Abstract
Epigenetic drugs, such as DNA methylation inhibitors (DNMTi) or histone deacetylase inhibitors (HDACi), are approved in monotherapy for cancer treatment. These drugs reprogram gene expression profiles, reactivate tumor suppressor genes (TSG) producing cancer cell differentiation and apoptosis. Epigenetic drugs have been shown to synergize with other epigenetic drugs or various anticancer drugs. To discover new molecular entities that enhance epigenetic therapy, we performed a high-throughput screening using FDA-approved libraries in combination with DNMTi or HDACi. As a screening model, we used YB5 system, a human colon cancer cell line, which contains an epigenetically silenced CMV-GFP locus, mimicking TSG silencing in cancer. CMV-GFP reactivation is triggered by DNMTi or HDACi and responds synergistically to DNMTi/HDACi combination, which phenocopies TSG reactivation upon epigenetic therapy. GFP fluorescence was used as a quantitative readout for epigenetic activity. We discovered that 45 FDA-approved drugs (4% of all drugs tested) in our FDA-approved libraries enhanced DNMTi and HDACi activity, mainly belonging to anticancer and antiarrhythmic drug classes. Transcriptome analysis revealed that combination of decitabine (DNMTi) with the antiarrhythmic proscillaridin A, produced profound gene expression reprogramming which was associated with down-regulation of 153 epigenetic regulators, including two known oncogenes in colon cancer (SYMD3 and KDM8). Also, we identified about 85 FDA-approved drugs that antagonized DNMTi and HDACi activity through cytotoxic mechanisms, suggesting detrimental drug interactions for patients undergoing epigenetic therapy. Overall, our drug screening identified new combinations of epigenetic and FDA-approved drugs, which can be rapidly implemented into clinical trials.
Keywords: Drug repurposing, Drug combination, High-throughput drug screening, Epigenetic therapy
Introduction
Epigenetic alterations play major roles in establishing and maintaining aberrant gene expression profiles in cancer cells. Epigenetics is defined by molecular mechanisms, such as DNA methylation, histone modifications, nucleosome occupancy and miRNA, that are involved in heritable gene expression patterns (1). Advances in epigenetics have revealed the importance and the diversity of epigenetic proteins encompassing more than 300 enzymes and regulators (2,3). In cancer, the epigenome is characterized by hundreds of epigenetic abnormalities occurring at DNA methylation and chromatin levels. These epigenetic aberrations are caused by altered expression or mutations in epigenetic enzymes and regulators, which are implicated in unlimited cell proliferation, loss of cell differentiation, resistance to apoptosis and metastasis (1,4,5). A well-known example of epigenetic reprogramming in cancer cells is the silencing of tumor suppressor genes (TSG) and the activation of oncogenes (5). TSG silencing involves promoter DNA hypermethylation and repressive chromatin marks catalyzed by DNA methyltransferases (DNMTs) and by histone deacetylases (HDAC), respectively (5,6).
Targeting epigenetic alterations in cancer cells is a new frontier in drug discovery, which is referred to as epigenetic therapy. The efficacy of epigenetic drugs, such as DNMT inhibitors (DNMTi) azacitidine and decitabine or HDAC inhibitors (HDACi) vorinostat, romidespin and belinostat, has led to their approval as monotherapy, in hematological malignancies (5). These drugs induce gene expression changes in cancer cells, which results in cancer cell differentiation, apoptosis and recognition by the immune system (5). Epigenetic drugs, when used in monotherapy, produce complete responses in a subset of patients and remissions are generally of short duration (7,8). Strategies are being explored to improve the efficacy of epigenetic drugs by increasing epigenetic drug specificity and using combination strategies.
It is well known that epigenetic drugs can produce synergistic responses when used in combination with other epigenetic drugs, chemotherapy, immunotherapy or targeted drugs. Early preclinical studies have focused on the combination of DNMTi and HDACi which produced concomitant synergistic reactivation of TSG and anticancer effects (9). The goal of this approach is to relieve epigenetic silencing mechanisms associated with TSG silencing to produce a robust epigenetic reprogramming in cancer cells. Several combinations of DNMTi and HDACi have been identified in preclinical studies and are currently tested in clinical trials (10,11). With the development of new epigenetic agents targeting histone methyltransferases, histone demethylases or bromodomains, novel synergistic combinations are being explored with DNMTi and/or HDACi (12–15). The combination of epigenetic drugs with standard chemotherapy, targeted drugs or immunotherapy is also a promising avenue. The rationale behind these combinatorial treatments is to use epigenetic drugs to lower apoptotic threshold, reverse drug resistance, or induce immunological responses (16–19). For example, synergistic combinations were reported with chemotherapeutic drugs (retinoic acids, cisplatin, carboplatin, and clofarabine); with targeted drugs such as erlotinib; and with immunotherapy (interleukin-2) (20–26). The potential of epigenetic drugs to synergize with a variety of anticancer approaches may be related to the high number of molecular targets associated with epigenetic dysregulation in carcinogenesis (3).
In multifactorial diseases such as cancer, there is strong rationale for the development of drug combinations over single-drug approaches, where combinational therapies are likely to be more effective than monotherapy (27). High-throughput screenings (HTS) based on phenotypic (cytotoxicity) or target-based assays are commonly used in drug discovery. Despite the availability of HTS approaches that can be used in combinatorial setting, the majority of HTS studies involve single drug screening, potentially missing the discovery of positive synergistic drug interactions. Innovative technologies suitable for combinatorial HTS are needed to accelerate drug development.
A promising strategy for drug development is to screen approved drugs for novel indications, a process called drug repositioning or repurposing (28). FDA-approved drug libraries can be used in combinatorial HTS which allows screening a wide diversity of molecular combinations. Conventional drug discovery takes an average of 13 years prior to approval and as much as US$ 1.8 billion. Drug repositioning may lead to faster approval and at lower costs, since FDA-approved drugs have successfully passed costly toxicological studies and their pharmacodynamic and pharmacokinetic properties are well-characterized.
To perform a combinatorial epigenetic HTS, we used YB5 cell-based system (29,30). This human colon cancer cell line is derived from SW48 cells, in which a single transgene was inserted containing green fluorescent protein (GFP) gene. GFP is driven by a cytomegalovirus (CMV) promoter, which is epigenetically silenced by promoter DNA hypermethylation and condensed chromatin marked by H3K27 trimethylation and loss of H3K9 acetylation (29,30). These epigenetic marks result in a stable silencing of GFP expression in 99.9% of YB5 cells. We previously demonstrated that DNMTi and HDACi trigger GFP expression, which phenocopies endogenous TSG reactivation induced by epigenetic therapy (29,30). After DNMT inhibition, GFP expression was dependent on both promoter DNA demethylation and chromatin gain of active marks (29). HDACi also reactivate GFP expression by switching chromatin repressive signals into active marks in the promoter region without changing DNA methylation (30). YB5 system is a model suitable for combinatorial HTS since GFP expression responds synergistically to the combination of DNMTi and HDACi, to a similar extent as endogenous TSG (29). Recently, YB5 cell-based system was used in a HTS to discover new epigenetic drugs among FDA-approved drug libraries in monotherapy. We have reported that a dozen of FDA-approved drugs exhibited unsuspected epigenetic and anticancer effects with promising repositioning potential (31).
Here, we used YB5 cells to screen FDA-approved drug libraries in sequential or simultaneous combination with DNMTi decitabine and HDACi trichostatin A (TSA) (32). This epigenetic HTS revealed new combinations between DNMTi or HDACi and FDA-approved drugs that can be rapidly tested into new clinical trials. We described specifically that one of these combinations produced a profound transcriptome cell reprogramming by targeting the down-regulation of epigenetic regulators with oncogenic activities in colon cancer. In addition, the results also revealed a list of FDA-approved drugs that antagonize DNMTi and HDACi activity, whose interaction should be carefully considered in patients treated with these epigenetic drugs.
Materials and Methods
Cell-based drug screening system
YB5 cell-based system was used as a platform for epigenetic drug screening. YB5 cells are derived from human colon cancer cell line SW48, as previously described (29,30). YB5 cells were authenticated at MD Anderson Cancer Center genomic core facility by DNA fingerprinting prior drug screening and validation experiments. YB5 cells have a single insertion of a DNA hypermethylated cytomegalovirus (CMV) promoter driving green fluorescent protein (GFP) gene. GFP expression is silenced in >99.9% of YB5 cells because CMV promoter has more than 90% cytosine DNA methylation, which is embedded into repressive chromatin with histone deacetylation, histone methylation repressive marks and nucleosome density around the transcriptional start site. YB5 cell line is cultured in L-15 medium supplemented with 10% fetal bovine serum and grown in log phase in 1% CO2 atmosphere, as previously described (29–31).
FDA-approved drug libraries and drug treatments
FDA-approved drug libraries were purchased at MS Discovery (US Drug collection library with 1040 drugs) or obtained from the NCI-Developmental Therapeutics Program (Combo Plate 3948/99 containing 77 drugs, NCI Oncology Drug sets with 89 drugs). A total of 1,206 drugs were screened. Because of redundancy between drug libraries, 1,118 unique FDA-approved drugs were screened in our libraries (Supplementary Fig. S1). Drugs are dissolved in DMSO in 96 well-plate format and were kept at −80°C before use. YB5 cells were grown in log-phase in 96 well-plates and treated with drug libraries (n≥1) with 3 different schedules (Fig. 1A). First schedule was a sequential combination with decitabine at 50 nM for 72h followed by treatment with FDA-approved drugs at 50 µM for 24h prior to flow cytometry analysis. The second HTS was a simultaneous combination with 50 nM decitabine concomitantly with FDA-approved drugs at 10 µM for 72h followed by 24h without drug treatment prior to flow cytometry analysis. The third HTS was a sequential combination of FDA-approved drugs at 10 µM for 72h followed by HDACi trichostatin A (TSA) at 0.2 µM for 24h prior flow cytometry analysis. Drugs and media were replaced every day. The results were compared with single drug screens with FDA-approved drug libraries (10 µM for 72h or 24h at 50 µM prior to flow cytometry analysis), as previously reported (31). Each experimental 96-well plate contained 80 different drug treatments. In addition, we included in each 96-well plate 16 controls for untreated cells (4 wells), for decitabine in monotherapy (50 nM, 72h, 4 wells), for TSA in monotherapy (0.2 µM, 24h, 4 wells), and 4 wells of sequential combination of decitabine (50 nM, 72h) followed by TSA (0.2 µM, 24h). For validation purposes, drugs were purchased at Sigma-Aldrich.
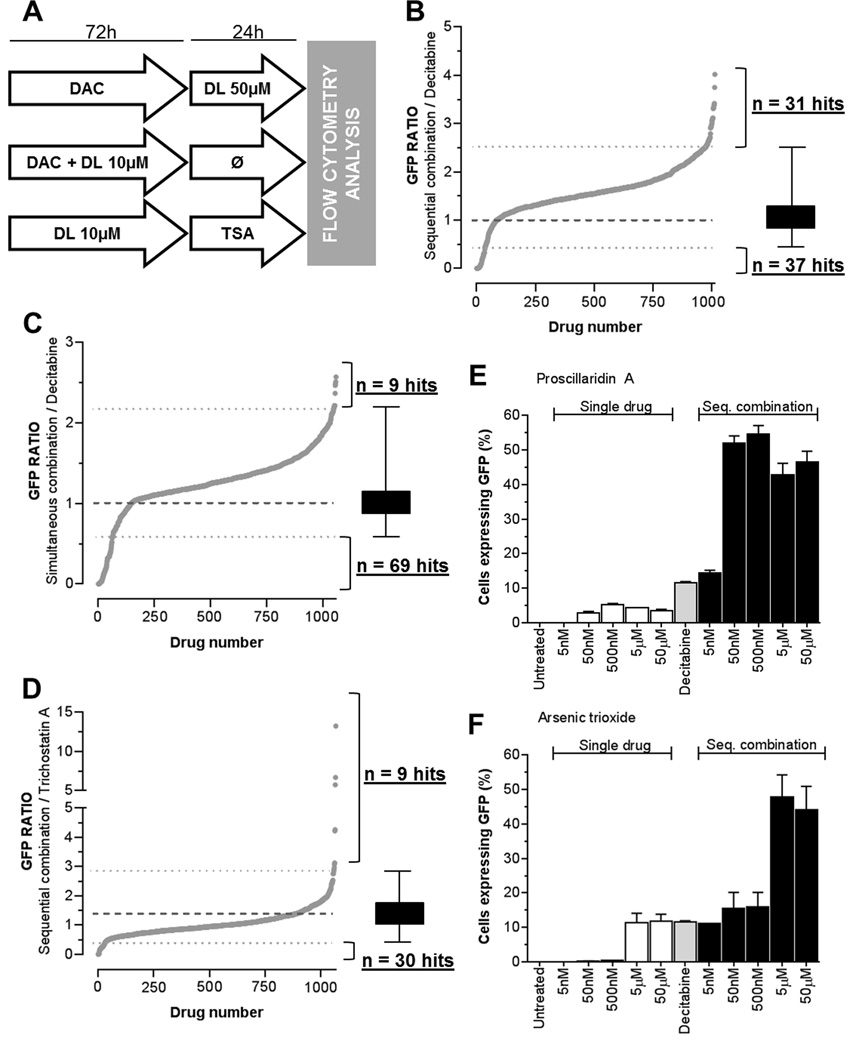
Figure 1.
Combinatorial epigenetic HTS using FDA-approved libraries with DNMTi or HDACi. A, Design of HTS testing the combination of FDA-approved drug libraries (DL, time of treatment and doses are indicated) with DNMTi (decitabine, DAC at 50 nM for 72h) and HDACi (TSA at 0.2 µM for 24h). GFP fluorescence was used as a quantitative readout for epigenetic effect and was measured by high-throughput flow cytometry (n≤1). GFP ratios were calculated by the percentage of GFP positive cells obtained after the combinatorial treatment divided by the percentage of GFP positive cells induced by either decitabine or TSA. GFP ratio of 1 marks the baseline epigenetic effect induced either by DAC or TSA alone (dotted line in bold type). Threshold for synergistic or antagonistic interaction was determined by the GFP ratios average value +/− 3 standard deviations for each HTS condition (dotted lines). GFP ratio and number of hits are shown for sequential treatment with decitabine (B), for simultaneous treatment with decitabine (C), and for sequential treatment with TSA (D). Validation experiments were performed using YB5 cells in sequential combination with decitabine (50nM, 72h) and a 24h treatment with selected hits such as proscillaridin A (E) and arsenic trioxide (F) at the doses indicated on the graph (n=3).
Flow cytometry for epigenetic GFP reactivation and data analysis
After drug treatments, YB5 cells were trypsinized in 96-well plates for 15 minutes and resuspended in L-15 media containing propidium iodide (PI) to stain for dead cells. Fluorescence obtained by GFP expression and PI staining were measured by flow cytometry using BD LSR II flow cytometer with a 96 well-plate adapter. A total of 10,000 cells were analyzed per well. Validations were performed using Millipore Guava flow cytometer (EMD, Millipore). All autofluorescent drugs (such as antimalarials) were removed from the analysis because autofluorescence creates a false positive signal that bleaches into GFP channel. Autofluoresent drugs were defined as those drugs producing more than 8% of the cells positive for both PI and GFP fluorescence, as previously described (31). All screening data were expressed as a GFP ratio which was calculated as follows: GFP ratio = GFP fluorescence of drug combination/GFP fluorescence of epigenetic drug alone (either decitabine or TSA). GFP signals of decitabine or TSA were obtained in the control wells in the same 96-well plate as the GFP signals obtained for the combination. PI fluorescence was plotted against GFP ratios to evaluate the effects of cytotoxic drugs in combination with epigenetic drugs.
Transcriptome, and gene ontology pathway analysis
For transcriptome analysis by RNA-sequencing, YB5 cells were treated with decitabine at 100 nM for 48h with or without 50 nM proscillaridin A for an additional 48h. RNA was isolated using Rneasy Mini Kit (Qiagen). Strand-specific RNA libraries were generated from 1µg of RNA using TruSeq stranded total RNA with Ribo-Zero Gold (Illumina). Sequencing was performed at Fox Chase Cancer Center genome facility (Temple University) using single end reads (50bp, average 50 million reads per sample) on the HiSeq2500 platform from Illumina. Sequenced reads were aligned to the hg19 genome assembly using TopHat2. Expression level and fold change of each treatment group was evaluated using edgeR (33). Differentially expressed genes were defined by at least two-fold change compared to control and a q-value lower than 0.1. Gene ontology analyses were performed using Metascape (34). RNA sequencing data were deposited in the Gene Expression Omnibus (GEO) database with the accession number GSE89154.
Statistical analysis
One-way ANOVA was used for statistical analysis and P value was evaluated by the Tukey–Kramer Multiple Comparison Test. Statistics and graphs were made using GraphPad Prism® software.
Results
Combinatorial drug screening results
YB5 system was used to screen among 1,118 FDA-approved drugs to discover combinations that enhances GFP reactivation induced by epigenetic drugs decitabine and TSA. Three combinations were tested: 1) sequential combination with decitabine at 50 nM for 72h, followed by 24h exposure with FDA-approved drugs at 50 µM before flow cytometry analysis; 2) simultaneous combination for 72h with decitabine (50 nM) and FDA-approved drugs (10 µM) followed by 24h without treatment prior to analysis; and 3) sequential combination with TSA, in which YB5 cells were treated with FDA-approved drugs at 10 µM for 72h followed by 24h exposure to TSA at 200 nM before flow cytometry analysis (Fig. 1A). Dose and schedule chosen for decitabine and TSA treatments were selected to allow the detection of synergistic interactions. Low doses of each epigenetic drug alone produced GFP reactivation in less than 20% of YB5 cells. Their sequential combination produced synergistic GFP reactivation in around 60% of the YB5 cells (Supplementary Fig. S2A). Sequential or simultaneous combinations with decitabine have been chosen to discover drugs that enhance its activity similarly to HDACi (9). Sequential combination with HDACi was performed to discover new drugs that produce synergistic interaction such as the one observed with decitabine (9). In this case, cells were treated with drug libraries followed by TSA. This sequence order was chosen since decitabine treatment followed by TSA produces synergistic responses (9). The opposite sequence order was not selected since HDACi followed by decitabine produced antagonistic effects due to HDACi-induced cell cycle arrest, which blocked the activity of S-phase specific drugs such as decitabine (35,36). For each drug combination, a GFP ratio was calculated using the percentage of YB5 cells expressing GFP in the combinatorial treatment divided by the percentage of YB5 cells expressing GFP after decitabine or TSA in monotherapy. Positive hits (synergistic) or negative hits (antagonistic) were determined as drug combinations producing a GFP ratio above or below GFP ratio of 1 plus or minus 3 standard deviations, respectively (Fig 1B–D).
Sequential HTS with decitabine identified 31 drugs that increased GFP expression induced by decitabine (up to 4-fold) and 37 drugs that antagonized and even completely abolished its activity (Fig. 1B). Among top hits, epigenetic drugs azacitidine (DNMTi) and vorinostat (HDACi) had GFP ratios of 3.75 and 3.31, respectively, which validated our combinatorial HTS. We identified as positive hits all 4 antiarrhythmic drugs present in the libraries, belonging to the cardiac glycoside sub-family (ouabain, digoxin, digitoxin and proscillaridin A) suggesting a class effect. In addition, the combination with arsenic trioxide produced a 3-fold increase in GFP ratio. Other drug combinations involving several antibacterials and antidiabetic drugs (phenformin and acetohexamide) induced decitabine activity with a GFP ratio up to 2.5-fold. Interestingly, out of the 31 positive combinations, 21 drugs (68%) did not induce GFP reactivation as single treatment, as previously described (31).
In simultaneous combinations with decitabine, 9 drugs increased decitabine effects (up to 2.5-fold), while 69 drugs reduced GFP expression (Fig. 1C). In this combination, all positive hits, except decitabine did not induce GFP reactivation in our single drug screen (31). The range of GFP ratio, among positive hits, was between 2.2 to 2.57-fold.
In combination with TSA, GFP expression was induced up to 12-fold by 9 drugs, whereas 30 drugs decreased GFP expression induced by TSA (Fig. 1D). Nine hits were identified, including decitabine and azacitidine with GFP ratios of 13.27 and 6.69, further validating the screening system. Among the other 7 hits, 3 anticancer drugs enhanced TSA activity. These drugs encompassed methotrexate, pemetrexed and sorafenib, which produced GFP ratios between 2.9 to 4.2. Interestingly, these anticancer drugs did not produce GFP reactivation alone in YB5 cells (31). Similarly to the HTS with decitabine, antiarrhythmic drugs, digitoxin and digoxin enhanced TSA epigenetic activity.
Validation experiments on selected combinations confirmed our HTS results. For instance, the sequential combination with decitabine and cardiac glycosides (proscillaridin A and digitoxin) or the anticancer drug arsenic trioxide (Fig. 1 E–F; Supplementary Fig. S2B–C). All FDA-approved drugs, that enhanced GFP expression induced by decitabine or TSA, are listed in Table 1. Altogether, these 3 HTS revealed that about 45 FDA-approved drugs enhanced the activity of epigenetic drugs such as DNMTi and HDACi. Some of these drugs represent interesting candidates for drug repositioning, particularly, the class of antiarrhythmic drugs.
Table 1.
Positive hits identified in combination screen with decitabine (sequential and simultaneous treatment) or TSA (sequential treatment). Drug names, drug function and GFP ratio are shown.
| Combination | Drug name | Drug function | GFP Ratio |
|---|---|---|---|
|
Sequential with decitabine |
Ronnel* | Insecticide | 4.02 |
| Azacitidine | Anticancer, DNA methylation inhibitor | 3.75 | |
| Ouabain | Antiarrhythmic, Na+/K+ channel blocker | 3.42 | |
| Cycloheximide | Antipsoriatic, protein synthesis inhibitor | 3.39 | |
| Proscillaridin | Antiarrhythmic, Na+/K+ channel blocker | 3.37 | |
| Lanatoside C | Antiarrhythmic, Na+/K+ channel blocker | 3.33 | |
| Altrenogest* | Progestin, estrus cycle suppression | 3.33 | |
| Vorinostat | Anticancer, histone deacetylase inhibitor | 3.31 | |
| Butylated hydroxytoluene* | Antioxidant | 3.12 | |
| Digoxin | Antiarrhythmic, Na+/K+ channel blocker | 3.11 | |
| Bromperidol* | Antipsychotic | 3.09 | |
| Arsenic trioxide | Anticancer | 3.08 | |
| Terbinafine hydrochloride* | Antifungal | 3.05 | |
| Oxybutynin chloride* | Anticholinergic | 3.00 | |
| Tacrine hydrochloride* | Anticholinesterase, K+ channel blocker | 2.98 | |
| Digitoxin | Antiarrhythmic, Na+/K+ channel blocker | 2.95 | |
| Amoxapine* | Antidepressant | 2.94 | |
| Ethinyl estradiol* | Estrogen | 2.92 | |
| Phenformin hydrochloride* | Antidiabetic | 2.91 | |
| Diloxanide furoate* | Amoebicide | 2.79 | |
| Acetohexamide | Antidiabetic | 2.72 | |
| Oxantel pamoate* | Anthelmintic | 2.71 | |
| Cyproheptadine* | Antipruritic | 2.69 | |
| Ticlopidine hydrochloride* | Antiplatelet | 2.68 | |
| Rolitetracycline* | Antibacterial | 2.67 | |
| Nialamide* | Antidepressant | 2.67 | |
| Rabeprazole sodium* | Gastric acid secretion inhibitor | 2.64 | |
| Toltrazuril* | Coccidiostat | 2.64 | |
| Lamotrigine* | Anticonvulsant | 2.63 | |
| Pyridoxine* | Vitamin B6 dietary supplement | 2.62 | |
| Benzoxiquine* | Antiinfective | 2.60 | |
|
Simultaneous with decitabine |
Trientine hydrochloride* | Chelating Agent | 2.57 |
| Decitabine | Anticancer, DNA methylation inhibitor | 2.51 | |
| Nitrofurazone* | Antiinfective | 2.49 | |
| Nitromide* | Antibacterial | 2.47 | |
| Triclosan* | Antiinfective | 2.37 | |
| Etidronate disodium* | Bone resorption inhibitor | 2.22 | |
| Netilmicin sulfate* | Antibacterial | 2.22 | |
| Arsanilic acid* | Antibacterial | 2.22 | |
| Levobunolol hydrochloride* | Antiglaucoma | 2.21 | |
|
Sequential with TSA |
Decitabine | Anticancer, DNA methylation inhibitor | 13.27 |
| Azacitidine | Anticancer, DNA methylation inhibitor | 6.69 | |
| Digitoxin | Antiarrhythmic, Na+/K+ channel blocker | 5.76 | |
| Methotrexate* | Anticancer | 4.26 | |
| Pemetrexed* | Anticancer | 4.22 | |
| Bromocriptine mesylate* | Antiparkinsonian | 3.13 | |
| Thiram | Antifungal | 3.08 | |
| Sorafenib* | Anticancer | 2.97 | |
| Digoxin | Antiarrhythmic, Na+/K+ channel blocker | 2.90 | |
Drug names marked by a star (*) indicate drugs without epigenetic activity in monotherapy, as previously published (31).
Decitabine and proscillaridin A combination reactivated genes associated with calcium signaling and decreased epigenetic genes with oncogenic activity
To further explore the repositioning potential of antiarrhythmic drugs in combination with decitabine, we selected the cardiac glycoside proscillaridin A, which was the most active in our validation experiments (Fig. 1E). To characterize the epigenetic effects of the combination between decitabine and proscillaridin A, we measured drug-induced transcriptome changes by RNA-sequencing. First, we defined the optimal experimental condition by treating YB5 cells with various doses and schedules of decitabine and proscillaridin A and measured GFP reactivation by flow cytometry. Maximal GFP reactivation was obtained after the sequential combination of decitabine at 100 nM for 48h followed by proscillaridin A at 50 nM for 48h (Supplemental Fig. S3). RNA-sequencing was performed for drug combination, single drug treatments and untreated cells.
Venn diagram analysis on up-regulated genes (fold change >2 and FDR <0.01) showed that proscillaridin A induced the reactivation of 5,622 genes while decitabine alone reactivated 248 genes (Fig. 2A). The combination produced the reactivation of 5,891 genes which were mainly caused by the effect of proscillaridin A treatment. Indeed, 87% of the genes (5,159 genes) were shared between the combination and the proscillaridin A treatment. Venn diagram analysis on down-regulated genes (fold change <0.5 and FDR <0.01) showed a potent effect of proscillaridin A alone which decreased the expression of 5,277 genes (Fig. 2B). Decitabine decreased only 2 genes in these conditions. The combinatorial treatment produced a down-regulation of 5,187 genes and the majority of them (95%, 4,928 genes) were down-regulated by proscillaridin A alone. Therefore, our transcriptome analysis showed that proscillaridin induced a potent reprogramming, which was mainly driving gene expression changes in the combination treatment for either up- or down-regulated genes.
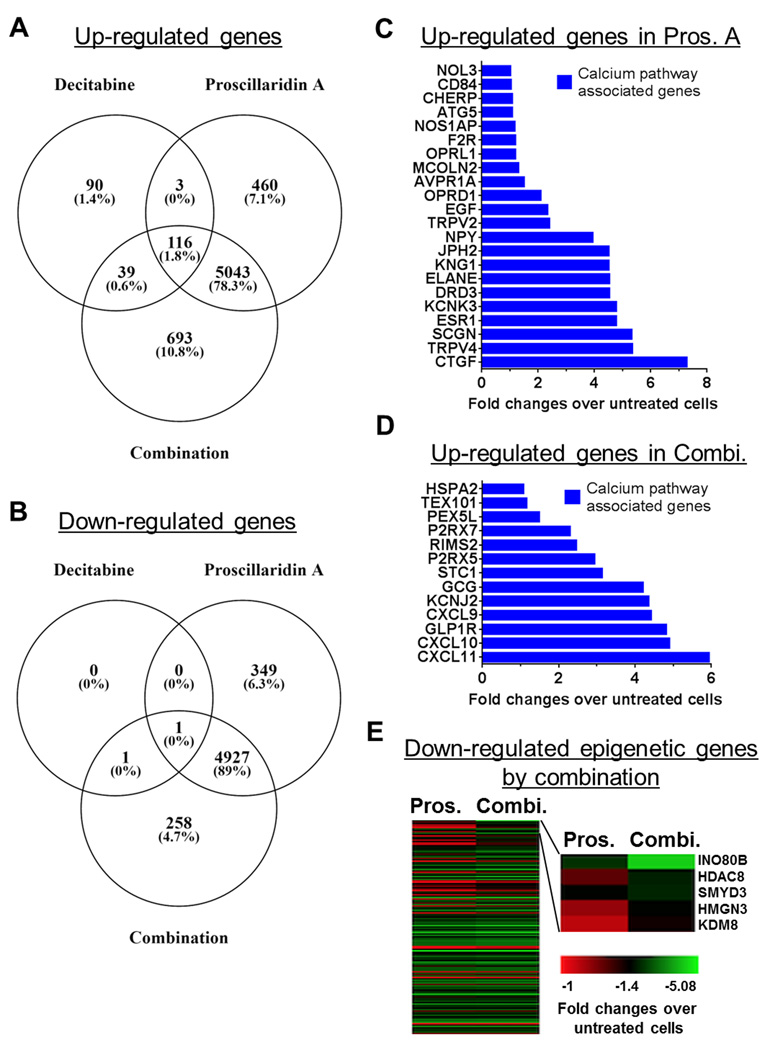
Figure 2.
Reprogramming colon cancer cells by massively down-regulating epigenetic genes. RNA-sequencing was performed on YB5 cells that were either untreated, or treated with decitabine (48h at 100 nM), proscillaridin A (48h at 50 nM), or their sequential combination (n=3). A, Venn diagram analysis of the number of up-regulated genes in treated cells versus untreated cells. B, Venn diagram analysis of the number of down-regulated genes in treated cells versus untreated cells. Metascape analysis was performed on both up and down-regulated gene data sets. C, Fold-changes in expression of genes exclusively up-regulated by proscillaridin A and belonging to calcium pathway associated genes by Metascape analysis. D, Fold-changes in expression of genes exclusively up-regulated by the combination and belonging to calcium pathway associated genes by Metascape analysis. E, Heat map of genes specifically down-regulated by the combination as compared to single drug treatment, belonging to epigenetic pathways by Metascape analysis. Fold-change ratios were calculated for down-regulated genes shared between proscillaridin A and the combination (n=4,928). Gene sets with ratio greater than 1 (n=2,146) were analyzed by Metascape. The results show that 153 epigenetic genes were specifically down-regulated (left panel). Most down-regulated genes are shown (right panel), which included 2 known oncogenes in colon cancer, SMYD3 and KDM8.
To determine the impact of gene expression changes, we performed gene ontology (GO) analysis using Metascape (34). First, we focused our analysis on up-regulated genes. Among 460 genes specifically up-regulated by proscillaridin A (non-overlapping to other treatments), Metascape analysis revealed that gene expression involved in metal ion transport was increased (Supplemental Fig. S4A). In this gene set, we identified a series of 22 up-regulated genes (from 2 to 7-fold), which were associated with GO-Terms associated with calcium pathways (Fig. 2C; list of GO-Term in Supplementary Table S1). The effects of proscillaridin A on the calcium signalling were corroborated by our recent report, demonstrating that epigenetic changes can be triggered by drugs targeting calcium signaling (31). Among 693 genes up-regulated (from 2 to 6-fold) specifically by the combination (non-overlapping to other treatments), Metascape analysis also showed an increase in expression in calcium ion transport genes. Specifically, 13 genes belonged to GO-Terms related to calcium pathways, further reinforcing the importance of calcium signaling in the epigenetic effects triggered by proscillaridin A alone or in combination with decitabine (Fig. 2D; Supplemental Fig. S4B, list of GO-Term in Supplementary Table S1).
Since most of the differentially expressed genes were shared between the treatment of proscillaridin A and the combination, we asked whether gene expression levels (i.e., fold changes) would differ between the combination and the treatment with proscillaridin A alone. We analyzed fold change ratios in gene expression between the combination and proscillaridin A. Among 5,043 genes, only two genes (ANKRD20A12P and TIMP3) had a more than 2-fold expression increase in the combination as compared to the single drug treatment. Among up-regulated genes shared between the 3 conditions (overlapping genes between decitabine, proscillaridin A and combination), less than 0.1% doubled their expression levels in the combinatorial treatments, also confirming that the effects observed in the combination were mostly driven by the effects of proscillaridin A treatment.
We then focused our analysis on down-regulated genes. Interestingly, the expression levels of almost half of these genes (2,163 genes) were more down-regulated specifically in the combination (up to 3.7-fold) as compared to proscillaridin A alone. Metascape analysis demonstrated that 153 of those were associated with GO-Terms belonging to epigenetic pathways (list of GO-Term in Supplementary Table S2). Most down-regulated genes specifically in the combination as compared to proscillaridin A alone belonged to chromatin modifiers such as INO80B, HDAC8, SMYD3, HMGN3, and KDM8 (Fig. 2E). Interestingly, SMYD3 and KDM8 were recently identified by others as potent oncogenes in colorectal cancer (37,38). These data highlight the epigenetic component of the combination between decitabine and proscillaridin A that down-regulates epigenetic pathways specifically involved in colon cancer development.
Epigenetic activity as single drugs is not a prerequisite to enhance DNMTi or HDACi
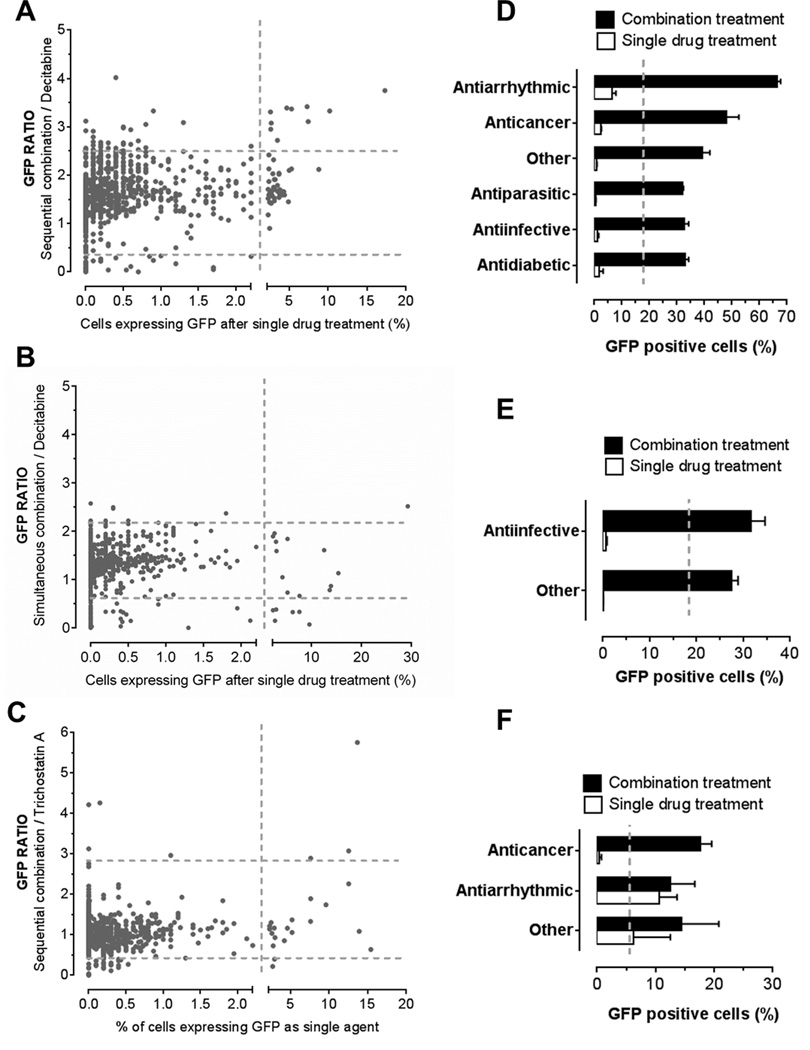
We next asked whether minimal epigenetic activity is a requirement for any drug to enhance epigenetic drug activity. To answer this question, we compared GFP ratios of combination treatment with decitabine and TSA to the percentage of GFP expressing cells measured in single drug HTS (31). Cut-off values for GFP positivity in single drug screening was established at 2.2% of YB5 cells expressing GFP as previously reported (shown by vertical grey dotted lines; Fig 3A–C) (31). We found that the number of positive hits in the combinatorial HTS was equally distributed above and below the cut-off for GFP positivity in single drug screening suggesting that epigenetic activity is not required for drugs to enhance DNMTi or HDACi.
Figure 3.
Synergy between the combination of FDA-approved drugs with DNMTi or HDACi is obtained regardless of epigenetic activity of FDA-approved drugs in monotherapy. GFP ratios in each HTS condition were plotted against percentages of YB5 cells expressing GFP after single drug treatments in A, sequential combination with decitabine; B, in simultaneous combination with decitabine; and C, sequential treatment with TSA. Horizontal dotted lines represent levels of 3 standard deviations above and below average GFP ratio in each screening condition. Vertical dotted lines represent threshold for GFP positivity calculated in the single drug screening as previously reported (31). Percentage of GFP positive cells was expressed by drug classes to compare the combination treatment to the single drug treatment in D, sequential combination with decitabine; E, simultaneous combination with decitabine; and F sequential combination with TSA. As a baseline level, vertical dotted lines represent percentage of GFP positive cells obtained after decitabine (in D and E) and TSA (F) treatment.
In sequential combination with decitabine (31 hits identified), most drugs producing synergistic GFP reactivation were drugs lacking the ability to induce GFP reactivation in monotherapy (Fig. 3A). Only 10 drugs (32%) induced GFP activity as single agent, as previously reported (31). In simultaneous combination with decitabine, all hits (n=9), except one (decitabine), did not produce GFP reactivation activity when used in monotherapy (Fig. 3B). In sequential combination with TSA, all 9 positive hits produced synergistic GFP ratio including 5 drugs, which produced GFP reactivation in monotherapy (Fig. 3C). Two of those, decitabine and azacitidine were excluded from the graph since their synergistic interaction with HDACi is well-characterized (29). Interestingly, pre-treatment with 4 FDA-approved drugs (methotrexate, pemetrexed, bromocriptine mesylate, and sorafenib) enhanced TSA-induced GFP reactivation but these drugs lacked epigenetic effects in monotherapy. Therefore, the data showed that synergistic responses obtained in our combinatorial HTS with decitabine or TSA are achievable by several types of FDA-approved drugs, where epigenetic activity in monotherapy is not required.
Identification of antiarrhythmic and anticancer drugs that synergize with epigenetic therapies
We then asked whether we could identify specific drug classes that enhance epigenetic activities of DNMTi or HDACi. We grouped the drugs by medical classes and compared the percentage of GFP positive cells in the combination treatments to the single drug screening previously published (31). In these analyzes, we removed epigenetic drugs contained in the libraries. In the sequential HTS with decitabine, drug classes producing the most striking synergistic responses belonged to the antiarrhythmic class, mainly represented by cardiac glycosides and the anticancer drugs (Fig. 3D). Interestingly, other groups of drugs, such as antiparasitics, antiinfectives and antidiabetics also produced potent GFP reactivation as compared to decitabine alone. Synergistic combination in simultaneous HTS with decitabine was achieved mainly by antiinfective drugs (Fig. 3E). In the combinatorial screen with TSA, anticancers and antiarrhythmics were drug classes producing synergistic GFP reactivation (Fig. 3F). In this case, the anticancer drug group encompassed two antifolate drugs (methotrexate and pemetrexed). Since folate contribute to production of S-adenosylmethionine, the donor moiety for methylation reactions, it is likely that antifolate drugs may influence DNA methylation content, thereby enhancing HDACi activity. Overall, the data suggest that several drug classes, mainly antiarrhythmic and anticancer drugs, synergize with epigenetic therapy suggesting new possible drug combinations that can be evaluated in clinical trials.
FDA-approved drugs producing cytotoxicity antagonize DNMTi or HDACi epigenetic effects
We found that some FDA-approved drugs blocked or interfered the epigenetic effects of DNMTi or HDACi (Fig. 1 B–D). This observation revealed potential antagonist drug interactions, which can be deleterious for cancer patients undergoing epigenetic therapy who are being prescribed several drugs simultaneously. Indeed, the HTS identified a series of 85 drugs that reduced or blocked GFP expression induced by decitabine or TSA (Table 2 and Supplementary Table S3–5). The use of these drugs in the clinics may decrease the epigenetic effects of decitabine or TSA in cancer patients.
Table 2.
Antagonistic hits identified in combination HTS with decitabine (sequential and simultaneous) or TSA (sequential). Drug names, drug function and GFP ratio are shown. Only top hits are shown in this table. Complete list of antagonistic drugs is shown in Supplemental Table S3–5.
| Combination | Drug name | Drug function | GFP Ratio |
|---|---|---|---|
|
Sequential with decitabine |
Fluphenazine hydrochloride | Antipsychotic | 0,00 |
| Penfluridol | Antipsychotic | 0,00 | |
| Toremiphene citrate | Anticancer | 0,00 | |
| Hexylresorcinol | Antiseptic | 0,00 | |
| Gentian violet | Antibacterial | 0,00 | |
| Econazole nitrate | Antifungal | 0,01 | |
| Tamoxifen citrate | Anticancer | 0,01 | |
| Thioridazine hydrochloride | Antipsychotic | 0,01 | |
| Selamectin | Anthelmintic | 0,02 | |
| Sertraline hydrochloride | Antidepressant | 0,02 | |
| Perhexiline maleate | Coronary vasodilator | 0,02 | |
| Gramicidin A | Antibacterial | 0,02 | |
| Mefloquine | Antimalarial | 0,04 | |
| Dioxybenzone | Sunscreen | 0,04 | |
| Estradiol valerate | Estrogen | 0,05 | |
|
Simultaneous with decitabine |
Methylbenzethonium chloride | Antiinfective | 0.00 |
| Valrubicin | Anticancer | 0.00 | |
| Benzethonium chloride | Antiseptic | 0.00 | |
| Gentian violet | Antibacterial | 0.00 | |
| Dactinomycin | Anticancer | 0.00 | |
| Epirubicin hydrochloride | Anticancer | 0.01 | |
| Mitomycin | Anticancer | 0.01 | |
| Nitrogen mustard | Anticancer | 0.01 | |
| Nilotinib | Anticancer | 0.02 | |
| Gemcitabine hydrochloride | Anticancer | 0.03 | |
| Plicamycin | Anticancer | 0.03 | |
| Cytarabine | Anticancer | 0.03 | |
| Mitoxantrone hydrochloride | Anticancer | 0.03 | |
| Rapamycin | Anticancer | 0.03 | |
| Cladribine | Anticancer | 0.03 | |
|
Sequential with TSA |
Valrubicin | Anticancer | 0.00 |
| Epirubicin hydrochloride | Anticancer | 0.00 | |
| Dactinomycin | Anticancer | 0.00 | |
| Plicamycin | Anticancer | 0.02 | |
| Mitoxantrone | Anticancer | 0.02 | |
| Teniposide | Anticancer | 0.09 | |
| Penicillin V potassium | Antibacterial | 0.11 | |
| Cycloheximide | Antipsoriatic | 0.15 | |
| Oxymetazoline hydrochloride | Nasal decongestant | 0.17 | |
| Bortezomib | Anticancer | 0.17 | |
| Fuchsin N | Anthelmintic | 0.18 | |
| Mechlorethamine | Anticancer | 0.19 | |
| Nitrogen mustard | Anticancer | 0.19 | |
| Mepenzolate bromide | Anticholinergic | 0.20 | |
| Benzethonium chloride | Antiseptic | 0.23 | |
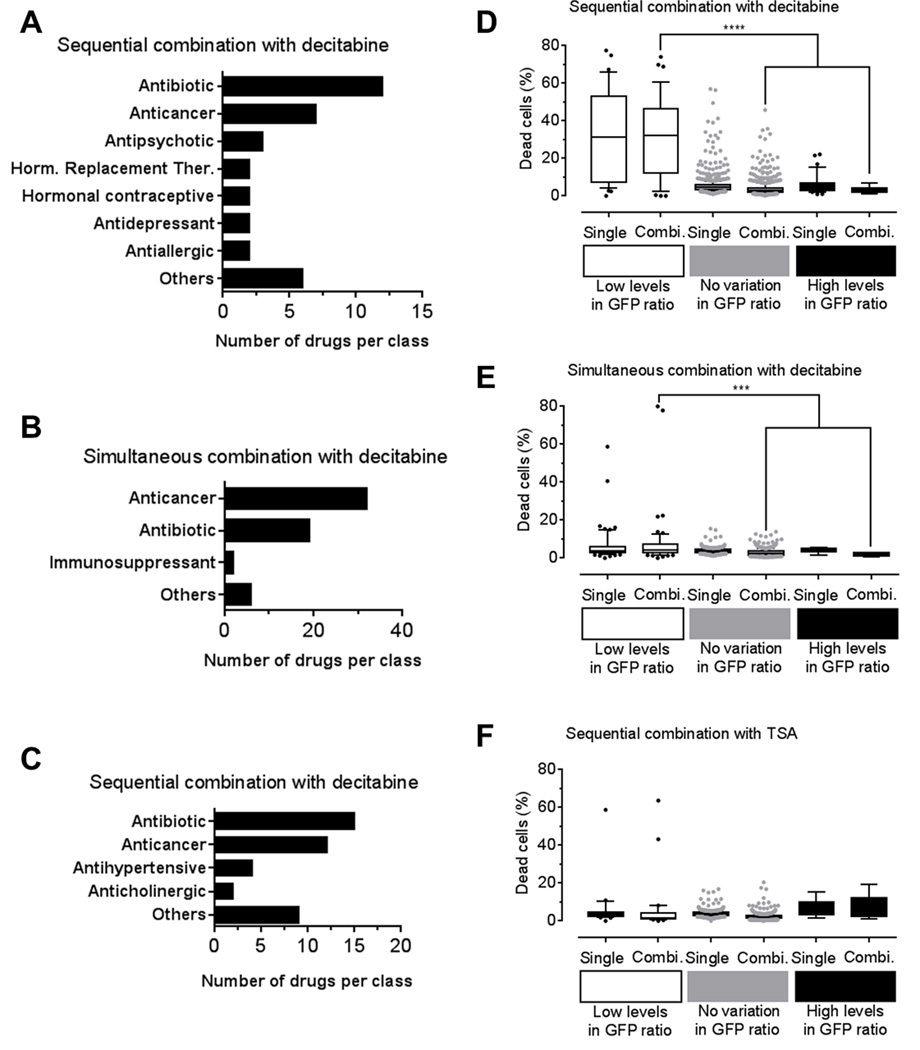
We analyzed the drugs that decreased GFP expression induced with DNMTi or HDACi by 3 standard deviations. Antagonistic drugs were grouped according to their drug functions. In sequential combination with decitabine, we identified 7 drug classes that repressed GFP expression, while 3 classes were identified in simultaneous combination (Fig. 4A–B). In sequential combination with TSA, we found 4 drug classes that repressed GFP reactivation (Fig. 4C). In all three combinatorial drug screens, antibiotics and anticancer drugs were commonly identified as antagonistic drug classes (Fig. 4A–C, Table 2 and Supplementary Table S3–S5). Interestingly, in all HTS, 3 drugs, nitrogen mustard, mechlorethamine, and dactinomycin, completely abolished GFP expression. These drugs belong to the cytotoxic chemotherapy drug class, which are known to trigger cell cycle block and apoptosis. These results confirm the notion that drugs inducing cytotoxicity or blocking cell cycle antagonize the effect of epigenetic drugs (5). The effect of cytotoxic drugs to GFP expressing cells could be due to a decreased ability to maintain GFP activation or alternatively to an increased cell toxicity. Since the percentage of GFP positive cells is specifically decreased when YB5 cells were exposed to cytotoxic drug combinations, it is possible that the epigenetic treatments reversed some drug-resistance mechanisms causing a lower apoptosis threshold specifically among GFP positive cell population. Such effects could be beneficial to cancer treatment by using epigenetic therapy to increase cancer cell sensitivity to chemotherapeutic agents without causing more side effects.
Figure 4.
Epigenetic combinatorial HTS reveals antagonist interactions with 85 FDA-approved drugs. Blockers of GFP-induction induced by epigenetic drugs were defined as those drugs producing GFP ratios more than 3 standard deviations below GFP ratio average value in each HTS condition. Drugs producing antagonistic interaction where grouped according to their drug classes in A, sequential combination with decitabine, B, simultaneous treatment with decitabine and C, sequential treatment with TSA. Percentage of dead cells (measured by propidium iodide staining) was shown for drug combinations with GFP ratios below (group name: in Low levels), in between (group name: No variation) and above (groupe name: High levels) 3 standard deviation intervals. As a control, percentage of dead cells was shown for the same drugs in the single HTS, as previously published (31). These analyzes were performed in HTS with D, sequential combination with decitabine, E, simultaneous combination with decitabine and F, sequential combination with TSA. Statistical analysis was done by One-way ANOVA followed by the Tukey–Kramer Multiple Comparison Test (P< 0.05).
To further analyze these results, we compared the percentage of dead cells (measured by propidium iodide staining) obtained after HTS either in monotherapy or in combination. Average percentage of dead cell was calculated for drug combinations producing GFP ratios below 3 standard deviations of the average GFP value (referred as low levels of GFP ratio), above 3 standard deviations of the average GFP value (referred as high levels of GFP ratio) and between 3 standard deviation limits of the average GFP value (referred as no variation in GFP ratio) in monotherapy or combinatorial HTS (31). In sequential combination with decitabine, there was no significant difference in dead cell percentage between single or combination groups suggesting that toxic drugs in monotherapy can produce synergistic GFP reactivation in combination with decitabine. Comparison among GFP ratio categories showed that drug combinations producing low GFP ratios exhibited a significantly high number of dead cells compared to other groups (P<0.001; Fig. 4D). Similar results were obtained in the simultaneous combination screen with decitabine, where drug combinations in low GFP ratio category were significantly more toxic as compared to the groups (P<0.05; Fig. 4E). However, the percentage of dead cells did not significantly change in the sequential treatment with TSA following FDA-approved drug treatments (Fig. 4F). Overall, these data validate in a large drug collection that cytotoxic drugs, such as anticancer drugs or antibiotics can blunt the epigenetic effects of DNMTi or HDACi.
Discussion
Drug discovery in oncology has focused on the development of single molecular entities with well-defined mechanism of action. However, cancer treatment is highly dependent on drug combinations. Thus, combinatorial drug screening is an approach that can be used to discover new combinations that are more likely to bring clinical benefits for oncology patients. In order to accelerate drug discovery, screening among FDA-approved drug libraries, a process called drug repositioning, has been shown to be an effective strategy in oncology (27). Drug repositioning has several advantages compared to classical drug development including cheaper, faster and safer preclinical and clinical validation steps (27).
Here, we described combinatorial HTS using FDA-approved libraries in association with DNMTi and HDACi to discover new epigenetic drug combinations in colon cancer. We used YB5 cell line system which responds quantitatively to epigenetic drug combinations by the induction of GFP, as a surrogate epigenetically silenced TSG (29–31). The rationale to target epigenetic abnormalities in colon cancer is justified by the importance of DNA methylation alterations (characterized by cases harboring high levels of DNA methylation called CpG Island Methylator Phenotype) and chromatin alterations in this malignancy (5,39). Combinations between DNMTi decitabine and antiarrhythmics or anticancer drugs were the most potent combination in this screen. Particularly, proscillaridin A and arsenic trioxide produced synergistic GFP reactivation in sequential combination (31). These drug combinations demonstrate promising activity which should be investigated in clinical trials against colon cancer. Interestingly, the combination of decitabine with arsenic trioxide is currently the focus of several clinical trials (NCT00671697, NCT02188706, NCT02190695) against acute myelogenous leukemia and myelodysplastic syndromes (40). We focused on the understanding of the epigenomics effects of the combination of DNMTi decitabine and proscillaridin A. This combination produced a potent epigenetic reprogramming in colon cancer cells, highlighted by hundreds of differentially expressed genes. Importantly, this combination is targeting calcium signaling which we previously linked to epigenetic therapeutic effects (31). In addition, this combination specifically down-regulated around 150 genes involved in epigenetic reprogramming which may explain why thousands of genes are differentially regulated by the treatment. More precisely, this combination induced a pronounced down-regulation of epigenetic modifiers with oncogenic properties in colon cancer such as SMYD3 and KDM8 (37,38).
A novel finding arising from these combinatorial HTS is that epigenetic synergy could be obtained in our model with drug combinations involving FDA-approved drugs lacking epigenetic activity. This finding paves the way for new epigenetic drug combinations and justifies our rationale for combinatorial HTS. Interestingly, we also noted that synergistic GFP reactivation was dependent on cell viability immediately after treatment. Indeed, drug combinations (at the selected dose level in these HTS) that produced elevated cell kills did not allow GFP reactivation.
We conclude that combinatorial HTS is a promising strategy for a drug discovery in oncology. Investigation of FDA-approved libraries in combination with epigenetic drugs allows us to propose new drug combinations for clinical trials. Here, we have focused on the combination of decitabine and proscillaridin A, which holds promising effects in down-regulating oncogenic signals carried by specific epigenetic modifiers in colon cancer. More studies are needed to explore these epigenetic combinations reported in colon cancer and other malignancies as a source for new epigenetic drug combinations. Finally, these 3 HTS also suggest potential detrimental drug interactions that should be carefully considered for patients treated with epigenetic drugs.
Supplementary Material
Acknowledgments
Financial Information
This work was supported by NIH grants CA100632 and CA046939 to J-P J. Issa. J-P J. Issa is an American Cancer Society Clinical Research professor supported by a generous gift from the F. M. Kirby Foundation.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
The authors declare no potential conflicts of interest.
References
- 1.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huston A, Arrowsmith CH, Knapp S, Schapira M. Probing the epigenome. Nat Chem Biol. 2015;11:542–545. doi: 10.1038/nchembio.1871. [DOI] [PubMed] [Google Scholar]
- 4.You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 2010;60:376–392. doi: 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- 6.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 7.Estey EH. Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia. Leukemia. 2013;27:1803–1812. doi: 10.1038/leu.2013.173. [DOI] [PubMed] [Google Scholar]
- 8.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 9.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 10.Kirschbaum M, Gojo I, Goldberg SL, Bredeson C, Kujawski LA, Yang A, et al. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol. 2014;167:185–193. doi: 10.1111/bjh.13016. [DOI] [PubMed] [Google Scholar]
- 11.Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momparler RL, Idaghdour Y, Marquez VE, Momparler LF. Synergistic antileukemic action of a combination of inhibitors of DNA methylation and histone methylation. Leuk Res. 2012;36:1049–1054. doi: 10.1016/j.leukres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Bhadury J, Nilsson LM, Muralidharan SV, Green LC, Li Z, Gesner EM, et al. BET and HDAC inhibitors induce similar genes and biological effects and synergize to kill in Myc-induced murine lymphoma. Proc Natl Acad Sci USA. 2014;111:2721–2730. doi: 10.1073/pnas.1406722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiskus W, Sharma S, Shah B, Portier BP, Devaraj SG, Liu K, et al. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014;28:2155–2164. doi: 10.1038/leu.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiskus W, Wang Y, Sreekumar A, Buckley KM, Shi H, Jillella A, et al. Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood. 2009;114:2733–2743. doi: 10.1182/blood-2009-03-213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owonikoko TK, Ramalingam SS, Kanterewicz B, Balius TE, Belani CP, Hershberger PA. Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells. Int J Cancer. 2010;126:743–755. doi: 10.1002/ijc.24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachmann PS, Piazza RG, Janes ME, Wong NC, Davies C, Mogavero A, et al. Epigenetic silencing of BIM in glucocorticoid poor-responsive pediatric acute lymphoblastic leukemia, and its reversal by histone deacetylase inhibition. Blood. 2010;116:3013–3022. doi: 10.1182/blood-2010-05-284968. [DOI] [PubMed] [Google Scholar]
- 18.Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Chiappinelli KB, Guzzetta AA, Easwaran H, Yen RW, Vatapalli R, et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5:587–598. doi: 10.18632/oncotarget.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin T, Si J, Raynal NJ, Wang X, Gharibyan V, Ahmed S, et al. Epigenetic synergy between decitabine and platinum derivatives. Clin Epigenetics. 2015;7:97. doi: 10.1186/s13148-015-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lainey E, Wolfromm A, Marie N, Enot D, Scoazec M, Bouteloup C, et al. Azacytidine and erlotinib exert synergistic effects against acute myeloid leukemia. Oncogene. 2013;32:4331–4342. doi: 10.1038/onc.2012.469. [DOI] [PubMed] [Google Scholar]
- 22.Gollob JA, Sciambi CJ, Peterson BL, Richmond T, Thoreson M, Moran K, et al. Phase I trial of sequential low-dose 5-aza-2'-deoxycytidine plus high-dose intravenous bolus interleukin-2 in patients with melanoma or renal cell carcinoma. Clin Cancer Res. 2006;12:4619–4627. doi: 10.1158/1078-0432.CCR-06-0883. [DOI] [PubMed] [Google Scholar]
- 23.Schwartsmann G, Schunemann H, Gorini CN, Filho AF, Garbino C, Sabini G, et al. A phase I trial of cisplatin plus decitabine, a new DNA-hypomethylating agent, in patients with advanced solid tumors and a follow-up early phase II evaluation in patients with inoperable non-small cell lung cancer. Invest New Drugs. 2000;18:83–91. doi: 10.1023/a:1006388031954. [DOI] [PubMed] [Google Scholar]
- 24.Glasspool RM, Brown R, Gore ME, Rustin GJ, McNeish IA, Wilson RH, et al. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in combination with carboplatin vs carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br J Cancer. 2014;110:1923–1929. doi: 10.1038/bjc.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadia TM, Faderl S, Ravandi F, Jabbour E, Garcia-Manero G, Borthakur G, et al. Final results of a phase 2 trial of clofarabine and low-dose cytarabine alternating with decitabine in older patients with newly diagnosed acute myeloid leukemia. Cancer. 2015;121:2375–2382. doi: 10.1002/cncr.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momparler RL, Dore BT, Momparler LF. Effect of 5-aza-2'-deoxycytidine and retinoic acid on differentiation and c-myc expression in HL-60 myeloid leukemic cells. Cancer Lett. 1990;54:21–28. doi: 10.1016/0304-3835(90)90086-d. [DOI] [PubMed] [Google Scholar]
- 27.Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology--patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12:732–742. doi: 10.1038/nrclinonc.2015.169. [DOI] [PubMed] [Google Scholar]
- 28.Blatt J, Corey SJ. Drug repurposing in pediatrics and pediatric hematology oncology. Drug Discov Today. 2013;18:4–10. doi: 10.1016/j.drudis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Si J, Boumber YA, Shu J, Qin T, Ahmed S, He R, et al. Chromatin remodeling is required for gene reactivation after decitabine-mediated DNA hypomethylation. Cancer Res. 2010;70:6968–6977. doi: 10.1158/0008-5472.CAN-09-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raynal NJ, Si J, Taby RF, Gharibyan V, Ahmed S, Jelinek J, et al. DNA methylation does not stably lock gene expression but instead serves as a molecular mark for gene silencing memory. Cancer Res. 2012;72:1170–1181. doi: 10.1158/0008-5472.CAN-11-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raynal NJ, Lee JT, Wang Y, Beaudry A, Madireddi P, Garriga J, et al. Targeting Calcium Signaling Induces Epigenetic Reactivation of Tumor Suppressor Genes in Cancer. Cancer Res. 2016;76:1494–1505. doi: 10.1158/0008-5472.CAN-14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monneret C. Histone deacetylase inhibitors. Eur J Med Chem. 2005;40:1–13. doi: 10.1016/j.ejmech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripathi S, Pohl MO, Zhou Y, Rodriguez-Frandsen A, Wang G, Stein DA, et al. Meta- and Orthogonal Integration of Influenza "OMICs" Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe. 2015;18:723–735. doi: 10.1016/j.chom.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffiths EA, Gore SD. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45:23–30. doi: 10.1053/j.seminhematol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Huang Q, Li Y, Song Y, Li Y. JMJD5 is a potential oncogene for colon carcinogenesis. Int J Clin Exp Pathol. 2015;8:6482–6489. [PMC free article] [PubMed] [Google Scholar]
- 38.Sarris ME, Moulos P, Haroniti A, Giakountis A, Talianidis I. Smyd3 Is a Transcriptional Potentiator of Multiple Cancer-Promoting Genes and Required for Liver and Colon Cancer Development. Cancer Cell. 2016;29:354–366. doi: 10.1016/j.ccell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch JS, Klco JM, Gao F, Procknow E, Uy GL, Stockerl-Goldstein KE, et al. Combination decitabine, arsenic trioxide, and ascorbic acid for the treatment of myelodysplastic syndrome and acute myeloid leukemia: a phase I study. Am J Hematol. 2011;86:796–800. doi: 10.1002/ajh.22092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.