Abstract
Cathelicidin LL-37 is one of the few human bactericidal peptides with potent antistaphylococcal activity. In this study we examined the susceptibility of LL-37 to proteolytic degradation by two major proteinases produced by Staphylococcus aureus, a metalloproteinase (aureolysin) and a glutamylendopeptidase (V8 protease). We found that aureolysin cleaved and inactivated LL-37 in a time- and concentration-dependent manner. Analysis of the generated fragments by mass spectroscopy revealed that the initial cleavage of LL-37 by aureolysin occurred between the Arg19-Ile20, Arg23-Ile24, and Leu31-Val32 peptide bonds, instantly annihilating the antibacterial activity of LL-37. In contrast, the V8 proteinase hydrolyzed efficiently only the Glu16-Phe17 peptide bond, rendering the C-terminal fragment refractory to further degradation. This fragment (termed LL-17-37) displayed antibacterial activity against S. aureus at a molar level similar to that of the full-length LL-37 peptide, indicating that the antibacterial activity of LL-37 resides in the C-terminal region. In keeping with LL-37 degradation by aureolysin, S. aureus strains that produce significant amounts of this metalloprotease were found to be less susceptible to LL-17-37 than strains expressing no aureolysin activity. Taken together, these data suggest that aureolysin production by S. aureus contributes to the resistance of this pathogen to the innate immune system of humans mediated by LL-37.
Staphylococcus aureus is a major human pathogen responsible for numerous cases of community- and hospital-acquired infections, ranging from relatively minor skin and wound infections to life-threatening diseases, including toxic shock syndrome, arthritis, endocarditis, osteomyelitis, and sepsis (22). Up to 60% of healthy individuals are permanently or transiently colonized by S. aureus. Nasal carriage is an important risk factor for serious systemic and internal organ infections that have high morbidity and mortality, especially in those patients with a suppressed immune system, undergoing surgery, or with intravenous devices (18). Furthermore, the worldwide spread of multidrug-resistant strains, including the emergence of strains with greatly reduced susceptibility to vancomycin (46), typically regarded as the antibiotic of last resort, indicates the danger of untreatable staphylococcal infections. The recent outbreaks of methicillin-resistant S. aureus causing community-acquired infections emphasize the continued problems caused by this organism.
In the past decade, a broad array of virulence factors mediating adherence to host tissues, damage to host cells, and strategies for evasion of the host defense mechanisms have been thoroughly characterized (31). Neutrophils play an important role in host defense against S. aureus. They employ both oxygen-dependent and -independent mechanisms to kill the pathogen. The importance of neutrophils in innate resistance to staphylococci is supported by the fact that neutropenic patients or those with chronic granulomatous disease (CGD) are particularly susceptible to S. aureus infections (21). Oxygen-independent killing mechanisms mediated by antimicrobial peptides or proteins (34, 39) play a more important role in killing staphylococci than previously thought. However, S. aureus has evolved the means to resist effector molecules of innate immunity, such as lysozyme and cationic antibacterial peptides, including the α-defensins of human neutrophils (HNP 1 to 3) (32, 33) and the β-defensin hBD2 (14, 49). The resistance of S. aureus to α-defensins, which account for 50% of the neutrophil granule proteins, likely contributes to the inability of neutrophils to effectively kill this pathogen (25). The bactericidal activity of neutrophils is reinforced by cathelicidin-derived peptides. The serine proteinases confined in the azurophil granules of neutrophils, especially elastase, may also play a major role in killing phagocytized bacteria, including S. aureus (5, 34, 43).
Beside the defensins, cathelicidins constitute the second major family of antibacterial peptides in mammals (for reviews see references 6 and 7). Cathelicidins contain an N-terminal domain called cathelin that is strictly conserved across species, followed by a C-terminal domain that comprises an antimicrobial peptide of a highly diverse structure (38, 47). The human cathelicidin hCAP-18 or FALL-39 is constitutively present in specific granules of neutrophils (42) and the testes (1), and it is inducibly expressed by keratinocytes (13) and in squamous epithelia (4, 10) in response to inflammatory challenge. In humans, hCAP-18 is processed by proteinase 3 to generate the active peptide LL-37 (42). On the other hand, the porcine and bovine cathelicidins are cleaved by neutrophil elastase-releasing antibacterial peptides referred to as protegrins and bactenacins, respectively (29, 38). In contrast to most defensins, cathelicidin-derived bactericidal peptides, including LL-37, possess considerable antistaphylococcal activity (44, 45) and, therefore, may significantly contribute to protection of the skin and mucosal surfaces of upper airways against colonization by S. aureus and other gram-positive pathogens. Indeed, the significance of cathelicidins for the innate host defense is apparent from the following observations: the mouse cathelicidin CRAMP protects the skin from invasive bacterial infections (27); proteolytic processing of porcine cathelicidins (protegrins) is essential for bacterial clearance from wounds (9); improved lung clearance of Pseudomonas aeruginosa is associated with overexpression of LL-37 (2, 3); and downregulation of LL-37 in atopic dermatitis predisposes patients to skin infection with S. aureus (28).
In addition to LL-37, human skin is also protected from S. aureus infection by the recently discovered β-defensin hBD-3 (14) as well as dermicidin, a novel antibiotic peptide secreted by sweat glands (36). Significantly, while dermicidin expression is constitutive, proinflammatory cytokines and contact with bacteria strongly induce hBD-3 expression in keratinocytes and airway epithelial cells. However, despite the formidable line of innate defense exerted by LL-37, dermicidin, and hBD-3, S. aureus and coagulase-negative staphylococci easily colonize nasal epithelium and skin, respectively. It is believed that the inherited resistance of Staphylococcus spp. to the bactericidal activity of α-defensins and hBD-2 is responsible for this ability (30). Here we show that proteinases from S. aureus, notably aureolysin, through degradation of LL-37 may also contribute to the ability of this pathogen to resist bactericidal peptides.
MATERIALS AND METHODS
Peptide synthesis.
Peptides were synthesized on an Applied Biosystems model 433A synthesizer on a 0.1- to 0.25-mmol scale, using either tert-butoxycarbonyl or 9-fluorenylmethoxy carbonyl chemistry as described previously (39, 40). Peptides were purified by preparative reversed-phase high-performance liquid chromatography (HPLC) using C18 silica columns and were obtained lyophilized as their trifluoroacetate salt. The purity and structural integrity of all peptides was confirmed by analytical HPLC and by mass spectrometry. The peptide concentration was determined by quantitative amino acid analysis.
Bacterial strains.
The following bacterial strains were used in this study: Escherichia coli (ATCC 33694), Bacillus subtilis M168 (ATCC 27370), a clinical isolate of Pseudomonas aeruginosa, Enterococcus faecalis (ATCC 29212), S. aureus strains Newman and SH1000 (15), DU5976 Newman sarA (23), DU5977 Newman sarA aur (23), and SH1000 sarA (this study), constructed by transducing the sarA::Kar mutation from PC1839 (8).
Proteinase purification.
The S. aureus metalloproteinase (aureolysin) and V8 proteinase (glutamylendopeptidase) were purified from culture medium of the V8 strain as described by Drapeau (11). Enzyme purity was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% Tricine gels. The protein concentration in each enzyme batch was determined by using a bicinchoninic acid (BCA) (Bio-Rad) kit with bovine serum albumin as a standard.
Zymography.
Culture media of S. aureus strains grown to mid-exponential phase were mixed with sample buffer (0.125 M Tris-HCl, 20% glycerol, 5% sodium dodecyl sulfate, 0.03% bromophenol blue, pH 6.8) and electrophoresed on 10% polyacrylamide gels containing 0.1% bovine gelatin using the Laemmli buffer system (19). To remove SDS, gels were incubated with 2.5% Triton X-100, followed by overnight incubation at 37°C in a buffer containing 50 mM Tris-HCl-5 mM CaCl2, pH 7.8. Aureolysin- and V8 protease-containing bands were visualized as clear zones against a dark background. To distinguish the origin of the proteolytic activity zone, media samples in some experiments were preincubated with either 1 mM 3,4-dichloroisocoumarin (inhibitor of the V8 proteinase) or 10 mM o-phenanthroline (inhibition of aureolysin), and the zymogram was developed in buffer containing the appropriate inhibitor.
Proteolytic activity assay.
Proteolytic activity in growth media of all strains of S. aureus was determined by using azocasein as the substrate. Briefly, 50-μl aliquots of the medium were added to 0.2 ml of a solution of 100 mM Tris-HCl-1 mM CaCl2, pH 7.8, supplemented with either 1 mM 3,4-dichloroisocoumarin (aureolysin activity) or 10 mM o-phenanthroline (V8 protease activity). After 30 min of preincubation at 37°C, 0.125 μl of 3% azocasein (wt/vol, in deionized water) was added and the mixture was incubated for 3 h at 37°C. The reaction was stopped by addition of 0.35 ml of ice-cold 6% (wt/vol) trichloroacetic acid (TCA). The samples were vortexed and kept on ice for 10 min, and then the undigested, precipitated azocasein was removed by centrifugation (14,000 × g, 10 min, at 4°C). From each sample, 200 μl of clear supernatant was transferred into wells of a microtiter plate. The optical density at 360 nm was measured against a blank sample containing all the reagents by using a plate reader (Spectromax 250, Molecular Devices), but with TCA added immediately after the substrate. The azocaseinolytic activity was calculated as the increase of milli-optical density at 360 nm (mOD360) during 3 h of incubation of the substrate with 50 μl of medium.
LL-37 and LL-17-37 degradation.
Peptide samples in acidified water at a concentration of 4 mg per ml were mixed with an equal volume of aureolysin or the V8 protease, serially diluted to the same molar concentration in 20 mM Tris-HCl-1 mM CaCl2, pH 7.8. The mixtures were incubated at 37°C. At specific time points, 10-μl aliquots were removed, diluted twofold in 0.01% acetic acid, and immediately used for assessing the residual bactericidal activity of the peptides, using either a radial diffusion or colony-counting procedure (see below). The diluted samples collected after different times of incubation with various enzyme concentrations were analyzed by molecular mass analysis. Based on the determined molecular mass of LL-37 and LL-17-37 degradation products, the cleavage sites of the peptides by the staphylococcal proteinases were determined.
To investigate LL-37 cleavage in the presence of human plasma, the peptide solution was mixed with an equal volume of proteases contained in serially diluted human plasma in phosphate-buffered saline (PBS) and were incubated at 37°C. The reaction was stopped by boiling samples in a reducing SDS-PAGE sample buffer and was analyzed for LL-37 degradation using the Western blot technique.
Western blot analysis.
Samples were resolved in SDS-PAGE (peptide gels) using the Tris-Tricine discontinuous buffer system (35). The electrophoresed gels were electroblotted (Trans-Blot Semi-Dry; Bio-Rad) onto polyvinylidene difluoride membranes (Amersham-Pharmacia Co.). Nonspecific binding sites on the membrane were blocked overnight in 5% dry milk and then were immunoblotted. The blots were probed with mouse antibodies against LL-37 (HyCult Biotechnology, Uden, The Netherlands). Immunoreactive peptides were detected by the procedure described in the Western Lightning Chemiluminescence Reagent Plus kit (Perkin Elmer Life Sciences, Boston, Mass.).
Antimicrobial assays. (i) Radial diffusion assays.
The two-stage radial diffusion assay was performed as described by Lehrer et al. (20). Briefly, the purified synthetic peptides, LL-37 and its truncated version, LL-17-37, either intact or after preincubation with proteinases, were serially diluted in acidified water (0.01% acetic acid) that contained 0.2% human serum albumin. The target bacteria were grown to mid-exponential phase in tryptic soy broth and were washed with 10 mM phosphate buffer (pH 7.4). Approximately 2 × 105 CFU per ml was incorporated into an agarose gel that contained 2% (wt/vol) low-melting-point agarose (Sigma) and 10 mM sodium phosphate buffer (pH 7.4) in a total volume of 15 ml, and it was poured into 12-cm-diameter petri dishes to form a thin (1.2 mm) underlying gel. A regularly spaced four-by-six array of wells of 3.2 mm in diameter was made in the underlying gel. Six 2.5-μl aliquots of peptide (containing 4, 3, 2, 1, 0.5, or 0.25 mg of peptide/ml) were added to the wells. After 3 h, 5 ml of overlay gel consisting of 6% tryptic soy broth agar and 10 mM sodium phosphate (pH 7.4) was poured. The plates were incubated overnight to allow surviving organisms to form microcolonies. Diameters of the zone of bacterial growth inhibition were measured to the nearest 0.1 mm before and/or after staining the gel with Coomassie brilliant blue and are expressed in units (1 U = 0.1 mm) after subtracting the diameter of the well. A linear relationship exists between the zone diameter and the base 10 logarithm of the peptide concentration. The MIC was determined by performing a least-mean-squares fit and solving for the x intercept.
(ii) Colony reduction assay.
Bacteria were grown as described above for the radial diffusion assays, washed twice in 10 mM sodium phosphate buffer (pH 7.4), and diluted to a final concentration of 2 × 105 to 5 × 105 CFU/ml in 10 mM sodium phosphate buffer (pH 7.4) with 0.2% (wt/vol) tryptic soy broth powder. Twofold serial dilutions of LL-37 or LL-17-37, either intact or after preincubation with aureolysin or the V8 protease, were prepared in acidified water (0.01% acetic acid), and 90-μl aliquots were transferred to a low-protein-binding polypropylene round-bottom microtiter plate (Costar, Cambridge, United Kingdom). To each of the wells, 10 μl of the bacterial suspension was added. The plates were incubated on a rotary shaker (300 rpm) at 37°C. After a 1-h incubation, 10-μl aliquots were removed and diluted 1:100 with the medium, and 10-μl samples were plated onto tryptic soy agar plates. Alternatively, 5-μl samples were spotted in duplicate on plates. The bactericidal activity was assessed next after counting colonies on the agar plates (CFU) and by visual inspection of the growth. The 50% lethal dose (LD50) and the minimal bactericidal concentrations (MBC) were defined as the concentration of the peptide at which 50% of the inoculum survived after the 1-h exposure or no visible colonies were seen on the plate, respectively. All experiments were performed at least in triplicate.
RESULTS
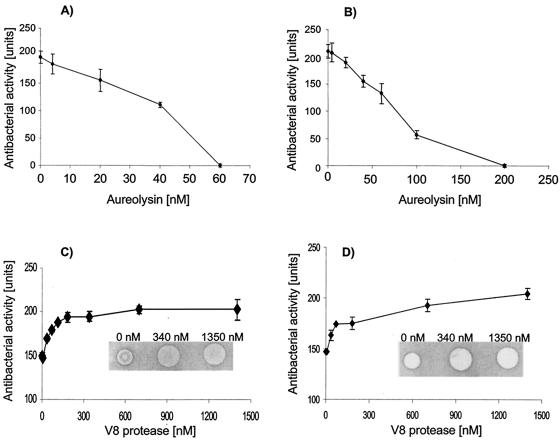
Degradation and inactivation of LL-37 by staphylococcal proteinases. LL-37 was incubated with purified aureolysin and V8 proteinase, either at a constant enzyme concentration over various time intervals or with varied concentrations of the proteinases for 45 min, and the peptide bactericidal and/or bacteriostatic activity was then determined by using the agar diffusion assay with E. coli and S. aureus (Newman strain) as the target bacteria. The data from these experiments indicated that aureolysin efficiently inactivated the antibacterial activity of LL-37 in a time- and concentration-dependent manners (Fig. 1A and B). In contrast, the antibacterial activity of LL-37 was not only resistant to inactivation by the V8 proteinase but, paradoxically, was enhanced (Fig. 1C and D).
FIG. 1.
Concentration-dependent effect of staphylococcal proteinases on the antibacterial activity of LL-37. LL-37 (3 mg/ml) was mixed with an equal volume of aureolysin (A and B) or the V8 protease (C and D) in 20 mM Tris, pH 7.8, with 1 mM CaCl2 to yield indicated concentrations of both proteinases. The digested samples were tested for antibacterial action against S. aureus strain Newman (A and C) and E. coli ATCC 33694 (B and D). The diameter of the bacterial growth inhibition zones was measured and converted into units (0.1 mm = 1 U of inhibition growth). Each point in the figure represents a mean ± standard deviation of at least two independent triplicate assays. Insets in panels C and D show representative results of bacterial growth inhibition by LL-37 alone and after preincubation with two different concentrations of the V8 protease.
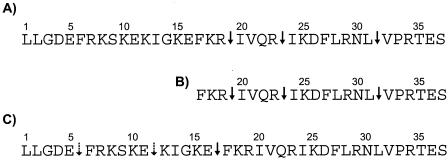
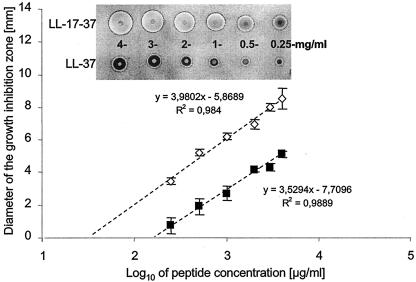
The rate of inactivation of the bactericidal activity by aureolysin correlated well with the rate of LL-37 degradation monitored by mass spectroscopy analysis. The peptide degradation apparently occurred by simultaneous hydrolysis of the Arg19-Ile20, Arg23-Ile24, and Leu31-Val32 peptide bonds (Fig. 2). In contrast, the V8 proteinase only hydrolyzed efficiently the Glu16-Phe17 peptide bond, releasing the C-terminal fragment which was refractory to further cleavage even after prolonged incubation (5 h) in the presence of the V8 proteinase. However, under this condition the N-terminal 16-mer peptide was further cleaved at the Glu5-Phe6 and Glu11-Lys12 peptide bonds. These data indicated that the bactericidal activity of LL-37 apparently resides in the C-terminal part of the peptide. To confirm this assumption, the peptide encompassing amino acid residues 17 to 37 of LL-37 was synthesized and tested for biological activity. Indeed, on a weight basis its bactericidal activity against E. coli, Bacillus subtilis, Pseudomonas aeruginosa, and Enterococcus faecalis was from 3- to 1.5-fold higher than that of intact LL-37 (Table 1). Similar results were obtained when the antistaphylococcal activity of the peptides was compared using the agarose diffusion assay (Fig. 3). However, because the truncated peptide is nearly one-half the molecular mass of the full-length peptide, the two peptides are essentially equivalent in activity on a molar basis. Thus, we conclude that the majority (if not total) bactericidal capacity of LL-37 can be localized to its C-terminal region.
FIG. 2.
Cleavage sites of LL-37 and its N-terminally truncated form (LL-17-37) by aureolysin (A and B) and the V8 proteinase (C). Solid and dotted arrows indicate primary and secondary cleavage sites, respectively.
TABLE 1.
Comparison of MBC of LL-37 and its N-terminally truncated derivative (LL-17-37) on different bacterial species
| Bacterial strain | MBC (μg/ml) for:
|
|
|---|---|---|
| LL-37 | LL-17-37 | |
| E. coli | 3 | 1.1 |
| B. subtilis | 1.6 | 0.8 |
| P. aeruginosa | 4 | 1.6 |
| E. faecalis | 6.3 | 4.5 |
FIG. 3.
Comparison of bacteriostatic and bactericidal activity of LL-37 and LL-17-37. The Newman strain of S. aureus in the logarithmic phase was mixed with basic agar and poured on the sterile petri dishes. After agar solidification, the wells were punched and 2.5-μl aliquots of the peptide at concentrations of 4, 3, 2, 1, 0.5, and 0.25 mg/ml were added. Plates were incubated for 3 h at 37°C, and then nutritious TSA was poured on the basic agar. Plates were incubated overnight at 37°C, dried, and stained with Coomassie brilliant blue, and the diameters of zones of inhibition growth were measured (inset) and plotted against the log of peptide concentration (in micrograms/milliliter). Each point represents a mean ± standard deviation of three independent duplicate assays. From the intercept with the x axis, the MIC was calculated at 30 and 152 μg/ml for LL-17-37 and LL-37, respectively. The inset shows representative results of bacterial growth inhibition by different concentrations of LL-37 and LL-17-37.
In accordance with the cleavage pattern of full-length LL-37, the LL-17-37 peptide was resistant to degradation by V8 proteinase and its antistaphylococcal activity remained intact even after 5 h of incubation with a high concentration of enzyme (1.4 μM). In contrast, the antibacterial activity of LL-17-37 was lost during incubation with aureolysin, and the kinetics of degradation were similar to those of the inactivation of intact LL-37 (data not shown). Mass spectroscopic analysis confirmed that the same peptide bonds were cleaved (Fig. 2B).
LL-37 was also susceptible to proteolytic degradation by aureolysin and the V8 protease in the presence of human plasma, as determined by the Western blot analysis (data not shown). In this case, the concentration of both V8 protease and aureolysin necessary to achieve complete cleavage of the peptide was somehow higher than that of buffer alone. This is apparently due to inhibition of the staphylococcal proteinase activity by human plasma inhibitors. Nevertheless, this result suggests that the LL-37 cleavage can occur in vivo.
Proteolytic activity produced by different isogenic mutants of S. aureus.
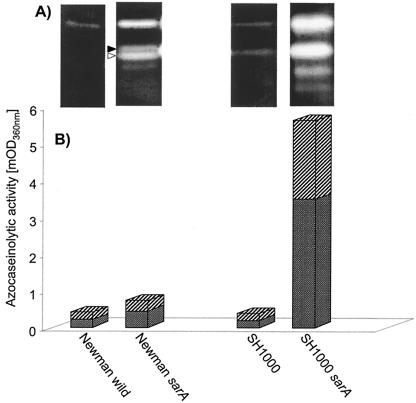
Metalloproteinase activity released by S. aureus into the environment may constitute one mechanism by which this pathogen defends itself against antimicrobial peptides. Because the expression of aureolysin is negatively regulated (8, 48) by the product of the staphylococcal accessory regulator gene (sarA), we used mutants of strains Newman and SH1000 deficient in this regulator. Before assessing the susceptibility of the S. aureus strains to the antistaphylococcal activity of LL-37 and LL-17-37, the proteolytic activity in media from the late-exponential cultures was analyzed by gelatin zymography (Fig. 4) and in an assay with azocasein as the substrate. The wild-type Newman strain produced very weak proteolytic activity which was visible on the gelatin zymography gels only after overnight incubation. In agreement with previously published data (23), this proteolytic activity was strongly enhanced in the sarA mutant (Fig. 4, lane 2). With this mutant, bands related to V8 proteinase and aureolysin activity, as distinguished by susceptibility to inhibition by 3,4-diisochlorocoumarin and EDTA, respectively, are clearly visible. Similarly, strain SH1000 (15) had a proteolytic phenotype similar to that of the Newman strain (Fig. 4, lanes 1 and 4). However, genetic inactivation of the sar operon in strain SH1000 resulted in release of high levels of proteolytic activity (Fig. 4, lane 3).
FIG. 4.
Proteolytic activity in media from the late-logarithmic phase of growth of isogenic mutants of the S. aureus strains Newman and SH1000. The activity was visualized either by gelatin-zymography analysis (A) or was quantified using azocasein as the substrate (B). The contribution of V8 proteinase (full arrowhead in panel A and stippled bars in panel B) and aureolysin (empty arrowhead and crossed bars) to total proteolytic activity was determined with the specific inhibitors 3,4-dichloroisocoumarin and o-phenanthroline, respectively.
Bactericidal effect of LL-17-37 on S. aureus isogenic mutants differing in level of proteinase production.
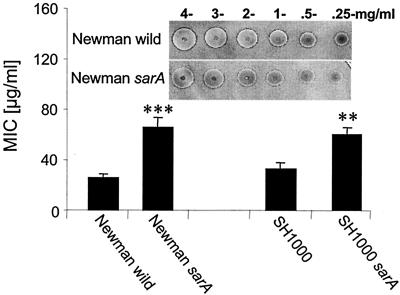
We wanted to test the hypothesis that the level of protease production by a given S. aureus strain could have an effect on its susceptibility to an antimicrobial peptide. Accordingly, we used peptide LL-17-37 for this purpose and incubated it with our test S. aureus strains that differed in levels of extracellular proteinase. As is shown in Fig. 5, a significant difference in the diameter of zones of bacterial growth was observed between the Newman mutant strain that produces a high level of aureolysin activity (Newman sarA) and the wild-type strain, which secretes it at a low level. The MBC for the proteolytic mutant was about 2.5 times higher than that of the aureolysin-negative mutant and its parental strain. A similar relation was observed for strains SH1000 and SH1000 sarA. Thus, we conclude that S. aureus could use endogenous extracellular proteases to decrease its level of susceptibility to an antimicrobial peptide.
FIG. 5.
Comparison of antistaphylococcal activity of LL-17-37 against S. aureus strains Newman and SH1000 and their sarA mutants. The bacteriostatic and bactericidal activity of LL-17-37 was determined using the radial diffusion assay. The diameter of the bacterial growth inhibition zones was measured before and after staining the agarose plates with Coomassie brilliant blue (inset) and was converted into units (0.1 mm = 1 U of inhibition growth). The units were plotted against the log of peptide concentration (in micrograms/milliliter), and the MIC was calculated. Each point represents a mean ± standard deviation of at least six independent duplicate assays. The statistically significant differences (Student's test) in MIC in comparison to that of the wild-type strain within the same group of isogenic mutants are indicated by asterisks: *, P < 0.01; **, P < 0.001.
DISCUSSION
Cationic peptides with antimicrobial activity constitute a formidable innate immunity defense barrier against infections (6). Nevertheless, some bacterial species have developed various mechanisms to circumvent this barrier. Through extensive modification of cell membrane phospholipids and cell wall teichoic and lipoteichoic acids that neutralize the cell surface negative charge (32, 33), S. aureus has gained a significant mechanism to protect itself against cationic peptides. In addition to these structural changes in the bacterial surface or membrane, it was recently shown that staphylokinase, a protein commonly released by S. aureus, binds and counteracts the bactericidal effect of α-defensins (16). A high frequency of staphylokinase-producing strains among isolates from the upper respiratory tract of healthy individuals may indicate that this mechanism is important in colonization of these mucosal membranes containing antimicrobial peptides. In this study we noted that an additional level of protection against antimicrobial peptides, at least LL-37, may depend on the proteolytic activity secreted by S. aureus.
The mass spectroscopy analysis of the LL-37 incubation mixture with S. aureus metalloproteinase (aureolysin) and the V8 protease indicated that the peptide is efficiently cleaved by both enzymes. The cleavage occurred even in the presence of human plasma, suggesting that LL-37 proteolytic processing by S. aureus proteases may take place in vivo. Surprisingly, however, only in the case of aureolysin was the cleavage of LL-37 correlated with the loss of bactericidal activity. In stark contrast, the V8 protease did not destroy the bactericidal action of LL-37 against both gram-negative and gram-positive bacteria. This was apparently related to the generation of the LL-17-37 C-terminal fragment of the intact peptide, which was refractory to further degradation by the V8 protease. N-terminally truncated LL-37 derivatives with increased bactericidal activity were recently described as being generated in human sweat by endogenous serine proteinases (26). Interestingly, the V8-like protease is commonly produced by Staphylococcus epidermidis (12, 24), which constitutes a normal human bacterial flora. We suggest that this activity may augment the LL-37-dependent antibacterial potential of human skin, protecting this niche from colonization by other bacterial species, including S. aureus.
Although S. aureus would remain sensitive to the bactericidal activity exerted by the truncated LL-17-37 peptide, it could still in theory defend itself against this peptide through the action of using aureolysin. This metalloproteinase efficiently inactivates LL-37 bactericidal activity through the cleavage of the peptide bonds located in the C-terminal portion of the peptide, and it would still be able to cleave LL-17-37 because this truncated peptide was inactivated at the same rate as intact peptide. Our results also suggest that the level of secreted aureolysin correlated with levels of S. aureus susceptibility to bactericidal activity of both LL-37 and LL-17-37. Thus, both the Newman and SH1000 strains with relatively weak proteolytic activity were more susceptible to LL-17-37 than their respective sarA mutants that produce greater amounts of aureolysin. To learn whether loss of aureolysin production by S. aureus altered bacterial susceptibility to LL-37, we also examined an aureolysin-deficient mutant of strain Newman sarA. We found that this mutant displayed a level of LL-37 susceptibility that was identical to that of wild-type strain Newman (data not shown). Thus, loss of aureolysin production by an overproducing strain (Newman sarA) and a concomitant increased susceptibility to LL-37 lends further support to our hypothesis that the action of aureolysin on LL-37 is yet another mechanism by which S. aureus resists antimicrobial peptides.
A protease-dependent deterrence of host defense based on cationic peptides has been long suggested as an important mechanism in bacterial virulence (17), but it was only experimentally verified relatively recently (37). Thus, the P. aeruginosa elastase, Proteus mirabilis metalloprotease, and Enterococcus faecalis gelatinase can degrade and inactivate LL-37. In the case of P. aeruginosa infection, degradation of LL-37 in human wound fluid enhanced bacterial survival. Interestingly, aureolysin, elastase, and gelatinase belong to family M4 (http://merops.sanger.ac.uk) of structurally related metallopeptidases of similar specificity, which in addition encompasses enzymes of other well-know human pathogens, including Vibrio cholerae, Listeria monocytogenes, Legionella pneumophila, and Clostridium perfringens. This may indicate that antibacterial peptide inactivation through proteolysis is widespread among pathogenic bacteria.
Acknowledgments
We thank L. Pucko for help with preparation of the manuscript.
This work was supported by grants 3 604B 008 22 and 3 P05A 092 22 awarded to M.S. and K.W., respectively, by the State Committee for Scientific Research (KBN, Warsaw, Poland) and grants AI43316 (W.M.S.), AI37945 (W.M.S. and R. Lehrer), and HL 26148 (J.T. and J.P.) from the National Institutes of Health. W.M.S. and P.S. were supported by a Senior Research Career Scientist Award from the VA Medical Research Service and a scholarship from the Polish Science Foundation (FNP, Warsaw, Poland), respectively. J.P. and M.P. are recipients of Subsydium Profesorskie awards from the Foundation for Polish Science (FNP). Also, part of this work was carried out with financial support from the Commission of the European Communities, specific RTD programme “Quality of Life and Management of Living Resources,” QLRT-2001-01250, and “Novel non-antibiotic treatment of staphylococcal diseases.”
This work does not necessarily reflect the views of the Commission of the European Communities and in no way anticipates the Commission's future policy in this area.
REFERENCES
- 1.Agerberth, B., H. Gunne, J. Odeberg, P. Kogner, H. G. Boman, and G. H. Gudmundsson. 1995. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 92:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals, R., D. J. Weiner, A. D. Moscioni, R. L. Meegalla, and J. M. Wilson. 1999. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 67:6084-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J. Clin. Investig. 103:1113-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belaaouaj, A., R. McCarthy, M. Baumann, Z. Gao, T. J. Ley, S. N. Abraham, and S. D. Shapiro. 1998. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4:615-618. [DOI] [PubMed] [Google Scholar]
- 6.Boman, H. G. 2003. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med. 254:197-215. [DOI] [PubMed] [Google Scholar]
- 7.Brodgen, K. A., M. Ackermann, P. B. McCray, and B. F. Tack. 2003. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob. Agents 22:465-478. [DOI] [PubMed] [Google Scholar]
- 8.Chan, P. F., and S. J. Foster. 1998. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J. Bacteriol. 180:6232-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, A. M., J. Shi, A. Ceccarelli, Y. H. Kim, A. Park, and T. Ganz. 2001. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood 97:297-304. [DOI] [PubMed] [Google Scholar]
- 10.Dorschner, R. A., V. K. Pestonjamasp, S. Tamakuwala, T. Ohtake, J. Rudisill, V. Nizet, B. Agerberth, G. H. Gudmundsson, and R. L. Gallo. 2001. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Investig. Dermatol. 117:91-97. [DOI] [PubMed] [Google Scholar]
- 11.Drapeau, G. R. 1976. Protease from Staphylococcus aureus. Methods Enzymol. 45:469-475. [DOI] [PubMed] [Google Scholar]
- 12.Dubin, G., D. Chmiel, P. Mak, M. Rakwalska, M. Rzychon, and A. Dubin. 2001. Molecular cloning and biochemical characterisation of proteases from Staphylococcus epidermidis. Biol. Chem. 382:1575-1582. [DOI] [PubMed] [Google Scholar]
- 13.Frohm, M., B. Agerberth, G. Ahangari, M. Ståhle-Bäckdahl, S. Lidén, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 14.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 15.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin, T., M. Bokarewa, T. Foster, J. Mitchell, J. Higgins, and A. Tarkowski. 2004. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J. Immunol. 172:1169-1176. [DOI] [PubMed] [Google Scholar]
- 17.Juretic, D., H. C. Chen, J. H. Brown, J. L. Morell, R. W. Hendler, and H. V. Westerhoff. 1989. Magainin 2 amide and analogues. Antimicrobial activity, membrane depolarization and susceptibility to proteolysis. FEBS Lett. 249:219-223. [DOI] [PubMed] [Google Scholar]
- 18.Kluytmans, J., A. van Belkum, and H. Verbrough. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Lehrer, R. I., M. Rosenman, S. S. S. L. Harwig, R. Jackson, and P. Eisenhauer. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137:167-173. [DOI] [PubMed] [Google Scholar]
- 21.Liese, J. G., V. Jendrossek, A. Jansson, T. Petropoulou, S. Kloos, M. Gahr, and B. H. Belohradsky. 1996. Chronic granulomatous disease in adults. Lancet 347:220-223. [DOI] [PubMed] [Google Scholar]
- 22.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 23.McAleese, F. M., E. J. Walsh, M. Sieprawska, J. Potempa, and T. J. Foster. 1991. Loss of clumping factor B fibrinogen binding activity by Staphylococcus aureus involves cessation of transcription, shedding and cleavage by metalloprotease. J. Biol. Chem. 276:29969-29978. [DOI] [PubMed] [Google Scholar]
- 24.Moon, J. L., A. Banbula, A. Oleksy, J. A. Mayo, and J. Travis. 2001. Isolation and characterization of a highly specific serine endopeptidase from an oral strain of Staphylococcus epidermidis. Biol. Chem. 382:1095-1099. [DOI] [PubMed] [Google Scholar]
- 25.Mullarky, I. K., C. Su, N. Frieze, Y. H. Park, and L. M. Sordillo. 2001. Staphylococcus aureus agr genotypes with enterotoxin production capabilities can resist neutrophil bactericidal activity. Infect. Immun. 69:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami, M., B. Lopez-Garcia, M. Braff, R. A. Dorschner, and R. L. Gallo. 2004. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J. Immunol. 172:3070-3077. [DOI] [PubMed] [Google Scholar]
- 27.Nizet, V., T. Ohtake, and X. Lauth. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 28.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 29.Panyutich, A., J. Shi, P. L. Boutz, C. Zhao, and T. Ganz. 1997. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect. Immun. 65:978-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 31.Peschel, A., and L. V. Collins. 2001. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides 22:1651-1659. [DOI] [PubMed] [Google Scholar]
- 32.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 33.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. M. van Kessel, and J. A. G. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor mprF is based on modification of membrane lipids with L-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 35.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 36.Schittek, B., R. Hipfel, B. Sauer, J. Bauer, H. Kalbacher, S. Stevanovic, M. Schirle, K. Schroeder, N. Blin, F. Meier, G. Rassner, and C. Garbe. 2001. Dermacidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 12:1133-1137. [DOI] [PubMed] [Google Scholar]
- 37.Schmidtchen, A., I.-M. Frick, E. Andersson, H. Tapper, and L. Björck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 38.Scocchi, M., B. Skerlavaj, D. Romeo, and R. Gennaro. 1992. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur. J. Biochem. 209:589-595. [DOI] [PubMed] [Google Scholar]
- 39.Shafer, W. M., J. Pohl, V. C. Onunka, N. Bangalore, and J. Travis. 1991. Human lysosomal cathepsin G and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J. Biol. Chem. 266:112-116. [PubMed] [Google Scholar]
- 40.Shafer, W. M., F. Hubalek, M. Huang, and J. Pohl. 1996. The bactericidal activity of a synthetic peptide (CG 117-136) of human lysosomal cathepsin G is dependent on arginine content. Infect. Immun. 64:4842-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinnar, A. E., K. L. Butler, and H. J. Park. 2003. Cathelicidin family of antimicrobial peptides: proteolytic processing and protease resistance. Bioorg. Chem. 31:425-436. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen, O., K. Arnljots, J. B. Cowland, D. F. Bainton, and N. Borregaard. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796-2803. [PubMed] [Google Scholar]
- 43.Sorensen, O. E., P. Follin, A. H. Johnsen, J. Calafat, G. S. Tjabringa, P. S. Hiemstra, and N. Borregaard. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97:3951-3959. [DOI] [PubMed] [Google Scholar]
- 44.Tkalcevic, J., M. Novelli, M. Phylactides, J. P. Iredale, A. W. Segal, and J. Roes. 2000. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12:201-212. [DOI] [PubMed] [Google Scholar]
- 45.Travis, S. M., N. N. Anderson, W. R. Forsyth, C. Espiritu, B. D. Conway, E. P. Greenberg, P. B. McCray, Jr., R. I. Lehrer, M. J. Welsh, and B. F. Tack. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner, J., Y. Cho, N.-N. Dinh, A. J. Waring, and R. I. Lehrer. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh, T. R., and R. A. Howe. 2002. The prevalence and mechanisms of vancomycin resistance in Staphylococcus aureus. Annu. Rev. Microbiol. 56:657-675. [DOI] [PubMed] [Google Scholar]
- 48.Zanetti, M. 2003. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:1-10. [DOI] [PubMed] [Google Scholar]
- 49.Ziebandt, A. K., H. Weber, J. Rudolph, R. Schmid, D. Hoper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics 1:480-493. [DOI] [PubMed] [Google Scholar]
- 50.Zucht, H. D., J. Grabowsky, M. Schrader, C. Liepke, M. Jurgens, P. Schulz-Knappe, and W. G. Forssmann. 1998. Human beta-defensin-1: a urinary peptide present in variant molecular forms and its putative functional implication. Eur. J. Med. Res. 3:315-323. [PubMed] [Google Scholar]