Abstract
Bioactive sphingolipids are regulators of immune cell function and play critical roles in inflammatory conditions including ulcerative colitis. As one of the major forms of inflammatory bowel disease, ulcerative colitis pathophysiology is characterized by an aberrant intestinal inflammatory response that persists causing chronic inflammation and tissue injury. Innate immune cells play an integral role in normal intestinal homeostasis but their dysregulation is thought to contribute to the pathogenesis of ulcerative colitis. In particular, neutrophils are key effector cells and are first line defenders against invading pathogens. While the activity of neutrophils in the intestinal mucosa is required for homeostasis, regulatory mechanisms are equally important to prevent unnecessary activation. In ulcerative colitis, unregulated neutrophil inflammatory mechanisms promote tissue injury and loss of homeostasis. Aberrant neutrophil function represents an early checkpoint in the detrimental cycle of chronic intestinal inflammation; thus, dissecting the mechanisms by which these cells are regulated both before and during disease is essential for understanding the pathogenesis of ulcerative colitis. We present an analysis of the role of sphingolipids in the regulation of neutrophil function and the implication of this relationship in ulcerative colitis.
Keywords: sphingolipids, sphingosine-1-phosphate, ceramide, ulcerative colitis, inflammatory bowel disease, neutrophils
1. Introduction
In the last three decades, it has become evident that sphingolipids are essential and critical regulators in the cell. The focus on bioactive sphingolipid research in signaling mechanisms has expanded from its origin in PKC regulation to multiple biological processes in immunity, inflammation, cellular growth, differentiation, proliferation, apoptosis, metabolism, and related pathologies (Abdel Hadi et al., 2016; Hannun et al., 1986; Hannun and Obeid, 2008; Pyne et al., 2016). Sphingolipids comprise a large class of structural and bioactive lipids that are metabolized in an interconnected network regulated by a large family of enzymes. Among the biologically active sphingolipids, sphingosine-1-phosphate (S1P) is one of the most characterized in immune and inflammatory processes. S1P regulates the differentiation of immune cells, production of inflammatory mediators and is an established regulator of immune trafficking. S1P can bind and activate five G-protein coupled S1P receptors (S1PR1-5) to mediate intracellular signaling and regulate cell function. The critical role of S1P and other sphingolipids in inflammation is underscored by the approval of FTY720 for the treatment of multiple sclerosis. FTY720 is a sphingosine analogue that alters lymphocyte trafficking by sequestering cells in secondary lymphoid organs preventing their migration towards sites of inflammation and delaying their return to the circulation. Upon phosphorylation by sphingosine kinase 2 (SK2), FTY720-phosphate functions as an agonist and modulator of G-protein coupled S1PRs, primarily S1PR1 in lymphocytes (Blankenbach et al., 2016). Modulators of S1PR signaling can have implication for diseases characterized by excessive lymphocyte recruitment such as inflammatory bowel disease (IBD) (Izzo et al., 2016; Rivera-Nieves, 2015). In fact, promising results in current clinical studies support the notion that sphingosine-1-phosphate (S1P) signaling and other sphingolipid metabolites may be involved in regulating inflammation in ulcerative colitis (UC) (Kunkel et al., 2013). The pathogenesis of UC is characterized by disruption of the innate and adaptive immune mechanisms that maintain intestinal homeostasis (Dieleman et al., 1998; Neurath, 2014). As effectors of innate immunity that can direct adaptive immune function, neutrophils represent an important research area in homeostasis of the gut. Neutrophils require regulation at multiple points to avoid collateral tissue damage during intestinal inflammation. In diseases like UC, characterized by high number of neutrophils in the intestinal lamina propria, the persistence of these cells contributes to tissue injury and the cycle of chronic inflammation that ensues. This review will highlight the current and expanding role of bioactive sphingolipids in UC. We summarize the mechanisms of neutrophil regulation in health and intestinal inflammation. Finally, we present an analysis of the scientific evidence supporting bioactive sphingolipids in neutrophil inflammatory responses, and discuss the implication of this relationship in the pathogenesis of UC.
2. Sphingolipid metabolism
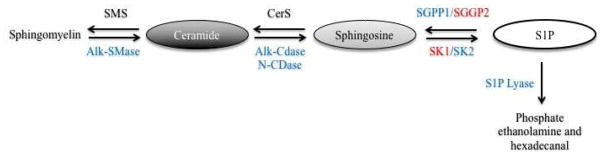
Ceramide is considered the central metabolite of the pathway and is generated either in a de novo fashion at the endoplasmic reticulum (ER) or through the metabolism of other sphingolipids in cellular compartments (Siow et al., 2015). Ceramide structure consists of a sphingoid backbone N-acylated by one of six ceramide synthases (CerS). De novo N-acylation first results in the generation of dihydroceramide species of varying acyl chain lengths before a desaturase catalyzes the final reaction in ceramide synthesis. Subsequently, ceramides are transported to the Golgi where ceramide is metabolized to sphingomyelin by sphingomyelin synthase (SMS), to ceramide-1-phosphate (C1P) by a ceramide kinase (CerK) or to glucosylceramide and complex glycosphingolipids by glucosylceramide synthase (Carroll et al., 2015). From here, these sphingolipid metabolites can be transported by membrane transport to different compartments to regulate cellular processes. The reverse reaction can also occur, where sphingomyelin, C1P and glycosphingolipids can be broken down by sphingomyelinase (SMase), ceramide phosphatase and glycosidases, respectively, to increase the pool of ceramide in the cell. Another axis of ceramide metabolism generates S1P, a critical regulator of immune function. In this pathway, ceramide is deacetylated by a ceramidase (CDase) to generate sphingosine. Sphingosine is rapidly phosphorylated by sphingosine kinases (SK1 and SK2) to generate S1P (Snider et al., 2010). Finally, S1P can be recycled back to sphingosine by the S1P phosphatase (SGPP) or irreversibly degraded to form hexadecanal and phosphate ethanolamine by the S1P lyase in a reaction that represents the exit point of the pathway. The cellular compartmentalization of enzymes allows interconnectivity in the rapid metabolism of sphingolipids and is a key regulatory aspect of sphingolipid biology (Hannun and Obeid, 2008). Therefore, understanding the axis of sphingolipid metabolism in different cellular compartments is critical to characterize sphingolipid mediated regulation of specific biological processes such as inflammation and the implications for therapeutic application.
3. Pathophysiology of ulcerative colitis
Inflammatory bowel disease comprises multiple disorders of the gastrointestinal tract, with ulcerative colitis (UC) and Crohn’s disease (CD) representing the two major forms. Both categories of IBD are characterized by chronic inflammation of the gastrointestinal tract and exhibit common and distinct clinical and pathological features that can make diagnosis in some cases inconclusive. Patients can present a range of clinical symptoms including severe abdominal pain, diarrhea, bloody stool, anemia and weight loss (Danese and Fiocchi, 2011). IBD patients can experience intermittent periods of active disease, albeit the presence of low-grade chronic inflammation can still persist. Clinical symptoms serve as the starting point for diagnosis and, along with histological and endoscopic assessment, serve to differentiate CD and UC. Crohn’s disease is characterized by discontinuous inflammatory lesions and deep penetrating ulcers that can occur at any location in the gastrointestinal track. Ulcerative colitis in contrast is distinguished by continuous inflammatory lesions in the intestinal mucosa of the distal colon and rectum. Currently, the global burden of UC is on the rise, with an increased incidence in several regions of the world, primarily in developing countries. In the United States, the incidence is approximate 20 per 100,000 person-years and the prevalence at approximately 250 cases per 100,000 persons (Muthas et al., 2016). Disease progression and chronic inflammation can result in ulceration, mucin depletion and loss of crypt architecture. Although IBD is considered idiopathic and standard criteria for clinical diagnosis is still lacking, an understanding of the pathophysiology points to a complex interplay of multiple factors. The current model suggests a loss of intestinal homeostasis as a result of persistent immune activation in the intestinal mucosa caused by host genetics, environmental pressure and perturbations in the gut microbiome (dysbiosis) (Elson et al., 2005). These factors manifest in a malicious pathological cycle where genetic mutations in immune or epithelial barrier regulators can increase the susceptibility that the host will mount an aberrant immune response against the intestinal microbiota. The inflammatory response directed against luminal antigens from the microbiota can compound more damage to the intestinal epithelial barrier, sustaining the cycle of inflammation (Abreu, 2010). Specifically how these factors relate to drive the disease is still not well understood and prolonged disease also increases the risk of dysplasia and colorectal cancer development (Arthur et al., 2012; Danese and Fiocchi, 2011). Furthermore, a complete makeup of the host genetics involved in UC is still not well characterized but significant research progress in the last decade has led to the identification of specific gene loci associated with disease predisposition. Prominent findings demonstrate a role for genes involved in innate responses such as nucleotide oligomerization domain 2 (NOD2) and autophagic regulator ATG16 as well as adaptive immune components of helper-T-cell types such as interleukin (IL)-10, IL-13, IL-17 and IL-23 (Liu and Stappenbeck, 2016; Ye and McGovern, 2016). Smoking, diet, infection, antibiotics and hygiene as well as other environmental factors may also contribute to UC pathogenesis by altering the gut microbiome and subsequently intestinal homeostasis (Basson et al., 2016; Zeng et al., 2016). Currently, there are multiple therapeutic agents in the clinic for treatment of UC. The guidelines for treatment take into consideration the severity of clinical symptoms; first line of treatment include immunosupressors that regulate myeloid function like 5-aminosalicylates, corticosteroids, and antibodies against TNF-α, calcineurin and integrins (Danese and Fiocchi, 2011; Hanauer, 2004; Lim and Hanauer, 2004). And while these treatment options are available, remission is not maintained in many patients underscoring the need to clearly understand the molecular mechanisms that regulate UC pathogenesis for disease prevention and development of additional innovative therapies.
4. Bioactive sphingolipid signaling in ulcerative colitis
The discovery of S1P as a regulator of immune cell trafficking and the development of S1PR modulators for the treatment of multiple sclerosis, opened many avenues of exploration for bioactive sphingolipids in inflammatory diseases (Snider et al., 2010). The premise that UC pathology is driven by an excessive recruitment of immune cells in the intestinal mucosa provided a strong rationale for investigating the role of SK/S1P in UC. Examination of clinical specimens from colitis patients found increased expression of SK1 in the intestinal epithelium (Degagne et al., 2014; Snider et al., 2010). The role of SK1 and S1P signaling has been investigated in multiple murine models of UC caused by acute or chronic intestinal injury and inflammation (Abdel Hadi et al., 2016; Snider et al., 2009) (Duan and Nilsson, 2009; Perse and Cerar, 2012). SK1 was identified as a regulator of disease mechanisms in dextran sulfate sodium (DSS)-induced colitis, as genetic deficiency or pharmacological inhibition protected mice from intestinal injury and systemic inflammation (Fig 1) (Snider et al., 2010) (Maines et al., 2008). Circulating S1P levels were also increased in DSS-induced colitis and no significant compensation from SK2 was observed (Snider et al., 2010). Mechanistically, protection was correlated with decreased colon levels of the enzyme COX-2, an inflammatory mediator in colitis shown in previous studies to be regulated by SK1 in response to TNF-α (Pettus et al., 2003). Reduced neutrophil infiltrate was also observed in the inflamed colon of SK1 knock out (KO) mice (Snider et al., 2010). Additional studies aimed at characterizing the tissue specific function of SK1 demonstrated that hematopoietic-derived SK1/S1P is responsible for neutrophilia in DSS-induced colitis while extra-hematopoietic SK1 promotes the induction of COX-2 (Snider et al., 2014). On the contrary, genetic deletion of SK2 in vivo provided no protection in DSS-induced colitis. SK2 deficiency in fact exacerbated tissue damage and production of inflammatory cytokines, suggesting that SK2 may have a protective role in UC (Fig 1) (Liang et al., 2013). While SK2 has been found to mediate phosphorylation of FTY720 for modulation of lymphocyte egress (Kharel et al., 2005), it seems plausible that S1P signaling in immune responses is multifaceted and thus several metabolic pathways of S1P regulation may exist in different tissues or even in different subcellular compartments driving these cellular processes. To characterize these pathways, multiple studies have aimed at investigating the role of various sphingolipid enzymes that regulate directly or indirectly the levels of S1P. Recently, it was shown that S1P lyase deletion in the epithelium exacerbated colitis and inflammation, demonstrating that epithelial production of S1P is detrimental during inflammation (Fig 1) (Degagne et al., 2014). Since S1P levels are also regulated by the activity of S1P phosphatases, the function of both isoforms, SGPP1 and SGPP2, was investigated in DSS-induced colitis. Only SGPP2 deficiency mitigated the inflammatory injury and decreased the levels of inflammatory cytokines, whereas SGPP1 had an opposite role (Fig 1). While SGPP1 is ubiquitously expressed, SGPP2 expression is mainly limited to the stomach and colon and interestingly, intestinal and circulating S1P were only regulated by SGPP2 (Huang et al., 2016).
Figure 1. Schematic depiction of the relative beneficial (blue) and detrimental (red) contributions of different bioactive sphingolipid metabolic enzymes in UC pathophysiology.
Sphingomyelin synthase (SMS), alkaline sphingomyelinase (Alk-SMase), ceramide synthase (CerS), alkaline ceramidase (Alk-CDase), neutral ceramidase (N-CDase), S1P phosphatase 1 and 2 (SGPP1 and SGPP2), sphingosine kinase 1 and 2 (SK1 and SK2).
FTY720 administration suppresses DSS-induced colitis and the activation of inflammatory pathways in SK2 KO mice, demonstrating that FTY720 immuno-modulatory function is not dependent on SK2. Modulation of S1P/S1PR1 signaling with FTY720 also attenuated inflammation in a model of spontaneous colitis using IL-10 KO mice due to sequestration of lymphocytes in secondary lymphoid organs. Lymphocyte sequestration resulted in decreased numbers of CD4+ T-helper cells in the inflamed intestine, accompanied by decreased production of IFN-γ while no regulation of T cell differentiation was observed (Fujii et al., 2006; Mizushima et al., 2004). Follow up observations have shown that in fact FTY720-mediated immunosuppression is also influenced by the induction of CD4+ CD25+ regulatory T cells and production of suppressor cytokines IL-10 and TGF-β (Daniel et al., 2007; Sawicka et al., 2005; Wang et al., 2004). The application of different models of colitis (DSS versus 2,4,6-trinitrobenzene sulfonic acid (TNBS)), strongly support the conclusion that FTY720 regulates different aspects of lymphocyte biology (Degagne and Saba, 2014). Additionally, FTY720 has been implicated in the regulation of dendritic cell development and modulation of effector functions such as antigen processing, Th-priming and cytokine production (Arlt et al., 2014; Idzko et al., 2002; Muller et al., 2005; Radeke et al., 2005). Recently, it was shown that regulation of S1PR1 expression represents another mechanism of altering sphingolipid signaling in colitis. S1PR1 expression is mainly regulated during chronic inflammation in T lymphocytes. Increased S1PR1 expression was also observed in the endothelium, and may represent an activation signal to recruit neutrophils and other cells from the circulation (Karuppuchamy et al., 2016).
Other pathways of S1P metabolism upstream of SK include the degradation of sphingomyelin to ceramide by SMases and the subsequent production of sphingosine by ceramidases. sphingomyelin is a dietary sphingolipid found in eggs, milk and meats and is a major component of eukaryotic membranes (Zeisel et al., 1986). Treatment of exogenous sphingomyelin in DSS-induced colitis and IL-10 KO mice results in aggravated tissue inflammation by promoting cell death of intestinal epithelial cells possibly due to increase ceramide synthesis and via release of lysosomal protease cathepsin D (Fischbeck et al., 2011). However, others have published that dietary sphingomyelin promotes an anti-inflammatory role in DSS-induced colitis in a PPAR-γ dependent manner (Mazzei et al., 2011). PPAR-γ is a nuclear receptor known to repress Toll-like receptor (TLR) inflammatory signaling by macrophages and colonic epithelial cells in UC (Bertin et al., 2013; Ogawa et al., 2005). While the role of dietary sphingomyelin in colitis remains to be clarified, regulation of sphingomyelin metabolic enzymes has been implicated in UC (Braun et al., 2009). Alkaline SMase (Alk-SMase) is expressed by intestinal epithelial cells and its activity was found reduced in patients with longstanding colitis (Sjoqvist et al., 2002). Consequently, other studies found that Alk-SMase activity increases with a diet rich in fiber and decreases with a high fat content diet (Cheng et al., 2004). In colitis patients, probiotic treatment also increases the activity of Alk-SMase and it correlates with a decreased disease index score (Fig 1) (Soo et al., 2008). Additionally, the role of ceramidases in UC has recently been investigated. These studies, however, have found that despite the expression of neutral CDase and alkaline CDase 3 in the intestinal mucosa, these enzymes do not contribute to S1P-mediated inflammation and actually may have a protective role in UC (Fig 1) (Snider et al., 2012; Wang et al., 2016). Thus it remains inconclusive which sphingolipid enzymes and metabolites function in connection to drive particular mechanistic signatures of inflammation in UC.
5. Neutrophil regulation and function in intestinal immunity and inflammation
Homeostatic regulation of neutrophil development
Neutrophils are polymorphonuclear (PMN) leukocytes best characterized for their effector function in acute inflammation. As one of the first leukocytes recruited to inflammatory sites, neutrophils are critical for host protection during infection and their deficiency or defective function results in severe immunopathologies (Bouma et al., 2010; Lanini et al., 2016). In humans, neutrophils constitute the major population of circulating leukocytes, approximately 50–70%, whereas in mice, neutrophils represent only 5–10% of blood leukocyte population (Fournier and Parkos, 2012; Kolaczkowska and Kubes, 2013; Mestas and Hughes, 2004). Neutrophils develop from a common myeloid progenitor in the medulla of the bone marrow and undergo a series of stages during maturation, starting with the myoblast and culminating with the PMN cell, in a process regulated by the transcription factors PU.1 and C/EBPα-ζ (Kolaczkowska and Kubes, 2013). For mobilization of neutrophils to the blood, retention factors in the bone marrow have to be inactivated. The constitutive production of CXCL12 by stromal cells in the bone marrow promotes a retention signal for neutrophils by interacting with its receptor CXCR4. Gain of function mutations in CXCR4 are observed in patients with warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome. WHIM patients suffer from severe neutropenia despite having normal to increased neutrophils in the bone marrow (Christopher and Link, 2007). At steady state, marginated pools of mature neutrophils can be found in other peripheral organs besides the bone marrow such as the spleen, liver and lung. It is suggested that these pools serve as reservoirs for rapid deployment during inflammation (Summers et al., 2010). Granulocyte colony stimulating factor (G-CSF or CSF-3) is a multifaceted regulator of innate immunity and the major extracellular regulator of granulopoiesis. G-CSF drives the production of hematopoietic stem cell (HSC) in the bone marrow and mobilizes HSC and neutrophils to the blood by negatively regulating CXCL12 production and CXCR4 surface expression (Christopher and Link, 2007). S1P has also been implicated in the regulation of HSC egress from the bone marrow (Bendall and Basnett, 2013). Furthermore, G-CSF orchestrates the differentiation, survival and proliferation of neutrophils and influences the priming of mature neutrophils for activation of effector functions. Deficiency of G-CSF or its receptor G-CSFR in mice results in severe neutropenia (Bendall and Bradstock, 2014). At steady state, the levels of G-CSF in the plasma are low to undetectable but rapidly increase during inflammatory stress (Roberts, 2005). G-CSF production is regulated in the bone marrow stroma and peripheral tissues by multiple cells, largely tissue myeloid cells, but also endothelial, epithelial, fibroblast and other mesenchymal cells (Bugl et al., 2012{Bendall, 2014 #160). While various pro-inflammatory stimuli including LPS, TNF-α, IL-1β, and VEGF can influence the production of G-CSF in vitro, the synthesis is primarily driven by the positive feedback axis of IL-23/IL-17A/G-CSF (Roberts, 2005). This model of granulopoiesis is sometimes refereed as the “turnstile model” and it suggests that the site of neutrophil clearance would function as the feedback sensor to regulate the production of neutrophils in the bone marrow (Fig 2) (Bugl et al., 2012). Under steady state, low numbers of neutrophils transmigrate into tissues but rapidly undergo apoptosis in the absence of inflammatory stimuli and are cleared by tissue resident phagocytes - mainly macrophages and dendritic cells. Engulfment of apoptotic neutrophils will initiate anti-inflammatory signals and down regulate the production of IL-23 by these tissue phagocytes, negatively regulating IL-17 synthesis by γδ and NK T cells and subsequently inhibiting G-CSF production. Mice deficient in adhesion molecules required for tissue transmigration exhibit neutrophilia since cells are unable to effectively exit the vasculature and thus create a dysfunctional feedback loop in G-CSF signaling. This phenotype is also observed in human leukocyte adhesion deficiencies (LAD) (von Vietinghoff and Ley, 2008). This feedback mechanism also mediates granulopoiesis and rapid mobilization of neutrophils during inflammation; delayed neutrophil apoptosis in combination with inflammatory cytokines and TLRs agonists activate the production of IL-23 by tissue myeloid cells that in a paracrine manner stimulate the turnstile model of IL-17A-driven G-CSF synthesis. The critical role of macrophages in regulating granulopoiesis is highlighted by the deficiency of splenic marginal zone and bone marrow stromal macrophages. The conditional macrophage deficient model was engineered by deleting the anti-apoptotic gene cellular FLICE-like inhibitory protein (c-FLIP) in myeloid cells using LysM-Cre recombinase technology. Deficiency in these pools of macrophages resulted in elevated numbers of neutrophils in the bone marrow, blood, peritoneum and spleen, and increased serum levels of G-CSF and IL-1β. Other studies targeting conditional deletion of conventional dendritic cells have reported similar findings (Birnberg et al., 2008). These data indicate that failure of neutrophil clearance at steady state by macrophages or dendritic cells dysregulates the feedback loop of G-CSF production (Gordy et al., 2011). Although G-CSF is considered the major driver of neutropoiesis, other not-yet identified pathways of neutrophil regulation exist, as evidenced by the presence of small number of neutrophils in G-CSF KO mice (Bugl et al., 2012). Recent data suggests that the commensal microflora also plays a critical role in neutrophil homeostasis. Germ-free mice display an even more drastic reduction in peripheral neutrophils than G-CSF KO mice, in a mechanism independent of G-CSF regulation (Hibbs et al., 2007).
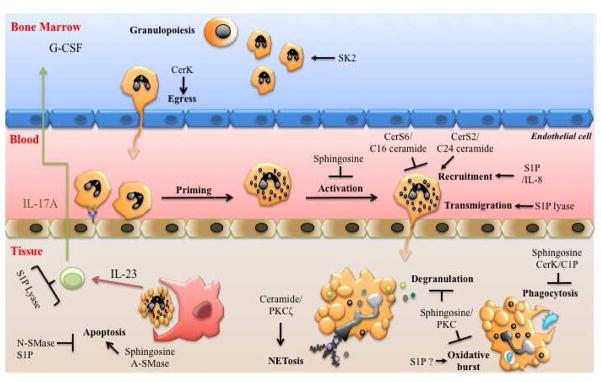
Figure 2. Bioactive sphingolipids regulate multiple aspects of neutrophil development and function.
Sphingosine kinase 2 (SK2) regulates the number of neutrophils in the bone marrow and ceramide kinase (CerK) promotes their egress to the blood. Neutrophil priming and/or activation in the periphery is inhibited by sphingosine. Ceramide synthase (CerS) 2 and CerS6 reciprocally regulate aspects of neutrophil recruitment to tissues. Chemotaxis is also regulated by sphingosine-1-phosphate (S1P) via interleukin (IL)-8 production. Transmigration through endothelial cell junctions is promoted by S1P lyase. In the tissue, various sphingolipid enzymes and metabolites can regulate neutrophil effector functions, such as phagocytosis, oxidative stress degranulation and production of neutrophil extracellular traps (NETosis). Finally, neutrophil apoptosis is inversely regulated by neutral and acid sphingomyelinases (N-SMase and A-SMase) via different metabolic pathways. Apoptotic neutrophils are recognized and engulfed by professional phagocytes to regulate the feedback loop of neutropoiesis via the IL-23/IL-17A/G-CSF signaling axis. Protein kinase C (PKC).
Neutrophil recruitment
Neutrophil recruitment from the vasculature to the tissue is regulated by multiple intrinsic and extrinsic factors. The endothelial adhesion molecules P-selectin and E-selectin capture free-flowing neutrophils in the vasculature though ligand binding. Once captured, neutrophils will roll along the vessel in the direction of the blood flow until inflammatory stimuli is sensed for priming and activation. Cytokines and chemokines originating from peripheral tissues line the lumen of the endothelium to prime and subsequently activate neutrophils. The class of ELR-CXC chemokines that includes CXCL8 (IL-8) in humans and CXCL1 (KC), CXCL2, and CXCL5 in mice plays a critical role in neutrophil chemotaxis. These chemokines are immobilized on the surface of endothelium to form an intravascular chemokine gradient that is sensed via the neutrophil receptor CXCR2. Priming and chemokine sensing induces conformational changes in cell surface-expressed integrins on neutrophils to increase binding affinity to receptors on the endothelium, primarily intracellular adhesion molecules (ICAM)-1 and -2. Ligation of integrins promotes crawling and subsequent transmigration of neutrophils through the vasculature cell wall, also known as diapedesis (Kolaczkowska and Kubes, 2013).
Neutrophil priming and activation
The activity of neutrophils is rapid yet very robust, thus it is regulated in a concerted manner to avoid unnecessary activation. At steady state, circulating peripheral neutrophils remain dormant until proper priming and activation signals are implemented. Priming serves to position neutrophils on “ready” mode and is induced by three major classes of pro-inflammatory stimuli: (a) pathogen derived molecular patterns (PAMPs) such as formylated peptide (fMLF), and LPS; (b) host derived danger associated molecular patterns (DAMPS) and other pro-inflammatory factors such as complement activation peptide C5a, leukotriene B4 (LTB4), and platelet activating factor (PAF); (c) growth factors and pro-inflammatory cytokines, primarily G-CSF, GM-CSF, TNF-α, IL-1β, IL-8, and IL-18. Adhesion and ligation of integrin receptors also has been shown in vitro to prime neutrophils (El-Benna et al., 2016{Bendall, 2014 #160; Wright et al., 2010). Intracellularly, priming induces the mobilization of primary granules with pre-formed receptors as cargo for a rapid increase of cell surface receptors to detect cytokines, chemokine and other activating signals. Priming will also stimulate de novo expression of genes critical for neutrophil bactericidal function and survival.
The activation alarm “is pressed” when sufficient danger is sensed in the form of infection or tissue injury, resulting in activation of the endothelium and a rapid emigration of neutrophils into tissue. Activation also induces neutrophil microbicidal and inflammatory mechanisms for pathogen clearance and is induced by various inflammatory stimuli. The threshold for activation is determined by the combinatorial stimulation of different agents. In vitro, some agonists, such as fMLF and C5a, serve dual roles in priming and activation depending on the concentration. Other potent activators of neutrophil effector function include opsonized particles, cell membrane derived arachidonic acid metabolites and the PKC activator phorbol 12-myristate 1-acetate (PMA) (Brandes et al., 2014; El-Benna et al., 2016; Singel and Segal, 2016). Activated neutrophils engage in microbicidal and inflammatory function, modulation of adaptive immunity, and reestablishment of homeostasis (Jones et al., 2016). A major mechanism of intracellular microbial killing is via production of reactive oxygen species (ROS) by NADPH oxidase (NOX) complex – also termed respiratory or oxidative burst - and through the activity of myeloperoxidase (MPO) in phagosomes (Panday et al., 2015). Phagocytic killing is also mediated by the production of antibacterial proteins such as defensins and lysozyme that can kill pathogens extracellularly when secreted from neutrophil granules (Coutinho et al., 2008; Kolaczkowska and Kubes, 2013). Neutrophil derived granules secrete proteases that have a role in proteolytic activation or degradation of cytokines and chemokines. Highly activated neutrophils can also kill pathogens extracellularly by releasing neutrophils extracellular traps (NETs). NETs are composed of decondensed chromatin, proteins and enzymes such as MPO. NETs immobilize pathogens to facilitate phagocytosis and also kill them through antibacterial components. In addition, NETs function as a scaffold for the trapping of viable, necrotic and apoptotic cells in a process referred as NETosis and by this mechanism are important regulators in the resolution of inflammation (Fig 2) (Hahn et al., 2016).
Neutrophil apoptosis
The dynamic relationship between neutrophil production in the bone marrow, release to the blood and clearance in the peripheral tissues is regulated in a feedback loop mechanism to maintain a homeostatic balance of cell numbers at steady state and during inflammatory resolution. At steady state, terminally differentiated neutrophils exhibit a short half-life of 6–8 hr and die spontaneously by apoptosis. Apoptotic neutrophils in tissues are recognized and cleared by professional phagocytes while senescent or damaged neutrophils in the vasculature are eliminated primarily in the liver, spleen and bone marrow (Christopher and Link, 2007; Kolaczkowska and Kubes, 2013). While resting neutrophils exhibit short life span, apoptosis in activated cells is delayed, rendering extended life span for the execution of effector functions. Nevertheless, proper regulation of neutrophil apoptosis is critical for intestinal homeostasis during infection and immune activation to avoid collateral tissue damage, as evidenced by the inappropriate regulation that contributes to chronic inflammation in IBD (Brannigan et al., 2000). Furthermore, apoptosis of activated neutrophils prevents other forms of cell death that can lead to the release of DAMPs and toxic agents that can perpetuate tissue inflammation and injury (Fournier and Parkos, 2012).
Neutrophil protective function in the intestinal mucosa
Gut homeostasis is regulated by the tripartite relationship between epithelial cells, the microflora and immune system. The intestinal epithelial cells form a physical barrier to separate the microflora in the gut lumen and mucosal immune cells in the lamina propria. The equilibrium among these three systems ensures proper immunity towards pathogenic invaders and tolerance to the commensal microflora. During infection, pathogen associated molecular patterns (PAMPs) sensing by lamina propria leukocytes and danger associated molecular patterns (DAMPs) released by damaged epithelial cells activate an inflammatory program to recruit neutrophils from vasculature. Neutrophils are recruited within minutes, transmigrating through the activated endothelium towards the chemokine gradient originating from the local inflammatory site. As part of the innate immune response in the gut, neutrophils mediate antimicrobial function and release monocyte chemoattractants to amplify the inflammatory response. Neutrophils can also influence adaptive immunity by promoting B cell humoral responses and regulating MHC expression and T cell function via IFN-γ (Mantovani et al., 2011; Silva, 2010). Clearance of infection and resolution of the neutrophil inflammatory response is critical to prevent excessive tissue injury and loss of epithelial barrier integrity. Proper function of neutrophils during resolution contributes to intestinal mucosal healing and the maintenance of tissue integrity. During the latter phase of the acute inflammatory response, neutrophils will switch their transcriptional profile to produce pro-resolution growth factors and lipid mediators such as lipoxins, resolvins and protectins. Through the production of resolvin 1 and protectin D1, neutrophils negatively regulate the recruitment of additional neutrophils to inflamed mucosa. Degradation of extracellular matrix through the release of proteases like matrix metalloproteinase 9 (MMP9) in combination with phagocytosis of dead cells and bacteria promotes tissue rebuilding. Intrinsically, infiltrated neutrophils will undergo apoptosis and are subsequently cleared by macrophages, signaling downregulation of neutropoiesis in the bone marrow (Jones et al., 2016).
Pathological role of neutrophils in UC
Neutrophil infiltration in the intestinal mucosa is a characteristic of UC pathophysiology and the degree of persistence and migration across the surface epithelium correlates with disease severity. The transmigration of activated neutrophils through epithelial paracellular and gap junctions, and their accumulation in the crypt lumen results in the formation of crypt abscesses, the characteristic histological lesions of UC. Breaching of the epithelial barrier increases permeability, facilitating the translocation of luminal bacteria into the lamina propria (Fournier and Parkos, 2012; Muthas et al., 2016). The persistence of activated neutrophils in the intestinal mucosa underscores the dysfunction in regulatory mechanisms of intestinal homeostasis. Evidence suggests that defective neutrophil function is implicated in UC pathophysiology. Various genes involved in the IL-23/IL-17A signaling axis (e.g. IL-23, STAT3, IL-12B) have been identified as susceptibility loci in IBD (Lees et al., 2011). Deletion of STAT3 in myeloid cells promotes increased influx of neutrophils and development of chronic enterocolitis (Lampinen et al., 2008). Another gene involved in increased susceptibility is IL-10, the critical regulator of inflammatory resolution. IL-10 KO mice exhibit elevated recruitment of activated neutrophils to the intestinal mucosa due to increased expression of the chemokine CXCL1 by epithelial cells. This data implies that development of spontaneous colitis in these mice is in part influenced by aberrant neutrophil regulation (Song et al., 1999).
However, the role of neutrophils in UC is controversial, likely perplexed by the complexity of IBD etiology and the multifaceted role of these cells in the intestines. The discrepancy arises from different studies describing, both, beneficial and detrimental roles for neutrophils in IBD. The role of neutrophils as effector cells of UC inflammation and pathogenesis is supported by studies using monoclonal antibody-mediated depletion of neutrophils in DSS-induced colitis. The results demonstrated that diminished neutrophil infiltrate partially protected from tissue injury caused by the production of reactive oxygen species (ROS) (Natsui et al., 1997). Blockade of neutrophil vascular transmigration with antibodies against adhesion protein Mac-1 also reduced the infiltrate and ulceration in TNBS-induced colitis (Palmen et al., 1995). In contrast, a follow up study found that depletion of neutrophils using anti-neutrophil serum worsened inflammation in a murine model of epithelial barrier injury using DSS- and TNBS-induced colitis. Increased bacterial translocation and morbidity concurred with the loss of neutrophils in the inflamed mucosa, highlighting the protective role of neutrophil against opportunistic infections in the gut. This study however, also found that neutrophil deficiency aggravated chronic T-cell mediated colitis induced by the adoptive transfer of CD4+CD45RBhigh lymphocytes (Kuhl et al., 2007). Moreover, depletion of macrophages and dendritic cells results in increase influx of intestinal neutrophils in DSS-induced colitis. Neutrophil-mediated tissue injury was inhibited in the absence of all mononuclear phagocytes, highlighting the coordinated function of myeloid cells in regulating intestinal inflammation (Fournier and Parkos, 2012; Qualls et al., 2006). Finally, delayed neutrophil apoptosis in human colonic mucosal tissue has been observed and may be induced by the elevated levels of G-CSF and GM-CSF in IBD (Noguchi et al., 2001) (Brannigan et al., 2000; Ina et al., 1999).
6. Sphingolipids in neutrophil regulation
Sphingolipids and oxidative burst: early connections
NOX activation is regulated by PKCδ (Bertram and Ley, 2011; Cosentino-Gomes et al., 2012) and the search for the biochemical mechanisms underlying this cellular process lead to one of the earliest reports on bioactive sphingolipids. In 1986 Wilson and colleagues identified the inhibitory role of sphingosine on oxidative burst, a process known to be regulated by PKC in human neutrophils (Fig 2) (Wilson et al., 1986). For this study, cytochrome C reduction was used as a functional readout of oxidative burst in response to various inducers. This report coincided with the pioneer discovery of sphingosine as an inhibitor of PKC and the bioactive nature of sphingolipids (Hannun et al., 1986). Additional studies also implicated sphingosine-mediated inhibition of PKC in the regulation of lactoferrin secretion from secondary granules (Wilson et al., 1987). Furthermore, while sphingosine is a major endogenous lipid in neutrophils, its cellular levels are negatively regulated during neutrophil activation (Wilson et al., 1988). Exogenous sphingosine was subsequently used as a tool to establish the role of PKC as an activator of Fcγ receptors (FcγR)-mediated endocytosis (Moraru et al., 1990).
The involvement of bioactive sphingolipids in neutrophil respiratory burst was also investigated in the context of ceramide. Intracellular ceramide level was increased in neutrophils stimulated with fMLP (Nakamura et al., 1994) and arachidonic acid- possibly through the activity of neutral SMase (Robinson et al., 1997). TNF-α mediated priming of fMLP responses coincided with the metabolism of sphingomyelin (Niwa et al., 2000). And although treatment with exogenous C2 ceramide displayed an inhibitory effect like sphingosine, C2 ceramide function was attributed to phospholipase D (PLD) inhibition and not to sphingosine generated downstream of ceramide catabolism (Lian et al., 1998; Mansfield et al., 2002; Nakamura et al., 1994). Modulation of NET formation by ceramide was recently investigated in response to the estrogen receptor agonist tamoxifen. Tamoxifen regulated the bactericidal activity of neutrophils by activating PKCζ in a ceramide-dependent manner (Fig 2). In accordance with previous observations, exogenous ceramide treatment decreased ROS production and oxidative burst but significantly increased NETosis. In this study, tamoxifen treatment increased C16 ceramide- and to a lesser extent C24 ceramide - by inhibiting glucosylceramide synthase (Corriden et al., 2015). This recent observation helped to clarify the relative contribution of ceramide and sphingosine on neutrophil effector functions upon activation and provided further insight into the different metabolic pathways and intracellular signaling targets involved.
Dissecting the role of SK/S1P in neutrophil function
Experiments with fMLP demonstrated increased activity of SK1 and production of S1P in primary human neutrophils and differentiated HL60 cells (Alemany et al., 1999; MacKinnon et al., 2002 #118). In neutrophils, intracellular [Ca2+] mobilization represents a crucial link between inflammatory stimuli and downstream activation of cellular responses (Salmon and Ahluwalia, 2011). [Ca2+] mobilization, degranulation and oxidative burst were blocked in SK1 knock down experiments and with the SK inhibitors N,N-dimethylsphingosine (DMS) and D-L-threo-dihydrosphingosine (DHS) (Itagaki and Hauser, 2003; MacKinnon et al., 2002; Schenten et al., 2011). The involvement of S1P in promoting neutrophil oxidative burst was corroborated in recent experiments using FTY720, which mediated inhibition of ROS production in a dose-dependent manner (Wang et al., 2015). More recently, another group demonstrated that SK1 KO neutrophils showed decreased antifungal activity and the phenotype is rescued with extracellular S1P (Farnoud et al., 2015).
In contrast to these observations, a follow up study showed that peritoneal neutrophils from SK1 KO mice exhibited no defect in recruitment in a model of thioglycollate-elicited peritonitis and in NOX activation upon fMLP stimulation. The discrepancies were not explained by compensation of SK2, SPP or SPL mRNA expression in these cells. Furthermore, SK1 KO mice were not protected in chronic inflammation, as demonstrated in a model of collagen-induced rheumatoid arthritis, leading the authors to conclude that SK1 is not required for neutrophil responses and inflammation (Michaud et al., 2006). This conclusion was supported by a separate group; bone marrow neutrophils from either SK1 and SK2 KO mice at steady state were quantified and analyzed by flow cytometry. Although SK2 KO mice had a reduction of 25% of cells, neutrophil numbers in SK1 KO were unchanged when compared to control mice. [Ca2+] mobilization was also examined in response to C5a and fMLP (among other inducers), demonstrating no difference in SK1 or SK2 KO. NOX activation, chemotaxis, degranulation and cytokine production were all unchanged in activated neutrophils from all strains. Interestingly, SK2 KO mice exhibited accelerated morbidity and mortality in a model of S. pneumoniae lung infection, suggesting a difference in the role of SK isoforms during infection (Zemann et al., 2007). In support of previous findings, DMS treatment significantly inhibited [Ca2+] mobilization and NOX activation in WT neutrophils. These results argue against a role for SK1 in neutrophil function, and although DMS was found to inhibit SK1 (Cuvillier et al., 1996; Yatomi et al., 1996), the inhibitor displays non-specific effects that may account for its role in neutrophil oxidative burst (Kim et al., 2005; Xu et al., 2002).
S1P is a pleotropic bioactive lipid involved in multiple pro-inflammatory responses. The level of S1P in cells is regulated by multiple metabolic enzymes and although SK1 genetic deficiency does not influence neutrophil function in mice thioglycollate-elicited neutrophils and bone marrow derived neutrophils (Michaud et al., 2006; Zemann et al., 2007), there is evidence of selective and compensatory regulation of S1P metabolic enzymes in neutrophil inflammatory responses (Mechtcheriakova et al., 2007). In contrast to previous work, the expression and activity of SK1 and SK2 is reciprocally regulated in neutrophils treated with LPS and TNF-α, and the same compensation is observed with S1PP1 and S1PP2 (Mechtcheriakova et al., 2007). This implies that neutrophils have evolved multiple mechanisms by which to regulate the levels of S1P, and different metabolic axis of regulation may exist in different subsets of neutrophil and during different inflammatory environments.
Sphingolipids in neutrophil phagocytosis
Phagocytic killing is one of the two most important antimicrobial mechanisms of neutrophils. The phagocytic process activates NOX mediated oxidative burst and functions in concert with humoral mechanisms in innate and adaptive immunity. Phagocytosis requires opsonization of particles or microorganisms by immunoglobulins (IgG) and complement proteins. The opsonins can ligate and activate two classes of receptors on the surface of neutrophils, Fcγ receptors that bind the Fc region of IgG and the complement receptors recognizing the C3b/C3bi fragments of the complement system. Ligation of these receptors activates actin polymerization and cytoskeleton rearrangement for pseudopodia formation and particle engulfment into a phagosome (Dale et al., 2008). Assessment of sphingolipids in neutrophil phagocytosis showed that elevated production of ceramide correlated with culmination of the oxidative burst. Moreover, exogenous short chain ceramide and sphingosine could inhibit IgG-induced phagocytosis. It was postulated that lipid remodeling triggered upon Fcγ receptor ligation functioned as a switch to turn off phagocytosis. In fact, ceramide produced by sphingomyelin degradation was found to act on the MAPK signaling pathway to negatively regulate IgG-opsonized erythrocytes (Suchard et al., 1997a; Suchard et al., 1997b). The MAPK pathway is required for the induction of phagocytosis in neutrophils. In line with this report, others showed that ceramide produced by sphingomyelin degradation regulates degranulation, a subsequent step in phagosome formation (Feldhaus et al., 2002). Increased expression of neutral SMase on the plasma membrane has also been observed during neutrophil phagocytosis (Hinkovska-Galcheva, V. et al., 1998; Shamseddine et al., 2015). The role of ceramide metabolism was further characterized by the identification of CK and C1P function in neutrophils (Fig 2) (Hinkovska-Galcheva, V.T. et al., 1998; Rile et al., 2003). CK activity on the plasma membrane was shown to increase upon fMLP priming and phagocytosis of IgG-opsonized erythrocytes. These studies found that exogenous C1P could regulate the fusion of liposomes and suggested a role for CK in regulating phagolysosome fusion (Hinkovska-Galcheva, V.T. et al., 1998). Fusion and release of granule content into the phagosome is another critical antimicrobial mechanism in neutrophils (Dale et al., 2008). Neutrophils express three different types of Fcγ receptors, the transmembrane FcγRI and FcγRIIa and the GPI-anchored FcγRIIIb (Pricop and Salmon, 2002). Ligation of FcγRIIIb receptor correlates with intracellular calcium [Ca2+] flux. The rise in [Ca2+] is required for actin cytoskeleton rearrangement and was inhibited by the SK inhibitor DMS (Chuang et al., 2000). These data demonstrated that various axis of ceramide metabolism could be activated simultaneously to regulate the complex processes involved in neutrophil phagocytosis.
Regulation of neutrophil migration and recruitment by sphingolipids
Regulation of leukocyte migration is one of the best characterized functions of S1P signaling (Maceyka and Spiegel, 2014). The increased levels of S1P in the bronchoalveolar lavage fluid of asthmatic patients suggested a pro-inflammatory role for this metabolite in pulmonary inflammation. S1P secreted from airway epithelial cells indirectly promoted neutrophil recruitment by increasing IL-8 production and ICAM-1 expression in A549 alveolar epithelial cells (Fig 2). Paracrine signaling by S1P was mediated by signaling via the extracellular matrix-regulated kinase 1/2 (ERK1/2) pathway and subsequent activation of PLD and [Ca2+] flux (Milara et al., 2009). The compartmentalization of S1P in blood and tissues is a critical regulatory mechanism of lymphocyte trafficking and is dependent on S1PR signaling (Degagne and Saba, 2014). Lymphocyte egress out of primary and secondary lymphoid organs is promoted by the high concentration of S1P in blood and lymph. The irreversible degradation of S1P by S1P lyase manipulates the compartmentalization of S1P in the blood. Interestingly, while S1P lyase deficiency results in lymphopenia, neutrophils are elevated in the blood (Schwab et al., 2005; Vogel et al., 2009). The role of S1P lyase in neutrophil migration was in investigated in S1P lyase KO mice. The high levels of neutrophils in the blood resulted from a defect in neutrophil migration to the peripheral tissues, partially explained by decreased expression of adhesion molecules. Importantly, decreased numbers of neutrophils in tissues disrupted the IL-23/IL-17/G-CSF signaling axis of neutropoiesis. This was demonstrated by increased numbers neutrophils in the bone marrow and spleen and inability to induce thioglycollate-mediated peritonitis in S1P lyase KO mice. Neutrophilia was partially rescued in S1PR4 and lyase double KO mice but an intrinsic defect in neutrophil migration towards chemotactic factors was also observed (Allende et al., 2011). Neutrophilia was also observed in SK1 deficient mice during pregnancy and correlated high levels of CXCL1 and CXCL2. This study however found that the combined loss of SK1 with heterozygocity of SK2 expression lead to increased neutrophil infiltrate into the fetomaternal interphase promoting oxidative damage and embryo death (Mizugishi et al., 2015). It remains to be answered how the S1P gradient in tissues could be altering neutrophil migration. In light of this question, some studies have shown that modulation of S1P signaling using FTY720 also correlated with decreased neutrophil recruitment in ishchemic lesions after cerebral stroke. Depletion of peripheral leukocytes in this model could explain the decreased migration of neutrophils (Czech et al., 2009). However, the precise mechanism by which FTY720 regulates neutrophil migration in this and other in vivo studies is largely unknown and awaits further investigation (Finley et al., 2013; Sun et al., 2016). The cumulative data does suggest that S1P signaling is altering multiple aspects of neutrophil biology, possibly in a tissue specific manner, and thus conditional KO models would be useful to characterize these pathways.
Moreover, different pools of ceramide were found to regulate neutrophil migration in murine autoimmune encephalomyelitis (EAE), a model of multiple sclerosis. Neutrophils are major effectors of inflammation in EAE, causing demyelination and axonal degeneration at early stages of the disease (Wu et al., 2010). C24 ceramide generated by CerS2 promoted increased mRNA expression of CXCR2 in neutrophils. The induction of CXCR2 by G-CSF was blocked in CerS2 KO mice (Barthelmes et al., 2015). The same group also reported that, on the contrary, CerS6 KO exacerbated the development of EAE by enhancing neutrophil migration and tissue infiltration. The production of C16 ceramide by CerS6 inhibited neutrophil adhesion, and the expression of CXCR2. They postulated that Cers6 functions to regulate a feedback regulatory loop to inhibit migration and protects from the onset of disease (Fig 2) (Eberle et al., 2015).
Sphingolipids in neutrophil apoptosis, a critical checkpoint for tissue homeostasis
Bioactive sphingolipids are established regulators of apoptosis in multiple cells and model systems. While S1P is implicated in cell survival and proliferation, ceramide and sphingosine are generally considered pro apoptotic. Both the intrinsic and extrinsic apoptotic pathways are regulated by sphingolipids. Ceramide generated de novo or upon the hydrolysis of sphingomyelin or complex sphingolipids can influence apoptotic events downstream of various stimuli (Tirodkar and Voelkel-Johnson, 2012). In neutrophils, ROS, Fas ligand and other pro-inflammatory mediators can activate the intrinsic and extrinsic apoptotic machineries. The intrinsic pathway regulates spontaneous apoptosis while the extrinsic pathway is regulated by stress. The stimuli present in the inflammatory microenvironment determines the biochemical pathways that initiate the apoptotic cascade, but ultimately both pathways converged in the activation of caspases. TNF-α signaling has dynamic effects in neutrophils depending on the local concentration, regulating apoptotic pathways, priming and promoting oxidative burst (Wright et al., 2010). Acute TNF-α treatment in neutrophils resulted in an early production of ceramide followed by generation of sphingosine at longer time points. Long term TNF-α treatment resulted in DNA fragmentation, a hallmark of apoptotic cell death (Tirodkar and Voelkel-Johnson, 2012). Interestingly, exogenous sphingosine and not ceramide was shown to induce DNA fragmentation in human primary neutrophils and differentiated myeloid leukemic HL-60 cells (Ohta et al., 1995; Ohta et al., 1994). In line with its role in pro survival, S1P production was detected in alveolar neutrophils in a model of acute lung injury. Inhibition of alveolar apoptosis is implicated in the pathogenesis of acute lung injury and is regulated by neutral SMase activity and the dependent production of S1P (Fig 2) (Lin et al., 2011). While these studies did not find a role for acid-SMase in stress-induced apoptosis, the enzyme has been shown to regulate spontaneous apoptosis triggered by ROS. Acid-SMase deficient neutrophils did not exhibit early production of ceramide and consequently showed delayed apoptosis in culture (Scheel-Toellner et al., 2004). The role of ceramide in regulating spontaneous apoptosis was further characterized using mass spectrometry. The early production of ceramide preceding spontaneous apoptosis was confirmed in these studies as a result of de novo synthesis of C16 and C24 ceramide and not though the salvage pathway (Seumois et al., 2007). It is possible that multiple pathways of ceramide regulation are activated in a stimulus and environment specific context.
CerK/C1P and neutrophils
Ceramide kinase (CerK) has been identified as a critical regulator of immune responses and its role in neutrophil biology may extend beyond phagolysosome formation (Hinkovska-Galcheva and Shayman, 2010). CerK deficiency in mice results in severe neutropenia. The decreased numbers of blood neutrophils coincided with elevated levels of lymphocytes and did not appear to be due to a migration defect since neutrophil recruitment was not affected in thioglycolate-induced peritonitis (Graf et al., 2008). Interestingly, CerK KO mice also exhibited elevated counts of neutrophils in the bone marrow suggesting the possibility that CerK deficiency regulates neutrophil bone marrow egress (Fig 2). C16 and C18 ceramide species were significantly increased in the serum of CerK KO, and this was marked by a concomitant downregulation of dihydroceramide. Since neutrophils are critical immune effectors in lung infection, the consequence of CerK deficiency was investigated in a model of pneumonia pulmonary infection and the results indicated that CerK KO mice exhibited increased morbidity and mortality. Decreased survival in CerK KO mice may be due to decreased numbers of neutrophils in the lungs or defected antimicrobial function by these cells. Although the elevated levels of ceramide in serum could promote spontaneous apoptosis in circulating neutrophils, is not clear from these studies if this would hold true in the bone marrow or in primed neutrophils (Graf et al., 2008). It was recently reported that C1P exerts anti-inflammatory effects in human neutrophils in a model of chronic obstructive pulmonary disease (COPD). Exogenous C1P treatment protected from tissue injury and significantly reduced the neutrophil infiltrate in the lungs. Mice also presented decreased levels of pro-inflammatory cytokines IL-1β, IL-6, MIP-1 and the neutrophil chemoattractant CXCL1. Mechanistically, the authors demonstrated that human neutrophils from COPD patients have increased levels of NFkB-driven IL-8 production and the phenotype is reversed with C1P treatment (Baudiss et al., 2015; Baudiss et al., 2016).
Sphingolipids in neutrophil related pathophysiology
Much effort has centered on investigating the role of ceramide metabolism in diseases where neutrophils are important regulators of inflammatory responses. The role of ceramide was evaluated in an in vivo model of allergic asthma, showing that inhibition of de novo ceramide generation using fumonicin B1 attenuated oxidative stress and epithelial cell apoptosis. Under severe oxidative stress caused by neutrophil activation, DNA damage in airway epithelial cells activates PARP, a nuclear enzyme and activator of apoptosis and necrosis (Masini et al., 2008). Fumonisin B1 administration in mice was also reported to reduce neutrophil infiltration assessed by MPO activity in a model of ischemia and reperfusion-induced shock (Cuzzocrea et al., 2008). Chronic airway inflammation is also characteristic of cystic fibrosis. The hereditary disease is caused by mutations in the cystic fibrosis transmembrane conductance regulator protein (CFTR). The defective function of the ion channel transporter contributes to the prevalence of opportunistic infections and neutrophil-mediated chronic inflammation in the lung. Ceramide accumulation has also been reported in airway epithelial cells of cystic fibrosis patients (Ulrich et al., 2010). Acid-SMase-mediated production of ceramide has been implicated in promoting pulmonary inflammation by recruiting activated neutrophils, and increasing susceptibility of bacterial infection (Becker et al., 2010). The pathology related to neutrophil inflammation is ameliorated in humans using a pharmacological inhibitor of acid-SMase or in the Cftr−/−/Smdp1−/− mouse model (Siegmann et al., 2014; Teichgraber et al., 2008). Acid-SMase-induction of ceramide not only targets epithelial cell apoptosis in the early phase of cystic fibrosis, it also plays an important role in the high susceptibility of Pseudomonas aeruginosa infection. Interestingly, during infection, neutrophils are targeted by the P. aeruginosa protein pyocyanin that activates the production of ROS, lysosome dysfunction and apoptosis in an acid -SMase-dependent manner (Prince et al., 2008). P. aeruginosa also accelerates the release of cytochrome c from mitochondria and the induction of apoptosis, this phenotype is inhibited in acid-SMase-deficient neutrophils and differentiated HL60 cells (Manago et al., 2015). Neutrophils represent double edge sword effectors in cystic fibrosis; while their recruitment in response to tissue damage has detrimental consequences, neutrophil inflammatory effector functions during P. aeruginosa are critical for host protection.
7. Conclusion and future perspectives
Progress in elucidating the mechanisms that underlie the development of UC is challenged by the complex and multifactorial pathobiology of the disease. It is now well recognized that sphingolipids are critical regulators of inflammation with significant potential as targets for therapeutic intervention in UC and other inflammatory conditions. S1P signaling is highlighted as an important regulatory pathway in inflammation, but accumulated evidence suggests that different pools of sphingolipid metabolites may contribute to different cellular biologies during the complex inflammatory profile in UC. Experimental data indicates that there is dysregulation of sphingolipid metabolism in UC, which could either take place at the onset of or is triggered upon inflammation. While both conditions may be operative, this is an important question that requires further clarification in order to appropriately translate experimental data into the clinic. The critical role of sphingolipids is further substantiated by the degree of functional compensation that exits among the metabolic enzymes. Clearly, tissue specific animal models will help our mechanistic understanding of the conditions that involve particular enzymes in different tissues and responses.
Neutrophils are an important component of innate myeloid responses in the gut and important drivers of inflammatory diseases. While neutrophil involvement in mucosal inflammation is a hallmark of UC, the conditions that promote a beneficial or detrimental role for these cells in the disease remain controversial. The degree of neutrophil density in the tissue may be an important determinant of their role in disease (Nathan, 2006). Calprotectin, a neutrophil derived protein complex, has been identified as a biomarker in UC and the levels correlate with the degree of neutrophil infiltrate in the gut (Muthas et al., 2016; Pruenster et al., 2016). The relevance of calprotectin as a metric of disease index in UC indicates that the complex regulation of neutrophil infiltration into the tissue may represent an important research area to block UC pathogenesis. Sphingolipids regulate multiple aspects of neutrophil biology and dysregulation of sphingolipid metabolism in UC is marked by alterations in neutrophil function. However, at present, the relationship between sphingolipid and neutrophils in UC requires further investigation. Deeper understanding of this relationship will clarify if bioactive sphingolipids regulate the threshold of neutrophil function in response to environmental cues in the gut. Alternatively, sphingolipids may impact UC pathobiology by regulating other aspects of neutrophil life cycle, such as development and differentiation in the bone marrow, regulation of marginalized pools or migration to tissues. Clearly, these are important research questions with potential for therapeutic intervention in UC and other diseases characterized by neutrophil dysfunction.
Acknowledgments
The original work in the laboratory of the authors was supported by a Veteran’s Administration Merit Award and a National Institute of Diabetes and Digestive and Kidney Diseases predoctoral grant # F31 DK105711-01 (MPE), Veteran’s Administration Merit Award (LMO), NIH Grants R01 GM097741 (LMO), and PO1CA097132 (LMO).
Footnotes
Disclosure
The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel Hadi L, Di Vito C, Riboni L. Fostering Inflammatory Bowel Disease: Sphingolipid Strategies to Join Forces. Mediators Inflamm. 2016;2016:3827684. doi: 10.1155/2016/3827684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Alemany R, Meyer zu Heringdorf D, van Koppen CJ, Jakobs KH. Formyl peptide receptor signaling in HL-60 cells through sphingosine kinase. J Biol Chem. 1999;274(7):3994–3999. doi: 10.1074/jbc.274.7.3994. [DOI] [PubMed] [Google Scholar]
- Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, Chen W, Saba JD, Proia RL. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2011;286(9):7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt O, Schwiebs A, Japtok L, Ruger K, Katzy E, Kleuser B, Radeke HH. Sphingosine-1-phosphate modulates dendritic cell function: focus on non-migratory effects in vitro and in vivo. Cell Physiol Biochem. 2014;34(1):27–44. doi: 10.1159/000362982. [DOI] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelmes J, de Bazo AM, Pewzner-Jung Y, Schmitz K, Mayer CA, Foerch C, Eberle M, Tafferner N, Ferreiros N, Henke M, Geisslinger G, Futerman AH, Grosch S, Schiffmann S. Lack of ceramide synthase 2 suppresses the development of experimental autoimmune encephalomyelitis by impairing the migratory capacity of neutrophils. Brain Behav Immun. 2015;46:280–292. doi: 10.1016/j.bbi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Basson A, Trotter A, Rodriguez-Palacios A, Cominelli F. Mucosal Interactions between Genetics, Diet, and Microbiome in Inflammatory Bowel Disease. Front Immunol. 2016;7:290. doi: 10.3389/fimmu.2016.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudiss K, Ayata CK, Lazar Z, Cicko S, Beckert J, Meyer A, Zech A, Vieira RP, Bittman R, Gomez-Munoz A, Merfort I, Idzko M. Ceramide-1-phosphate inhibits cigarette smoke-induced airway inflammation. Eur Respir J. 2015;45(6):1669–1680. doi: 10.1183/09031936.00080014. [DOI] [PubMed] [Google Scholar]
- Baudiss K, de Paula Vieira R, Cicko S, Ayata K, Hossfeld M, Ehrat N, Gomez-Munoz A, Eltzschig HK, Idzko M. C1P Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Preventing NF-kappaB Activation in Neutrophils. J Immunol. 2016;196(5):2319–2326. doi: 10.4049/jimmunol.1402681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KA, Riethmuller J, Zhang Y, Gulbins E. The role of sphingolipids and ceramide in pulmonary inflammation in cystic fibrosis. Open Respir Med J. 2010;4:39–47. doi: 10.2174/1874306401004010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall LJ, Basnett J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr Opin Hematol. 2013;20(4):281–288. doi: 10.1097/MOH.0b013e3283606090. [DOI] [PubMed] [Google Scholar]
- Bendall LJ, Bradstock KF. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25(4):355–367. doi: 10.1016/j.cytogfr.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Bertin B, Dubuquoy L, Colombel JF, Desreumaux P. PPAR-gamma in ulcerative colitis: a novel target for intervention. Curr Drug Targets. 2013;14(12):1501–1507. doi: 10.2174/13894501113149990162. [DOI] [PubMed] [Google Scholar]
- Bertram A, Ley K. Protein kinase C isoforms in neutrophil adhesion and activation. Arch Immunol Ther Exp (Warsz) 2011;59(2):79–87. doi: 10.1007/s00005-011-0112-7. [DOI] [PubMed] [Google Scholar]
- Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29(6):986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Blankenbach KV, Schwalm S, Pfeilschifter J, Meyer Zu Heringdorf D. Sphingosine-1-Phosphate Receptor-2 Antagonists: Therapeutic Potential and Potential Risks. Front Pharmacol. 2016;7:167. doi: 10.3389/fphar.2016.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma G, Ancliff PJ, Thrasher AJ, Burns SO. Recent advances in the understanding of genetic defects of neutrophil number and function. Br J Haematol. 2010;151(4):312–326. doi: 10.1111/j.1365-2141.2010.08361.x. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N, Schroder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Brannigan AE, O’Connell PR, Hurley H, O’Neill A, Brady HR, Fitzpatrick JM, Watson RW. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock. 2000;13(5):361–366. doi: 10.1097/00024382-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, Lehmann WD, Fuellekrug J, Stremmel W, Ehehalt R. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis. 2009;15(11):1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- Bugl S, Wirths S, Muller MR, Radsak MP, Kopp HG. Current insights into neutrophil homeostasis. Ann N Y Acad Sci. 2012;1266:171–178. doi: 10.1111/j.1749-6632.2012.06607.x. [DOI] [PubMed] [Google Scholar]
- Carroll B, Donaldson JC, Obeid L. Sphingolipids in the DNA damage response. Advances in Biological Regulation. 2015;58:38–52. doi: 10.1016/j.jbior.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ohlsson L, Duan RD. Psyllium and fat in diets differentially affect the activities and expressions of colonic sphingomyelinases and caspase in mice. Br J Nutr. 2004;91(5):715–723. doi: 10.1079/BJN20041107. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14(1):3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Chuang FY, Sassaroli M, Unkeless JC. Convergence of Fc gamma receptor IIA and Fc gamma receptor IIIB signaling pathways in human neutrophils. J Immunol. 2000;164(1):350–360. doi: 10.4049/jimmunol.164.1.350. [DOI] [PubMed] [Google Scholar]
- Corriden R, Hollands A, Olson J, Derieux J, Lopez J, Chang JT, Gonzalez DJ, Nizet V. Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat Commun. 2015;6:8369. doi: 10.1038/ncomms9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR. Cell signaling through protein kinase C oxidation and activation. Int J Mol Sci. 2012;13(9):10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho HD, Lobo KM, Bezerra DA, Lobo I. Peptides and proteins with antimicrobial activity. Indian J Pharmacol. 2008;40(1):3–9. doi: 10.4103/0253-7613.40481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Di Paola R, Genovese T, Mazzon E, Esposito E, Crisafulli C, Bramanti P, Salvemini D. Anti-inflammatory and anti-apoptotic effects of fumonisin B1, an inhibitor of ceramide synthase, in a rodent model of splanchnic ischemia and reperfusion injury. J Pharmacol Exp Ther. 2008;327(1):45–57. doi: 10.1124/jpet.108.139808. [DOI] [PubMed] [Google Scholar]
- Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A, Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun. 2009;389(2):251–256. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112(4):935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178(4):2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- Degagne E, Pandurangan A, Bandhuvula P, Kumar A, Eltanawy A, Zhang M, Yoshinaga Y, Nefedov M, de Jong PJ, Fong LG, Young SG, Bittman R, Ahmedi Y, Saba JD. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J Clin Invest. 2014;124(12):5368–5384. doi: 10.1172/JCI74188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degagne E, Saba JD. S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer. Clin Exp Gastroenterol. 2014;7:205–214. doi: 10.2147/CEG.S43453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan RD, Nilsson A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog Lipid Res. 2009;48(1):62–72. doi: 10.1016/j.plipres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Eberle M, Ebel P, Mayer CA, Barthelmes J, Tafferner N, Ferreiros N, Ulshofer T, Henke M, Foerch C, de Bazo AM, Grosch S, Geisslinger G, Willecke K, Schiffmann S. Exacerbation of experimental autoimmune encephalomyelitis in ceramide synthase 6 knockout mice is associated with enhanced activation/migration of neutrophils. Immunol Cell Biol. 2015;93(9):825–836. doi: 10.1038/icb.2015.47. [DOI] [PubMed] [Google Scholar]
- El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273(1):180–193. doi: 10.1111/imr.12447. [DOI] [PubMed] [Google Scholar]
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Farnoud AM, Bryan AM, Kechichian T, Luberto C, Del Poeta M. The Granuloma Response Controlling Cryptococcosis in Mice Depends on the Sphingosine Kinase 1-Sphingosine 1-Phosphate Pathway. Infect Immun. 2015;83(7):2705–2713. doi: 10.1128/IAI.00056-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaus MJ, Weyrich AS, Zimmerman GA, McIntyre TM. Ceramide generation in situ alters leukocyte cytoskeletal organization and beta 2-integrin function and causes complete degranulation. J Biol Chem. 2002;277(6):4285–4293. doi: 10.1074/jbc.M106653200. [DOI] [PubMed] [Google Scholar]
- Finley A, Chen Z, Esposito E, Cuzzocrea S, Sabbadini R, Salvemini D. Sphingosine 1-phosphate mediates hyperalgesia via a neutrophil-dependent mechanism. PLoS One. 2013;8(1):e55255. doi: 10.1371/journal.pone.0055255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbeck A, Leucht K, Frey-Wagner I, Bentz S, Pesch T, Kellermeier S, Krebs M, Fried M, Rogler G, Hausmann M, Humpf HU. Sphingomyelin induces cathepsin D-mediated apoptosis in intestinal epithelial cells and increases inflammation in DSS colitis. Gut. 2011;60(1):55–65. doi: 10.1136/gut.2009.201988. [DOI] [PubMed] [Google Scholar]
- Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5(4):354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- Fujii R, Kanai T, Nemoto Y, Makita S, Oshima S, Okamoto R, Tsuchiya K, Totsuka T, Watanabe M. FTY720 suppresses CD4+CD44highCD62L- effector memory T cell-mediated colitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(2):G267–274. doi: 10.1152/ajpgi.00496.2005. [DOI] [PubMed] [Google Scholar]
- Gordy C, Pua H, Sempowski GD, He YW. Regulation of steady-state neutrophil homeostasis by macrophages. Blood. 2011;117(2):618–629. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf C, Zemann B, Rovina P, Urtz N, Schanzer A, Reuschel R, Mechtcheriakova D, Muller M, Fischer E, Reichel C, Huber S, Dawson J, Meingassner JG, Billich A, Niwa S, Badegruber R, Van Veldhoven PP, Kinzel B, Baumruker T, Bornancin F. Neutropenia with impaired immune response to Streptococcus pneumoniae in ceramide kinase-deficient mice. J Immunol. 2008;180(5):3457–3466. doi: 10.4049/jimmunol.180.5.3457. [DOI] [PubMed] [Google Scholar]
- Hahn J, Knopf J, Maueroder C, Kienhofer D, Leppkes M, Herrmann M. Neutrophils and neutrophil extracellular traps orchestrate initiation and resolution of inflammation. Clin Exp Rheumatol. 2016;34(4 Suppl 98):6–8. [PubMed] [Google Scholar]
- Hanauer SB. Review article: aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):60–65. doi: 10.1111/j.1365-2036.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Loomis CR, Merrill AH, Jr, Bell RM. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261(27):12604–12609. [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, Dunn AR. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178(10):6435–6443. doi: 10.4049/jimmunol.178.10.6435. [DOI] [PubMed] [Google Scholar]
- Hinkovska-Galcheva V, Kjeldsen L, Mansfield PJ, Boxer LA, Shayman JA, Suchard SJ. Activation of a Plasma Membrane–Associated Neutral Sphingomyelinase and Concomitant Ceramide Accumulation During IgG-Dependent Phagocytosis in Human Polymorphonuclear Leukocytes. Blood. 1998;91(12):4761–4769. [PubMed] [Google Scholar]
- Hinkovska-Galcheva V, Shayman JA. Ceramide-1-phosphate in phagocytosis and calcium homeostasis. Adv Exp Med Biol. 2010;688:131–140. doi: 10.1007/978-1-4419-6741-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkovska-Galcheva VT, Boxer LA, Mansfield PJ, Harsh D, Blackwood A, Shayman JA. The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion. J Biol Chem. 1998;273(50):33203–33209. doi: 10.1074/jbc.273.50.33203. [DOI] [PubMed] [Google Scholar]
- Huang WC, Liang J, Nagahashi M, Avni D, Yamada A, Maceyka M, Wolen AR, Kordula T, Milstien S, Takabe K, Oravecz T, Spiegel S. Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans. FASEB J. 2016;30(8):2945–2958. doi: 10.1096/fj.201600394R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, Dichmann S, Mockenhaupt M, Gebicke-Haerter P, Di Virgilio F, Girolomoni G, Norgauer J. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002;16(6):625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- Ina K, Kusugami K, Hosokawa T, Imada A, Shimizu T, Yamaguchi T, Ohsuga M, Kyokane K, Sakai T, Nishio Y, Yokoyama Y, Ando T. Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in Inflammatory Bowel disease. J Gastroenterol Hepatol. 1999;14(1):46–53. doi: 10.1046/j.1440-1746.1999.01807.x. [DOI] [PubMed] [Google Scholar]
- Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003;278(30):27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo R, Figliuzzi MM, Monteleone G. Sphingosine-1-phosphate receptor: a novel therapeutic target in ulcerative colitis. Expert Rev Clin Immunol. 2016:1–3. doi: 10.1080/1744666X.2016.1217774. [DOI] [PubMed] [Google Scholar]
- Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28(2):137–145. doi: 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Karuppuchamy T, Behrens EH, Gonzalez-Cabrera P, Sarkisyan G, Gima L, Boyer JD, Bamias G, Jedlicka P, Veny M, Clark D, Peach R, Scott F, Rosen H, Rivera-Nieves J. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharel Y, Lee S, Snyder AH, Sheasley-O’neill SL, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Pearson-White S, Lynch KR. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280(44):36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim YW, Inagaki Y, Hwang YA, Mitsutake S, Ryu YW, Lee WK, Ha HJ, Park CS, Igarashi Y. Synthesis and evaluation of sphingoid analogs as inhibitors of sphingosine kinases. Bioorg Med Chem. 2005;13(10):3475–3485. doi: 10.1016/j.bmc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kuhl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, Loddenkemper C, Heimesaat MM, Grollich K, Zeitz M, Farkas S, Hoffmann JC. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133(6):1882–1892. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12(9):688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampinen M, Sangfelt P, Taha Y, Carlson M. Accumulation, activation, and survival of neutrophils in ulcerative colitis: regulation by locally produced factors in the colon and impact of steroid treatment. Int J Colorectal Dis. 2008;23(10):939–946. doi: 10.1007/s00384-008-0509-x. [DOI] [PubMed] [Google Scholar]
- Lanini LL, Prader S, Siler U, Reichenbach J. Modern Management of Phagocyte defects. Pediatr Allergy Immunol. 2016 doi: 10.1111/pai.12654. [DOI] [PubMed] [Google Scholar]
- Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- Lian JP, Huang R, Robinson D, Badwey JA. Products of sphingolipid catabolism block activation of the p21-activated protein kinases in neutrophils. J Immunol. 1998;161(8):4375–4381. [PubMed] [Google Scholar]
- Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, Takabe K, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23(1):107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WC, Hanauer SB. Controversies with aminosalicylates in inflammatory bowel disease. Rev Gastroenterol Disord. 2004;4(3):104–117. [PubMed] [Google Scholar]
- Lin WC, Lin CF, Chen CL, Chen CW, Lin YS. Inhibition of neutrophil apoptosis via sphingolipid signaling in acute lung injury. J Pharmacol Exp Ther. 2011;339(1):45–53. doi: 10.1124/jpet.111.181560. [DOI] [PubMed] [Google Scholar]
- Liu TC, Stappenbeck TS. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu Rev Pathol. 2016;11:127–148. doi: 10.1146/annurev-pathol-012615-044152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510(7503):58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AC, Buckley A, Chilvers ER, Rossi AG, Haslett C, Sethi T. Sphingosine kinase: a point of convergence in the action of diverse neutrophil priming agents. J Immunol. 2002;169(11):6394–6400. doi: 10.4049/jimmunol.169.11.6394. [DOI] [PubMed] [Google Scholar]
- Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, Upson JJ, Smith CD. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53(4):997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manago A, Becker KA, Carpinteiro A, Wilker B, Soddemann M, Seitz AP, Edwards MJ, Grassme H, Szabo I, Gulbins E. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid Redox Signal. 2015;22(13):1097–1110. doi: 10.1089/ars.2014.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield PJ, Hinkovska-Galcheva V, Shayman JA, Boxer LA. Granulocyte colony-stimulating factor primes NADPH oxidase in neutrophils through translocation of cytochrome b(558) by gelatinase-granule release. J Lab Clin Med. 2002;140(1):9–16. doi: 10.1067/mlc.2002.124551. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, Comhair SA, Li D, Cuzzocrea S, Matuschak GM, Salvemini D. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. J Pharmacol Exp Ther. 2008;324(2):548–557. doi: 10.1124/jpet.107.131565. [DOI] [PubMed] [Google Scholar]
- Mazzei JC, Zhou H, Brayfield BP, Hontecillas R, Bassaganya-Riera J, Schmelz EM. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: importance of peroxisome proliferator-activated receptor gamma expression. J Nutr Biochem. 2011;22(12):1160–1171. doi: 10.1016/j.jnutbio.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakova D, Wlachos A, Sobanov J, Kopp T, Reuschel R, Bornancin F, Cai R, Zemann B, Urtz N, Stingl G, Zlabinger G, Woisetschlager M, Baumruker T, Billich A. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal. 2007;19(4):748–760. doi: 10.1016/j.cellsig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580(19):4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Milara J, Mata M, Mauricio MD, Donet E, Morcillo EJ, Cortijo J. Sphingosine-1-phosphate increases human alveolar epithelial IL-8 secretion, proliferation and neutrophil chemotaxis. Eur J Pharmacol. 2009;609(1–3):132–139. doi: 10.1016/j.ejphar.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Inoue T, Hatayama H, Bielawski J, Pierce JS, Sato Y, Takaori-Kondo A, Konishi I, Yamashita K. Sphingolipid pathway regulates innate immune responses at the fetomaternal interface during pregnancy. J Biol Chem. 2015;290(4):2053–2068. doi: 10.1074/jbc.M114.628867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima T, Ito T, Kishi D, Kai Y, Tamagawa H, Nezu R, Kiyono H, Matsuda H. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis. 2004;10(3):182–192. doi: 10.1097/00054725-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Moraru II, Laky M, Stanescu T, Buzila L, Popescu LM. Protein kinase C controls Fc gamma receptor-mediated endocytosis in human neutrophils. FEBS Lett. 1990;274(1–2):93–95. doi: 10.1016/0014-5793(90)81337-n. [DOI] [PubMed] [Google Scholar]
- Muller H, Hofer S, Kaneider N, Neuwirt H, Mosheimer B, Mayer G, Konwalinka G, Heufler C, Tiefenthaler M. The immunomodulator FTY720 interferes with effector functions of human monocyte-derived dendritic cells. Eur J Immunol. 2005;35(2):533–545. doi: 10.1002/eji.200425556. [DOI] [PubMed] [Google Scholar]
- Muthas D, Reznichenko A, Balendran CA, Bottcher G, Groth Clausen I, Karrman Mardh C, Ottosson T, Uddin M, MacDonald TT, Danese S, Berner Hansen M. Neutrophils in Ulcerative Colitis: A review of selected biomarkers and their potential therapeutic implications. Scand J Gastroenterol. 2016:1–45. doi: 10.1080/00365521.2016.1235224. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Abe A, Balazovich KJ, Wu D, Suchard SJ, Boxer LA, Shayman JA. Ceramide regulates oxidant release in adherent human neutrophils. J Biol Chem. 1994;269(28):18384–18389. [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Natsui M, Kawasaki K, Takizawa H, Hayashi SI, Matsuda Y, Sugimura K, Seki K, Narisawa R, Sendo F, Asakura H. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J Gastroenterol Hepatol. 1997;12(12):801–808. doi: 10.1111/j.1440-1746.1997.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Niwa M, Kozawa O, Matsuno H, Kanamori Y, Hara A, Uematsu T. Tumor necrosis factor-alpha-mediated signal transduction in human neutrophils: involvement of sphingomyelin metabolites in the priming effect of TNF-alpha on the fMLP-stimulated superoxide production. Life Sci. 2000;66(3):245–256. doi: 10.1016/s0024-3205(99)00587-1. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Hiwatashi N, Liu ZX, Toyota T. Increased secretion of granulocyte-macrophage colony-stimulating factor in mucosal lesions of inflammatory bowel disease. Digestion. 2001;63(Suppl 1):32–36. doi: 10.1159/000051908. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122(5):707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Sweeney EA, Masamune A, Yatomi Y, Hakomori S, Igarashi Y. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: a possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 1995;55(3):691–697. [PubMed] [Google Scholar]
- Ohta H, Yatomi Y, Sweeney EA, Hakomori S, Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 1994;355(3):267–270. doi: 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- Palmen MJ, Dijkstra CD, van der Ende MB, Pena AS, van Rees EP. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin Exp Immunol. 1995;101(2):351–356. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12(1):5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17(11):1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- Pricop L, Salmon JE. Redox regulation of Fcgamma receptor-mediated phagocytosis: implications for host defense and tissue injury. Antioxid Redox Signal. 2002;4(1):85–95. doi: 10.1089/152308602753625889. [DOI] [PubMed] [Google Scholar]
- Prince LR, Bianchi SM, Vaughan KM, Bewley MA, Marriott HM, Walmsley SR, Taylor GW, Buttle DJ, Sabroe I, Dockrell DH, Whyte MK. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J Immunol. 2008;180(5):3502–3511. doi: 10.4049/jimmunol.180.5.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruenster M, Vogl T, Roth J, Sperandio M. S100A8/A9: From basic science to clinical application. Pharmacol Ther. 2016 doi: 10.1016/j.pharmthera.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, Jiang HR, Ubhi S, Pyne S. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Advances in Biological Regulation. 2016;60:151–159. doi: 10.1016/j.jbior.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80(4):802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- Radeke HH, von Wenckstern H, Stoidtner K, Sauer B, Hammer S, Kleuser B. Overlapping signaling pathways of sphingosine 1-phosphate and TGF-beta in the murine Langerhans cell line XS52. J Immunol. 2005;174(5):2778–2786. doi: 10.4049/jimmunol.174.5.2778. [DOI] [PubMed] [Google Scholar]
- Rile G, Yatomi Y, Takafuta T, Ozaki Y. Ceramide 1-phosphate formation in neutrophils. Acta Haematol. 2003;109(2):76–83. doi: 10.1159/000068491. [DOI] [PubMed] [Google Scholar]
- Rivera-Nieves J. Strategies that target leukocyte traffic in inflammatory bowel diseases: recent developments. Curr Opin Gastroenterol. 2015;31(6):441–448. doi: 10.1097/MOG.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23(1):33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- Robinson BS, Hii CS, Poulos A, Ferrante A. Activation of neutral sphingomyelinase in human neutrophils by polyunsaturated fatty acids. Immunology. 1997;91(2):274–280. doi: 10.1046/j.1365-2567.1997.d01-2227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon MD, Ahluwalia J. Pharmacology of receptor operated calcium entry in human neutrophils. Int Immunopharmacol. 2011;11(2):145–148. doi: 10.1016/j.intimp.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Sawicka E, Dubois G, Jarai G, Edwards M, Thomas M, Nicholls A, Albert R, Newson C, Brinkmann V, Walker C. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J Immunol. 2005;175(12):7973–7980. doi: 10.4049/jimmunol.175.12.7973. [DOI] [PubMed] [Google Scholar]
- Scheel-Toellner D, Wang K, Assi LK, Webb PR, Craddock RM, Salmon M, Lord JM. Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem Soc Trans. 2004;32(Pt 5):679–681. doi: 10.1042/BST0320679. [DOI] [PubMed] [Google Scholar]
- Schenten V, Melchior C, Steinckwich N, Tschirhart EJ, Brechard S. Sphingosine kinases regulate NOX2 activity via p38 MAPK-dependent translocation of S100A8/A9. J Leukoc Biol. 2011;89(4):587–596. doi: 10.1189/jlb.0510304. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309(5741):1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Seumois G, Fillet M, Gillet L, Faccinetto C, Desmet C, Francois C, Dewals B, Oury C, Vanderplasschen A, Lekeux P, Bureau F. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J Leukoc Biol. 2007;81(6):1477–1486. doi: 10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- Shamseddine AA, Airola MV, Hannun YA. Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Advances in Biological Regulation. 2015;57:24–41. doi: 10.1016/j.jbior.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmann N, Worbs D, Effinger F, Bormann T, Gebhardt M, Ulrich M, Wermeling F, Muller-Hermelink E, Biedermann T, Tighe M, Edwards MJ, Caldwell C, Leadbetter E, Karlsson MC, Becker KA, Gulbins E, Doring G. Invariant natural killer T (iNKT) cells prevent autoimmunity, but induce pulmonary inflammation in cystic fibrosis. Cell Physiol Biochem. 2014;34(1):56–70. doi: 10.1159/000362984. [DOI] [PubMed] [Google Scholar]
- Silva MT. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J Leukoc Biol. 2010;87(5):805–813. doi: 10.1189/jlb.1109767. [DOI] [PubMed] [Google Scholar]
- Singel KL, Segal BH. NOX2-dependent regulation of inflammation. Clin Sci (Lond) 2016;130(7):479–490. doi: 10.1042/CS20150660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow D, Sunkara M, Morris A, Wattenberg B. Regulation of de novo sphingolipid biosynthesis by the ORMDL proteins and sphingosine kinase-1. Advances in Biological Regulation. 2015;57:42–54. doi: 10.1016/j.jbior.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Sjoqvist U, Hertervig E, Nilsson A, Duan RD, Ost A, Tribukait B, Lofberg R. Chronic colitis is associated with a reduction of mucosal alkaline sphingomyelinase activity. Inflamm Bowel Dis. 2002;8(4):258–263. doi: 10.1097/00054725-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Snider AJ, Ali WH, Sticca JA, Coant N, Ghaleb AM, Kawamori T, Yang VW, Hannun YA, Obeid LM. Distinct roles for hematopoietic and extra-hematopoietic sphingosine kinase-1 in inflammatory bowel disease. PLoS One. 2014;9(12):e113998. doi: 10.1371/journal.pone.0113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 2009;23(1):143–152. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Orr Gandy KA, Obeid LM. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie. 2010;92(6):707–715. doi: 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Wu BX, Jenkins RW, Sticca JA, Kawamori T, Hannun YA, Obeid LM. Loss of neutral ceramidase increases inflammation in a mouse model of inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2012;99(3–4):124–130. doi: 10.1016/j.prostaglandins.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Ito K, Denning TL, Kuninger D, Papaconstantinou J, Gourley W, Klimpel G, Balish E, Hokanson J, Ernst PB. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J Immunol. 1999;162(4):2275–2280. [PubMed] [Google Scholar]
- Soo I, Madsen KL, Tejpar Q, Sydora BC, Sherbaniuk R, Cinque B, Di Marzio L, Cifone MG, Desimone C, Fedorak RN. VSL#3 probiotic upregulates intestinal mucosal alkaline sphingomyelinase and reduces inflammation. Can J Gastroenterol. 2008;22(3):237–242. doi: 10.1155/2008/520383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchard SJ, Hinkovska-Galcheva V, Mansfield PJ, Boxer LA, Shayman JA. Ceramide inhibits IgG-dependent phagocytosis in human polymorphonuclear leukocytes. Blood. 1997a;89(6):2139–2147. [PubMed] [Google Scholar]
- Suchard SJ, Mansfield PJ, Boxer LA, Shayman JA. Mitogen-activated protein kinase activation during IgG-dependent phagocytosis in human neutrophils: inhibition by ceramide. J Immunol. 1997b;158(10):4961–4967. [PubMed] [Google Scholar]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WY, Dimasi DP, Pitman MR, Zhuang Y, Heddle R, Pitson SM, Grimbaldeston MA, Bonder CS. Topical Application of Fingolimod Perturbs Cutaneous Inflammation. J Immunol. 2016;196(9):3854–3864. doi: 10.4049/jimmunol.1501510. [DOI] [PubMed] [Google Scholar]
- Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tummler B, Lang F, Grassme H, Doring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14(4):382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- Tirodkar TS, Voelkel-Johnson C. Sphingolipids in apoptosis. Exp Oncol. 2012;34(3):231–242. [PubMed] [Google Scholar]
- Ulrich M, Worlitzsch D, Viglio S, Siegmann N, Iadarola P, Shute JK, Geiser M, Pier GB, Friedel G, Barr ML, Schuster A, Meyer KC, Ratjen F, Bjarnsholt T, Gulbins E, Doring G. Alveolar inflammation in cystic fibrosis. J Cyst Fibros. 2010;9(3):217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ, Sun W, Swaffield J, Oravecz T. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One. 2009;4(1):e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181(8):5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Xu R, Snider AJ, Schrandt J, Li Y, Bialkowska AB, Li M, Zhou J, Hannun YA, Obeid LM, Yang VW, Mao C. Alkaline ceramidase 3 deficiency aggravates colitis and colitis-associated tumorigenesis in mice by hyperactivating the innate immune system. Cell Death Dis. 2016;7:e2124. doi: 10.1038/cddis.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Graeler MH, Goetzl EJ. Physiological sphingosine 1-phosphate requirement for optimal activity of mouse CD4+ regulatory T Cells. FASEB J. 2004;18(9):1043–1045. doi: 10.1096/fj.04-1555fje. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fan H, Xie R, Yang J, Ren Y, Yang Y, Li W. The Effect of Sphingosine 1-Phosphate/Sphingosine 1-Phosphate Receptor on Neutrophil Function and the Relevant Signaling Pathway. Acta Haematol. 2015;134(1):49–56. doi: 10.1159/000369291. [DOI] [PubMed] [Google Scholar]
- Wilson E, Olcott MC, Bell RM, Merrill AH, Jr, Lambeth JD. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986;261(27):12616–12623. [PubMed] [Google Scholar]
- Wilson E, Rice WG, Kinkade JM, Jr, Merrill AH, Jr, Arnold RR, Lambeth JD. Protein kinase C inhibition by sphingoid long-chain bases: effects on secretion in human neutrophils. Arch Biochem Biophys. 1987;259(1):204–214. doi: 10.1016/0003-9861(87)90487-5. [DOI] [PubMed] [Google Scholar]
- Wilson E, Wang E, Mullins RE, Uhlinger DJ, Liotta DC, Lambeth JD, Merrill AH., Jr Modulation of the free sphingosine levels in human neutrophils by phorbol esters and other factors. J Biol Chem. 1988;263(19):9304–9309. [PubMed] [Google Scholar]
- Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49(9):1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- Wu F, Cao W, Yang Y, Liu A. Extensive infiltration of neutrophils in the acute phase of experimental autoimmune encephalomyelitis in C57BL/6 mice. Histochem Cell Biol. 2010;133(3):313–322. doi: 10.1007/s00418-009-0673-2. [DOI] [PubMed] [Google Scholar]
- Xu CB, Zhang Y, Stenman E, Edvinsson L. D-erythro-N,N-dimethylsphingosine inhibits bFGF-induced proliferation of cerebral, aortic and coronary smooth muscle cells. Atherosclerosis. 2002;164(2):237–243. doi: 10.1016/s0021-9150(02)00100-4. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35(2):626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- Ye BD, McGovern DP. Genetic variation in IBD: progress, clues to pathogenesis and possible clinical utility. Expert Rev Clin Immunol. 2016;12(10):1091–1107. doi: 10.1080/1744666X.2016.1184972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr. 1986;116(1):50–58. doi: 10.1093/jn/116.1.50. [DOI] [PubMed] [Google Scholar]
- Zemann B, Urtz N, Reuschel R, Mechtcheriakova D, Bornancin F, Badegruber R, Baumruker T, Billich A. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol Lett. 2007;109(1):56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]