Abstract
Hematopoiesis depends on a supportive microenvironment. Preclinical studies in mice have demonstrated that osteoblasts influence the development of blood cells, particularly erythrocytes, B lymphocytes, and neutrophils. However, it is unknown whether osteoblast numbers or function impact blood cell counts in humans. We tested the hypothesis that men with low BMD or greater BMD loss have decreased circulating erythrocytes and lymphocytes and increased myeloid cells. We performed a cross-sectional analysis and prospective analysis in the Osteoporotic Fractures in Men (MrOS), a multi-site longitudinal cohort study. 2571 community-dwelling men (≥65 years) who were able to walk without assistance, did not have a hip replacement or fracture and had complete blood counts (CBCs) at the third study visit were analyzed. Multivariable (MV)-adjusted logistic regression estimated odds of white blood cell subtypes (highest and lowest quintile vs middle), and anemia (clinically defined) associated with BMD by DXA scan (at visit 3), annualized percent BMD change (baseline to visit 3), and high BMD loss (>0.5%/year, from baseline to visit 3) at the femoral neck (FN) and total hip (TH). MV adjusted models included age, BMI, cancer history, smoking status, alcohol intake, corticosteroid use, self-reported health, thiazide use and physical activity. At visit 3 greater TH BMD loss (per standard deviation) was associated with increased odds of anemia, high neutrophils, and low lymphocytes. Annualized BMD loss of >0.5% was associated with increased odds of anemia, high neutrophils, and low lymphocytes. Similar results were observed for FN BMD regarding anemia and lymphocytes. We concluded that community-dwelling older men with declining hip BMD over about 7 years had increased risks of anemia, lower lymphocyte count, and higher neutrophil count, consistent with pre-clinical studies. Bone health and hematopoiesis may have greater interdependency than previously recognized.
Introduction
Accumulating molecular and clinical research evidence suggests that hematopoiesis and bone metabolism are interconnected. In the bone marrow, the production of blood cells depends upon a supportive microenvironment of hematopoietic and non-hematopoietic cells (1). Non-hematopoietic stromal cells include bone-forming osteoblasts and their precursors, which influence the differentiation of hematopoietic stem cells (HSCs) into mature hematopoietic lineages (2) (3).
Disorders of hematopoiesis, such as sickle cell anemia and thalassemia, can affect the skeletal system. Patients with sickle cell anemia have increased rates of skeletal complications, including osteopenia, osteoporosis, fractures, avascular necrosis, vertebral bone deformities, and bone and joint pain (4). Thalassemia, a disease of defective hemoglobin production and impaired erythropoiesis, is associated with chronic bone marrow hyperplasia, decreased bone mineral density and increased risk of fractures (5). In Italy, women with anemia had lower trabecular and cortical bone density than women without anemia, based on peripheral quantitative computed tomography (pQCT) near the time of blood draw; men with anemia also had reduced cortical bone density (6). In the Women’s Health Initiative, postmenopausal women with anemia had a 38–81% increased risk of incident fracture at any skeletal site, with the highest risk being at the hip, which is composed mainly of cortical bone, after controlling for multiple covariates (7).
Perturbations in bone metabolism can influence hematopoiesis. In pre-clinical studies, osteoblasts and their precursors have been implicated in erythroid, myeloid and lymphoid development (8–15). Signaling mediated through regulators of osteoblast function such as the parathyroid hormone receptor or Gsα, a stimulatory G protein α subunit, in osteoblast lineage cells regulate numbers of hematopoietic stem cells, B lymphocytes, and neutrophils in mice (11, 14–16). Interactions between osteoblasts and hematopoietic cells in humans have been relatively unexplored. We recently reported increases in circulating hematopoietic stem cells in postmenopausal women with osteoporosis during administration of a recombinant parathyroid hormone analog (teriparatide); one of the first demonstrations that bone-targeting medications can influence the human hematopoietic niche (17).
These studies support the possibility of a clinically relevant relationship between bone health and blood homeostasis. We tested the specific a priori hypotheses that low bone mass and loss of bone mineral density (BMD), assessed by dual-energy X-ray absorptiometry (DXA), are associated with altered numbers of circulating blood cells, specifically white blood cell (WBC) subtypes and hemoglobin, in a well-characterized longitudinal cohort of older, relatively healthy men. We hypothesized that low BMD and greater bone loss would relate to decreased erythrocytes and lymphocytes and increased cells of myeloid lineage. We also assessed the relationship of bone mass and BMD with platelet count in exploratory analyses.
Materials and Methods
Study population
The Osteoporotic Fractures in Men (MrOS) study is a prospective observational study of community dwelling older men recruited from six sites across the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA) as previously described (18, 19). The Institutional Review Board at each site approved the study and all participants provided written informed consent. In brief, eligible men were 65 years or older, able to walk without assistance, and without bilateral hip replacement surgery. Initially, 5994 men were enrolled from March 2000 to April 2002 and completed most baseline measurements. Of these men, 4681 completed the 3rd clinic visit between March 2007 and March 2009, with a mean of 6.9 years between visit 1 and visit 3. A subset of 3636 men had complete blood counts (CBC), including white blood cell (WBC) count with differential counts of WBC subtypes measured at visit 3. Two men with extreme total WBC values (over 50,000 total WBC count) were excluded from all analyses. Men with prevalent fracture (defined as self-reported fractures after age 50 but prior to MrOS study initiation as well as self-reported and adjudicated incident fractures from MrOS visit 1 to visit 3) at visit 3 (N= 1023) were excluded as they were felt to be a different and heterogeneous group from those without fracture, due to altered bone remodeling and different bone health treatments, potentially affecting results. Of the remaining 2611 men that were included in descriptive analysis, 25 men did not have DXA at visit 3. Therefore, 2586 men at visit 3 comprised the analytic study population (Figure 1). Four men were excluded from neutrophil analysis only due to blood counts suggestive of acute illness (total WBC over 17,000 with preponderance of neutrophils).
Figure 1.

Participant flowchart for the analytical cohort.
Pertinent participant characteristics
In MrOS, demographic information, medical history, and tobacco and alcohol use were ascertained at baseline and follow-up visits using standardized questionnaires and in-person participant interviews. The Physical Activity Scale for the Elderly (PASE) questionnaire was used to assess physical activity (20). At each visit, weight (kg) was measured on balance beams or digital scales while standing height (cm) was measured on Harpenden stadiometers, then used to calculate body mass index (BMI) as kg/m2. Medications were brought in by participants and verified by study staff. All medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA, USA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA, USA)(21).
Bone mineral density
Bone mineral density (BMD) was measured at baseline and visit 3 at the total hip (TH), femoral neck (FN) and lumbar spine (LS) using dual-energy X-ray absorptiometry (DXA) on Hologic QDR 4500-W densitometers (Hologic Inc., Waltham, MA, USA). A central quality-control lab, certification of DXA operators, and standardized procedures for scanning were used to ensure reproducibility of DXA measurements at all six clinical sites (19).
Complete blood counts
Fasting blood samples were collected at visit 3. Complete blood counts (CBC), and WBC subtypes were performed at local Quest Diagnostic laboratories for 5 study sites and Stanford Outreach for 1 site. Measurements were standardized across labs and included hemoglobin concentration and counts of platelet, total WBC, and WBC subtypes: neutrophils, lymphocytes and monocytes. We defined anemia as hemoglobin less than 12 g/dL and thrombocytopenia (or low platelet count) as less than 150,000/μL platelets. Each of the WBC subtypes were categorized by quintiles: “low” (1st, or lowest, quintile), “middle” (middle 2nd thru 4th quintiles), and “high” (highest quintile), based on their corresponding distributions in the entire analytic study population.
Statistical Analyses
The analytic study baseline for all analyses was visit 3 in MrOS. We compared pertinent participant characteristics and BMD at 3 skeletal sites (FN, TH and LS), according to counts of WBC subtypes, hemoglobin concentration, and platelet count, using ANOVA F-test for continuous variables, chi-square test for dichotomous variables, and Mantel-Haenszel test for 3-category variables. Participant characteristics included age, BMI, use of: testosterone, corticosteroids, hydrochlorothiazide, and bone medications, cancer history (including non-melanoma skin cancer), rheumatoid arthritis history, activity score, alcohol intake, smoking status.
Initially, we evaluated cross-sectionally at visit 3 the odds of an altered number of a hematopoietic cell type in association with BMD at the FN, TH and LS. We conducted two multivariable logistic regression models to estimate the odds ratio for each of the following: anemia, thrombocytopenia, and low and high counts of each WBC subtype, associated with one standard deviation lower BMD value. The first model adjusted for age and study site, the 2nd was the ‘full’ multivariable (MV)-adjusted model adjusted for the potential confounders or putative risk factors for bone loss: age, study site, BMI, smoking status, alcohol use, physical activity, cancer history, self-reported health corticosteroid use, and thiazide use.
Next, we conducted analogous logistic regression models to estimate the odds ratio for each of the following: anemia, thrombocytopenia, and low and high numbers of each WBC subtype, associated with one standard deviation decrease in annualized percent BMD loss at the FN, TH and LS. We calculated BMD loss (from visit 1 to visit 3) and converted it to an annualized percentage. In addition, we estimated the odds ratio for altered numbers of hematopoietic cell type associated with having “high BMD loss” compared to not having high BMD loss. High BMD loss was defined as an annualized decline in BMD of more than 0.5% from visit 1 to visit 3.
All p-values reported were two-sided, and all analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC, U.S.A.).
Results
Participant characteristics at visit 3
Our cohort consisted of 2,586 men with a mean age of 78.9 years at visit 3 and a mean follow-up time of 6.8 years since their first study visit. Participant characteristics are shown in Table 1a and 1b. Participants with anemia, high neutrophil count, thrombocytopenia, or high monocyte count, were slightly older and less active than those without. Participants with anemia and high neutrophil count also had slightly shorter height, lower alcohol intake, had a higher frequency of corticosteroid use, and less often had excellent or good self-reported health. Physical activity was lowest in the anemia group. A high proportion of participants with low lymphocytes had a history of cancer (46%), but age, height, physical activity and corticosteroid use was similar to those who had middle and high lymphocytes. Osteoporosis medication use was ≤ 6% in all groups across the cohort.
Table 1a.
Characteristics of MrOS participants by Hgb (normal vs anemia), and platelet counts (normal vs thrombocytopenia).
| Covariates | Hgb (N=2611) | Platelets (N=2590) | ||||
|---|---|---|---|---|---|---|
| Normal (N=2442) | Anemia (N=169) | p | Normal (N=2276) | Thrombocytopenia (N=314) | p | |
| Age | 78.8 (5.0) | 81.8 (5.5) | <0.001 | 78.8 (5.1) | 79.7 (5.1) | 0.007 |
| BMI | 27.2 (3.7) | 26.5 (4.1) | 0.024 | 27.2 (3.7) | 26.9 (4.1) | 0.29 |
| Height | 173.5 (6.8) | 171.7 (7.6) | 0.004 | 173.3 (6.8) | 173.8 (6.9) | 0.19 |
| Hx of Cancer | 922 (38%) | 69 (41%) | 0.43 | 857 (38%) | 126 (40%) | 0.40 |
| Health Status - Excellent/good | 2155 (88%) | 117 (69%) | <0.001 | 1990 (88%) | 265 (84%) | 0.12 |
| Smoking Status | 45 (2%) | 3 (2%) | 0.95 | 41 (2%) | 6 (2%) | 0.89 |
| Activity Score | 135.9 (67.7) | 108.6 (67.7) | <0.001 | 135.8 (68.1) | 124.9 (66.9) | 0.008 |
| RA | 170 (7%) | 23 (14%) | 0.001 | 173 (8%) | 18 (6%) | 0.23 |
| Alcohol Intake | 1569 (65%) | 93 (55%) | 0.012 | 1459 (64%) | 192 (62%) | 0.36 |
| Corticosteroid Use | 255 (11%) | 29 (17%) | 0.007 | 255 (11%) | 23 (7%) | 0.037 |
| Thiazide Use | 492 (20%) | 38 (23%) | 0.47 | 475 (21%) | 50 (16%) | 0.041 |
| Anti-OP Use | 122 (5%) | 7 (4%) | 0.62 | 109 (5%) | 19 (6%) | 0.33 |
| FN BMD | 0.78 (0.13) | 0.77 (0.14) | 0.18 | 0.78 (0.13) | 0.77 (0.12) | 0.24 |
| Total hip BMD | 0.96 (0.14) | 0.93 (0.16) | 0.006 | 0.96 (0.15) | 0.95 (0.14) | 0.17 |
| Lumbar spine BMD | 1.27 (0.31) | 1.31 (0.34) | 0.18 | 1.28 (0.30) | 1.28 (0.35) | 0.99 |
| FN BMD Δ | −0.34 (0.86) | −0.75 (1.08) | <0.001 | −0.35 (0.89) | −0.47 (0.88) | 0.027 |
| Total hip BMD Δ | −0.33 (0.70) | −0.87 (0.97) | <0.001 | −0.36 (0.72) | −0.43 (0.79) | 0.11 |
| LS BMD Δ | 1.22 (1.95) | 1.45 (3.74) | 0.43 | 1.22 (2.16) | 1.31 (1.73) | 0.39 |
| FN BMD Loss >0.5% | 920 (39%) | 94 (56%) | <0.001 | 869 (39%) | 136 (45%) | 0.06 |
| TH BMD Loss >0.5% | 801 (34%) | 100 (60%) | <0.001 | 768 (35%) | 124 (41%) | 0.036 |
| LS BMD Loss >0.5% | 367 (16%) | 30 (19%) | 0.26 | 360 (17%) | 35 (18%) | 0.037 |
Definition of anemia: Hgb < 12g/dL; thrombocytopenia: <150k/uL.
Table 1b.
Characteristics of MrOS participants by WBC subtypes.
| Covariates | Neutrophils (N=2602) | Lymphocytes (N=2606) | Monocytes (N=2606) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (N=522) | Middle (N=1560) | High (N=520) | p | Low (N=518) | Middle (N=1567) | High (N=521) | p | Low (N=519) | Middle (N=1566) | High (N=521) | p | |
| Age | 78.0 (4.7) | 79.0 (5.1) | 79.7 (5.2) | <0.001 | 79.3 (5.0) | 78.8 (5.1) | 79.1 (5.2) | 0.12 | 78.3 (4.9) | 78.8 (4.9) | 80.1 (5.6) | <0.001 |
| BMI | 26.4 (3.6) | 27.3 (3.8) | 27.3 (3.9) | <0.001 | 26.5 (3.8) | 27.1 (3.7) | 27.7 (4.0) | <0.001 | 26.5 (3.7) | 27.2 (3.7) | 27.4 (3.9) | <0.001 |
| Height | 173.8 (7.1) | 173.4 (6.7) | 172.7 (6.9) | 0.033 | 173.7 (7.1) | 173.3 (6.7) | 173.3 (6.9) | 0.42 | 173.7 (7.0) | 173.4 (6.7) | 172.9 (6.9) | 0.18 |
| Hx of Cancer | 212 (41%) | 597 (38%) | 177 (34%) | 0.029 | 237 (46%) | 569 (36%) | 181 (35%) | <0.001 | 202 (39%) | 602 (38%) | 183 (35%) | 0.21 |
| Health Status – Excellent/good | 467 (89%) | 1375 (88%) | 421 (81%) | <0.001 | 451 (87%) | 1371 (88%) | 445 (86%) | 0.43 | 456 (88%) | 1386 (89%) | 425 (82%) | 0.003 |
| Smoking Status | 4 (1%) | 22 (1%) | 22 (4%) | <0.001 | 4 (1%) | 28 (2%) | 16 (3%) | 0.006 | 8 (2%) | 25 (2%) | 15 (3%) | 0.11 |
| Activity Score | 144 (71) | 135 (67) | 124 (68) | <0.001 | 132 (66) | 136 (69) | 131 (69) | 0.25 | 139 (66) | 136 (69) | 123 (67) | <0.001 |
| RA | 34 (7%) | 115 (7%) | 44 (9%) | 0.23 | 36 (7%) | 123 (8%) | 34 (7%) | 0.80 | 36 (7%) | 107 (7%) | 50 (10%) | 0.10 |
| Alcohol Intake | 359 (69%) | 1003 (65%) | 294 (57%) | <0.001 | 328 (64%) | 1005 (65%) | 325 (63%) | 0.81 | 335 (65%) | 1002 (64%) | 321 (62%) | 0.23 |
| Corticosteroid Use | 42 (8%) | 150 (10%) | 91 (18%) | <0.001 | 53 (10%) | 178 (11%) | 53 (10%) | 0.97 | 42 (8%) | 150 (10%) | 92 (18%) | <0.001 |
| Thiazide Use | 91 (18%) | 297 (19%) | 140 (27%) | <0.001 | 100 (19%) | 316 (20%) | 112 (22%) | 0.38 | 84 (16%) | 332 (21%) | 112 (22%) | 0.033 |
| Anti-OP Use | 30 (6%) | 74 (5%) | 24 (5%) | 0.39 | 32 (6%) | 73 (5%) | 23 (4%) | 0.19 | 24 (5%) | 77 (5%) | 27 (5%) | 0.68 |
| FN BMD | 0.79 (0.13) | 0.79 (0.13) | 0.77 (0.13) | 0.016 | 0.78 (0.13) | 0.78 (0.13) | 0.79 (0.14) | 0.07 | 0.77 (0.13) | 0.79 (0.13) | 0.78 (0.14) | 0.17 |
| Total hip BMD | 0.95 (0.14) | 0.96 (0.15) | 0.95 (0.15) | 0.11 | 0.95 (0.15) | 0.96 (0.14) | 0.97 (0.15) | 0.047 | 0.94 (0.14) | 0.96 (0.15) | 0.95 (0.15) | 0.002 |
| Lumbar spine BMD | 1.27 (0.34) | 1.28 (0.30) | 1.28 (0.30) | 0.70 | 1.27 (0.32) | 1.27 (0.30) | 1.30 (0.32) | 0.22 | 1.24 (0.29) | 1.29 (0.31) | 1.29 (0.30) | 0.007 |
| FN BMD Δ | −0.32 (0.87) | −0.34 (0.85) | −0.51 (0.99) | <0.001 | −0.51 (0.89) | −0.34 (0.87) | −0.33 (0.92) | <0.001 | −0.40 (0.85) | −0.36 (0.87) | −0.37 (0.95) | 0.64 |
| Total hip BMD Δ | −0.32 (0.72) | −0.34 (0.70) | −0.48 (0.82) | <0.001 | −0.49 (0.80) | −0.34 (0.70) | −0.32 (0.75) | <0.001 | −0.41 (0.71) | −0.34 (0.72) | −0.40 (0.77) | 0.06 |
| LS BMD Δ | 1.23 (2.15) | 1.28 (2.15) | 1.09 (1.93) | 0.24 | 1.07 (2.13) | 1.25 (1.95) | 1.33 (2.50) | 0.12 | 1.02 (1.85) | 1.25 (1.95) | 1.38 (2.70) | 0.019 |
| FN BMD Loss >0.5% | 196 (38%) | 592 (39%) | 223 (44%) | 0.042 | 240 (48%) | 589 (39%) | 183 (36%) | <0.001 | 211 (42%) | 605 (40%) | 196 (39%) | 0.48 |
| TH BMD Loss >0.5% | 163 (32%) | 518 (34%) | 217 (43%) | <0.001 | 211 (42%) | 526 (35%) | 162 (32%) | <0.001 | 200 (39%) | 507 (33%) | 192 (39%) | 0.77 |
| LS BMD Loss >0.5% | 76 (15%) | 227 (15%) | 92 (19%) | 0.10 | 102 (21%) | 224 (15%) | 70 (14%) | 0.004 | 89 (18%) | 218 (15%) | 89 (18%) | 0.95 |
Definition of WBC subtypes: neutrophils: low <2705cells/uL, high > 4605 cells/uL; lymphocytes: low <1080 cells/uL, high >1885 cells/uL; monocytes: low <350 cells/uL, high >607 cells/uL. All cutpoints were based on quintile distribution with low group as the lowest (1st) quintile, middle group as 2–4th quintiles, and high group as the highest (5th) quintile.
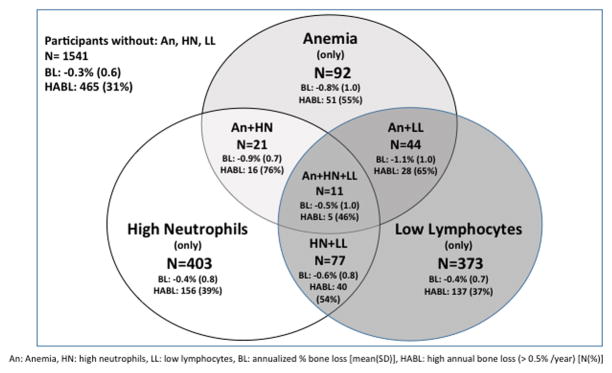
TH BMD was lower in participants with anemia (0.93 g/cm2) and low lymphocytes (0.95 g/cm2), while FN BMD was lower in participants with high neutrophil count (0.77 g/cm2) than in their respective comparison groups. The mean annualized BMD loss in our cohort was −0.37% at the TH and −0.36% at the FN. Participants with anemia, high neutrophil count, or low lymphocyte count had significantly more BMD loss at the TH (−0.87%, −0.48%, −0.49%) and FN (−0.75%, −0.51%, −0.51%) respectively and had a larger proportion with high BMD loss (> 0.5% annualized loss) at the TH and FN than those in the comparison groups. A relative minority of participants had co-existing anemia, high neutrophil count or low lymphocyte count as shown in Figure 2. TH BMD loss was higher when two of these conditions co-existed. Participants with thrombocytopenia had more BMD loss at the FN than those without (−0.47% vs. −0.35%). Overall there was BMD gain of 1.23% at the LS in our cohort. Participants with low monocyte count had lower TH BMD (0.94 g/cm2) and LS BMD (1.24 g/cm2) and less LS BMD gain than those with middle and high monocyte counts (1.02% vs. 1.25% & 1.38% respectively).
Figure 2.
Bone variables of interest at total hip in selected participant subgroups.
The number and characteristics of participants with anemia (An), high neutrophils (HN), and low lymphocytes (LL) and those in which these conditions co-exist are shown inside the diagram while participants with none of these conditions are shown outside of the diagram. BL indicates annualized percent bone loss [mean(SD)], HABL indicates high annual bone loss defined as > 0.5% per year [N(%)].
Risk of altered hematopoietic cell numbers by BMD
Age- and site-adjusted and multivariable-adjusted associations of absolute BMD values and hematopoietic cell counts are presented in Table 2. Lower BMD at the hip was associated with increased risk for high neutrophil count and low monocyte count. Age- and site-adjusted analyses revealed that every one standard deviation (SD) decrease in BMD at the FN was associated with decreased odds of having high lymphocyte count and increased odds of having high neutrophil and low monocyte count, while decrease in BMD at the TH was associated with decreased odds of having high lymphocyte count and increased odds of having low monocyte count. However, in MV-adjusted models only the association of monocytes with TH BMD remained significant (OR 1.15, 95% CI 1.02–1.29).
Table 2.
Associations of site-specific BMD* to hematopoietic cell types at visit 3, N=2571
| Anemia | Low Platelets | WBC subtype | ||||||
|---|---|---|---|---|---|---|---|---|
| neutrophils | lymphocytes | monocytes | ||||||
| low | high | low | high | low | high | |||
| Femoral neck | ||||||||
| Age & Site adj. | 1.02 (0.87, 1.20) | 1.06 (0.93, 1.19) | 1.01 (0.91, 1.12) | 1.14 (1.03, 1.27) | 1.01 (0.91, 1.12) | 0.88 (0.80, 0.97) | 1.13 (1.02, 1.25) | 0.99 (0.89, 1.09) |
| MTV adj.** | 0.92 (0.78, 1.09) | 1.02 (0.90, 1.17) | 0.93 (0.83, 1.04) | 1.12 (1.00, 1.25) | 0.95 (0.85, 1.07) | 0.92 (0.83, 1.02) | 1.05 (0.94, 1.18) | 0.98 (0.88, 1.09) |
| Total hip | ||||||||
| Age & Site adj. | 1.13 (0.96, 1.33) | 1.07 (0.94, 1.21) | 1.10 (0.99, 1.22) | 1.08 (0.97, 1.20) | 1.04 (0.94, 1.16) | 0.89 (0.80, 0.98) | 1.23 (1.11, 1.37) | 1.01 (0.91, 1.12) |
| MTV adj.** | 1.02 (0.86, 1.22) | 1.02 (0.89, 1.17) | 1.01 (0.90, 1,13) | 1.05 (0.93, 1.17) | 0.99 (0.88, 1.10) | 0.93 (0.83, 1.04) | 1.15 (1.02, 1.29) | 1.01 (0.90, 1.13) |
| Lumbar Spine | ||||||||
| Age & Site adj. | 0.89 (0.76, 1.04) | 1.03 (0.91, 1.17) | 1.04 (0.94, 1.15) | 0.98 (0.89, 1.09) | 1.00 (0.90, 1.11) | 0.93 (0.84, 1.03) | 1.14 (1.02, 1.27) | 1.04 (0.94, 1.16) |
| MTV adj.** | 0.86 (0.74, 1.00) | 1.01 (0.89, 1.15) | 0.99 (0.89, 1.10) | 0.98 (0.88, 1.09) | 0.96 (0.86, 1.07) | 0.96 (0.86, 1.07) | 1.08 (0.97, 1.21) | 1.05 (0.94, 1.17) |
OR and 95% CI per 1SD decrease of BMD
Adjusted for age, site, BMI, smoking status, alcohol use, physical activity, history of cancer, self-reported health, corticosteroid use, and thiazide use.
Risk of altered hematopoietic cell counts by change in BMD
Age- and site-adjusted and MV-adjusted associations of annualized percent change in BMD and hematopoietic cell counts are presented in Table 3. Loss of BMD at the FN and TH was associated with anemia, high neutrophils and low lymphocytes. In separate MV-adjusted models, greater annualized BMD loss (per 1-SD of annualized BMD loss), at the FN was associated with increased odds of anemia (OR 1.33; 95% CI 1.15–1.55), high neutrophil count (OR 1.16; 95% CI 1.04–1.29) and low lymphocyte count (OR 1.21; 95% CI 1.09–1.34). Greater BMD loss at the TH was associated with increased odds of anemia (OR 1.50; 95% CI 1.30–1.73) high neutrophil count (OR 1.13; 95% CI 1.01–1.25) and low lymphocyte count (OR 1.22; 95% CI 1.09–1.35).
Table 3.
Associations of site-specific annualized BMD % change* (visit 1 to visit 3) to hematopoietic cell types at visit 3, N=2571
| Anemia | Low Platelets | WBC subtype | ||||||
|---|---|---|---|---|---|---|---|---|
| neutrophils | lymphocytes | monocytes | ||||||
| Low | high | Low | high | Low | high | |||
| Femoral neck | ||||||||
| Age & Site adj. | 1.45 (1.26, 1.68) | 1.13 (1.00, 1.27) | 1.00 (0.90, 1.11) | 1.19 (1.07, 1.31) | 1.22 (1.10, 1.35) | 0.97 (0.87, 1.07) | 1.07 (0.97, 1.19) | 0.98 (0.89, 1.09) |
| MTV adj.** | 1.33 (1.15, 1.55) | 1.09 (0.96, 1.23) | 0.98 (0.88, 1.09) | 1.16 (1.04, 1.29) | 1.21 (1.09, 1.34) | 0.98 (0.88, 1.09) | 1.03 (0.93, 1.15) | 0.95 (0.85, 1.06) |
| Total hip | ||||||||
| Age & Site adj. | 1.60 (1.40, 1.83) | 1.07 (0.95, 1.21) | 1.02 (0.92, 1.14) | 1.16 (1.05, 1.28) | 1.23 (1.11, 1.36) | 0.96 (0.86, 1.07) | 1.14 (1.03, 1.26) | 1.03 (0.92, 1.14) |
| MTV adj.** | 1.50 (1.30, 1.73) | 1.02 (0.91, 1.16) | 1.00 (0.89, 1.12) | 1.13 (1.01, 1.25) | 1.22 (1.09, 1.35) | 0.98 (0.88, 1.09) | 1.09 (0.98, 1.21) | 0.99 (0.89, 1.10) |
| Lumbar Spine | ||||||||
| Age & Site adj. | 0.87 (0.76, 1.00) | 0.98 (0.87, 1.10) | 1.05 (0.94, 1.17) | 1.07 (0.95, 1.21) | 1.05 (0.94, 1.18) | 0.99 (0.90, 1.10) | 1.09 (0.97, 1.23) | 0.99 (0.89, 1.09) |
| MTV adj.** | 0.89 (0.77, 1.02) | 0.97 (0.86, 1.09) | 1.03 (0.92, 1.14) | 1.09 (0.97, 1.23) | 1.03 (0.92, 1.15) | 1.00 (0.90, 1.12) | 1.06 (0.94, 1.19) | 0.99 (0.89, 1.10) |
OR and 95% CI per 1SD decrease of annualized BMD % change
Adjusted for age, site, BMI, smoking status, alcohol use, physical activity, history of cancer, self-reported health corticosteroid use, and thiazide use.
Risk of accelerated BMD loss with hematopoietic cell counts
The relationship between high BMD loss (>0.5% annualized BMD decline) and hematopoietic cell counts are presented in Table 4. High BMD loss at the FN and TH was associated with increased odds of having anemia, high neutrophil count and low lymphocyte count. In MV-adjusted models, men with high BMD loss at the FN had an 79% increased odds of anemia (OR 1.79; 95% CI 1.28–2.49) and a 49% increased odds of low lymphocyte count (OR 1.49; 95% CI 1.21–1.84), while high BMD loss at the TH was associated with a 2.10–fold increased odds of anemia (OR 2.10; 95% CI 1.49–2.95), a 34% increased odds of high neutrophil count (OR 1.34; 95% CI 1.08–1.68), a 36% increased odds of low lymphocyte count (OR 1.36; 95% CI 1.09–1.70), and a 29% increased odds of low monocyte count (OR 1.29; 95% CI 1.04–1.61). In addition, high BMD loss at the LS was associated with a 33% decreased odds of thrombocytopenia (OR 0.67; 95% CI 0.45–0.98) and a 43% increased odds of high monocyte count (OR 1.43; 95% CI 1.06–1.91) in MV-adjusted models.
Table 4.
Associations of site-specific High BMD loss (>0.5% annualized) (visit 1 to visit 3) to hematopoietic cell types at visit 3, N=2571
| Anemia | Low Platelets | WBC subtype | ||||||
|---|---|---|---|---|---|---|---|---|
| Neutrophils | lymphocytes | monocytes | ||||||
| Low | High | Low | high | Low | high | |||
| Femoral neck | ||||||||
| Age & Site Adj. | 1.93 (1.40, 2.67) | 1.23 (0.96, 1.57) | 0.99 (0.81, 1.23) | 1.20 (0.97, 1.48) | 1.49 (1.21, 1.84) | 0.86 (0.69, 1.06) | 1.12 (0.91, 1.38) | 0.92 (0.74, 1.13) |
| MTV adj. | 1.79 (1.28, 2.49) | 1.17 (0.91, 1.50) | 0.97 (0.78, 1.20) | 1.16 (0.93, 1.43) | 1.49 (1.21, 1.84) | 0.88 (0.71, 1.09) | 1.06 (0.85, 1.31) | 0.87 (0.70, 1.08) |
| Total hip | ||||||||
| Age & Site Adj. | 2.37 (1.70, 3.30) | 1.23 (0.95, 1.58) | 0.98 (0.79, 1.22) | 1.40 (1.13, 1.72) | 1.38 (1.12, 1.71) | 0.86 (0.69, 1.07) | 1.38 (1.11, 1.70) | 1.12 (0.90, 1.39) |
| MTV adj. | 2.10 (1.49, 2.95) | 1.14 (0.88, 1.48) | 0.96 (0.76, 1.20) | 1.34 (1.08, 1.68) | 1.36 (1.09, 1.70) | 0.87 (0.69, 1.09) | 1.29 (1.04, 1.61) | 1.05 (0.84, 1.31) |
| Lumbar Spine | ||||||||
| Age & Site Adj. | 1.23 (0.80, 1.91) | 0.69 (0.47, 1.01) | 1.02 (0.76, 1.36) | 1.22 (0.92, 1.61) | 1.37 (1.04, 1.80) | 1.00 (0.74, 1.35) | 1.18 (0.89, 1.56) | 1.48 (1.11, 1.97) |
| MTV adj. | 1.18 (0.75, 1.84) | 0.67 (0.45, 0.98) | 0.96 (0.71, 1.29) | 1.23 (0.92, 1.64) | 1.30 (0.99, 1.71) | 1.00 (0.74, 1.36) | 1.10 (0.83, 1.46) | 1.43 (1.06, 1.91) |
MTV: Adjusted for age, site, BMI, smoking status, alcohol use, physical activity, history of cancer, self-reported health, corticosteroid use, and thiazide use.
Discussion
We evaluated the relationship of BMD, and BMD change, to numbers of various hematopoietic cell types, in a community-based cohort of older men enrolled in a prospective observational study. High BMD loss (defined as > 0.5% annual decline) at the hip, a site composed mainly of cortical bone, was associated with a higher risk of anemia. BMD loss at the hip was also associated with low lymphocytes and separately, high neutrophils. To our knowledge, this is the first study to report associations between bone density measurements by DXA and counts of white blood cell subtypes in humans. Our study is also one of the few to observe associations between bone density and anemia in elderly men. We found that the rate of BMD loss, and less so low absolute BMD, relates to altered hematopoiesis. Further study of the relationship between the skeletal system and hematopoiesis and their reciprocity may have important clinical implications, since hematopoietic cell counts would be simple and relatively inexpensive adjuncts to bone health assessment.
Overall, annualized loss of BMD appeared to correlate better with hematopoietic cell counts than absolute BMD. Absolute bone density is a cross-sectional snapshot of bone health while annualized BMD loss measures a trend across time. Further studies are needed to evaluate why the rate of bone density loss may be a better clinical marker of bone health as it relates to hematopoiesis.
We observed that high BMD loss at the hip was associated with a greater than 2-fold risk of anemia. These observations are consistent with the findings for cortical bone density and anemia in men participating in the In-Chianti study (6). While we did not find that reduced absolute BMD is associated with anemia in cross-sectional analysis, it may be that the DXA scan we used is less sensitive at uncovering differences in bone density than the pQCT used in the Italian study. It is plausible that deteriorating bone would provide a less supportive environment for hematopoiesis, resulting in anemia, however we cannot categorically determine whether bone is affecting hematopoiesis or vice versa from the current study. Alternatively, anemia may be a marker for disability and lower muscle strength (22) which are known to be associated with decreased BMD (23), perhaps due to decreased mechanical stimuli on bone. In our study, the relationship between BMD loss and anemia persisted despite controlling for physical activity and BMI, which are frequently linked to disability and lower muscle mass (24–26), but the possibility of residual confounding from decreased muscle strength in participants with anemia cannot be completely excluded.
We also observed that high BMD loss was associated with increased neutrophil counts, decreased monocyte counts and decreased lymphocyte counts. With aging there is a well-documented increase in myeloid cells accompanied by a decline in lymphocytes. Several mechanisms have been proposed, including inflammation, intrinsic bias of hematopoietic stem cells favoring myeloid over lymphoid lineage commitment, and extrinsic effects of an aging bone marrow microenvironment (27–30). No previous studies have evaluated counts WBC subtypes and clinical assessments of bone health. WBC subtype counts can be considered measures of inflammation and the inflammatory biomarkers IL-6 and CRP have been associated with lower bone mineral density (31) and bone loss (32). Older populations are known to have increased co-morbidities and multi-morbidity has been associated with IL-6 and CRP levels specifically in the MIDUS longitudinal study (33). One potential explanation for our findings therefore could be increased co-morbidity with associated increases in inflammation and bone loss. Increasing levels of co-morbidity are related to lower self-reported health (34), which was associated with high bone loss (data not shown), and neutrophil and monocyte counts in our study. We found that high bone loss was associated with high neutrophils and low monocytes independent of self-reported health. The increase in neutrophils may be related to the chronic inflammation that occurs with aging regardless of general health or co-morbidities, but it is unclear why neutrophils and monocytes, which are both myeloid derived cells, have differing relationships with bone loss, although cells of the osteoblastic lineage might affect differentiation of neutrophils and monocytes differently (35).
In animal models, cells at distinct stages of maturation along the osteoblast lineage interact with specific populations of hematopoietic cells (35). For example, mesenchymal stem cells (MSCs) constitute a critical niche for HSCs (13, 36–38). In contrast, B lymphocyte and erythrocyte lineages are dependent upon osteoblast progenitors (8–10, 13–15) while terminally differentiated osteoblasts and osteocytes influence myelopoiesis (11). Based on mouse models of altered bone metabolism (9, 11, 14, 15), we predicted that poor bone health would lead to decreased erythrocytes and lymphocytes and increased cells of myeloid lineage such as neutrophils. Overall, our results are consistent with these predictions. Whether B lymphocytes, erythrocytes and myeloid cells are supported by cells of the osteoblast lineage at distinct stages of differentiation in humans has not been determined. As noted in Figure 2, there was relatively little overlap between men with decreased lymphocytes, decreased erythrocytes or increased myeloid cells. We therefore explored descriptively whether greater BMD loss was more prevalent in men with more than one altered hematopoietic cell count. This hypothesis was not tested further because of limited sample size of men with multiple altered cell lines. However, we suspect that no one unifying mechanism exists to explain the observed associations of BMD loss and hematopoiesis.
Our study has several strengths and limitations. The analyses were performed in a large prospective observational cohort of community-dwelling older men. The cohort was multi-site across the U.S. and well characterized in detail, from extensive data collection using standardized questionnaires, interviews, exams, DXA, blood draws and assay methods. However, the relevance to other racial/ethnic groups or women is as yet unknown due to the predominantly Caucasian (about 90%) and exclusively male cohort. While there is intra-individual variability in blood counts, the large sample size of the study population should temper this. Because CBC was measured only at visit 3, temporality of the development of altered blood counts and associations between change in BMD and change in blood cell counts could not be achieved. We did not have information about acute infection or illness at the time of blood draw, and while we did exclude participants who had patterns suggestive of acute illness it is possible that some of the cell populations analyzed were affected in some instances. Performing multiple comparisons can increase the chance findings statistically (false positives), so we were careful not to make conclusions based on findings that were not consistent with a priori hypotheses or were not consistent in our analyses. Our results revealed similar patterns of associations at both hip sites, and agreed with previous literature. Spine BMD generally increased in our study, which could be due to artifacts such as arthritis or aortic calcification; this may have biased our results towards the null, making it hard to detect small differences at this site. We did not have information on the type of anemia or testosterone level at visit 3 for our participants, which would have been informative for that analysis.
In summary, older Caucasian men with BMD loss had an increased risk of anemia, low lymphocyte count, or high neutrophil count; the directions of these associations are consistent with findings from animal studies. High BMD loss, particularly at the hip, were more strongly related to these altered blood counts than absolute BMD, suggesting that rate of BMD loss influences hematopoiesis. Future translational studies are warranted to confirm our findings and to further map the bone-blood interaction and potential clinical implications.
Acknowledgments
Author’s Roles: Study design: RV, JYW, JL and ARH. Study conduct: EO and SC. Data collection: EO and SC. Data analysis: LYL. Data interpretation: RV, JYW, JL and ARH. Drafting manuscript: RV. Revising manuscript content: all authors. Approving final version of manuscript: All authors. RV and JYW take responsibility for the integrity of the data analysis.
Funding/Support:
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. RV was supported by NIDDK grant T32 DK007217. JYW was supported by NIH grant DP2 OD008466.
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts to report.
Contributor Information
Rodrigo J. Valderrábano, Division of Endocrinology, Stanford University School of Medicine, Stanford, CA.
Li-Yung Lui, San Francisco Coordinating Center, California Pacific Medical Center, San Francisco, CA.
Jennifer Lee, Division of Endocrinology, Stanford University School of Medicine, Stanford, CA. Palo Alto Veteran Affairs Health Care System, Palo Alto, CA.
Steven R. Cummings, San Francisco Coordinating Center, California Pacific Medical Center, San Francisco, CA.
Eric S. Orwoll, Department of Medicine, Bone and Mineral Unit, Oregon Health and Science University, Portland, OR.
Andrew R. Hoffman, Division of Endocrinology, Stanford University School of Medicine, Stanford, CA. Palo Alto Veteran Affairs Health Care System, Palo Alto, CA.
Joy Y. Wu, Division of Endocrinology, Stanford University School of Medicine, Stanford, CA.
References
- 1.Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–37. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- 2.Wu JY, Kronenberg HM. Bone Marrow Hematopoietic Niches. Osteoimmunology: Interactions of the Immune and Skeletal Systems. 2015:103–19. [Google Scholar]
- 3.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osunkwo I. An update on the recent literature on sickle cell bone disease. Curr Opin Endocrinol Diabetes Obes. 2013;20(6):539–46. doi: 10.1097/01.med.0000436192.25846.0b. [DOI] [PubMed] [Google Scholar]
- 5.Vogiatzi MG, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Olivieri N, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res. 2009;24(3):543–57. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesari M, Pahor M, Lauretani F, Penninx BW, Bartali B, Russo R, et al. Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporos Int. 2005;16(6):691–9. doi: 10.1007/s00198-004-1739-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Thomson CA, Aickin M, Nicholas JS, Van Wyck D, Lewis CE, et al. The relationship between incidence of fractures and anemia in older multiethnic women. J Am Geriatr Soc. 2010;58(12):2337–44. doi: 10.1111/j.1532-5415.2010.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–64. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 9.Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149(1):63–74. doi: 10.1016/j.cell.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schepers K, Hsiao EC, Garg T, Scott MJ, Passegue E. Activated Gs signaling in osteoblastic cells alters the hematopoietic stem cell niche in mice. Blood. 2012;120(17):3425–35. doi: 10.1182/blood-2011-11-395418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulzele K, Krause DS, Panaroni C, Saini V, Barry KJ, Liu X, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2013;121(6):930–9. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu VW, Saez B, Cook C, Lotinun S, Pardo-Saganta A, Wang YH, et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J Exp Med. 2015;212(5):759–74. doi: 10.1084/jem.20141843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panaroni C, Fulzele K, Saini V, Chubb R, Pajevic PD, Wu JY. PTH Signaling in Osteoprogenitors Is Essential for B-Lymphocyte Differentiation and Mobilization. J Bone Miner Res. 2015;30(12):2273–86. doi: 10.1002/jbmr.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–81. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 17.Yu EW, Kumbhani R, Siwila-Sackman E, DeLelys M, Preffer FI, Leder BZ, et al. Teriparatide (PTH 1–34) treatment increases peripheral hematopoietic stem cells in postmenopausal women. J Bone Miner Res. 2014;29(6):1380–6. doi: 10.1002/jbmr.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary clinical trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Pahor M, Chrischilles E, Guralnik J, Brown S, Wallace R, Carbonin P. Drug data coding and analysis in epidemiologic studies. European journal of epidemiology. 1994;10(4):405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 22.Penninx BW, Pahor M, Cesari M, Corsi AM, Woodman RC, Bandinelli S, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52(5):719–24. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 23.Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12(10):1547–51. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease C, Prevention. Physical activity among adults with a disability--United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(39):1021–4. [PubMed] [Google Scholar]
- 25.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mechanisms of ageing and development. 1999;107(2):123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 26.Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up study of NHANES I. JAMA. 1994;271(14):1093–8. [PubMed] [Google Scholar]
- 27.Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol. 2011;29(10):928–33. doi: 10.1038/nbt.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108(50):20012–7. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 30.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6(3):265–78. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005;16(10):1263–71. doi: 10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- 32.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86(5):2032–42. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 33.Friedman EM, Ryff CD. Living well with medical comorbidities: a biopsychosocial perspective. J Gerontol B Psychol Sci Soc Sci. 2012;67(5):535–44. doi: 10.1093/geronb/gbr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorem GF, Schirmer H, Emaus N. Health Impact Index. Development and Validation of a Method for Classifying Comorbid Disease Measured against Self-Reported Health. PLoS One. 2016;11(2):e0148830. doi: 10.1371/journal.pone.0148830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panaroni C, Tzeng YS, Saeed H, Wu JY. Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches. Curr Osteoporos Rep. 2014;12(1):22–32. doi: 10.1007/s11914-014-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]