Abstract
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) are potent inhibitors of human immunodeficiency virus type 1 (HIV-1); however, currently marketed NNRTIs rapidly select resistant virus, and cross-resistance within the class is extensive. A parallel screening strategy was applied to test candidates from a series of diarylpyrimidines against wild-type and resistant HIV strains carrying clinically relevant mutations. Serum protein binding and metabolic stability were addressed early in the selection process. The emerging clinical candidate, TMC125, was highly active against wild-type HIV-1 (50% effective concentration [EC50] = 1.4 to 4.8 nM) and showed some activity against HIV-2 (EC50 = 3.5 μM). TMC125 also inhibited a series of HIV-1 group M subtypes and circulating recombinant forms and a group O virus. Incubation of TMC125 with human liver microsomal fractions suggested good metabolic stability (15% decrease in drug concentration and 7% decrease in antiviral activity after 120 min). Although TMC125 is highly protein bound, its antiviral effect was not reduced by the presence of 45 mg of human serum albumin/ml, 1 mg of α1-acid glycoprotein/ml, or 50% human serum. In an initial screen for activity against a panel of 25 viruses carrying single and double reverse transcriptase amino acid substitutions associated with NNRTI resistance, the EC50 of TMC125 was <5 nM for 19 viruses, including the double mutants K101E+K103N and K103N+Y181C. TMC125 also retained activity (EC50 < 100 nM) against 97% of 1,081 recent clinically derived recombinant viruses resistant to at least one of the currently marketed NNRTIs. TMC125 is a potent next generation NNRTI, with the potential for use in individuals infected with NNRTI-resistant virus.
Successful long-term treatment of human immunodeficiency virus type 1 (HIV-1) by antiretrovirals is often hindered by incomplete viral suppression and the resulting emergence of drug resistance. There is now widespread resistance to all available classes of antiretrovirals, and cross-resistance within classes is extensive, often severely limiting the treatment options available (17, 20). Although currently marketed nonnucleoside reverse transcriptase inhibitors (NNRTIs) are highly selective and extremely potent, they rapidly select for resistant virus. Moreover, single mutations can lead to dramatic reductions in susceptibility, often to all available inhibitors within the class (2, 11). This broad cross-resistance prevents the consecutive use of currently marketed NNRTIs in treatment regimens (1). Next-generation agents with activity against NNRTI-resistant isolates would therefore offer new treatment options.
To increase the likelihood of identifying new compounds active against NNRTI-resistant strains and that have interesting drug-like properties, we developed the following strategies. The structure-activity relationship, traditionally limited to activity against the wild-type virus, was expanded to include concurrent evaluation of several NNRTI-resistant strains. To facilitate our understanding of the effect of new chemical substituents on the binding of the compounds to wild-type and resistant reverse transcriptase (RT), a panel of mutants was constructed harboring various NNRTI resistance-associated mutations within exactly the same genetic background. A second step was to include assessment of metabolic stability early in the discovery process, thereby establishing a structure-metabolism relationship. One of the early lead compounds was indeed prone to metabolism (22), leading to a short half-life in plasma when tested in vivo. The combination of structure-activity relationship and structure-metabolism relationship allowed concurrent optimization of structures for broad-spectrum anti-HIV activity and metabolic stability. Finally, functional protein-binding assays and a large panel of NNRTI-resistant, clinically derived recombinant viruses were used to assess the activity of selected lead compounds.
Our strategy ensured a data-driven and rapid selection process that, along with structural insights, paved the road for the discovery of the imidoyl thiourea (ITU), diaryltriazines (DATA), and the diarylpyrimidine (DAPY) series (21-23). Here we report the pharmacological characteristics of TMC125 (also known as R165335) (Fig. 1), a DAPY compound identified by using this screening strategy.
FIG. 1.
Chemical structure of TMC125. The chemical formula for C20H15BrN6O or 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzonitrile is shown.
MATERIALS AND METHODS
Cells and viruses.
MT4 cells are human T-lymphoblastoid cells that are highly sensitive to HIV infection, producing a rapid and pronounced cytopathic effect. Peripheral blood mononuclear cells (PBMC) for use in drug susceptibility assays were purified from HIV-negative donors and activated as previously described (13). Mature monocyte/macrophages (M/Ms) were separated out from freshly isolated PBMC by adhesion as described by Perno and Yarchoan (27). All cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and antibiotics in a humidified incubator with a 5% CO2 atmosphere at 37°C. Virus stocks of HIV-1 and HIV-2 strains were produced in MT4 cells, except for HIV-1 Ba-L, which was cultured in M/Ms.
Site-directed mutants.
Mutant RT coding sequences were generated from a pGEM vector containing the HIV-1 LAI (clone HXB2) protease (PR) and RT coding sequence by using a QuikChange site-directed mutagenesis kit (Stratagene), and high-pressure liquid chromatography-purified primers (Genset Oligos). Plasmids were sequenced to confirm that they contained the desired mutations. Mutant viruses were created by recombination of the mutant PR-RT sequence with a PR-RT deleted HIV-1 HXB2 proviral clone (18).
Recombinant clinical isolates.
Recombinant viruses derived from clinical samples were constructed as previously described by cotransfection of MT4 cells with sample-derived viral PR and RT coding sequences and an HIV-1 HXB2-derived proviral clone deleted in the PR and RT coding region (18).
Drug sensitivity assays.
The antiviral activity of compounds against laboratory-adapted strains, site-directed mutants, and clinical sample derived recombinant viruses was tested by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay as previously described (18, 26). Briefly, various concentrations of the test compounds were added to wells of a flat-bottom microtiter plate. Subsequently, virus and MT4 cells were added to a final concentration of 200 50% cell culture infectious dose (CCID50)/well and 30,000 cells/well, respectively. In order to determine the toxicity of the test compound, mock-infected cell cultures containing an identical compound concentration range were incubated in parallel with the virus-infected cell cultures. After 5 days of incubation (37°C, 5% CO2), the viability of the cells was determined by using MTT. The results of drug susceptibility assays were expressed as an EC50 defined as the concentration of drug at which there was 50% infection compared to the drug-free control. In some cases, a fold change in susceptibility was calculated by dividing the EC50 for the tested virus by the EC50 for the wild-type virus (HIV-1 LAI) tested in parallel. Toxicity results are expressed as the CC50, which was defined as the concentration of drug at which the cell viability was reduced by 50% compared to the drug-free control.
PBMC-based drug susceptibility assays were carried out as previously described (13). Briefly, phytohemagglutinin-stimulated PBMC from healthy seronegative donors were incubated with serial dilutions of the test compounds and infected with virus at a multiplicity of infection (MOI) of 0.001 CCID50 per cell. Infected cells were washed and incubated in media containing the same concentration of drug. The production of virus was quantified after 7 days by using a p24 enzyme-linked immunosorbent assay (ELISA; NEN Life Science Products).
To test the drug sensitivity of HIV-1 Ba-L, adherent M/Ms cultured in 48-well plates were exposed to various concentrations of drug for 1 h before incubation with 300 50% tissue culture infective doses of virus/ml for 2 h. Cells were then washed and cultured for 15 days in the same concentration of drug. The production of virus was quantified by using a p24 ELISA as described above (27).
Genotyping and subtype determination.
Genotypic analysis was performed by automated population-based full-sequence analysis (ABI Prism BigDye terminator cycle sequencing). Sequencing results are reported as amino acid changes compared to the wild-type (HXB2) reference sequence (19). Subtypes were determined by heteroduplex mobility assay (HMA) (12) or sequencing.
Mechanism of action studies.
For time-of-addition assays, a high MOI of HIV-1 LAI was used to infect MT4-LTR-EGFP cells, which were then incubated on ice for 30 min. Before transfer of the cells to 37°C, two washing steps at 4°C were performed to remove unadsorbed virus and to synchronize the infection. From 1 h postinfection onward, the compounds to be tested were added to parallel cultures at different time points. After 24 h, cultures were examined for fluorescence, and supernatants were tested for p24 concentration.
Inhibition of RT was assessed by using a poly-r(A) RT-scintillation proximity assay (SPA; Amersham Biosciences). This assay consists of a biotinylated DNA/RNA primer template, which is annealed to streptavidin-SPA beads. The radiolabeled compound [3H]TTP is incorporated, and extension of the primer is proportional to the amount of RT present. This assay mimics the RNA-dependent DNA-polymerase reaction during reverse transcription. The IC50 is the concentration of compound that inhibits RT activity by 50%.
Metabolic stability assay.
Aliquots of human liver microsomal fractions, prepared as previously described (5), were incubated with TMC125 at a final concentration of 30 μM and a cofactor solution (1 mg of glucose-6-phosphate/ml, 1 mg of MgCl2 · 0.6H2O/ml, and 0.5 U of glucose-6-phosphate dehydrogenase/ml in 0.5 M phosphate buffer [pH 7.4]). After 5 min at 37°C, the reactions were initiated by adding a 1.25-mg/ml solution of NADP in homogenization buffer (1.15% KCl in 0.01 M phosphate buffer [pH 7.4]). After 15, 30, and 120 min of incubation at 37°C with constant shaking, reactions were terminated by the addition of an equal volume of dimethyl sulfoxide. The degree of metabolism of TMC125 was determined by measurement of the residual parent compound in the reaction mixture by using a liquid chromatography-mass spectrometry (LC-MS) method. Residual anti-HIV activity was also measured by using a colorimetric anti-HIV assay as described previously (26).
Antiviral activity in the presence of HS proteins.
MT4 cells were infected with HIV-1 LAI at an MOI of 0.001 to 0.01 CCID50 per cell. After 1 h of incubation, cells were washed and plated into a 96-well plate containing serial dilutions of TMC125 in the presence of 10% FCS, 10% FCS plus 1 mg of α1-acid glycoprotein (AAG)/ml, 10% FCS plus 45 mg of human serum albumin (HSA)/ml or 50% human serum (HS). After 5 to 6 days, the EC50 was determined by cell viability assay using resazurin (14). Briefly, this involved adding a 1/10 volume of resazurin solution [0.1 mg of resazurin/ml, 1 mM K4Fe(CN)6, and 1 mM K3Fe(CN)6 in 0.1 mM potassium phosphate buffer; pH 7.4] and measuring the fluorescence of the formed resofurin after 5 or 25 h of incubation at 37°C. In some cases, data were confirmed by quantifying the level of HIV replication by p24 ELISA.
Combination with other antiretrovirals.
Combinations of compounds were tested against HIV-1 LAI in MT4 cells at three different molar ratios: 3×, 1×, and 1/3× the estimated EC50 ratio of the compounds. Drugs tested in combination with TMC125 included the nucleoside RT inhibitors (NRTIs) abacavir, didanosine, lamivudine, stavudine, zalcitabine, and zidovudine; the nucleotide RT inhibitor tenofovir; the PR inhibitors (PIs) amprenavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir; and the currently marketed NNRTIs delavirdine, efavirenz, and nevirapine. A combination index (CI) at 50% protection was calculated by using a formula derived from the classical isobologram model for combinations (7). Each combination was tested nine times. A drug combination was scored as synergistic when the mean CI was <0.8 and as antagonistic when the mean CI was >1.2. A mean CI between 0.8 and 1.2 was scored as additive. Control combinations were the sham combination zidovudine-zidovudine and the combination zidovudine-emivirine, which has previously been reported to be synergistic (6).
Compounds.
Marketed anti-HIV compounds were extracted and purified by high-pressure liquid chromatography from the commercial formulation. Emivirine was synthesized and purified according to published methods (29).
RESULTS
Lead optimization process.
Compounds were simultaneously optimized for activity against wild-type HIV-1 and selected NNRTI-resistant mutants and for increased metabolic stability (Table 1). Increased potency against one NNRTI-resistant mutant generally correlated with increased potency against the other mutants. However, increased potency against NNRTI-resistant mutants did not correlate consistently and to the same magnitude with increased potency against the wild-type virus. Although the activity of TMC125 against wild-type virus was similar to the activity of the other compounds, it was more active than the other tested compounds against all of the NNRTI-resistant mutants. This increased activity was particularly notable for the double mutant L100I+K103N. TMC125 was selected for further study on the basis of its increased antiviral activity and of the improved metabolic stability compared to the other compounds tested (Table 1).
TABLE 1.
Antiviral activity and metabolic stability of ITU, DATA, and DAPY analogs
Compound numbers refer to the numbers in the publications by Ludovici et al. (21, 22). The roman numerals in parentheses indicate the specific publication in which the compound was introduced (II, reference 22; III, reference 21).
That is, after 2 h of incubation with human or rat microsomes. *, As assayed by bioassay for residual anti-HIV activity; †, as assessed by LC-MS.
NT, not tested.
Anti-HIV activity of TMC125.
The activity of TMC125 against three different strains of HIV-1 (LAI, SF2, and the monocytotropic HIV-1 Ba-L) and one strain of HIV-2 (ROD) was compared to the activity of the three currently marketed NNRTIs: delavirdine, efavirenz, and nevirapine (Table 2). The EC50 values for TMC125 against HIV-1 LAI (in both MT4 cells and PBMC), HIV-1 SF, and HIV-1 Ba-L were in the nanomolar range (1.4 to 4.8 nM) and were similar to those obtained for efavirenz (1.0 to 3.4 nM). Delavirdine and nevirapine were at least 10-fold less potent than TMC125 or efavirenz against HIV-1 in all of the virus-cell combinations tested, including combinations with wild-type HIV-1. In contrast to the other three NNRTIs tested, TMC125 also showed activity in the micromolar range against HIV-2 (EC50 = 3.5 μM). In this assay, TMC125 did not show cytotoxicity (CC50 > 100 μM) as opposed to efavirenz (CC50 = 42 μM).
TABLE 2.
Anti-HIV activity of TMC125 in comparison with other NNRTIs
| Compound | MT4 CC50 (μM) | Median EC50, nM (range)a
|
||||
|---|---|---|---|---|---|---|
| MT4
|
PBMC
|
M/M, Ba-L (R5) | ||||
| LAI (HIV-1) | ROD (HIV-2) | LAI (X4) | SF2 (X4-R5) | |||
| Nevirapine | >100 | 76.3 (30.8-202.0) | >100,000 | 22.1 | 129.8 | 45.0 (18.0-65.0) |
| Delavirdine | 73 | 16.1 (8.3-531.8) | >100,000 | ND | ND | ND |
| Efavirenz | 42 | 1.0 (0.2-3.2) | >41,000 | 1.2 | 3.4 | 2.0 (0.9-3.0) |
| TMC125 | >100 | 1.4 (0.3-1.6) | 3,479 | 1.4 | 4.8 | 2.0 (0.8-4.0) |
Median and range of several determinations for MT4 LAI and M/M Ba-L experiments. For MT4 ROD and PBMC experiments, the result of a single determination is shown. ND, not determined.
Activity of TMC125 against NNRTI-resistant viruses.
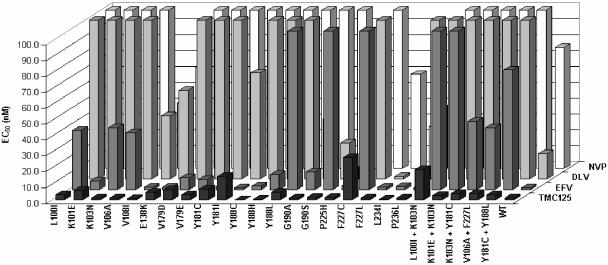
An initial screen for activity against NNRTI-resistant HIV-1 was carried out on a panel of viruses constructed by site-directed mutagenesis to carry a selection of single (n = 20) and double (n = 5) RT amino acid substitutions known to be associated with resistance to marketed and investigational NNRTIs. Compared to currently prescribed NNRTIs, TMC125 showed excellent activity against this panel of mutant viruses with EC50 values of <5 nM (Fig. 2) and a fold change in EC50 values of <4 for 18 of the viruses, including four carrying double amino acid substitutions. TMC125 EC50 values of >10 nM and fold resistance values of >10 were observed for only three of the viruses tested (L100I+K103N, Y181I, and F227C). These three variants are present at very low frequency in the clinical population. An analysis of a database of over 74,000 clinical isolates showed that the prevalence of Y181I is 0.51%, that of F227C is nearly 0 (17 isolates), and the combination of L100I and K103N is present in only 3% of these isolates.
FIG. 2.
Antiviral activity of TMC125 and current NNRTIs tested against a panel of 25 HIV-1 strains harboring one or two NNRTI resistance-associated mutations. HIV-1 strains were constructed by site-directed mutagenesis as described in Materials and Methods. NVP, nevirapine; DLV, delavirdine, EFV, efavirenz.
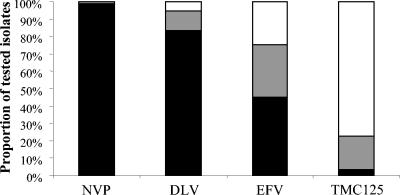
To further evaluate the antiviral activity of TMC125 on the virus populations currently present in patients, drug sensitivity assays were performed on a broad, random selection of recent clinical samples. Of the 2,157 samples tested, 1,081 samples (50.1%) showed a ≥10-fold change in EC50 compared to wild-type for at least one of the current NNRTIs. In this subset of 1,081 samples, 98, 88, and 81% were resistant to NVP, DLV, and EFV, respectively, whereas only 20% showed the same fold change for TMC125. The distribution of the EC50 concentrations within the resistant samples is illustrated in Fig. 3, which compares TMC125 and the three approved NNRTIs. TMC125 inhibited 98% of all samples and 97% of the NNRTI-resistant strains, with an EC50 of <100 nM. For efavirenz, a compound equally potent against wild-type HIV-1, these percentages were 77 and 54%, respectively. Moreover, TMC125 inhibited 77% of NNRTI-resistant strains with an EC50 of <10 nM, whereas this was the case for only 23% for efavirenz.
FIG. 3.
Antiviral activity of different NNRTIs tested against a panel of 1,081 resistant recombinant clinical isolates. Bars: ▪, EC50 > 100 nM;  , EC50 > 10 nM and < 100 nM; □, EC50 < 10 nM. NVP, nevirapine; DLV, delavirdine; EFV, efavirenz.
, EC50 > 10 nM and < 100 nM; □, EC50 < 10 nM. NVP, nevirapine; DLV, delavirdine; EFV, efavirenz.
Activity of TMC125 against a range of HIV-1 subtypes.
TMC125 was tested in parallel with nevirapine, delavirdine, and efavirenz for activity against a panel of 32 clinically derived recombinant viruses (M.-P. de Béthune, K. Hertogs, L. Heyndrickx, J. Vingerhoets, K. Fransen, H. Azijn, L. Michiels, W. Janssens, A. Scholliers, B. Larder, S. Bloor, R. Pauwels and G. van der Groen, Antivir. Ther. 4[Suppl. 1]:33, abstr. 49, 1999). These viruses represented HIV-1 group M subtypes A, B, C, D, F, and H, as well as circulating recombinant forms (CRFs) CRF01_AE, CRF02_AG, CRF05_DF, and HIV-1 group O. The strains were from different geographic origins. The results are summarized in Table 3. All of the group M viruses tested were sensitive to TMC125 with EC50 values of <5 nM and fold changes in EC50 values of <4. Seven of the group M viruses carried mutations in the RT coding region at positions associated with NNRTI resistance (positions 98, 101, 106, and 179). Except for one virus carrying a V179I substitution, which showed slightly reduced sensitivity to delavirdine (a fivefold change), all of these viruses remained sensitive to all of the inhibitors tested. The one group O virus tested naturally harbored amino acids at positions 98 (G), 179 (E), and 181 (C), which in group M HIV-1 strains are associated with NNRTI resistance. This virus displayed significantly reduced sensitivity to nevirapine (89-fold change), delavirdine (144-fold change), and efavirenz (42-fold change) but only moderately reduced sensitivity to TMC125 (10-fold change).
TABLE 3.
Activity of NNRTIs against recombinant viruses of different subtypes
| Pol subtypea | nb | Mean activity (range)c
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| NVP
|
DLV
|
EFV
|
TMC125
|
||||||
| EC50 | FC | EC50 | FC | EC50 | FC | EC50 | FC | ||
| CRF02_AG | 4 | 13 (5.6-19) | 0.75 (0.4-1.3) | 9.3 (5.6-16) | 1.1 (0.6-1.9) | 0.7 (<0.3-1.1) | 0.9 (<0.4-1.2) | 1.3 (0.9-1.9) | 1.1 (0.7-1.5) |
| CRF01_AE | 5 | 19 (12-98) | 1.3 (0.8-7) | 29 (20-170) | 3.3 (2.3-20) | 1.2 (0.6-1.9) | 1.3 (0.7-2.1) | 1.8 (1-4.3) | 1.4 (0.8-3.3) |
| B | 8 | 29 (11-70) | 2.1 (0.8-5) | 28 (5.7-52) | 3.2 (0.7-6) | 1.2 (<0.3-2.6) | 1.1 (0.3-2.9) | 1.5 (0.9-3) | 1.2 (0.7-2.3) |
| C | 4 | 13 (7.7-53) | 0.9 (0.6-3.8) | 34 (22-46) | 3.9 (2.6-5.3) | 0.6 (<0.3-1.4) | 0.6 (0.4-1.5) | 1.5 (1.0-2.4) | 1.2 (0.8-1.9) |
| D | 3 | 12 (7.0-15) | 0.9 (0.5-1.0) | 9.7 (7.7-20) | 1.1 (0.9-2.3) | 0.4 (<0.3-0.4) | 0.4 (0.3-0.5) | 1.7 (1.2-1.7) | 1.3 (1.0-1.3) |
| CRF05_DF | 3 | 33 (18-43) | 1.7 (0.9-3.1) | 6.1 (5.6-12) | 0.6 (0.6-1.3) | 0.4 (<0.3-0.4) | 0.8 (0.3-0.9) | 0.9 (0.8-1.0) | 0.7 (0.7-0.8) |
| F | 1 | 30 | 2.1 | 27 | 3.1 | 0.9 | 1.0 | 1.3 | 1.0 |
| H | 3 | 15 (5.0-19) | 1.1 (0.4-1.4) | 18 (2.8-22) | 2.0 (0.3-2.6) | 0.6 (<0.3-0.7) | 0.5 (0.3-0.8) | 1.1 (0.9-1.8) | 0.9 (0.7-1.4) |
| O | 1 | >1,300 | 89 | >1,300 | 140 | 38 | 42 | 13 | 9.9 |
Polymerase subtype as determined by full sequencing of the protease and RT (first 400 amino acids) genes. For some of the strains, mutations at amino acid positions implicated in NNRTI resistance were found; subtype B, two strains (A98S/A + K101R and V179I); subtype CRF05_DF, two strains (V106I and V179I); subtype H, three strains (V179I and twice K101Q + V179I); and subtype O, one strain (A98G + Y179E + Y181C).
n = the number of strains tested in each subtype.
EC50, median EC50 expressed in nanomolar units; FC, fold change in EC50, calculated as the EC50 for the tested virus divided by the EC50 for the wild-type virus (HIV-1 HXB2) tested in parallel, according to the Antivirogram method.
Mechanism of action assays.
A time-of-addition study was carried out in order to define the stage in the HIV life cycle at which TMC125 acts. The binding inhibitor DS5000, the NRTI zidovudine, the PI saquinavir, and the NNRTI efavirenz were used as controls. DS5000 had no inhibitory effect when added 1 h postinfection, whereas zidovudine lost activity from 4 h postinfection on. Saquinavir, which acts at the end of the viral life cycle, retained activity even when added 20 h postinfection (data not shown). The time-of-addition versus activity profile for TMC125 was very similar to that of efavirenz and did not overlap with zidovudine, with a significant loss of activity when drug addition was delayed for 7 h postinfection. In a single experiment measuring the effect of the compounds on RT enzymatic activity, TMC125 gave an IC50 of 38 nM, in comparison to an IC50 for efavirenz of 16 nM.
Metabolic stability assays.
The metabolic stability of compounds was tested by incubation with human microsomal fractions. The residual compound remaining was determined by LC-MS, and residual anti-HIV activity was assessed in an in vitro assay. The results of the LC-MS analysis correlated well with those of the antiviral assay (Table 1). Although compound 6 (the lead compound) was rapidly degraded, minimal degradation was seen for TMC125 after 30 min of incubation, with no reduction in concentration and only a 2% reduction in antiviral activity (data not shown). After 120 min, there was a 15% decrease in TMC125 concentration and a 7% decrease in antiviral activity.
Antiviral activity of TMC125 in the presence of HS proteins.
In uninfected individuals, the average HSA concentration is 40 mg/ml, and the AAG concentration ranges from 0.4 to 0.8 mg/ml. Studies have shown that AAG levels in HIV-infected individuals may be elevated to 0.8 to 1.8 mg/ml (25). For this reason, a functional assay was performed to determine the antiviral activity of TMC125 against HIV-1 LAI in the presence of 45 mg of HSA/ml or 1 mg of AAG/ml, as well as 50% HS. The ratio between the EC50 in the presence of the protein and the EC50 in the absence of the protein was calculated (Table 4). The antiviral effect of TMC125 was not significantly affected by the presence of 50% HS (twofold increase in EC50) or by the presence of HSA (threefold increase in EC50). AAG had no measurable affect on the activity of TMC125. Nevirapine gave similar results, whereas the activity of efavirenz was significantly reduced in the presence of HSA (20-fold increase in EC50), as described previously (8).
TABLE 4.
Influence of human proteins in serum on the anti-HIV potency of selected NNRTIs
| Compound | Mediana EC50 with human serum protein/EC50 in 10% FCS
|
||
|---|---|---|---|
| AAG (1 mg/ml) | HSA (45 mg/ml) | 50% HS | |
| Nevirapine | 1 | 3 | 1 |
| Efavirenz | 4 | 20 | 6 |
| TMC125 | 1 | 3 | 2 |
Median of at least four determinations.
Combination assays.
Both the positive control zidovudine-emivirine and the combination of TMC125-zidovudine gave mean CIs that were significantly less than 1 (0.57 ± 0.04 [P < 0.0001] and 0.71 ± 0.08 [P < 0.005], respectively), suggesting synergy between TMC125 and zidovudine. All other combinations tested gave mean CIs that were between 0.8 and 1.2 (data not shown), indicating an additive effect with no evidence of synergy or antagonism.
DISCUSSION
We have applied a novel parallel screening strategy to simultaneously optimize a series of compounds for activity against wild-type HIV-1, selected NNRTI mutants, and increased metabolic stability. Interestingly, increased potency against the wild-type virus was not predictive for increased potency against NNRTI mutants. This highlights the importance of including NNRTI mutants in first-line screening protocols to facilitate the identification and selection of broad-spectrum NNRTIs.
TMC125 was found to be a highly potent inhibitor of HIV-1, with activity in the nanomolar range comparable to that of the commonly prescribed NNRTI, efavirenz. Unlike efavirenz and the majority of marketed and investigational NNRTIs, TMC125 also showed some activity against HIV-2, which, although not clinically relevant, is a reflection of its broader spectrum of activity. The cytotoxicity of TMC125 was low in a variety of human cell lines (data not shown), resulting in a selectivity index of >70,000 (HIV-1 LAI in MT4 cells). Mechanism-of-action studies confirmed that the activity of TMC125 is principally due to the inhibition of HIV RT. TMC125 acted at the same stage of replication as efavirenz and was an active inhibitor of recombinant HIV-1 RT with an IC50 comparable to that of efavirenz. No inhibitory activity of TMC125 was seen in assays monitoring the entry of HIV into the host cell or the enzymatic activity of HIV-1 PR (data not shown). Crystallography studies have shown that TMC125 binds and adapts to changes in the NNRTI binding pocket (9).
Since orally administered drugs pass through the liver before reaching the systemic circulation, molecules stable in the presence of liver enzymes are more likely to exhibit higher systemic exposure on oral administration than molecules that are rapidly metabolized. The most important metabolic pathway is the cytochrome P450 system (CYP), found in high concentrations on the endoplasmic reticulum of hepatocytes. The stability of compounds in the presence of human liver microsomes should be considered early in any drug selection procedure. The limited decrease in concentration (15%) and in antiviral activity (7%) measured after 120 min of incubation of TMC125 with human liver microsomal fractions suggests a slow hepatic metabolism and therefore good metabolic stability.
Binding of some antiretrovirals to HS proteins is known to inhibit their entry into cells (4) and can reduce their activity in vivo. TMC125, like other NNRTIs, is highly bound to HS proteins (>99% [unpublished observations]). However, in the functional assay described here, the activity of TMC125 was not significantly affected by the presence of either HS proteins at physiological concentrations (1 mg of AAG or 45 mg of HSA/ml) or 50% HS. Of the other NNRTIs tested, nevirapine showed a similar pattern in the presence of protein, while the activity of efavirenz was strongly affected.
Although the HIV-1 group M subtype B is predominant in Europe and the United States, recent studies have indicated that the prevalence of non-B subtypes and CRFs is increasing to significant levels, particularly in immigrants and heterosexually infected individuals (3, 28). For this reason, it is essential to determine a drug candidate's activity against other subtypes and groups. TMC125 showed comparable activity to a range of clinically derived recombinant viruses representing HIV-1 group M subtypes A through H, including several CRFs. TMC125 also showed significantly improved activity against group O viruses compared to the other NNRTIs tested.
Combination therapy is the current standard of care for antiretroviral therapy. Since mixtures of antiretroviral agents may be synergistic, additive, or antagonistic, it is important to test compounds of interest in combination with all of the currently prescribed antiretrovirals. Zidovudine has been previously shown to act in synergy with other NNRTIs, for example, delavirdine (6) and emivirine (5). TMC125 in combination with zidovudine was synergistic, whereas all other combinations tested (NRTI, NNRTI, and PI) were additive with no evidence of antagonism.
One of the greatest challenges facing clinicians treating HIV today is overcoming the increasing levels of resistance and cross-resistance that develop at each successive therapeutic failure. The extensive cross-resistance that exists between currently approved NNRTIs has severely limited the sequential use of these potent agents. Although not fully predictive of the in vivo situation, in vitro selection of resistant HIV is a valuable tool for better understanding of emerging resistance and drug susceptibility. In vitro selection for TMC125 resistance at both a high and a low MOI is in progress. The data show that TMC125 has an increased genetic barrier to resistance compared to other NNRTIs: multiple mutations are required before there is a decrease in susceptibility to TMC125, whereas only one mutation is needed to confer high-level resistance to the currently approved NNRTIs, including efavirenz (unpublished data; J. Vingerhoets, H. Azijn, E. Fransen, K. Andries, R. Pauwels and M.-P. de Bethune, Abstr. Antivir. Ther. 7:S8, 2002; M.-P. de Béthune, H. Azijn, K. Andries, P. Janssen, and R. Pauwels, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1681, 2001). In the meantime, we have assessed the potential activity of TMC125 against a very large pool of NNRTI-resistant viruses generated by site-directed mutagenesis or clinically derived. The data presented here show that TMC125 remains active against a broad panel of NNRTI-resistant recombinant viruses chosen to represent a range of mutations selected by currently marketed and investigational NNRTIs. For example, L234I, F227L, and V106A+F227L are associated with resistance to capravirine (formerly S-1153 and AG-1549), whereas V106A, E138K, and F227L are associated with resistance to the new Glaxo-SmithKline benzophenone compounds (GW4751, GW4511, and GW3011) (J. Chan, R. Ferris, G. Roberts, S. Short, K. Weaver, R. Hazen, K. Creech, M. St. Clair, R. Dornsife, G. Freeman, J. Tidwell, K. Romines, L. Schaller, J. Cowan, and L. Boone, Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 6, 2003). Importantly, TMC125 inhibited not only viruses with single amino acid substitutions in the RT enzyme but also several common double mutants such as K101E+K103N and K103N+Y181C (10, 11, 24). Activity was slightly reduced against virus carrying L100I+K103N (EC50 = 19.4 nM), but to a much lesser extent than the activity of the other NNRTIs tested, leaving the potential for in vivo efficacy. High-throughput cell-based screening assays allowed for the testing of TMC125 against a panel of more than 1,000 NNRTI-resistant recombinant viruses derived from recent clinical samples. TMC125 showed a high level of activity against this panel of resistant viruses, with EC50 values of <100 nM for 97% of the samples. Attempts to crystallize TMC125 bound to wild-type RT have failed thus far due to poor resolution (K. Das, A. D. Clark, P. L. Boyer, D. W. Ludovici, M.-P. de Béthune., K. Andries, P. Lewi, E. Arnold, S. H. Hughes, B. L. De Corte, R. W. Kavash, M. J. Kukla, R. Pauwels, M. de Jonge, F. Daeyaert, L. Koymans, M. Vinkers, J. Heeres, and P. A. Janssen, Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 613, 2003). A crystal structure has been obtained with the K103N mutant in which the mode of binding of TMC125 appears to be different from other DAPY and DATA compounds. The lack of success with crystallizing TMC125 with the wild-type enzyme is possibly due to the drug having several alternative docking modes in the wild-type enzyme. Interestingly, this binding mode flexibility may explain the drug's potent activity against resistant viruses (9).
We have shown in a 7-day Phase IIa proof of concept study (TMC125-C208) that treatment with TMC125 of antiretroviral-naive HIV-infected subjects resulted in a very rapid decline in viral load (mean 1.99 log10 decrease in HIV-1 RNA) compared to placebo treatment. Two of twelve TMC125-treated subjects achieved a viral load of <50 HIV-1 RNA copies/ml, and eight of twelve reached a viral load of <400 copies/ml (16). The excellent in vitro activity of TMC125 against a wide range of NNRTI-resistant viruses suggests that this compound could be used to treat NNRTI-experienced patients. This is the first time that it has been considered possible to effectively use an NNRTI sequentially after NNRTI failure. This potential has been confirmed in a separate clinical study (TMC125-C207) in which 16 subjects who were failing on an NNRTI-containing regimen and whose virus displayed between 10- and 500-fold resistance to efavirenz had their NNRTI substituted for TMC125. Treatment with TMC125 was associated with a rapid and substantial drop in viral load (median 0.89 log10 decrease in HIV-1 RNA over 7 days) (15).
We conclude that TMC125 is a potent next-generation NNRTI that holds promise for the treatment of NNRTI-resistant virus. Our results validate the parallel screening approach that was designed and applied to the identification of TMC125 as a drug candidate. TMC125 has the potential to significantly improve treatment options for HIV-infected individuals.
Acknowledgments
We thank Hilde Bohets for performing the metabolic stability experiments, Dirk Jochmans for the combination experiments, Chih Y. Ho and Robert W. Kavash for the synthesis of the compound, and Carlo F. Perno for assessment of the activity of TMC125 against HIV-1/Ba-L in primary M/Ms.
REFERENCES
- 1.Antinori, A., M. Zaccarelli, A. Cingolani, F. Forbici, M. G. Rizzo, M. P. Trotta, S. Di Giambenedetto, P. Narciso, A. Ammassari, E. Girardi, A. De Luca, and C. F. Perno. 2002. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res. Hum. Retrovir. 18:835-838. [DOI] [PubMed] [Google Scholar]
- 2.Bacheler, L., S. Jeffrey, G. Hanna, R. D'Aquila, L. Wallace, K. Logue, B. Cordova, K. Hertogs, B. Larder, R. Buckery, D. Baker, K. Gallagher, H. Scarnati, R. Tritch, and C. Rizzo. 2001. Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J. Virol. 75:4999-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balotta, C., G. Facchi, M. Violin, S. Van Dooren, A. Cozzi-Lepri, F. Forbici, A. Bertoli, C. Riva, D. Senese, P. Caramello, G. Carnevale, G. Rizzardini, L. Cremonini, L. Monno, G. Rezza, C. F. Perno, G. Ippolito, A. d'Arminio-Monforte, A. M. Vandamme, and M. Moroni. 2001. Increasing prevalence of non-clade B HIV-1 strains in heterosexual men and women, as monitored by analysis of reverse transcriptase and protease sequences. J. Acquir. Immune Defic. Syndr. 27:499-505. [DOI] [PubMed] [Google Scholar]
- 4.Bilello, J. A., P. A. Bilello, K. Stellrecht, J. Leonard, D. W. Norbeck, D. J. Kempf, T. Robins, and G. L. Drusano. 1996. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 40:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohets, H., K. Lavrijsen, J. Hendrickx, J. van Houdt, V. van Genechten, P. Verboven, W. Meuldermans, and J. Heykants. 2000. Identification of the cytochrome P450 enzymes involved in the metabolism of cisapride: in vitro studies of potential co-medication interactions. Br. J. Pharmacol. 129:1655-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan, T. M., D. L. Taylor, C. G. Bridges, J. P. Leyda, and A. S. Tyms. 1995. The inhibition of human immunodeficiency virus type 1 in vitro by a non-nucleoside reverse transcriptase inhibitor MKC-442, alone and in combination with other anti-HIV compounds. Antivir. Res. 26:173-187. [DOI] [PubMed] [Google Scholar]
- 7.Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. [DOI] [PubMed] [Google Scholar]
- 8.Corbett, J. W., S. S. Ko, J. D. Rodgers, S. Jeffrey, L. T. Bacheler, R. M. Klabe, S. Diamond, C.-M. Lai, S. R. Rabel, J. A. Saye, S. P. Adams, G. L. Trainor, P. S. Anderson, and S. K. Erickson-Viitanen. 1999. Expanded-spectrum nonnucleoside reverse transcriptase inhibitors inhibit clinically relevant mutant variants of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 43:2893-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das, K., A. D. Clark, P. J. Lewi, J. Heeres, M. R. De Jonge, L. M. Koymans, H. M. Vinkers, F. Daeyaert, D. W. Ludovici, M. J. Kukla, B. De Corte, R. W. Kavash, C. Y. Ho, H. Ye, M. A. Lichtenstein, K. Andries, R. Pauwels, M. P. De Béthune, P. L. Boyer, P. Clark, S. H. Hughes, P. A. Janssen, and E. Arnold. 2004. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 47:2550-2560. [DOI] [PubMed] [Google Scholar]
- 10.Deeks, S. G. 2001. International perspectives on antiretroviral resistance: nonnucleoside reverse transcriptase inhibitor resistance. J. Acquir. Immune Defic. Syndr. 26(Suppl. 1):S25-S33. [DOI] [PubMed] [Google Scholar]
- 11.Delaugerre, C., R. Rohban, A. Simon, M. Mouroux, C. Tricot, R. Agher, J. M. Huraux, C. Katlama, and V. Calvez. 2001. Resistance profile and cross-resistance of HIV-1 among patients failing a non-nucleoside reverse transcriptase inhibitor-containing regimen. J. Med. Virol. 65:445-448. [PubMed] [Google Scholar]
- 12.Delwart, E. L., E. G. Shpaer, J. Louwagie, F. E. McCutchan, M. Grez, H. Rubsamen-Waigmann, and J. I. Mullins. 1993. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science 262:1257-1261. [DOI] [PubMed] [Google Scholar]
- 13.Division of AIDS, National Institute of Allergy and Infectious Diseases. 1997. DAIDS virology manual for HIV Laboratories. Publication NIH-97-3828. U.S. Department of Health and Human Services, Washington, D.C.
- 14.Fields, R. D., and M. V. Lancaster. 1993. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 11:48-50. [PubMed] [Google Scholar]
- 15.Gazzard, B., A. Pozniak, W. Rozenbaum, P. Yeni, S. Staszewski, K. Arasteh, K. De Dier, M. Peeters, B. Woodfall, J. Stebbing, and G. A. E. van't Klooster. 2003. An open-label assessment of TMC 125: a new, next generation NNRTI, for 7 days in HIV-1-infected individuals with NNRTI resistance. AIDS 17:F49-F54. [DOI] [PubMed] [Google Scholar]
- 16.Gruzdev, B., A. Rakhmanova, E. Doubovskaya, A. Yakovlev, M. Peeters, A. Rinehart, K. De Dier, P. Baede-Van Dijk, W. Parys, and G. van't Klooster. 2003. A randomized, double-blind, placebo-controlled trial of TMC125 as 7-day monotherapy in antiretroviral naive, HIV-1-infected subjects. AIDS 17:2487-2494. [DOI] [PubMed] [Google Scholar]
- 17.Harrigan, P. R., and B. A. Larder. 2002. Extent of cross-resistance between agents used to treat human immunodeficiency virus type 1 infection in clinically derived isolates. Antimicrob. Agents Chemother. 46:909-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hertogs, K., M. P. de Bethune, V. Miller, T. Ivens, P. Schel, A. Van Cauwenberge, C. Van Den Eynde, V. Van Gerwen, H. Azijn, M. Van Houtte, F. Peeters, S. Staszewski, M. Conant, S. Bloor, S. Kemp, B. Larder, and R. Pauwels. 1998. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob. Agents Chemother. 42:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larder, B. A., A. Kohli, P. Kellam, S. D. Kemp, M. Kronick, and R. D. Henfrey. 1993. Quantitative detection of HIV-1 drug resistance mutations by automated DNA sequencing. Nature 365:671-673. [DOI] [PubMed] [Google Scholar]
- 20.Leigh-Brown, A. J., S. D. Frost, W. C. Mathews, K. Dawson, N. S. Hellmann, E. S. Daar, D. D. Richman, and S. J. Little. 2003. Transmission fitness of drug-resistant human immunodeficiency virus and the prevalence of resistance in the antiretroviral-treated population. J. Infect. Dis. 187:683-686. [DOI] [PubMed] [Google Scholar]
- 21.Ludovici, D. W., B. L. De Corte, M. J. Kukla, H. Ye, C. Y. Ho, M. A. Lichtenstein, R. W. Kavash, K. Andries, M. P. de Bethune, H. Azijn, R. Pauwels, P. J. Lewi, J. Heeres, L. M. Koymans, M. R. de Jonge, K. J. Van Aken, F. F. Daeyaert, K. Das, E. Arnold, and P. A. Janssen. 2001. Evolution of anti-HIV drug candidates. 3. Diarylpyrimidine (DAPY) analogues. Bioorg. Med. Chem. Lett. 11:2235-2239. [DOI] [PubMed] [Google Scholar]
- 22.Ludovici, D. W., R. W. Kavash, M. J. Kukla, C. Y. Ho, H. Ye, B. L. De Corte, K. Andries, M. P. de Bethune, H. Azijn, R. Pauwels, H. E. Moereels, J. Heeres, L. M. Koymans, M. R. de Jonge, K. J. Van Aken, F. F. Daeyaert, P. J. Lewi, K. Das, E. Arnold, and P. A. Janssen. 2001. Evolution of anti-HIV drug candidates. 2. Diaryltriazine (DATA) analogues. Bioorg. Med. Chem. Lett. 11:2229-2234. [DOI] [PubMed] [Google Scholar]
- 23.Ludovici, D. W., M. J. Kukla, P. G. Grous, S. Krishnan, K. Andries, M. P. de Bethune, H. Azijn, R. Pauwels, E. De Clercq, E. Arnold, and P. A. Janssen. 2001. Evolution of anti-HIV drug candidates. 1. From alpha-anilinophenylacetamide (α-APA) to imidoyl thiourea (ITU). Bioorg. Med. Chem. Lett. 11:2225-2228. [DOI] [PubMed] [Google Scholar]
- 24.Moyle, G. 2001. The emerging roles of non-nucleoside reverse transcriptase inhibitors in antiretroviral therapy. Drugs 61:19-26. [DOI] [PubMed] [Google Scholar]
- 25.Oie, S., M. A. Jacobson, and D. I. Abrams. 1993. Alpha 1-acid glycoprotein levels in AIDS patients before and after short-term treatment with zidovudine (ZDV). J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 6:531-533. [PubMed] [Google Scholar]
- 26.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 27.Perno, C. F., and R. Yarchoan. 1993. Culture of HIV in monocytes and macrophages, p. 12.4.1-12.4.11. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, vol. 3. John Wiley & Sons, Inc., New York, N.Y. [Google Scholar]
- 28.Perrin, L., L. Kaiser, and S. Yerly. 2003. Travel and the spread of HIV-1 genetic variants. Lancet Infect. Dis. 3:22-27. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka, H., H. Takashima, M. Ubasawa, K. Sekiya, N. Inouye, M. Baba, S. Shigeta, R. T. Walker, E. De Clercq, and T. Miyasaka. 1995. Synthesis and antiviral activity of 6-benzyl analogs of 1-[(2-hydroxyethoxy)methyl]-5-(phenylthio)thymine (HEPT) as potent and selective anti-HIV-1 agents. J. Med. Chem. 38:2860-2865. [DOI] [PubMed] [Google Scholar]