Abstract
Background
Nodal status has long been considered pivotal to oncologic care, staging, and management. This has resulted in rudimentary metrics determined for adequate lymph node yield in colon and rectal cancers for accurate cancer staging. In the era of neoadjuvant treatment the implications of lymph node yield and status on patient outcomes remains unclear.
Patient and Methods
The study included 1,680 locally advanced rectal cancer patients from the National Comprehensive Cancer Network (NCCN) prospective oncology database stratified into 3 groups based on preoperative therapy received: No neoadjuvant therapy, neoadjuvant chemoradiation, neoadjuvant chemotherapy. Clinicopathologic characteristics and survival were compared between groups with univariate and multivariate analyses undertaken.
Results
The 3 groups’ clinicopathologic characteristics demonstrated statistically significant differences and heterogeneity between groups. The neoadjuvant chemoradiation group demonstrated the statistically lowest median lymph node yield (15) compared to 17 and 18 for no neoadjuvant, and neoadjuvant chemotherapy, respectively (p<0.0001). Neoadjuvant treatment did impact survival with chemoradiation demonstrating increased median overall survival of 42.7 compared to 37.3 and 26.6 months for neoadjuvant chemotherapy and no neoadjuvant therapy, respectively (p<0.0001). Patients with <12 lymph node yield had improved median overall survival of 43.3 months compared to 36.6 months in patients with ≥12 lymph nodes (p=0.009). Multivariate analysis demonstrated that neither node yield nor status were predictors for overall survival.
Discussion
This analysis reiterates that nodal yield in rectal cancer is multi-factorial with neoadjuvant therapy being a significant factor. Node yield and status were not significant predictors of overall survival. A nodal metric may not be clinically relevant in the era of neoadjuvant therapy and guidelines for perioperative therapy may need reconsideration.
Background
Nodal status has long been considered a key pathologic factor in cancer management and care. Previous studies have demonstrated an association between lymph node yield and status with regard to patient outcomes in colorectal cancer.1-4As such in 1990, at the World Congress of Gastroenterology, it was first recommended that at least 12 lymph nodes be examined after radical resection to optimally stage patients and acknowledged the impact of preoperative therapy on nodal yield.5 Subsequently, several major organizations including the American Joint Committee on Cancer (AJCC), Union for International Cancer Control, and College of American Pathologists recommended at least 12 lymph nodes be examined for accurate staging in colorectal cancer becoming a well-accepted metric.
Since that time, there have several large population based studies demonstrating inadequate lymph node harvest and evaluation being undertaken in colorectal cancer.6-7 This has been more prevalent with rectal cancer and a number of studies have determined limitations to this including variability related to surgical techniques, pathologic assessment, patient factors, institutions, and the effect of neoadjuvant therapy.8-12
Regarding surgical technique, it is well accepted that a total mesorectal excision (TME) be undertaken to help achieve optimal oncologic outcomes. Proficient TME allows for harvest of the traditional primary nodal drainage basin as well as to optimize the circumferential resection margin. If an incomplete TME is undertaken, this can account for ‘understaging’ (‘Nx’) with inadequate surgical lymph node yield, as well as potentially negatively impact outcome with disease recurrence.
Pathologic techniques have been implicated as a factor in lymph node yield due to inadequate specimen analysis and processing. Patient related factors such as age and obesity have been associated with decreased lymph node yield.13-16 Institutional related factors demonstrate variation and high non-compliance with the ≥12 lymph node metric.17-18
In the last decade, the standard of care for rectal cancer care has been based on the German Rectal Cancer Group trial.19 This phase III trial compared neoadjuvant chemoradiation to the then standard postoperative chemoradiation therapy and demonstrated a statistically significant improvement in local recurrence with reduced treatment toxicity, but without a difference in overall survival. Since neoadjuvant chemoradiation was established as the standard of care for clinical stage II/III rectal cancer, studies have demonstrated its’ association with decreased lymph node yield.20-22
Recently, long-term results for the European Organisation for the Research and Treatment of Cancer (EORTC) 22921 study examined the utility of fluorouracil-based adjuvant chemotherapy following preoperative therapy for rectal cancer.23 This study concluded that adjuvant chemotherapy demonstrated no statistically significant improvement in disease-free or overall survival in patients after neoadjuvant treatment. This naturally prompts question as to the utility of the current standard of care of 6 months of perioperative systemic therapy.
Given the unclear utility of adjuvant therapy after preoperative therapy has been administered, the clinical importance of pathologic nodal yield and status must be questioned given their impact on the treatment decisions made. Hence, an established lymph node metric and nodal status for rectal cancer may or may not be clinically relevant in the era of neoadjuvant chemoradiation administered for locally advanced rectal cancer.
Methods
The National Comprehensive Cancer Network (NCCN) is a not-for-profit alliance comprised of 25 of the nation’s leading cancer centers whose goal is to improve the quality of multi-disciplinary cancer care to patients. The NCCN has compiled a prospective NCCN Oncology Outcomes Database for Colorectal Cancers abstracted from 8 participating NCCN institutions: City of Hope Comprehensive Cancer Center (CCC), Memorial Sloan-Kettering Cancer Center, The University of Texas MD Andersons Cancer Center, Dana Farber Cancer Institute, Fox Chase Cancer Center, Roswell Park Cancer Center, Robert H. Lurie CCC at Northwestern University, and The Ohio State University CCC – James Cancer Hospital and Solove Research Institute. The data collection process was approved by the respective institutions’ Institutional Review Boards.
A cohort of 1680 clinical stage II/III primary rectal cancer patients who underwent surgical intervention excluding pelvic exenteration, subtotal colectomy, total proctocolectomy, transanal excision, Hartman’s procedure, and rectosigmoid resections was compiled. The patient cohort was divided into 3 groups based on the type of neoadjuvant therapy received prior to surgery: No neoadjuvant (N = 111), neoadjuvant chemoradiation (N = 1364), and neoadjuvant chemotherapy (N = 205). This analysis is inclusive of data from September 2005 to September 2013.
The clinicopathologic characteristics were analyzed and compared using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables among the 3 groups. Lymph node analysis for group comparisons was undertaken using Kruskal-Wallis test. Clinicopathologic characteristics effect on lymph node were analyzed using Kruskal-Wallis test or Wilcoxon test as appropriate.
Overall survival (OS) was defined from date of diagnosis to date of death. Patients alive at the date of last observation were censored for survival analysis. Survival curves were estimated using Kaplan-Meier method. Survival curves were compared by the log-rank tests for the univariate analysis. Multivariable Cox regression models were fit to the overall survival using all the variables with p<0.2 in the univariate analysis. Variables with p>0.05 were removed sequentially from the Cox regression model. All statistical analyses were conducted using SAS for Windows® Version 9.2 (SAS Institute Inc., Cary, NC).
Results
The 3 groups’ clinicopathologic characteristics were compared as illustrated in Table 1 demonstrating heterogeneity between groups with statistical significance demonstrated for median age, age at diagnosis, Charlson comorbidity index, pathologic TNM stage, type of surgery undertaken, median overall survival, lymph node yield, and node positivity.
Table 1.
Patient clinicopathologic characteristics stratified by Neoadjuvant therapy. NCCN institutions(+++) include Fox Chase Cancer Center, City of Hope, MD Anderson Cancer Center, Roswell Park Cancer Institute, Dana-Farber Cancer Institute, Ohio State University/Arthur James Cancer Hospital, Memorial Sloan Kettering Cancer Center, and Robert Lurie Comprehensive Cancer Center.
| Variable | Category | No Neoadjuvant (N=111) |
Neoadjuvant Chemoradiation (N=1364) |
Neoadjuvant Chemo Only (N=205) |
P-value* |
|---|---|---|---|---|---|
| Median age | 64 (38-93) | 57 (19-89) | 56 (25-89) | <.0.0001 | |
| Age at diagnosis | <65 years | 61 (55%) | 1004 (74%) | 148 (72%) | 0.0001 |
| ≥65 | 50 (45%) | 360 (26%) | 57 (28%) | ||
| Gender | Male | 58 (52%) | 813 (60%) | 111 (54%) | 0.13 |
| Female | 53 (48%) | 551 (40%) | 94 (46%) | ||
| Charlson comorbidity |
0 or 1 | 93 (84%) | 1249 (92%) | 184 (90%) | 0.02 |
| 2+ | 18 (16%) | 115 ( 8%) | 21 (10%) | ||
| Pathologic TNM Stage+ |
0 | 5 (5%) | 272 (20%) | 19 (9%) | <0.0001 |
| I | 57 (51%) | 359 (26%) | 13 (6%) | ||
| II | 32 (29%) | 324 (24%) | 51 (25%) | ||
| III | 17 (15%) | 409 (30%) | 122 (60%) | ||
| Type of Surgery |
LAR | 90 (81%) | 952 (70%) | 175 (85%) | <0.0001 |
| APR | 15 (14%) | 332 (24%) | 26 (13%) | ||
| Proctectomy | 6 ( 5%) | 80 ( 6%) | 4 ( 2%) | ||
| Adjuvant chemo Therapy |
Yes | 0(0%) | 1364(100%) | 205 (100%) | <0.0001 |
| No | 111 (100%) | 0 (0%) | 0 (%) | ||
| NCCN Insitution+++ |
A | 12 (11%) | 66 ( 5%) | 8 ( 4%) | Not |
| B | 15 (14%) | 111 ( 8%) | 24 (12%) | compared | |
| C | 4 ( 4%) | 70 ( 5%) | 3 ( 1%) | ||
| D | 24(22%) | 351 (26%) | 42 (20%) | ||
| E | 4 ( 4%) | 74 ( 5%) | 11 ( 5%) | ||
| F | 30 (27%) | 140 (10%) | 26 (13%) | ||
| G | 11 (10%) | 452 (33%) | 85 (41%) | ||
| H | 11 (10%) | 100 ( 7%) | 6 ( 3%) | ||
| Median OS in months (95% CI) |
26.6 (23.1, 33.3) | 37.3 (36.6, 39.2) | 42.7 (36.1, 47.0) | <0.0001 | |
| Lymph Nodes Removed |
|||||
| Mean ± SD | 19.9 ± 10.0 | 16.3 ± 7.0 | 19.4 ± 8.3 | ||
| Median (Range) | 17 (4-64) | 15 (1-69) | 18 (2-61) | <0.0001 | |
| Lymph Nodes Positive |
|||||
| Mean ± SD | 0.8 ± 4.4 | 0.9 ± 2.1 | 2.2 ± 3.6 | <0.0001 | |
| Median (Range) | 0 (0-44) | 0 (0-16) | 1 (0-28) |
The treatment groups demonstrated statistical significance with regards to median age and age at diagnosis. The no neoadjuvant group had an increased median age of 64 years, compared to 57 and 56 years, for neoadjuvant chemoradiation and the neoadjuvant chemotherapy groups, respectively (p<00001). Likewise, the age of diagnosis in the no neoadjuvant treatment demonstrated a higher proportion of patients ≥65 years with 45% compared to 26% and 28% for neoadjuvant chemoradiation and neoadjuvant chemotherapy, respectively (p = 0.0001). In examining the comorbidities of the group, the Charlson comorbidity index was utilized and the no neoadjuvant treatment had a higher proportion of patients with Charlson comorbidity index >2 with 16% compared to 8% and 10% for neoadjuvant chemoradiation and neoadjuvant chemotherapy, respectively (p = 0.02).
Pathologic staging demonstrated statistically significant differences between the 3 groups. The neoadjuvant chemoradiation demonstrated a greater proportion of pathologic Stage 0 patients with 20% compared to 5% and 9% for no neoadjuvant treatment and neoadjuvant chemotherapy, respectively. In examining the groups for pathologic Stage 3 patients, this comprised 30% of patients in the neoadjuvant chemoradiation group compared to 60% and 15% in the neoadjuvant chemotherapy and no neoadjuvant therapy groups, respectively.
The neoadjuvant chemoradiation group demonstrated the lowest median lymph node yield with 15 compared to 17 and 18 for no neoadjuvant, and neoadjuvant chemotherapy, respectively (p<0.0001). In analyzing the number of positive nodes between groups, there was a statistically significant difference seen with the neoadjuvant chemotherapy group demonstrating increased positive nodes with mean of 2.2 ± 3.6 compared to the 0.9 ± 2.1, and 0.8 ± 4.4, respectively (p<0.0001). In comparing median overall survival, there was improved survival with neoadjuvant chemotherapy and neoadjuvant chemoradiation of 42.7 months and 37.3 months, respectively compared to 26.6 months with no neoadjuvant therapy (p<0.0001).
As illustrated in Table 2, univariate analysis demonstrated the factors associated with an effect on lymph node yield included tumor grade, clinical T-stage, surgery type, as well as neoadjuvant chemoradiation. Multivariate analysis revealed surgery type, tumor grade, clinical T-stage, and neoadjuvant treatment were predictors of overall survival with neither node number nor status being statistically significant. When examining nodal status irrespective of nodal yield, patients with positive lymph node status, though not statistically significant, had increased overall survival of 38.4 months compared to 36.9 months for node negative status (p=0.09).
Table 2.
Univariate and Multivariate analysis of clinical and pathologic factors related to median overall survival (OS).
| Variable | Category | N | Median OS (months) |
95%CI | Univariate Analysis P-value |
Multivariate Analysis P- value |
|---|---|---|---|---|---|---|
| Age at diagnosis | <50 years | 483 | 37.3 | 36.2 – 40.3 | 0.70 | Not included |
| 50-64 | 730 | 37.3 | 35.9 – 40.1 | |||
| 65-74 | 318 | 36.8 | 34.6 – 40.0 | |||
| 75+ | 149 | 37.9 | 33.1 – 44.8 | |||
| Gender | Male | 982 | 38.4 | 36.9 – 40.9 | 0.17 | 0.19 |
| Female | 649 | 36.4 | 35.2-37.5 | |||
| Charlson comorbidity |
0 | 1251 | 37.3 | 36.4 – 38.9 | 0.30 | Not included |
| 1 | 275 | 40.9 | 35.1 – 45.5 | |||
| 2+ | 154 | 36.4 | 33.1 – 40.3 | |||
| Pathologic TNM Stage+ |
0 | 296 | 37.7 | 35.7 – 41.2 | 0.55 | Not included |
| I | 429 | 37.0 | 35.4 – 40.4 | |||
| II | 407 | 36.8 | 34.6 – 39.5 | |||
| III | 548 | 38.0 | 36.4 – 40.8 | |||
| Type of Surgery |
LAR | 1217 | 38.3 | 36.8 – 40.3 | <0.0001 | <0.001 |
| APR | 373 | 36.7 | 35.1 – 40.4 | |||
| Proctectomy | 90 | 34.9 | 27.1 – 36.2 | |||
| Tumor Grade At Diagnosis |
I | 81 | 44.6 | 36.9 – 52.3 | 0.02 | 0.02 |
| II | 1222 | 37.0 | 36.0 – 38.5 | |||
| III | 109 | 42.0 | 37.2 – 49.4 | |||
| Unknown | 268 | 36.0 | 32.4 – 38.6 | |||
| Clinical T Stage+ | Tx | 206 | 36.8 | 32.6 – 40.6 | 0.03 | 0.03 |
| T0/T1/T2 | 143 | 40.3 | 35.6 – 45.5 | |||
| T3 | 1234 | 37.5 | 36.6 – 39.2 | |||
| T4 | 97 | 31.5 | 26.2 – 36.4 | |||
| Node Status (Lymph Nodes Positive) |
Negative | 1144 | 36.9 | 35.7 – 38.5 | 0.09 | 0.31 |
| Positive | 536 | 38.4 | 36.7 – 42.1 | |||
| Time from the last dose to surgery (N=500) |
≤ 8 weeks | 314 | 22.3 | 20.9 – 23.6 | 0.25 | Not included |
| Node Stage | > 8 weeks | 186 | 17.9 | 15.9 – 22.2 | ||
| Adjuvant Chemo Therapy |
Yes | 1569 | 37.7 | 36.8 – 39.5 | >0.0001 | Redundant* |
| No | 111 | 26.6 | 23.1 – 33.3 | |||
| Neoadjuvant Treatment | No Neoadjuvant | 111 | 26.6 | 23.1-33.3 | <0.0001 | <0.001 |
| Neoadjuvant Chemoradiation |
1364 | 37.3 | 36.6 – 39.2 |
Adjuvant chemotherapy is redundant because all the patients who received chemotherapy neoadjuvantly received chemotherapy in the adjuvant setting
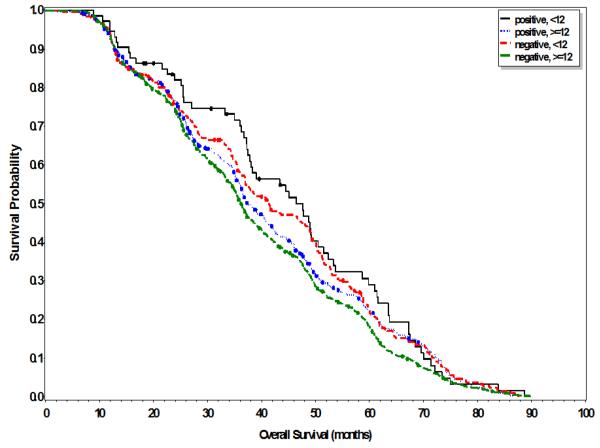
Sub-group analyses were undertaken examining the impact of both nodal yield and status on overall survival as illustrated in Table 3 and Figure 1. Patients with <12 lymph node yield had improved median overall survival of 43.3 months compared to 36.6 months in patients with ≥12 lymph nodes (p=0.009). Further analysis of patients with <12 lymph node yield revealed that patients with positive nodal status had increased median overall survival of 46.5 months compared to 41.5 months for negative nodal status, though not statistically significant (p=0.46). Similarly, patients with ≥12 lymph node yield and positive nodal status had increased median overall survival of 37.4 months compared to 36.3 months in patients with negative nodal status with trend towards but not statistically significant (p=0.07). In examining those patients receiving neoadjuvant chemoradiation, patients with <12 lymph node yield had improved overall survival of 43.4 months compared to 36.8 months in those with ≥12 lymph nodes (p=0.02).
Table 3.
Univariate analysis examining impact of nodal yield and nodal status on median overall survival (OS)
| Group | Category | N | Median OS (months) |
95%CI | P-value |
|---|---|---|---|---|---|
| All Groups | <12 nodes | 323 | 43.3 | 37.8 – 48.7 | 0.009 |
| ≥12 nodes | 1357 | 36.6 | 35.7 – 37.8 | ||
| Lymph nodes <12 | Negative | 249 | 41.5 | 36.9 – 48.9 | 0.46 |
| Positive | 74 | 46.5 | 37.5 – 51.5 | ||
| Lymph nodes ≥12 | Negative | 895 | 36.3 | 35.1 – 37.3 | 0.07 |
| Positive | 462 | 37.4 | 36.0 – 40.8 | ||
| Chemoradiation Group | <12 nodes | 288 | 43.4 | 37.5 – 48.9 | 0.02 |
| ≥12 nodes | 1076 | 36.8 | 35.9 – 38.3 |
Figure 1.
Overall survival stratified by nodal yield (<12 and ≥12) and status (negative/positive)
Discussion
Lymph node status remains an essential factor for cancer staging and guiding treatment decisions. In examining the factors associated with lymph node yield in rectal cancer, modifiable factors include surgical and pathology measures. Surgical vigilance with optimal oncologic surgical technique with TME is desired. However, despite the highly qualified surgeons from participating NCCN institutions, there still remain some patients in which a ≥ 12 lymph node yield was not achieved irrespective of neoadjuvant treatment as shown in Table 1 with nodal yields ranging from 1-69 in this patient series.
The College of American Pathologists (CAP) understands the importance of lymph node yield as reflected in their suggested rectal specimen assessment protocols. This requires meticulous tissue assessment, but is not necessarily standardized with technique variance in grossing, fixation, processing, embedding, staining, and ultimately pathologist review all potentially affecting lymph node yield. They acknowledge the challenges of nodal assessment, particularly in the elderly, obese, and patients treated neoadjuvantly.
Neoadjuvant therapy is a well-accepted non-modifiable factor (with the exception of deferring it) affecting lymph node yield and was again demonstrated in this analysis with statistical significance seen between groups with the neoadjuvant chemoradiation group having the lowest median lymph node yield (15). In examining the pathologic staging of the groups, the neoadjuvant chemoradiation group not only had a greater stage 0 (pathologic complete response) rate at 20% versus 9% in the neoadjuvant chemotherapy, but also had 30% with pathologic stage 3 disease versus 60% in the neoadjuvant chemotherapy. This difference could be reflective of response to the neoadjuvant treatment given but may also relate to earlier clinical staging.
Again, the EORTC 22921 study concluded that fluorouracil based adjuvant chemotherapy demonstrated no statistically significant improvement in disease-free or overall survival in patients after neoadjuvant treatment. With clinical stage II/III rectal cancer patients who can tolerate multimodality therapy and receive neoadjuvant chemoradiation, the benefit of adjuvant therapy is unclear, and hence the clinical importance of nodal status and in particular yield remains in question.
This study has several limitations requiring acknowledgement. This is a retrospective analysis undertaken utilizing the NCCN patient database. The NCCN database is comprised of a specific patient population derived from 8 participating NCCN institutions. These institutions do comprise some of the leading tertiary care cancer institutions in the country. This certainly introduces several biases warranting mention. Given the specialized nature of NCCN treatment centers, this lends to potential referral bias such that the NCCN patient sample may not be truly representative of the overall patient population. Additionally, the specialized nature of NCCN institutions introduces selection bias in the clinician expertise, patients treated, as well as the level and type of cancer care available and administered to this specific patient sample. This is exemplified in the group stratification based on therapy that is likely determined by patient characteristics and clinician recommendations despite equivalent preoperative clinical staging (clinical stage II/III).
As is often encountered with many large population database studies, the NCCN patient database has inherent limitations as to the data available for analysis. There exists missing or even desired but unavailable data for which data imputation and analysis is not reasonable to pursue for scientifically sound statistics and conclusions thus leaving some questions unanswered. Finally, as in all retrospective analyses, the factors examined and analyzed cannot be considered causative, but rather associated with the obtained results and drawn following conclusions.
Acknowledging the limitations of this large, but retrospective analysis, this analysis demonstrated no association between increased lymph node harvest or nodal status on patient survival irrespective of the patient therapies undertaken. The current NCCN guidelines (version 2.2016) discussion based on previous studies acknowledges the impact of neoadjuvant therapy on decreased lymph node yield.24 This study interestingly demonstrated that patients with <12 lymph nodes harvested had improved overall survival irrespective of nodal status. Hence, a nodal metric in rectal cancer may not be clinically relevant in the setting of the current standard of care with neoadjuvant treatment playing a major role in the multimodality treatment of rectal cancer.
Nodal yield and status after neoadjuvant therapy may more importantly be a surrogate marker of disease response and not ideal to base adjuvant therapy decisions on, particularly given the EORTC conclusions showing no demonstrable benefit to 5-FU based adjuvant therapy. Preoperative clinical staging may in fact be more representative of cancer biology from which to base adjuvant therapy decision making on to potentially impact and improve patient outcomes. That being said, in patients who were not significantly downstaged by neoadjuvant treatment and had positive lymph node status, one could anticipate potentially adverse outcomes based on their pathologic staging and lack of response to neoadjuvant therapy. Given the impact of neoadjuvant therapy on rectal cancer outcomes, ongoing (e.g. CALGB/Alliance NCCTG-N1048, PROSPECT) and future prospective randomized trials are prudent and needed to further determine the relevance of lymph node yield and status to patient outcomes as well as optimize therapeutic approaches in order to advance the multi-modality care of rectal cancer.
Acknowledgments
No research support
Footnotes
No Disclaimers
References
- 1.Le Voyer TE, Sigurdson ER, Hanlan AL, et al. Colon Cancer Survival is Associated with Increasing Number of Lymph Nodes Analyzed: A Secondary Survey of Intergroup Trial INT-0089. JCO. 2003 Aug 1;21(15) doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 2.Swanson RS, Compton CC, Stewart AK, Bland KI. The Prognosis of T3N0 Colon Cancer is Dependent on the Number of Lymph Nodes Examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 3.Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of Number of Nodes Retrieved on Outcome in Patients with Rectal Cancer. J Clin Oncol. 2001;19:157–63. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 4.Cserni G, Vinh-Hung V, Burzykowski T. Is there a Minimum Number of Lymph Nodes that should be Histologically Assessed for a Reliable Nodal Staging of T3N0M0 Colorectal Carcinomas? J Surg Oncol. 2002;81:63–69. doi: 10.1002/jso.10140. [DOI] [PubMed] [Google Scholar]
- 5.Fielding LP, Arsenault PA, Chapuis PH, et al. Clinicopathologic Staging for Colorectal Cancer: An International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT). Working Party Report to the World Congresses of Gastroenterolgoy, Sydney 1990. Journal of Gastroenterology and Hepatology. 1991 Jul-Aug;6(4):35–44. doi: 10.1111/j.1440-1746.1991.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 6.Baxter NN, Virnig DJ, Rothenberger DA, et al. Lymph Node Evaluation in Colorectal Cancer Patients: A Population-Based Study. Journal of National Cancer Institute. 2005;97(3):219–225. doi: 10.1093/jnci/dji020. [DOI] [PubMed] [Google Scholar]
- 7.Wright FC, Law CH, Last L, et al. Lymph Node Retrieval and Assessment in Stage II Colorectal Cancer: A Population Based Study. Ann Surg Oncol. 2003;10:903–909. doi: 10.1245/aso.2003.01.012. [DOI] [PubMed] [Google Scholar]
- 8.American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed Springer; New York, NY: 2002. Colon and Rectum. [Google Scholar]
- 9.Taflampas P, Christodoulakis M, Gourtsoyianni S, et al. The effect of Preoperative Chemoradiotherapy on Lymph Node Harvest after Total Mesorectal Excision for Rectal Cancer. Dis Colon Rectum. 2009 Aug;52(8):1470–4. doi: 10.1007/DCR.0b013e3181a0e6ac. [DOI] [PubMed] [Google Scholar]
- 10.McDonald JR, Renehan AG, O’Dwyer ST, Haboubi NY. Lymph Node Harvest in Colon and Rectal Cancer: Current Considerations. World J Gastorintest Surg. 2012 Jan 27;4(1):9–19. doi: 10.4240/wjgs.v4.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans MD, Barton K, Rees A, et al. The Impact of Surgeon and Pathologist on Lymph Node Retrieval in Colorectal Cancer and its Impact on Survival for Patients with Dukes’ Stage B Disease. Colorectal Dis. 2008;10:157–164. doi: 10.1111/j.1463-1318.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Bentrem DJ, Stewart AK, et al. Lymph Node Evaluation as a Colon Cancer Quality Measure: A National Hospital Report Card. J Natl Cancer Inst. 2008 Sep 17;100(18):1310–7. doi: 10.1093/jnci/djn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller ED, Robb BW, Cummings OW, Johnstone PA. The Effects of Preoperative Chemoradiotherapy on Lymph Node Sampling in Rectal Cancer. Dis Colon Rectum. 2012 Sep;55(9):1002–7. doi: 10.1097/DCR.0b013e3182536d70. [DOI] [PubMed] [Google Scholar]
- 14.Shen SS, Haupt BX, Ro JY, et al. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med. 2009;133:781–786. doi: 10.5858/133.5.781. [DOI] [PubMed] [Google Scholar]
- 15.Baxter NN, Morris AM, Rothenberger DA, Tepper JE. Impact of Preoperative Radiation for Rectal Cancer on Subsequent Lymph Node Evaluation: A Population –Based Analysis. Int J Radiat Oncol Biol Phys. 2005;61:426–431. doi: 10.1016/j.ijrobp.2004.06.259. [DOI] [PubMed] [Google Scholar]
- 16.Gorog D, Nagy P, Peter A, Perner F. Influence of Obesity on Lymph Node Recovery from Rectal Cancer Specimens. Pathol Oncol Res. 2003;9(3):180–183. doi: 10.1007/BF03033734. [DOI] [PubMed] [Google Scholar]
- 17.Nathan H, Shore AD, Anders RA, et al. Variation in Lymph Node Assessment after Colon Cancer Resection: Patient, Surgeon, Pathologist, or Hospital? J Gastrointest Surg. 2011 Mar;15(3):471–9. doi: 10.1007/s11605-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SL, Ji H, Hollenbeck BK, et al. Lymph Node Examination Rates and Survival after Resection for Colon Cancer. JAMA. 2007 Nov 14;298(18):2149–54. doi: 10.1001/jama.298.18.2149. [DOI] [PubMed] [Google Scholar]
- 19.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med. 2004 Oct 21;351(17):1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 20.De la Fuente SG, Manson RJ, Ludwig KA, Mantyh CR. Neoadjuvant Chemoradiation for Rectal Cancer Reduces Lymph Node Harvest in Proctectomy Specimens. J Gastrointest Surg. 2009 Feb;13(2):269–274. doi: 10.1007/s11605-008-0717-2. [DOI] [PubMed] [Google Scholar]
- 21.Marks JH, Valsdottir EB, Rather AA, et al. Fewer than 12 Lymph Nodes can be Expected in a Surgical Specimen after High-Dose Chemoradiation Therapy for Rectal Cancer. Dis Colon Rectum. 2010 Jul;53(7):1023–1029. doi: 10.1007/DCR.0b013e3181dadeb4. [DOI] [PubMed] [Google Scholar]
- 22.Rullier A, Laurent C, Capdepont M, et al. Lymph Nodes after Preoperative Chemoradiotherapy for Rectal Carcinoma: Number, Status, and Impact on Survival. Am J Surg Pathol. 2008 Jan;32(1):45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 23.Bosset JF, Calais G, Mineur L, et al. for the EORTC Radiation Oncology Group Fluorouracil-based Adjuvant Chemotherapy after Preoperative Chemoradiotherapy in Rectal Cancer: Long-term Results of the EORTC 22921 Randomised Study. Lancet Oncol. 2014 Jan 16; doi: 10.1016/S1470-2045(13)70599-0. Epub. [DOI] [PubMed] [Google Scholar]
- 24.Benson AB, Venook AP, Bekaii-Saab T, et al. [Accessed March 28, 2016];National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Rectal Cancer. (Version 2). 2016 Available at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.