Abstract
Background
Cognitive bias is a common characteristic of major depressive disorder (MDD) and is posited to remain during remission and contribute to recurrence risk. Attention bias may be related to enhanced amygdala activity or altered amygdala functional connectivity in depression. The current study examined attention bias, brain activity for emotional images, and functional connectivity in post-menopausal women with and without a history of major depression.
Methods
Attention bias for emotionally valenced images was examined in 33 postmenopausal women with (n=12) and without (n=21) a history of major depression using an emotion dot probe task during fMRI. Group differences in amygdala activity and functional connectivity were assessed using fMRI and examined for correlations to attention performance.
Results
Women with a history of MDD showed greater attentional bias for negative images and greater activity in brain areas including the amygdala for both positive and negative images (pcorr < 0.001) than women without a history of MDD. In all participants, amygdala activity for negative images was correlated with attention facilitation for emotional images. Women with a history of MDD had significantly greater functional connectivity between the amygdala and hippocampal complex. In all participants amygdala – hippocampal connectivity was positively correlated with attention facilitation for negative images.
Limitations
Small sample with unbalanced groups.
Conclusions
These findings provide evidence for negative attentional bias in euthymic, remitted depressed individuals. Activity and functional connectivity in limbic and attention networks may provide a neurobiological basis for continued cognitive bias in remitted depression.
Keywords: attention, depression, functional MRI, cognition, brain imaging/neuroimaging
1. Introduction
Major depressive disorder (MDD) is a complex disorder that impacts multiple systems in the brain and alters emotional and cognitive processes that are integral to healthy daily functioning and quality of life. The incidence and prevalence of MDD in women is 2–3 times higher than in men (Kessler et al., 2003). In men new onset rates and 12 month prevalence of MDD remain fairly constant from puberty to old age, but increase in women at puberty and remain higher than men until menopause (Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, 2005; Kessler, 2003). Although new-onset MDD in women generally declines in following the menopause transition, women with previous depressive episodes remain at high risk for recurrence(Freeman et al., 2014). Identifying trait-like factors that remain during remission may help identify novel targets for treatments and interventions aimed at reducing depression recurrence in these women.
Cognitive bias for negative emotional information may contribute to mood disorder risk. Beck’s model posits that greater attention or memory for negative events contributes to the development, maintenance, and recurrence of depression by influencing schemas about the self and the world(Beck, 2005; Beck and Haigh, 2014). Consistent with this cognitive model depressed and dysthymic individuals show enhanced memory (Hallion and Ruscio, 2011) and attention(Duque and Vazquez, 2015; Isaac et al., 2014; Sears et al., 2011; Wiens and Syrjänen, 2013) for negative information. Attention bias may present as enhanced initial orientation of attention to emotionally-valenced stimuli or difficulty disengaging from stimuli that have captured attention. Previous studies of attention bias in currently depressed and at-risk individuals have demonstrated difficulty disengaging attention from emotionally-valenced stimuli (Gotlib et al., 2004; Joormann et al., 2007). Studies examining biased attentional orientation toward emotional-stimuli have provided inconsistent results, however orientation can be affected by stimulus type and duration and many studies have used stimuli better suited to assess attention bias related to anxiety than depression (Gotlib and Joormann, 2010).
A causative link between cognitive bias and mood is suggested by the finding that modulating attention bias also affects anxiety and depressive symptoms (Hallion and Ruscio, 2011). Experimentally, attention bias modification has been shown to be able to both decrease depressive symptoms (by reducing negative bias) and increase depressive symptoms (by increasing negative bias)(Hallion and Ruscio, 2011), indicating that bias in attention may contribute directly to depressive symptoms rather than being a consequence of depressed mood.
An important component of the cognitive model of depression is that cognitive bias is not only related to current mood-state or stress but also to mood-independent cognitive schemas and altered cognitive processing of emotional information that remain during remission and euthymia (Beck and Haigh, 2014; Sears et al., 2011). Mood-congruent cognitive biases have been reliably found in currently depressed individuals and are associated with the severity of depressive symptoms (Gaddy and Ingram, 2014). Cognitive bias in euthymic, remitted depressed individuals may indicate a trait cognitive vulnerability for depression (Joormann and Gotlib, 2007; Sears et al., 2011) that contributes to the high risk of recurrence (Keller and Berndt, 2002; Kessler et al., 2003; Solomon et al., 1997).
Brain activity or functional connectivity differences that remain in remission may represent a neurobiological basis for continued cognitive biases and vulnerability to depression. Altered activity in fronto-limbic circuits has been associated with attention bias, negative mood, and rumination in currently depressed individuals. De Raedt and Koster (2010) posit a model of depression vulnerability in which decreased dorsal prefrontal activity and increased amygdala activity contributes to negative mood and risk for future depressive episodes (De Raedt and Koster, 2010). This model is supported by studies demonstrating that both currently depressed (Arnone et al., 2012), and at risk populations (Zhong et al., 2011) show greater amygdala activity to negative emotional stimuli than never depressed healthy controls. Attention bias for negative information in remitted depressed individuals may be related to greater amygdala responses for negative stimuli and altered functional connectivity.
To assess attention bias in euthymic post-menopausal women with remitted depression we examined performance and functional brain activity in participants with and without a history of depression during an emotion dot probe (EDP) task. We used fMRI during the EDP task to examine brain activity associated with differences in attentional bias for emotionally-valenced images. We examined resting-state functional connectivity before the EDP task to assess differences in intrinsic amygdala functional connections to other brain areas between the groups. This intrinsic functional connectivity, measured before the task and in the absence of negative-mood induction, may represent stable, trait-like differences in amygdala function (unrelated to task or mood). Altered functional interaction between brain regions responsible for emotional processing may predispose processing of negative emotional information in women with past MDD history despite being euthymic and reveal a neurobiological basis for cognitive vulnerability to depression recurrence in these women.
We hypothesized that participants with a history of MDD would show attention bias for negative images that would not be seen in participants without a history of MDD, and that attention bias would be associated with greater amygdala activity for negative images and altered amygdala functional connectivity.
2. Methods
2.1 Participants
Thirty three right handed postmenopausal women between the ages of 45–75 were included in this study (Past MDD: n=12; No MDD: n=21). Participants were recruited for a larger study examining the effects of estradiol replacement on stress responding in postmenopausal women with and without a history of depression. Participants were recruited through notices in local media and direct mailings which described the study as having a focus on cognition after menopause. Potential participants completed a screening visit before approval for study inclusion. The results reported here include data from all women who completed baseline study visits (pre-estradiol treatment). No participants received estradiol treatment prior to or during participation in the procedures described here. None of the participants were taking ovarian hormones and were at least one year without such treatment. This study was approved by the Vanderbilt University Institutional Review Board and informed consent was obtained from all participants.
2.2 Cognitive Screening
All participants were cognitively assessed using: the Wechsler Abbreviated Scale of Intelligence (WASI-II) (Wechsler, 2013), the Mini Mental State Exam (MMSE) (Folstein et al., 1975), Mattis Dementia Rating Scale (DRS-2) (Mattis, 1988), Brief Cognitive Rating Scale, and the Global Deterioration Scale (GDS) (Reisberg et al., 1982). Participants were required to have a GDS score of 1–2 and a MMSE score of greater than 25. No participant scored below 123 on the Mattis scale or below 90 on the WASI; participants were of average or above intelligence with no evidence of dementia or mild cognitive impairment.
2.3 MDD History Screening
Participants were screened for current and past depression, mania and dysthymia using the partial Structured Clinical Interview for DSM-IV-TR (SCID) (Spitzer et al., 1992). Participants were excluded for a history of premenstrual dysphoric disorder, or any axis I diagnosis (current or past) other than MDD.
Criteria for never depressed participants were: no current or past episodes that met SCID criteria for MDD, dysthymia, or mania, a current score less than 7 on the Beck Depression Inventory (BDI), and less than 15 on Beck Anxiety Inventory (BAI) (Beck, 1978).
Criteria for prior history of MDD were: at least one episode, in the last ten years that met criteria for MDD on the SCID, no MDD episodes in the last year, current BDI score less than 7, and current BAI less than 15.
2.4 Emotion Dot Probe (EDP) Task
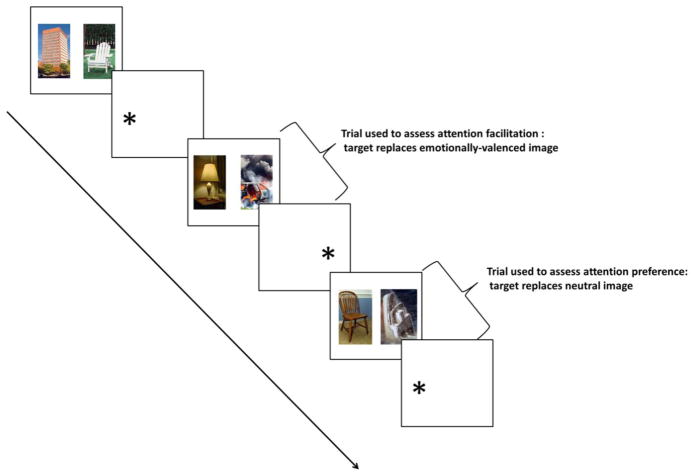
The EDP task is a spatial attention task that measures attention bias (Kimonis et al., 2006; Muñoz Centifanti et al., 2013). The EDP task used in this study was a picture variant using images from the International Affective Pictures System (IAPS) (Lang, P.J., Bradley, M.M., & Cuthbert, 2008) and included neutral, positive, and negative (threat and distress) images (Figure 1). Trials of the EDP consisted of a fixation cross presented in the middle of the screen, followed by a brief presentation (500 ms) of a picture pair with one image each on the right and left of the screen. After the picture presentation, a target (asterisk) appeared either on the right or left of the screen (replacing one of the images) and the participant was instructed to indicate by finger button press the side of the screen on which the target appeared as quickly as possible (available response duration was 1750 ms). The time between the target’s appearance and the subject’s response was used for the calculations of reaction time; with incorrect trials excluded (Supplementary Table 1).
Figure 1.
Emotion Dot Probe (EDP) Task
The EDP was adapted for MRI and run as an event related design (1 run) with three trial types relevant to the presented data: “Neutral” trials (60 trials): neutral-neutral pair; “Negative” trials(60 trials): neutral-negative (valence: mean = 2.58, SD = 0.92; arousal: mean = 5.56, SD = 0.88) pair; and “Positive” trials (60 trials): neutral-positive (valence: mean = 7.53, SD = 0.48; arousal: mean = 4.72, SD = 0.91) pair. There were no trials in which negative and positive images were presented together and the presentation order of trial types was randomized. In 2/3 (40 trials) of “Negative” or “Positive” trials the target replaced the emotionally valenced image.
Attention facilitation is the primary outcome measure of the EDP task and represents bias in orienting attention towards emotionally-valenced images (Kimonis et al., 2006). For each emotional trial type, attention facilitation was defined as the difference in reaction time between “Negative” or “Positive” trials and “Neutral” trials. A shorter reaction time for “Negative” or “Positive” trials indicated attention facilitation for negative or positive images.
In 1/3 (20 trials) of “Negative” and “Positive” trials the target replaced the neutral image. These trials were used for the measurement of attention preference (in other literature referred to as attention bias) which represents difficulty disengaging attention from the location of emotionally-valenced images once the target replaces the neutral image (Kimonis et al., 2006). Attention preference was measured as the difference in reaction time for trials in which the target replaced the neutral image and trials in which the target replaced the emotionally valenced image. Only correct trials were included in performance measure calculations (Supplementary Table 1).
2.5 EDP Analysis
The effect of valence on overall EDP performance was assessed using a multivariate analysis of variance (MANOVA) including EDP performance measures (facilitation and preference) as dependent variables with trial valence (positive and negative) and group (MDD Hx− and MDD Hx+)as independent variables.
A separate MANOVA including valenced EDP performance measures (negative facilitation, positive facilitation, negative preference, and positive preference) as dependent variables with group (MDD Hx− and MDD Hx+) and trait anxiety (STAI) as independent variables was conducted. This analysis was designed to assess the effects of MDD history separately from the effects of trait anxiety (which is often high in individuals with MDD), and to assess any interactive effect between MDD history and trait anxiety.
2.6 Self-rated Measures
Before the EDP, participants completed self-rated measures including: the State – Trait Anxiety Inventory (STAI) (Spielberger, 1983), the Stress Arousal Checklist (SACL) (King et al., 1983), and the Profile of Mood States (POMS) (McNair et al., 1989). Following the EDP, participants completed a second SACL and POMS, and a visual analogue scale (VAS) for task perception including: the extent of their personal involvement, how stressful, new, uncontrollable, and unpredictable the task was, and whether they anticipated negative consequences. These measures have been previously used to assess the stressfulness of laboratory tasks and procedures (Kirschbaum et al., 1999; Kudielka et al., 2004). Independent t-tests were run for group differences in STAI, VAS, and for difference score on the SACL and POMS (pre to post EDP).
2.7 Imaging Parameters
Participants were scanned on a Philips 3.0 Tesla Achieva scanner, with an 8 channel head coil. All participants received the following MR sequences:
Sagittal T1-weighted 3D Turbo Field Echo Sensitivity Encoding (TFE SENSE) sequence perpendicular to the anterior commissure (AC) -posterior commissure (PC) line, repetition time (TR) of 9.9 ms, echo time (TE) of 4.6 ms, a flip angle of 8°, number signal averages (NSA) 1.0, a field of view (FOV) of 256 mm, a 256 × 256 matrix, and 1.0 mm slice thickness with no gap for 140 contiguous slices.
T2- weighted Gradient and Spin Echo (GRASE) sequence parallel to the AC-PC line, TR 2470 ms, TE 80 ms, NSA 3.0, FOV of 230 mm, 0.7 mm slice thickness with 5.0 mm gap for 28 slices
Echoplanar Blood Oxygenation Level Dependent (EpiBOLD) functional resting-state scan with transverse orientation, TR 1500 ms, TE 35 ms, flip angle 90°, 1 NSA for FOV 240 mm, 80 X 80 matrix, and 5.0 mm slice thickness with no gap, for 24 slices.
EpiBOLD functional sequence during the EDP with transverse orientation, TR 2500 ms, TE 35 ms, flip angle 90°, 1 NSA for, FOV 240, 240 X 128 matrix, and 4.0 mm slice thickness with no gap, with ascending interleaved acquisition, for 35 contiguous slices.
2.8 Imaging Analysis
fMRI data was processed using Statistical Parametric Mapping (SPM8) (Wellcome Department of Cognitive Neurology, 2008). Preprocessing included: realignment of the functional runs and correction for bulk-head motion, coregistration of functional and anatomical images for each participant, segmentation of the anatomical image, normalization of the anatomical and functional images to the standard MNI template, and spatial smoothing with a Gaussian filter (8 mm at full width, half maximum).
At first level analysis, T-maps were created from linear contrasts for the task conditions “Negative – Neutral” and “Positive – Neutral”; these T-maps were used in the whole-brain (masked for gray matter) 2nd - level analysis of participant group effects with two-sample t-tests. 2nd level t-tests were conducted to examine differences in brain activity for the contrast of task conditions “Negative-Neutral” and “Positive-Neutral” between the two participant groups (MDD Hx + and MDD Hx−). Average percent signal change (task condition – fixation time between trials) was calculated for each significant cluster from the second level group comparison using MARSBAR (Brett et al., 2002). Percent signal change was used for correlation analyses with behavioral measures.
The preprocessed functional images had a voxel size of 2 X 2 X 2 mm and cluster threshold correction was used to control for multiple comparisons (from voxel wise p = 0.005) to corrected p < 0.001 with k = 58 (corrected voxel-wise p = 0.000001) from alpha simulation in REST toolbox for SPM (Song et al., 2011).
2.9 Functional Connectivity Analysis
Each participant completed a resting scan (eyes open with fixation cross) before the EDP task scan. Images from the resting scan were preprocessed in SPM8 including: realignment of the functional runs and correction for bulk-head motion, coregistration of functional and anatomical images for each participant, segmentation of the anatomical image, normalization of the anatomical and functional images to the standard MNI template, and spatial smoothing with a Gaussian filter (6 mm at full width, half maximum). To evaluate resting state functional connectivity to amygdala, we entered right and left amygdala (anatomical ROI from the human AAL atlas defined in WFU PickAtlas) as seeds in a resting state functional connectivity analysis performed using the MATLAB-based functional connectivity toolbox Conn (Whitfield-Gabrieli and Nieto-Castanon, 2012). Each subject’s normalized structural and functional images and T1W tissue maps were used as input into Conn. The resulting BOLD time series were band-pass filtered (0.01–0.1 Hz) to further reduce noise and increase sensitivity. Volumes that exceed 2 mm of motion were identified using the ART toolbox in SPM. The output matrices of SPM movement were entered into CONN as first-level covariates; the mean BOLD time series from each seed was entered as a predictor in a multiple regression general linear model at each voxel.
Individual subject functional connectivity maps (in beta-weight units) were entered into a whole-brain (masked for gray matter) second level random effects analysis of participant group effects with two-sample t-tests. Cluster threshold correction was used to control for multiple comparisons (from voxel wise p = 0.005) to corrected p < 0.001 with k = 32 (corrected voxel-wise p = 0.000002) from alpha simulation in REST toolbox for SPM (Song et al., 2011).
3. Results
3.1 Participants
Participants in this study were 33 postmenopausal women. Twenty one women had no history of depression (MDD Hx−), and 12 had a past history of depression (MDD Hx+). Depression history was assessed using the SCID: time since first episode (M =17.81 years, SD = 10.85), number of episodes (M = 2.1, SD = 0.95), time since last episode (M = 4.1 years, SD = 2.38). There was no significant difference in age between the participant groups (Table 1). All participants scored within the normal range for age on the DRS, WASI IQ, and MMSE, and no participants endorsed significant impairments on the BCRS or GDS. There were no significant differences between groups on these measures nor on the NEO, BDI, or BAI, state anxiety, or trait anxiety inventories (Table 1), additionally all participants scored within the non-clinical range on these measures consistent with a euthymic state.
Table 1.
Participant Screening
| MDD Group
|
|||||
|---|---|---|---|---|---|
| MDD Hx − (n = 21) | MDD Hx + (n = 12) | ||||
|
| |||||
| Mean | Standard Deviation | Mean | Standard Deviation | p value | |
| Age | 60.64 Range: (53.03–68.89) |
6.81 | 62.42 Range: (53.93–74.80) |
5.79 | 0.45 |
| Dementia Rating Scale | 141.45 Range: (130 – 144) |
2.52 | 137.00 Range: (92 – 144) |
14.32 | 0.16 |
| WASI IQ | 118.10 Range: (98–136) |
10.17 | 115.55 Range: (100 – 140) |
12.36 | 0.54 |
| Global Deterioration Scale | 1.73 Range: (1–2) |
.46 | 1.75 Range: (1 – 2) |
.45 | 0.89 |
| Brief Cognitive Rating Scale | 9.36 Range: (2–12) |
1.14 | 9.83 Range: (8 – 12) |
1.34 | 0.29 |
| Mini Mental State Exam | 29.09 Range: (26–30) |
1.06 | 29.58 Range: (28–30) |
.67 | 0.16 |
| NEO - Neuroticism | 12.05 | 5.45 | 15.75 | 6.63 | 0.09 |
| NEO - Extraversion | 32.82 | 3.97 | 31.75 | 6.63 | 0.56 |
| NEO - Openness | 31.18 | 5.98 | 32.33 | 9.08 | 0.66 |
| NEO - Agreeableness | 38.77 | 5.00 | 38.33 | 5.05 | 0.81 |
| NEO - Conscientiousness | 36.32 | 5.57 | 35.33 | 8.21 | 0.68 |
| Beck Depression Inventory | 2.91 | 3.21 | 2.17 | 2.62 | 0.50 |
| Beck Anxiety Inventory | 2.68 | 2.95 | 3.08 | 3.26 | 0.72 |
Abbreviations: MDD = major depressive disorder; WASI = Wechsler Abbreviated Scale of Intelligence; NEO = Neuroticism-Extraversion-Openness Five Factor Inventory.
Two of the participants in the MDD HX+ group (and no participants in the MDD Hx−) were currently taking antidepressants during study participation. Participants taking antidepressant medication were required to have been on the same medication and dosage for at least 3 months. The effect of removing the 2 participants taking antidepressant medication from analysis was examined; all results remained significant and the direction of effects was not changed.
3.2 Self-rated Measures
There were no differences between participant groups on any of the subjective measures (Table 2). In an analysis including all participants, SACL arousal significantly decreased after the EDP compared to before the EDP (t (35) = 4.65, p < 0.001); there was no significant change in SACL stress (t (35) = −0.88, p = 0.38) or POMS total mood disturbance (p = 0.13). There was no significant difference between groups in the change in SACL arousal (t (34) = 0.52, p = 0.61), SACL stress (t (34) = 0.38, p = 0.71), or POMS total mood disturbance (t (34) = 0.80, p = 0.43) pre and post EDP. The EDP did not increase subjective stress or negative mood, and there was no difference between the MDD Hx+ and MDD Hx− groups in these self-rated measures.
Table 2.
Subjective Measures
| MDD Group
|
|||||
|---|---|---|---|---|---|
| MDD Hx− (n = 21) | MDD Hx+ (n = 12) | ||||
|
| |||||
| Mean | Standard Deviation | Mean | Standard Deviation | p value | |
| STAI - Trait Anxiety | 25.23 | 6.36 | 24.17 | 5.56 | 0.63 |
| STAI - State Anxiety | 26.91 | 6.83 | 28.75 | 5.38 | 0.43 |
| Pre SACL - Stress | 1.29 | 2.19 | .83 | 1.34 | 0.45 |
| Pre SACL - Arousal | 10.43 | 1.83 | 9.25 | 3.55 | 0.22 |
| Pre POMS - Total Mood Disturbance | −13.14 | 13.10 | −10.50 | 14.83 | 0.60 |
| Post SACL - Stress | 1.95 | 3.91 | 1.08 | 1.51 | 0.47 |
| Post SACL - Arousal | 7.36 | 3.76 | 6.67 | 3.58 | 0.60 |
| Post POMS - Total Mood Disturbance | −9.32 | 17.10 | −1.75 | 16.34 | 0.22 |
| VAS - Involved | 81.68 | 22.70 | 83.42 | 14.72 | 0.81 |
| VAS - Stressful | 19.77 | 22.16 | 22.42 | 25.17 | 0.75 |
| VAS - New | 75.86 | 27.12 | 68.58 | 40.07 | 0.53 |
| VAS - Uncontrollable | 26.41 | 33.00 | 23.92 | 23.02 | 0.82 |
| VAS - Unpredictable | 52.00 | 33.82 | 40.08 | 29.06 | 0.31 |
| VAS - Anticipate | 12.05 | 16.33 | 13.17 | 10.97 | 0.83 |
Abbreviations: STAI = State-Trait Anxiety Inventory; SACL = Stress Arousal Checklist; POMS = Profile of Mood States; VAS = visual analog scale.
3.3 Emotion Dot Probe Performance
In a MANOVA including valence and MDD history there was a main effect of valence on attention preference (F (1) = 10.62, p < 0.01) with greater overall preference for negative images than positive images (t (66) = 3.63, p <0.001), but not facilitation (F (1) = 0.01, p = 0.91). There was no significant interaction between MDD history and valence on attention facilitation (F (1) = =0.95, p = 0.33) or preference (F (1) = 0.38, p = 0.54). There were no significant differences in median reaction time between groups for any trial type (Supplementary Table 3).
The MDD Hx− group had significantly longer reaction times for trials in which the target replaced positive (t (20) = −3.99, p < 0.005) or negative (t (20) = −5.09, p < 0.005) images compared to neutral images. The MDD Hx+ group did not show a significant difference in reaction times for targets that replace positive (t (11) = −0.54, p = 0.60) or negative (t (11) = 0.05, p = 0.96) image compared to neutral images.
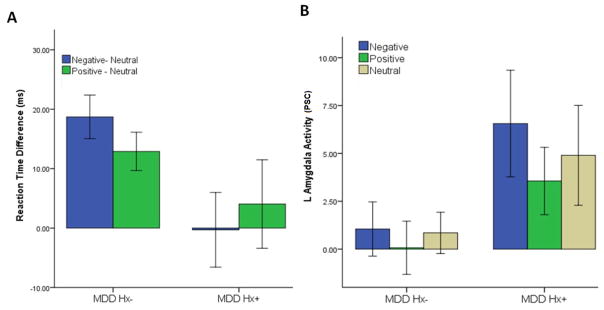
In a MANOVA including MDD history and trait anxiety (STAI), there was a main effect of MDD history on facilitation for negative images (F(1) = 8.20, p < 0.05) with the MDD Hx+ group showing greater facilitation for negative images (mean = −0.30 ms, SD = 16.08) than the MDD Hx− group (mean = 18.71 ms, SD = 13.65) (Figure 2). There was no significant main effect of MDD history on facilitation for positive images or on preference for either negative or positive images (Supplementary Table 1). There was no significant main effect of trait anxiety or a significant interaction effect of trait anxiety and MDD history on any facilitation or preference measures (Supplementary Table 2).
Figure 2. EDP Performance and Amygdala Activity.
A) MDD HX− group (n=21) showed greater reaction time for both negative and positive images than neutral images during the EDP. The MDD Hx+ (n=12) group did not show a reaction time difference between negative and neutral images. The difference in reaction time between neutral and negative images was significantly greater in the MDD Hx− group compared to the MDD Hx+ group (t (34) = 2.78, p < 0.01). B) Amygdala activity during the EDP was greater in the MDD HX+ during both negative positive images than the MDD Hx− group (corr p < 0.001). Error bars: +/− 1 SD.
3.4 Task fMRI
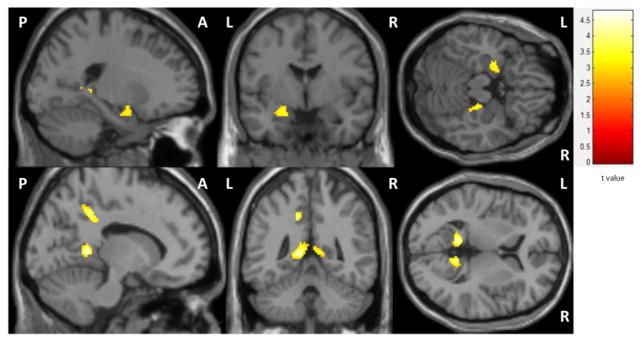
During negative image trials (“Negative”- “Neutral”) the MDD Hx+ group had significantly greater activity in the left amygdala, right para/hippocampus, and bilateral precuneus than the MDD HX− group (Figure 3). During positive image trials (“Positive”- “Neutral”), the MDD Hx+ group had greater activity in the left amygdala and bilateral precuneus than the MDD Hx− group. There was no difference in activity during trials that contained only neutral images.
Figure 3. EDP Brain Activity.
Negative images - neutral images: MDD HX+ > -MDD HX− (corr p < 0.001).MDD Hx+ group had greater activity in the left amygdala (MNI: −22, −2, −16) and right hippocampal complex (MNI: 22,−28,−14), and bilateral precuneus (MNI: −12, −46, 6; 10, −50, 8) during negative images than the MDD Hx− group.
Left amygdala activity for negative images was positively correlated with attention facilitation for both negative and positive images in an analysis of all participants, with the correlation stronger for negative facilitation (negative facilitation: r = 0.44, p < 0.01, positive facilitation: r = 0.34, p < 0.05) (Supplementary Figure 1). There was no association between amygdala activity for positive images and attention facilitation for either negative or positive images. Activity in the right parahippocampus and bilateral precuneus was not significantly correlated with negative or positive facilitation.
3.5 Functional Connectivity
Seeding right or left amygdala in a single t-test for all participants resulted in positive connectivity maps that included bilateral limbic regions (amygdala and hippocampus), temporal pole, inferior and superior temporal regions, basal ganglia, middle cingulum, insula, and inferior frontal regions. The mean number of volumes that exceeded 2 mm of motion per participant was 1.51 (SD = 0.46) with no significant difference between groups (t (31) = −0.11, p = 0.91). There was one outlier participant per group for volumes exceeding the motion threshold; removing these data from the analysis resulted in a mean number of volumes that exceeded 2 mm of motion per participant of 1.00 (SD = 0.31) with no significant difference between groups (t (29) = 0.43, p = 0.67), and did not alter the functional connectivity results, thus these data were included in all analyses.
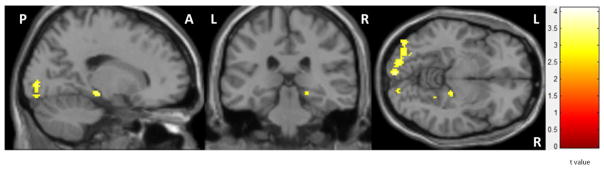
A second level independent t-test comparison of participant groups showed greater connectivity in the MDD Hx+ group between left amygdala and right parahippocampus/hippocampus and bilateral calcarine areas (corrected p < 0.001) (Figure 4). In an analysis of all participants, left amygdala resting connectivity to the right parahippocampus/hippocampus was correlated with negative facilitation (r = 0.36, p<0.05) and amygdala activity during the EDP task (negative images: r = 0.47, p < 0.01; positive images; r = 0.38, p < 0.05; neutral images: r = 0.47, p < 0.01) (Supplementary Figure 2). Left amygdala connectivity to precuneus did not significantly correlate with amygdala activity or performance during the EDP task. There were no significant differences between groups for right amygdala connectivity.
Figure 4. Functional Connectivity with Left Amygdala.
Greater resting functional connectivity between left amygdala and right hippocampal complex (MNI: 18, −28, −10) and calcarine areas (MNI: 12, −98, 4) in MDD HX+ compared to MDD HX− (corr p < 0.001).
4. Discussion
The principal finding of this study is that women with a history of MDD showed greater attention facilitation for negative images than women without a history of MDD. Women with a history of MDD also showed greater amygdala activity for negative images and greater amygdala-hippocampal functional connectivity than women without a history of MDD. Amygdala activity and connectivity to the hippocampal complex were positively correlated with attention facilitation for negative images. These findings suggest that participants without a history of MDD avoided orienting attention to emotionally-valenced images, while participants with a history of MDD showed attention bias or an inability to suppress preferential orienting to emotionally-valenced images.
The difference in attention facilitation for negative images between the participant groups during euthymic mood supports the hypothesis that cognitive bias is a trait characteristic of depression vulnerability rather than a consequence of negative mood. Beck’s cognitive model and recent studies in individuals with other risk factors for depression suggest that cognitive biases may be present even before MDD onset and contribute to increased risk for mood and anxiety disorders (Beck and Haigh, 2014; Joormann and Gotlib, 2007; Zhong et al., 2011). Although this study was not designed to determine whether attention bias is a risk factor or consequence of MDD history, the results of this study indicate that attention bias for negative information can be seen in individuals with past MDD without inducing a negative mood state. Attention bias for negative information may be an on-going vulnerability for MDD recurrence independent of mood state.
In contrast to well accepted findings of bias for positive emotional information in never depressed individuals (Isaac et al., 2014; Joormann and Gotlib, 2007; Sears et al., 2011) this study found that women without a history of MDD showed attentional facilitation away from both negative and positive images (toward neutral images). The results of this study are also counter previous findings that depressed individuals show difficulty disengaging attention from emotional stimuli rather than biased attention orienting (Gotlib et al., 2004). This study used shorter presentation durations and more complex visual stimuli than previous studies that found positivity bias in never depressed participants. As this study used a modified version of the EDP task with IAPS images, replication using an emotion-faces version of the task with longer image durations may be necessary for better comparison with previous literature. It may be that attentional avoidance of negative and positive images in never depressed participants represents an effect of arousal rather than valence. Both negative and positive images are higher in arousal ratings than neutral images in the IAPS. Because of the short stimuli presentation durations, participants may have adopted a strategy of avoiding highly arousing images in an attempt to avoid negative images
Participants in the current study may have avoided directing attention to highly arousing images; a similar study found that older adults showed attentional avoidance for both positive and negative images compared to neutral images (Demeyer and De Raedt, 2013). However, participants with a history of MDD had equivalent reaction times during neutral and emotionally valenced image trials suggesting that they were less able to direct attention away from the both negative and positive images compared to neutral images.
Participants with a history of MDD also showed greater limbic and visual region activity for emotionally valenced images than participants without a history of MDD. In all participants left amygdala activity was more strongly correlated with attention facilitation for negative images than positive images. These results suggest that there is a stronger relation between amygdala activity and attention for negative information than positive information. The amygdala has direct bi-directional projections to early visual areas and likely enhances attention to emotional information through feedback mechanisms to visual regions (Pourtois et al., 2013). Resting functional connectivity analyses in the current study indicated stronger connectivity between left amygdala and areas important for the integration of visual perception and attention (Cavanna and Trimble, 2006) in participants with a history of MDD. Lateralization of these findings to the left amygdala may be related to the left lateralization of amygdala activity for emotionally-valenced information (Cahill et al., 2001) and greater overall functional connectivity in left compared to right amygdala(Kilpatrick et al., 2006) in women. Attention networks in individuals with a history of MDD may be predisposed to attentional gain for negative emotional information through amygdala feedback to visual regions.
4.1 Limitations
The depression history of participants in this study was fairly homogenous with no participants having highly recurrent depressive episodes. We did not include participants who reported current or past diagnosed anxiety disorder and the participant groups had similar low scores on the BAI and STAI. The current results did not show an effect of trait anxiety or interaction with MDD history; however the participants in this study had low anxiety symptoms. As depression and anxiety are highly co-morbid, future studies should seek to include a wider range of anxiety symptoms and examine the separate effects of depression and anxiety vulnerability on emotional attention. The results of this study should be interpreted with consideration to relatively small sample size for the MDD Hx+ group and the unbalanced sample size between the two groups. These results may be best utilized for generating hypotheses for future work to include larger sample sizes and more specific characterization of depression and anxiety history.
Additionally, this study only included postmenopausal women as participants, which limits the interpretation of these results for young women and men. Although sex differences in attention bias in depression have not been previously examined, prior studies suggest sex differences in neural activity for emotional processing that may interact with gonadal hormone levels (Kret and De Gelder, 2012). Future studies should examine whether the association of depression history and cognitive bias differs by sex or hormonal status.
4.2 Conclusions
Cognitive models predict that enhanced amygdala sensitivity and attentional processing of negative information increases depression risk by supporting negative cognitive schemas. Greater attention to negative information may contribute to the perception that negative information is more salient or predictive of future events than neutral or positive information, thus enhancing the depressogenic effects of negative life events and stressors.
Greater amygdala activity and attention facilitation for emotionally-valenced images in women with a history of MDD compared to women with no MDD history may be relevant to the continued greater risk of depressive episodes associated with past depression in postmenopausal women. Altered amygdala activity and connectivity in remitted women with a history of MDD may contribute to a cognitive vulnerability for depressive symptoms that remains during euthymic remission.
The current findings of positive correlations between negative attention facilitation and amygdala activity and resting connectivity in attention networks provide evidence that differences in activity and functional connectivity in limbic and attention networks may provide a neurobiological basis for cognitive bias in remitted depressed individuals. If the results of this study are confirmed future work may further define neurobiological markers of depression recurrence risk and identify individuals who may benefit from cognitive training treatment approaches. Women with a history of MDD who show attention bias for negative emotional information may be at a greater risk for depression recurrence and benefit from cognitive training approaches. The effect of cognitive bias modification training on EDP performance, associated brain activity, and risk for depression recurrence should be examined in prospective longitudinal studies.
Supplementary Material
Correlation between left amygdala activity during negative trials and A) negative facilitation during the EDP task (r = 0.44, p < 0.01), B) positive facilitation during the EDP task (r = 0.34, p < 0.05).
Correlation between left amygdala and right hippocampus resting functional connectivity and left amygdala activity for A) negative images (r = 0.47, p < 0.01), B) positive images (r = 0.38, p < 0.05), and C) neutral images (r = 0.47, p < 0.01).
Highlights.
MDD history was associated with attention bias for negative images.
MDD history was associated with greater amygdala activity for emotional images.
Amygdala activity was correlated with attention facilitation for emotional images.
Amygdala-hippocampal connectivity was greater in participants with MDD history.
Amygdala connectivity correlated with attentional facilitation for negative images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, Williams SR, Deakin JFW, Anderson IM. Increased amygdala responses to sad but not fearful faces in major depression: Relation to mood state and pharmacological treatment. Am J Psychiatry. 2012;169:841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953–9. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory. Depression. 1978;2006:2–4. [Google Scholar]
- Beck AT, Haigh EAP. Advances in cognitive theory and therapy: The generic cognitive model. Annu Rev Clin Psychol. 2014;10:1–24. doi: 10.1146/annurev-clinpsy-032813-153734. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:497. http://dx.doi.org/10.1016/S1053-8119(02)90010-8. [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-Related Difference in Amygdala Activity during Emotionally Influenced Memory Storage. Neurobiology of Learning and Memory. 2001 doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006 doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cogn Affect Behav Neurosci. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Demeyer I, De Raedt R. Attentional Bias for Emotional Information in Older Adults: The Role of Emotion and Future Time Perspective. PLoS One. 2013:8. doi: 10.1371/journal.pone.0065429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque A, Vazquez C. Double attention bias for positive and negative emotional faces in clinical depression: evidence from an eye-tracking study. J Behav Ther Exp Psychiatry. 2015;46:107–114. doi: 10.1016/j.jbtep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Boorman DW, Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA psychiatry. 2014;71:36–43. doi: 10.1001/jamapsychiatry.2013.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy MA, Ingram RE. A meta-analytic review of mood-congruent implicit memory in depressed mood. Clin Psychol Rev. 2014 doi: 10.1016/j.cpr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Neubauer Yue D, Joormann J. Attentional Biases for Negative Interpersonal Stimuli in Clinical Depression. J Abnorm Psychol. 2004;113:127–135. doi: 10.1037/0021-843X.113.1.127. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A Meta-Analysis of the Effect of Cognitive Bias Modification on Anxiety and Depression. Psychol Bull. 2011;137:940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Isaac L, Vrijsen JN, Rinck M, Speckens A, Becker ES. Shorter gaze duration for happy faces in current but not remitted depression: Evidence from eye movements. Psychiatry Res. 2014;218:79–86. doi: 10.1016/j.psychres.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–5. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Keller MB, Berndt ER. Depression treatment: a lifelong commitment? Psychopharmacol Bull. 2002 [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. Journal of Affective Disorders. 2003:5–13. doi: 10.1016/S0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1097/00132578-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Fazekas H, Loney BR. Psychopathy, aggression, and the processing of emotional stimuli in non-referred girls and boys. Behav Sci Law. 2006;24:21–37. doi: 10.1002/bsl.668. [DOI] [PubMed] [Google Scholar]
- King MG, Burrows GD, Stanley GV. Measurement of stress and arousal: validation of the stress/arousal adjective checklist. Br J Psychol. 1983;74(Pt 4):473–479. doi: 10.1111/j.2044-8295.1983.tb01880.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–62. doi: 10.1097/00006842-199903000-00006. 0033-3174/99/6102-0154. [DOI] [PubMed] [Google Scholar]
- Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/S0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. 2008 doi: 10.1016/j.epsr.2006.03.016. [DOI] [Google Scholar]
- Mattis S. Dementia rating scale. Ressources Inc; Odessa, F.L: 1988. Psychol. Assess. [Google Scholar]
- McNair D, Lorr M, Droppleman LF. In: Profile of mood states (POMS) McNair Douglas M, Lorr Maurice, Droppleman Leo F., editors. 1989. [DOI] [Google Scholar]
- Muñoz Centifanti LC, Kimonis ER, Frick PJ, Aucoin KJ. Emotional reactivity and the association between psychopathy-linked narcissism and aggression in detained adolescent boys. Dev Psychopathol. 2013;25:473–485. doi: 10.1017/S0954579412001186. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biol Psychol. 2013 doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, De Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Sears CR, Newman KR, Ference JD, Thomas CL. Attention to emotional images in previously depressed individuals: An eye-tracking study. Cognit Ther Res. 2011;35:517–528. doi: 10.1007/s10608-011-9396-5. [DOI] [Google Scholar]
- Solomon Da, Keller MB, Leon aC, Mueller TI, Shea MT, Warshaw M, Maser JD, Coryell W, Endicott J. Recovery from major depression. A 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry. 1997;54:1001–6. doi: 10.1001/archpsyc.1997.01830230033005. http://dx.doi.org/10.1001/archpsyc.1997.01830230033005. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory (STAI) Mind Gard. 1983;94061:261–3500. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID) - History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WASI -II: Wechsler abbreviated scale of intelligence - second edition. J Psychoeduc Assess. 2013;31:337–41. doi: 10.1177/0734282912467756. [DOI] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wiens S, Syrjänen E. Directed attention reduces processing of emotional distracters irrespective of valence and arousal level. Biol Psychol. 2013;94:44–54. doi: 10.1016/j.biopsycho.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Wang W, Yao S. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011;88:233–242. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between left amygdala activity during negative trials and A) negative facilitation during the EDP task (r = 0.44, p < 0.01), B) positive facilitation during the EDP task (r = 0.34, p < 0.05).
Correlation between left amygdala and right hippocampus resting functional connectivity and left amygdala activity for A) negative images (r = 0.47, p < 0.01), B) positive images (r = 0.38, p < 0.05), and C) neutral images (r = 0.47, p < 0.01).