adolescent F0-Nicotine on F1 generation learning for chunk-boundary elements, same-generation F1-adolescent nicotine effects were observed. F1-Nicotine groups had significantly slower acquisition (i.e., fewer correct responses) compared to F1-Control groups' performance when spacing was held constant. This effect was observed as follows: F1-Nicotine Massed made fewer correct responses than F1-Control Massed on blocks 1, 9, and 13 (but more on blocks 2 and 21); and F1-Nicotine Massed groups made fewer correct responses than F1-Control Spaced groups on blocks 1, 7, 10-16, 20-22, 40-43, and 45. Thus, rats that received F1-Nicotine made fewer correct choices when learning chunk-boundary elements than rats that did not.
3.2.2. Spacing Effects on Chunk-Boundary Element Acquisition
Spacing effects on chunk-boundary element acquisition were observed as Massed groups' significantly slower acquisition (i.e., fewer correct responses) compared to Spaced groups' performance with F1-nicotine exposure held constant. This effect was observed as follows: F1-Nicotine Massed groups made fewer correct responses than F1-Nicotine Spaced groups on blocks 1, 4-17, 20-22, 26, 30, 32, and 39-43; F1-Control Massed groups made fewer correct responses than F1-Control Spaced groups on blocks 6, 12-15, 18, 20-22, 39, 40 and 42. Thus, rats that received a few sessions of massed training at the beginning of serial pattern learning made fewer correct choices when learning chunk-boundary elements than rats that received spaced training.
3.3. Evidence for Transgenerational Adolescent Nicotine Effects and Spacing Effects on Violation Element Learning
To assess the effects of F0 or F1 adolescent nicotine exposure and spacing on acquisition of the violation element, we conducted a 2 (spacing of training) × 2 (F0 exposure) × 2 (F1 exposure) × 49 (block) repeated measures ANOVA. The ANOVA found significant main effects of spacing, F(1,52) = 11.34, p = .001, η2 = 0.16, and block, F(48,2496) = 123.78, p < .001, η2 = 0.66. The analysis also found significant interactions for spacing × block, F(48,2496) = 3.99, p < .001, η2 = 0.02, and spacing × F0 exposure × block, F(48,2496) = 2.49, p < .001, η2 = 0.01. No other main effect or interactions were significant (p > .05) and accounted for very little of the variance3. Thus, parental F0 generation drug exposure and spacing of training significantly affected acquisition of the violation element. Planned comparisons based on the appropriate error term from the ANOVA identified the significant differences (p < 0.05) that follow.
3.3.1. Evidence of Transgenerational Effects of Parental F0 Generation Adolescent Nicotine on Offspring F1 Generation Violation Element Acquisition
Fig. 4 shows acquisition of the violation element for F1-generation rats as a function of spacing of training and F0 adolescent nicotine exposure for the 49 blocks training. A transgenerational effect of Parental F0 adolescent nicotine exposure was observed as F0-Nicotine Massed rats' slower acquisition (i.e., fewer correct responses) compared to F0-Control Massed rats' performance (when spacing was held constant). This effect was observed as F0-Nicotine Massed rats significantly fewer correct responses than F0-Control Massed rats on 22 blocks of training, namely, blocks 26-42 and 44-48.
Fig. 4.
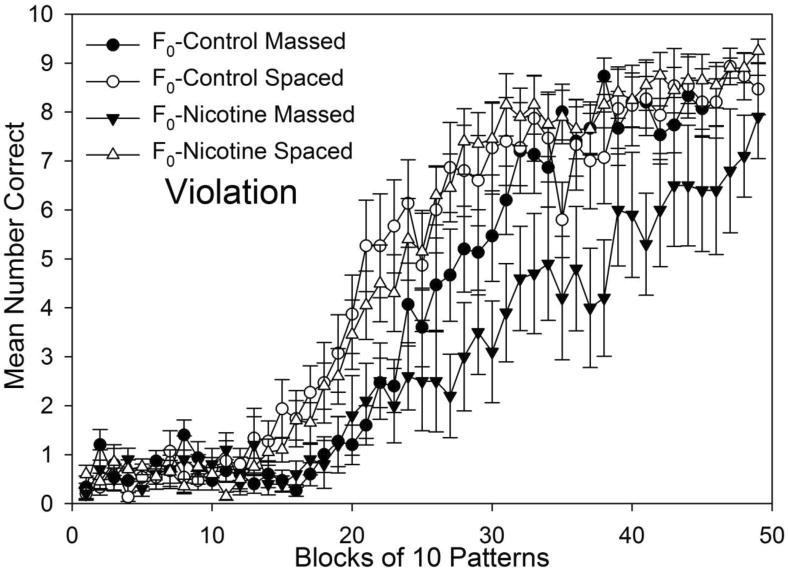
Acquisition of the violation element grouped by F0 exposure and spacing of training for all 49 blocks of training. Mean number of correct responses were averaged for each block of 10 patterns of training. Evidence for transgenerational effects of parental F0 adolescent nicotine exposure on offspring F1 adult learning was observed as significantly fewer F0 Nicotine Massed group (filled triangles) correct responses compared to F0-Control Massed group (filled circles) on 22 training blocks, namely, blocks 26-42 and 44-48. See the text for a complete list of blocks where groups differed significantly. Error bars: ± SEM.
It should be noted that the foregoing results were based on post hoc comparisons following an omnibus ANOVA including all groups' data, but other lower-order analyses are possible4. To further assess the claim that parental F0 generation exposure to nicotine during adolescence produced transgenerational effects on learning in adult offspring, an additional analysis was conducted to compare the violation element acquisition curves of the F0-Nicotine Massed group and the F0-Control Massed group, as shown in Fig. 5. A curve parallelism F-test, conducted in SigmaPlot (SigmaPlot Ver. 7.0, Systat Software Inc.), indicated that F0 exposure to adolescent nicotine significantly slowed violation element acquisition, F(3,90) = 39.14, p < .001, thus providing additional evidence of a transgenerational impairment of violation element learning in the offspring F1 generation caused by adolescent nicotine exposure in the parental F0 generation.
Fig. 5.
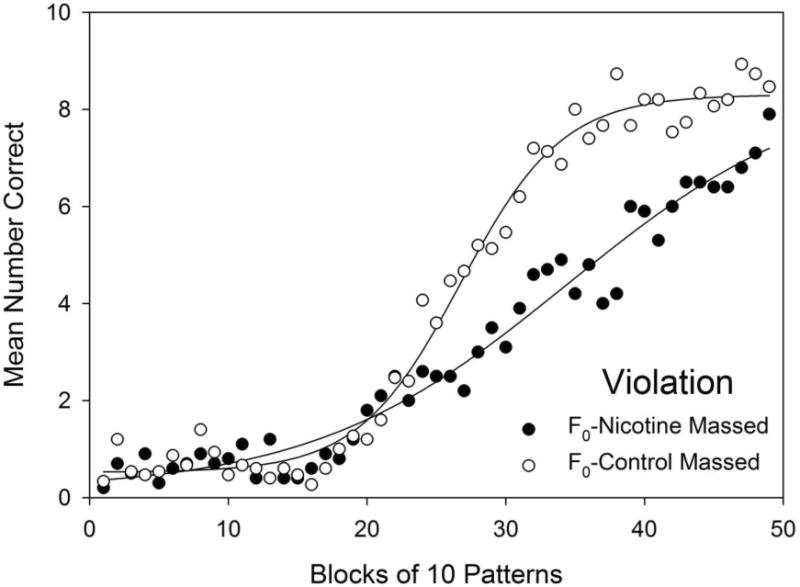
Fitted acquisition curves for the violation element for the F0-Nicotine Massed and the F0-Control Massed groups for the 49 blocks training.
3.3.2. Spacing Effects on Violation Element Acquisition
Spacing effects on violation element acquisition were observed as Massed groups' significantly slower acquisition (i.e., fewer correct responses) compared to Spaced groups' performance with F0-nicotine exposure held constant. This spacing effect was observed as follows: Examining the effects of spacing in the data presented in Fig. 4, the F0-Nicotine Massed group made significantly fewer correct responses than the F0-Nicotine Spaced group (open triangles) on 30 blocks, namely, on blocks 18 and 20-48. The F0-Control Massed group (filled circles) made fewer correct responses than the F0-Control Spaced group (open circles) on 13 blocks, namely, on blocks 17, 19-24, 26-28, 30, 35, and 38. Taken together, these results also indicate that F0 nicotine exposure exacerbated the negative effects of massed training. To determine whether massed training resulted in delayed onset of learning, slower learning, or both, an additional analysis was conducted comparing violation element acquisition curves of the F0-Nicotine Massed group and the F0-Nicotine Spaced group, as shown in Fig. 6. A curve parallelism F-test, conducted in SigmaPlot (SigmaPlot Ver. 7.0, Systat Software Inc.), revealed that massed training significantly slowed acquisition compared to spaced training, F(3,90) = 53.47, p < .001.
Fig. 6.
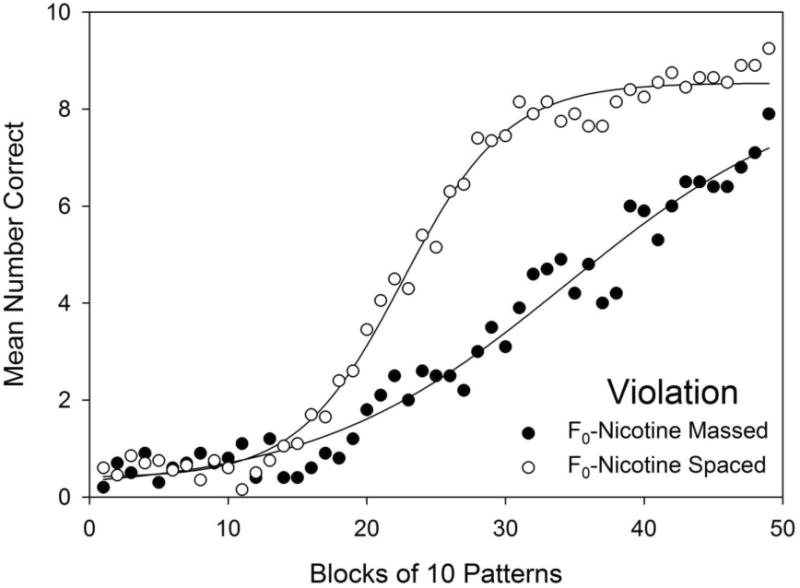
Fitted acquisition curves for the violation element for the F0-Nicotine Massed and the F0-Nicotine Spaced groups for the 49 blocks training.
4. Discussion
The results of the current study support the claim that adolescent nicotine exposure in one generation of rats can impair the cognitive capacity of rats in a subsequent generation. Half the rats of the parental F0 generation were exposed to nicotine during adolescence and half were not, then half the female rats of the offspring F1 generation were exposed to nicotine during adolescence and half were not. We used a serial multiple choice (SMC) task to train the female offspring to perform a complex serial pattern of responses. The pattern was composed of three pattern element types that in past research have been shown to be differentially sensitive to behavioral and pharmacological manipulations. As in past research, we observed a same-generation F1 adolescent nicotine exposure impairment on pattern learning we have reported before (Pickens et al., 2013; Renaud et al., 2015). More importantly, we also observed a transgenerational learning impairment caused by parental F0 generation nicotine exposure in offspring F1 generation female rats as slower learning of one of the three element types, namely, the violation element, albeit at twice the daily dose used in Pickens et al. (2013). We also observed that this impairment was exacerbated by massed training conditions that made learning the violation element more difficult even in vehicle control rats. Thus, adolescent nicotine exposure in one generation produced a cognitive impairment observed in the next generation.
The specificity of the adolescent nicotine impairment as an effect on violation element learning without significant impairment for learning of other element types is consistent with our previous results in the SMC paradigm. Specifically, Pickens et al. (2013) and Renaud et al. (2015) observed no effects of adolescent nicotine on within-chunk or chunk-boundary element learning in adulthood in female rats. Pickens et al. (2013) also showed that the adolescent nicotine impairment of violation element learning was a sex-specific effect; whereas same-generation adolescent nicotine impaired learning the violation element more in female rats, it impaired learning chunk-boundary elements more in male rats. The results of the current study thus suggest that transgenerational effects of adolescent nicotine exposure in this case may involve the same underlying cognitive systems as same-generation adolescent nicotine effects. Thus, a novel prediction of this hypothesis would be that transgenerational effects of adolescent nicotine exposure in male rats should be expressed more as an impairment of chunk-boundary learning with less impairment of violation element learning, the same effects as observed after same-generation adolescent nicotine exposure reported by Pickens et al. (2013).
With regard to the cognitive and neural systems affected in the transgenerational effect observed in the current study, our previous research has shown that serial pattern learning in the SMC task recruits different and separable cognitive systems for learning violation element learning, chunk-boundary element learning, and within-chunk element learning (Muller & Fountain, 2010; 2016), as noted in the Introduction. Specifically, learning to anticipate violation elements depends on multiple-item memory, that is, learning to use multiple pattern elements to cue the proper next response on the violation trial (Kundey & Fountain, 2010; Muller & Fountain, 2010; Muller & Fountain, 2016). Although the specific neural system or systems required for multiple-item learning have not been identified, rats' ability to learn to anticipate violation elements, and to a lesser extent chunk-boundary elements, depends on intact muscarinic cholinergic and NMDAr systems (Chenoweth & Fountain, 2015; 2016; Fountain & Rowan, 2000; Fountain, Rowan, & Wollan, 2013). More generally, hippocampus appears to play a key role in tracking sequences of stimuli (Agster, Fortin, & Eichenbaum, 2002; Fortin, Agster, & Eichenbaum, 2002). We are on relatively firm ground to speculate that adolescent nicotine exposure likely impairs systems that depend on the concurrent use of multiple cues (i.e., compound or configural cues) to anticipate the violation element of the serial pattern used in the current experiment, but we are on much less firm ground to specify the location or nature of these effects in the brain. Clearly, much work is left to be done in this area.
An unanticipated result in the current study was that serial pattern learning in the SMC task is strongly affected by spacing of training. Whereas adolescent nicotine exposure affected only violation element acquisition, massed training slowed acquisition to some extent for all element types, and massed training also potentiated the effects of F0 adolescent nicotine exposure on F1 generation learning. It should be noted that the spacing effect is one of the most replicable effects in experimental psychology (Crystal & Babb, 2008; McDaniel, Fadler, & Pashler, 2013; Sisti, Glass, & Shors, 2007). On the positive side, observing spacing effects in our complex SMC paradigm – which was explicitly modeled after a human sequential learning and memory paradigm (Restle & Brown, 1970a, 1970b) – opens the door to using the SMC paradigm as an animal model of human spacing phenomena that are relevant to a host of issues in learning, memory, and education.
Although the current study provides evidence for transgenerational effects of adolescent nicotine exposure that result in cognitive impairments in offspring, future studies should investigate the biological processes that give rise to these effects with special attention directed toward distinguishing the roles of epigenetic mechanisms involving DNA methylation versus other transgenerational mechanisms. Because the effects of adolescent nicotine on maternal care and on direct exposure of the germ line were not controlled, the results of the current study do not support the strong claim that the effects observed were based on epigenetic mechanisms. Therefore, although the research reported in this paper indicates that adolescent nicotine exposure in one generation of rats can impair the cognitive capacity of rats in the next generation, research directed toward identifying and describing the mechanisms underlying this effect is sorely needed to provide a sound basis for better evaluating the threat posed by transgenerational and epigenetic effects of adolescent nicotine exposure.
Supplementary Material
Fig. S1: Daily mean weights for all groups for all 35 days of injections. The F0-Control F1- Control group weighed more than the F0-Nicotine F1-Nicotine group on days 13-15, and 17-35. The F0-Nicotine F1-Control weighed more than the F0-Nicotine F1-Nicotine group on days 8-35. The F0-Control F1-Control weighed more than the F0-Control F1-Nicotine group on days 13-15, and 17-35. The F0-Nicotine F1-Control group weighed more than the F0-Control F1-Nicotine on days 4-35. The F0-Nicotine F1-Control weighed less on days 16 and 19 and more on days 34 and 35 than the F0-Control F1-Control. Error bars: ± SEM.
Highlights.
Transgenerational effects of adolescent nicotine on adult cognition were assessed in rats.
F0 generation breeder rats received either adolescent nicotine or no nicotine exposure.
Female F1 generation offspring received either adolescent nicotine or no nicotine exposure.
F0 and F1 generation nicotine produced cognitive deficits in the F1 generation.
Adolescent nicotine in one generation produced a cognitive impairment in the next generation.
Acknowledgments
The project described was supported in part by Award Number R15DA023349 from the National Institute on Drug Abuse to S. B. Fountain. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. We thank Megan Miller for assistance with injections and data collection and Megan Miller and Jessica Sharp for their helpful comments on an earlier draft of this paper.
Footnotes
Effects on within-chunk element acquisition involving F0 exposure as a factor were not significant (p > .05) and accounted for very little of the variance: F0 exposure, F(1,52) = 2.36, p = .131, η2 = 0.03; F0 exposure × F1 exposure, F(1,52) = .086, p = .770, η2 = 0.001; F0 exposure × spacing, F(1,52) = 2.32, p = .133, η2 = 0.03; F0 exposure × block, F(48,2496) = 10.44, p = .334, η2 = 0.005; F0 exposure × F1 exposure × spacing, F(1,52) = 1.43, p = .237, η2 = 0.02; F0 exposure × spacing × block, F(48,2496) = .825, p = .203, η2 = 0.006; and F0 exposure × F1 exposure × spacing × block, F(48,2496) = 3.04, p = .947, η2 = 0.004.
Effects on chunk-boundary element acquisition involving F0 exposure as a factor were not significant (p > .05) and accounted for very little of the variance: F0 exposure, F(1,52) = .854, p = .360, η2 = 0.011; F0 exposure × F1 exposure, F(1,52) = 2.338, p = .132, η2 = 0.030; F0 exposure × spacing, F(1,52) = .001, p = .978, η2 < 0.001; F0 exposure × block, F(48,2496) = .968, p = .535, η2 = 0.002; F0 exposure × F1 exposure × spacing, F(1,52) = 1.641, p = .206, η2 = 0.021; F0 exposure × F1 exposure × block; F(48,2496) = 1.308, p = .077, η2 = 0.002; and F0 exposure × F1 exposure × spacing × block F(48,2496) = 1.075, p = .336, η2 = 0.002.
Effects of other factors on violation element acquisition were not significant (p > .05) and accounted for very little of the variance: F0 exposure, F(1,52) = 2.226, p = .142, η2 = 0.031; F0 exposure × F1 exposure, F(1,52) = 2.652, p = .109, η2 = 0.037; F0 exposure × spacing, F(1,52) = 2.030, p = .160, η2 = 0.028; F0 exposure × block, F(48,2496) = 3.988, p = .467, η2 = 0.021; F0 exposure × F1 exposure × spacing, F(1,52) = .065, p = .799, η2 = 0.001; F0 exposure × F1 exposure × block, F(48,2496) = 1.254, p = .114, η2 = 0.007; and F0 exposure × F1 exposure × spacing × block, F(48,2496) = .563, p = .993, η2 = 0.003.
A lower-order F0 Exposure × F1 Exposure ANOVA was conducted on the violation element data of a subset of groups, namely, only those that received massed training. This analysis did not include data from groups that received spaced training. No significant effects were found (p > .05). Similarly, a lower-order F0 Exposure × F1 exposure × block ANOVA was conducted on the violation element data of a subset of groups, namely, only those that received massed training. The only significant effect was for block of training, F(48,1488)= 92.15, p < .001, η2 = 0.73. The failure to detect effects of F0 or F1 exposure in these analyses, under the conditions of low power because of small group size, could be due to effects that were detectable in the larger analysis only when exposure effects in massed and spaced groups could be assessed together in the same analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. J Neurosci. 2002;22(13):5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12097529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Gapp K, Saab BJ, Mansuy IM. Transgenerational epigenetic effects on brain functions. Biol Psychiatry. 2013;73(4):313–320. doi: 10.1016/j.biopsych.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Chenoweth AM, Fountain SB. Central muscarinic cholinergic involvement in serial pattern learning: Atropine impairs acquisition and retention in a serial multiple choice (SMC) task in rats. Neurobiol Learn Mem. 2015;123:18–27. doi: 10.1016/j.nlm.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Chenoweth AM, Fountain SB. Strategy breakdown following muscarinic blockade in rats. Neurobiol Learn Mem. 2016;131:83–86. doi: 10.1016/j.nlm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34(2):299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Crystal JD, Babb SJ. Spatial memory in rats after 25 hours. Learning and Motivation. 2008;39(4):278–284. doi: 10.1016/j.lmot.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5(5):458–462. doi: 10.1038/nn834. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11976705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SB. Rule abstraction, item memory, and chunking in rat serial-pattern tracking. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:96–105. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Sensitivity to violations of “run” and “trill” structures in rat serial-pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 1995a;21:78–81. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Coding of hierarchical versus linear pattern structure in rats and humans. Journal of Experimental Psychology: Animal Behavior Processes. 1995b;21(3):187–202. doi: 10.1037//0097-7403.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Differential impairments of rat serial-pattern learning and retention induced by MK-801, an NMDA receptor antagonist. Psychobiology. 2000;28:32–44. [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Experimental Brain Research. 2008;187:651–656. doi: 10.1007/s00221-008-1346-4. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Muller MD, Kundey SMA, Pickens LRG, Doyle KE. The organization of sequential behavior: Conditioning, memory, and abstraction. In: Wasserman EA, Zentall TR, editors. Handbook of Comparative Cognition. Oxford: Oxford University Press; 2012. pp. 594–614. [Google Scholar]
- Fountain SB, Rowan JD, Wollan MO. Central cholinergic involvement in sequential behavior: impairments of performance by atropine in a serial multiple choice task for rats. Neurobiol Learn Mem. 2013;106:118–126. doi: 10.1016/j.nlm.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. Transgenerational epigenetic effects on animal behaviour. Prog Biophys Mol Biol. 2013;113(3):447–454. doi: 10.1016/j.pbiomolbio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Kundey SMA, Fountain SB. Blocking in rat serial pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:307–312. doi: 10.1037/a0016523. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Fadler CL, Pashler H. Effects of spaced versus massed training in function learning. Journal of Experimental Psychology: Learning, Memory & Cognition. 2013 doi: 10.1037/a0032184. [DOI] [PubMed] [Google Scholar]
- Muller MD, Fountain SB. Concurrent cognitive processes in rat serial pattern learning: Item memory, serial position, and pattern structure. Learning and Motivation. 2010;41:252–272. doi: 10.1016/j.lmot.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Fountain SB. Concurrent cognitive processes in rat serial pattern learning: II. Discrimination learning, rule learning, chunk length, and multiple-item memories. J Exp Anal Behav. 2016;105(1):155–175. doi: 10.1002/jeab.186. [DOI] [PubMed] [Google Scholar]
- Pickens LRG, Rowan JD, Bevins RA, Fountain SB. Sex differences in adult cognitive deficits after adolescent nicotine exposure in rats. Neurotoxicol Teratol. 2013;38:72–78. doi: 10.1016/j.ntt.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud SM, Pickens LR, Fountain SB. Paradoxical effects of injection stress and nicotine exposure experienced during adolescence on learning in a serial multiple choice (SMC) task in adult female rats. Neurotoxicol Teratol. 2015;48:40–48. doi: 10.1016/j.ntt.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restle F, Brown ER. Organization of serial pattern learning. In: Bower GH, editor. Psychology of learning and motivation. 1. New York: Academic Press; 1970a. Reprinted from: In File. [Google Scholar]
- Restle F, Brown ER. Serial pattern learning. Journal of Experimental Psychology. 1970b;83:120–125. [Google Scholar]
- Rowan JD, McCarty MK, Kundey SMA, Osburn CD, Renaud SM, Kelley BM, Matoushek AW, Fountain SB. Adolescent exposure to methylphenidate impairs serial pattern learning in the serial multiple choice (SMC) task in adult rats. Neurotoxicol Teratol. 2015;51:21–26. doi: 10.1016/j.ntt.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: Learning over time enhances memory and the survival of new neurons. Learning & Memory. 2007;14(5):368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RF, McDonald CG, Bergstrom HC, Ehlinger DG, Brielmaier JM. Adolescent nicotine induces persisting changes in development of neural connectivity. Neurosci Biobehav Rev. 2015;55:432–443. doi: 10.1016/j.neubiorev.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Spaeth AM, Barnet RC, Hunt PS, Burk JA. Adolescent nicotine exposure disrupts context conditioning in adulthood in rats. Pharmacology Biochemistry and Behavior. 2010;96(4):501–506. doi: 10.1016/j.pbb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempowski NK, Carman HM, Fountain SB. Temporal phrasing and overshadowing in rat serial-pattern learning. Learning and Motivation. 1999;30:74–100. [Google Scholar]
- Szyf M. The dynamic epigenome and its implications in toxicology. Toxicol Sci. 2007;100(1):7–23. doi: 10.1093/toxsci/kfm177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Daily mean weights for all groups for all 35 days of injections. The F0-Control F1- Control group weighed more than the F0-Nicotine F1-Nicotine group on days 13-15, and 17-35. The F0-Nicotine F1-Control weighed more than the F0-Nicotine F1-Nicotine group on days 8-35. The F0-Control F1-Control weighed more than the F0-Control F1-Nicotine group on days 13-15, and 17-35. The F0-Nicotine F1-Control group weighed more than the F0-Control F1-Nicotine on days 4-35. The F0-Nicotine F1-Control weighed less on days 16 and 19 and more on days 34 and 35 than the F0-Control F1-Control. Error bars: ± SEM.


