Abstract
Background
Altered hypothalamic-pituitary-adrenal (HPA) function is common in youth with major depressive disorder (MDD) but variability in the strength and direction of HPA alterations has prompted a search for symptom-based subtypes with unique neuroendocrine signatures. This study investigated the extent to which depressive symptom composites were differentially associated with cortisol responses to psychosocial stress.
Methods
This study examined salivary cortisol responses to the Trier Social Stress Test (TSST) in 145 adolescents who varied in their risk for MDD: 38 had current MDD; 35 were healthy but at high risk for MDD based on having one or both parents with unipolar MDD; and 72 were healthy youth with no personal or family history of a psychiatric disorder. Multilevel models examined within-person change in cortisol levels during a 2-hour resting phase prior to the TSST and both linear and quadratic changes in cortisol levels following the TSST.
Results
Anticipatory cortisol reactivity was lower in MDD youth compared to low-risk youth, and in youth with higher compared to lower depressive symptom severity. Whereas affective symptoms were associated with increased anticipatory cortisol reactivity and more rapid recovery to the TSST, neurovegetative symptoms were associated with decreased anticipatory cortisol reactivity and slower recovery.
Limitations
The cross-sectional design does not permit inferences regarding temporal relations between cortisol responses and depressive symptom composites.
Conclusions
The present findings suggest that heterogeneity among studies examining HPA reactivity in depressed youth may be driven, in part, by differences in depressive symptom composites across samples.
Keywords: major depressive disorder, depressive symptoms, adolescence, stress, TSST
1. Introduction
Hypothalamic-pituitary-adrenocortical (HPA) alterations in youth with major depressive disorder (MDD) are not universal (for reviews, see Lopez-Duran et al., 2009; Stetler and Miller, 2011). Heterogeneity in stress responses in healthy individuals has spurred the development of criteria for distinguishing cortisol “responders” from “nonresponders” (Miller et al., 2013). These individual differences are especially pronounced in depressed youth (for a review, see Lopez-Duran et al., 2009) and have likely contributed to inconsistent findings on the relation between depression and HPA reactivity in youth. Some efforts to explain why only 40% to 60% of depressed individuals exhibit HPA alterations (for a review, see Parker et al., 2003) have proposed depressive subtypes with unique neuroendocrine signatures and distinct etiologies (for a review, see Antonijevic, 2006). These efforts, driven in part by growing dissatisfaction with descriptive phenomenological approaches to psychiatric diagnosis, examined whether neuroendocrine abnormalities might adhere more closely to specific depressive symptom clusters than to depressive diagnoses (Halbreich, 2006). The present study adopted a symptom-based approach to investigate the determinants of cortisol stress reactivity in adolescents who varied in their depressive symptom presentation.
1.1 Psychosocial Stressors and Cortisol Reactivity in Youth
Adolescence is a developmental period characterized by elevated stress levels, due to changes in academic environment, social activities, and physical development (Ge et al., 2003; Wigfield et al., 1991). Further, youth who experienced stressful life events were more likely to develop MDD (Grant et al., 2004). Interpersonal stressors predicted first onsets and recurrences of MDD (Sheets and Craighead, 2014), and the HPA axis appeared to play a role in this increased vulnerability (Colich et al., 2015; Rao et al., 2008). For example, cortisol reactivity to psychosocial stressors predicted depressive symptom trajectories in formerly depressed emerging adults (Morris et al., 2012) and development of MDD in girls (Colich et al., 2015). Therefore, cortisol reactivity may be especially useful as a risk marker for depression. Further supporting this view, Stroud et al. (2009) proposed that changes in the stress response over the adolescent transition may account, in part, for the growing risk for MDD during adolescence (Hankin et al., 1998).
Most research to date examining relations between depressive symptoms and HPA activity has focused on diurnal cortisol secretion (Rao et al., 1996) and responses to a pharmacologic challenge (for reviews, see O’Keane et al., 2012; Stetler and Miller, 2011). Psychosocial stress paradigms complement these approaches by probing suprahypothalamic circuits involved in the HPA response to stress. Studies of cortisol reactivity to psychosocial stress in child and adolescent samples, however, have yielded inconsistent findings. Greater cortisol responses have been reported for depressed compared to non-depressed adolescents (Rao et al., 2008), dysphoric post-pubertal youth compared to non-dysphoric youth (Hankin et al., 2010), and adolescents with more recent onset of depressive symptoms (Booij et al., 2013). In contrast, blunted cortisol responses have been reported for dysphoric pre-pubertal youth compared to non-dysphoric youth (Hankin et al., 2010), adolescents with more severe depression severity (Harkness et al., 2011), adolescents with internalizing symptoms (Spies et al., 2011), children with higher self-reported depressive symptoms (Dieleman et al., 2010), children with current, past or sub-syndromal MDD (Suzuki et al., 2013), and adolescents with more chronic depressive symptoms (Booij et al., 2013). Taken together, these findings suggest that alterations in cortisol reactivity are not limited to youth with depressive diagnoses and may be determined by a variety of factors including depression severity and developmental timing. Recent evidence suggests that prepubertal girls with blunted cortisol responses and postpubertal girls with elevated cortisol responses were at greatest risk for MDD onset (Colich et al., 2015). The present study considered associations between pubertal stage and cortisol response parameters.
1.2 Depressive Symptom Composites
MDD is a syndrome characterized by heterogeneity in symptom presentation: there are 227 possible symptom combinations that satisfy the diagnostic criteria (van Loo et al., 2012). Although youth with MDD must present with at least one core affective symptom (depressed or irritable mood and/or anhedonia), they may exhibit different combinations of neurovegetative symptoms (i.e., sleep or appetite disturbances, fatigue, psychomotor retardation) or cognitive symptoms (i.e., impaired concentration, worthlessness, guilt, suicidal ideation). Heterogeneity in the presentation of depressive symptoms may help to explain why youth exhibit increased or decreased cortisol reactivity to psychosocial stressors. Research in depressed preschoolers suggested that those with anhedonia have higher cortisol reactivity than those without anhedonia (Luby et al., 2004) but did not differ from hedonically depressed or non-depressed preschoolers in cortisol levels at the end of a stress task (Luby et al., 2003). Research on neurovegetative symptoms indicated that higher fatigue was associated with lower cortisol reactivity to a laboratory stressor in non-depressed adults (Lennartsson et al., 2015), and relations between sleep disturbances and cortisol reactivity to laboratory stressors differed for boys and girls (Pesonen et al., 2012). To our knowledge, no studies have examined relations between cognitive depressive symptoms and cortisol reactivity in depressed youth. However, self-esteem has been implicated as a vulnerability factor for depression and is closely tied to one of the cognitive depressive symptoms – feelings of worthlessness (Beck, 1991; Roberts and Monroe, 1999). Research in healthy adults indicated that higher self-esteem was associated with lower anticipatory cortisol reactivity to a stress task (Turan, 2015) and lower cortisol reactivity to interpersonal rejection (Ford and Collins, 2010).
1.3 Maltreatment History
Exposure to childhood maltreatment (including physical, sexual or emotional abuse, physical or emotional neglect, and exposure to domestic violence) can produce enduring changes in HPA function and can increase vulnerability to MDD (for a review see Heim et al., 2008). Neurodevelopmental traumatology models posit that the long-term negative sequelae of childhood maltreatment are caused, in part, by the adverse effects of stress response dysregulation on brain development during critical vulnerability periods (for a review, see De Bellis et al., 2011). Previous studies indicated that adolescents with a history of maltreatment exhibit elevated cortisol reactivity and delayed cortisol recovery to psychosocial stress tasks (MacMillan et al., 2009), except in cases of more severe depressive symptoms (Harkness et al., 2011). The importance of accounting for childhood maltreatment in studies examining associations between HPA reactivity and MDD is underscored by researchers who posit that MDD in the context of maltreatment may represent a distinct subtype (for a review, see Heim et al., 2004; Rao et al., 2008). Accordingly, the present study accounted for the effects of maltreatment history while examining the association between depressive symptoms and cortisol response parameters.
1.4 The Present Study
The present study sought to examine the extent to which specific depressive symptoms (affective, cognitive, neurovegetative) predict cortisol reactivity to a psychosocial stressor, thereby accounting for the mixed findings regarding depression and cortisol reactivity. As a first step, our aim was to replicate prior research on the associations between MDD status (currently depressed, at high risk for depression, or low risk for depression) and cortisol reactivity in youth. We expected that our largely post-pubertal sample of adolescents with MDD would exhibit greater cortisol responses than adolescents at high- or low-risk for depression. Second, based on evidence that alterations in cortisol reactivity were present in both syndromal and sub-syndromal depressed youth (Suzuki et al., 2013), we examined the association of depression severity and cortisol responses. We hypothesized that higher overall depression severity, regardless of diagnostic status, would be associated with more blunted anticipatory cortisol reactivity and recovery in adolescents (Harkness et al., 2011).
Third, our primary study aim was to examine affective, neurovegetative and cognitive depressive symptom composites as predictors of cortisol stress responses. This method of categorizing depressive symptoms conceptually has proven useful in prospective studies (Kouros et al., 2016) and limits the number of predictors tested per model. Based on prior work in depressed adolescents (Hankin et al., 2010; Rao et al., 2008), we hypothesized that affective symptoms would be associated with a robust cortisol response pattern in adolescents marked by increased anticipatory reactivity and more rapid cortisol recovery. Although no studies, to our knowledge, have examined associations between the neurovegetative symptom cluster and cortisol reactivity in youth, we nonetheless hypothesized that neurovegetative symptoms would be associated with a pattern of blunted anticipatory cortisol reactivity and recovery in adolescents based on research examining fatigue in adults (Lennartsson et al., 2015). Given the lack of research on cognitive symptoms and cortisol reactivity in youth, no specific hypotheses were made. However, based on studies in adults (Ford and Collins, 2010; Turan, 2015), we expected that higher levels of cognitive symptoms would be associated with blunted anticipatory cortisol reactivity. We expected relations between depressive symptom composites and cortisol outcomes to hold after accounting for pubertal status, given that this sample was primarily post-pubertal (Colich et al., 2015).
2. Method
2.1. Participants
This study is part of a larger ongoing, programmatic investigation of the onset and course of depression in adolescents. Participants were 145 youth, ages 12 to 17 years old, who varied in their risk for MDD: 38 had current MDD; 35 were adolescents who had no personal history of a psychiatric disorder but were at high risk for MDD based on having one or both parents with unipolar MDD (high-risk youth); and 72 were adolescents with no personal or family history of a psychiatric disorder (low-risk youth). Participants with depression who had a lifetime history of mania, hypomania, schizophrenia, schizoaffective disorder, autism, or with a family history of bipolar disorder were excluded from the study. All participants were medically healthy and free from psychotropic medication (for a minimum of 8 weeks but most of the depressed youth were psychotropic-naive), other medications that can impact HPA activity, and nicotine and alcohol or illicit drug use, as determined by physical examination, laboratory investigations, breath carbon monoxide levels, and urine cotinine and drug screens. Written informed consent/assent was obtained from all participants and all procedures were approved by the institutional review board.
2.2. Measures
Depression diagnosis
The diagnosis of MDD and other psychiatric disorders was based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997), which was administered by a trained research staff member and reviewed with the Principal Investigator (UR). A random set of assessments (25%) were independently coded for reliability (κ’s ≥ 0.85). The K-SADS-PL was administered to both the adolescent and parent and summary scores were tabulated after re-interviewing the youth and parent to resolve discrepancies.
Depressive symptoms
The presence and severity of individual symptoms of depression in the last two weeks was assessed with the Children’s Depression Rating Scale-Revised Version (CDRS-R; Poznanski and Mokros, 1996). The CDRS-R is a semi-structured interview that was administered separately to mothers and adolescents by the same interviewer. The interviewer uses information obtained from both the mother and the adolescent to rate each of 17 depressive symptoms on a 7- or 5-point severity scale (Poznanski and Mokros, 1996). The CDRS has good internal consistency, construct validity, inter-rater reliability, and is sensitive to changes in specific symptoms over time (Mayes et al., 2010); inter-rater reliability scores in this sample (based on 25% of independently coded assessments) were ≥ 0.85. The total score was used to index depressive symptom severity. Composite mean scores were computed for affective symptoms (symptoms of difficulty having fun, social withdrawal, irritability, depressed feeling, excessive weeping, depressed facial affect, listless speech), cognitive symptoms (symptoms of impaired school work, excessive guilt, low self-esteem, morbid ideation, suicidal ideation), and neurovegetative symptoms (symptoms of sleep disturbance, appetite disturbance, excessive fatigue, physical complaints, hypoactivity).
Family history of psychopathology
History of psychiatric disorders in family members was determined by a semi-structured interview: The Family History-Research Diagnostic Criteria (FH-RDC; Andreasen et al., 1977). The adolescent’s mother was interviewed regarding lifetime psychiatric disorders in all first-degree relatives (including self, spouse, and all offspring). The FH-RDC was used to determine depression-risk status for the non-depressed youth.
Psychosocial stressor and cortisol assay
A standardized psychosocial stress protocol– the Trier Social Stress Test (TSST) modified for pediatric samples– was used to induce HPA reactivity (Buske-Kirschbaum et al., 1997). Participants completed semi-structured diagnostic interviews and questionnaires on a different day than the TSST. Youth were not given details regarding the TSST; instead, they were informed that they would be completing a challenging task similar to tasks they had likely performed in school. Participants were instructed not to drink caffeinated drinks after 12 pm on the day of the TSST and not to eat or drink anything other than water prior to 1 hour before arrival to the laboratory and during the laboratory visit. After arrival to the laboratory and an initial rest period for 30 minutes, baseline saliva samples were collected at 30-min intervals for 2 hours prior to the stress task (five samples). Participants arrived at the laboratory at 4:30 pm and the stress protocol (with baseline pre-stress saliva sampling) began at 5:00pm. During this rest period, participants either read or watched TV with a neutral content. After a 5-min preparation period, youth completed a 5-min public-speaking task and a 5-min mental arithmetic task; both tasks were performed in front of an audience and a video-recorder. Post-stress saliva samples were collected immediately after the task and at 10-min intervals for 60 minutes (seven samples). Salivary cortisol levels were determined in duplicate using a commercially available enzyme immunoassay kit (Enzyme-Linked ImmunoSorbent Assay, ALPCO diagnostics, Salem, NH). The intra- and inter-assay coefficients of variation for the assays were less than 10%. Samples from the same subject were analyzed in the same assay.
Childhood Maltreatment
The presence of maltreatment occurring before age 12 was determined by the Childhood Adversity Interview (CAI; Henry and Hammen, 1998), which is a semi-structured interview that was administered by trained raters to youth and parent. The presence of maltreatment was determined by a score of ≥3 (1 = none, 5 = most severe) on at least one of the following subscales: physical abuse/assault, sexual abuse/assault, and witnessing domestic violence. The CAI has demonstrated good inter-rater and test-retest reliability (Espejo et al., 2007); inter-rater reliability scores in this sample (based on 25% independently coded assessments) were ≥ 0.85.
Pubertal maturation
Tanner staging was determined based on pictorial representations of breast, genital, and pubic hair development provided by youth self-report (Marshall and Tanner, 1969, 1970). Tanner stages 1 and 2 reflect development up to the onset of puberty and Tanner stages 3 to 5 reflect post-pubertal development. A summary Tanner score was calculated for each participant as her/his highest stage for breast/genital and pubic hair development.
2.3 Data Analytic Plan
Cortisol data were log-transformed to reduce skewness. Depressive symptom composites were computed for all youth based on their mean scores for affective, cognitive, and neurovegetative symptoms. Preliminary analyses examining depressive symptom composite measures revealed no problems with multicollinearity (VIF’s < 4.2, tolerances > .30) (Cohen et al., 2003). To examine within- and between-person change in cortisol responses simultaneously, we specified a piecewise multilevel model (MLM) using hierarchical linear models (HLM v. 6; Raudenbush et al., 2004). At Level 1, within-person change was modeled to examine (1) linear change in cortisol levels during acclimation to the laboratory environment (resting), (2) instantaneous rate of change in cortisol levels immediately prior to the TSST (anticipatory reactivity), and (3) quadratic change in cortisol levels post-TSST (recovery). These within-person estimates were aggregated at Level 2 (between-person model) to provide the average level of resting, anticipatory reactivity and recovery estimates for the sample.
A piecewise MLM approach was adopted to allow simultaneous modeling of both pre- and post-TSST cortisol slopes (Llabre et al., 2001; MacMillan et al., 2009; Willett et al., 1998). We created two dummy coded vectors that were centered at time = 0 (final pre-stress saliva sample): a pre-TSST vector, d1, coded as ‘1’ for time < 0 and as ‘0’ for time ≥ 0; a post-TSST vector, d2, coded as ‘1’ for time > 0 and as ‘0’ for time ≤ 0. The time vector was coded as follows (in minutes relative to the final pre-stress saliva sample): −120, −90, −60, −30, 0, +15, +25, +35, +45, +55, +65, +75. The interaction of time and d1 yielded the Level 1 ‘resting slope’ and the time X d2 interaction yielded the Level 1 ‘anticipatory reactivity slope’. Finally, the Level 1 ‘recovery’ parameter was computed as the product of anticipatory reactivity slope with itself. A resting cortisol slope was included based on prior work suggesting that this ‘arrival index’ may convey important information regarding how individuals habituate or acclimate to a novel environment (Balodis et al., 2010). A baseline model without predictors indicated significant level 1 parameter variance components for resting slope and anticipatory reactivity slope but not for quadratic change; hence, subsequent MLMs specified random intercepts, resting slopes, and anticipatory reactivity slopes.
Prior to testing primary hypotheses regarding depressive symptom severity and depressive symptom composites, between-group differences based on MDD status (MDD, high-risk, low-risk) in cortisol response outcomes were tested by entering group status as a Level 2 predictor of resting, anticipatory reactivity, and recovery. Next, to address the depressive symptom severity hypothesis, participants’ CDRS-R total score was included at Level 2 to test the extent to which overall depressive symptom severity was associated with pre- and post-TSST changes in cortisol levels. Finally, to address depressive symptom composite hypotheses, all three depressive symptom composites (affective, neurovegetative, cognitive) were entered simultaneously at Level 2 to test the extent to which each composite was uniquely associated with pre- and post-TSST changes in cortisol. The impact of pubertal status on cortisol outcomes was examined in preliminary analyses and the impact of maltreatment (Maltx) status on pre- and post-TSST changes in cortisol levels was controlled for in all models.
The two-level MLM was as follows:
- Level 1 Model:
- Level 2 Model:
The parameter β00 represents the cortisol trajectory’s intercept (i.e., baseline cortisol level) immediately prior to the TSST, β10 represents the rate of change in cortisol levels during acclimation to the laboratory environment (i.e., linear cortisol change from arrival to the laboratory up until the start of the TSST; resting), β20 represents the instantaneous rate of change in cortisol levels (i.e., linear slope/anticipatory reactivity) immediately prior to the TSST, and β30 represents how this rate of change accelerates or decelerates over the course of the TSST (i.e., curvature/recovery). For example, an individual may exhibit elevated anticipatory cortisol reactivity (i.e., a positive value for β20), but this rate of increase could slow over time such that cortisol levels reach a peak and eventually decline (i.e., a negative value for β30). The cross-level interactions of depressive symptom composite scores and the resting, anticipatory reactivity and recovery slopes tested the extent to which change in cortisol levels pre- and post-TSST differed depending on depressive symptom composites. Significant interactions were probed, and simple slopes were calculated using Preacher and colleagues’ online calculator (Preacher, Curran, & Bauer, 2006). Three youth were missing depressive symptom composite scores and one youth was missing cortisol data for the TSST; missing data at Level 1 was handled using maximum-likelihood estimation.
3. Results
Demographic and clinical characteristics of the sample (Table 1) and correlations between study variables (Table 2) are presented for MDD, high-risk, and low-risk youth. Group differences were found for ethnic composition and for mean scores on all depressive symptom composites, with the depressed group manifesting higher levels of all symptom composites. Preliminary analyses revealed that neither age, pubertal status (Tanner stage), sex, nor ethnic composition were significantly associated with changes in cortisol levels (i.e., resting, anticipatory reactivity, recovery).
Table 1.
Descriptive Statistics for Currently Depressed Youth and Youth at High or Low Risk for MDD.
| Current MDD (n = 38) | High Risk for MDD (n = 35) | Low Risk for MDD (n = 72) | Group Differences | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | X2 | |
|
|
||||
| Sex | 2.09 | |||
| Male | 18 (47) | 22 (63) | 36 (50) | |
| Female | 20 (53) | 13 (37) | 36 (50) | |
| Race | 2.75 | |||
| Caucasian | 18 (47) | 10 (29) | 29 (40) | |
| Ethnicity | ||||
| Hispanic | 12 (32) | 16 (46) | 15 (21) | 7.69* |
| Non-Hispanic | 26 (68) | 19 (54) | 57 (79) | |
| Maltreatment History | 16 (42) | 12 (34) | 18 (25) | 3.50 |
|
|
||||
| M (SD) | M (SD) | M (SD) | F | |
|
|
||||
| Age | 14.3 (1.7) | 14.5 (1.9) | 14.6 (1.8) | 0.41 |
| Tanner stage | 4.4 (0.7) | 4.3 (0.8) | 4.3 (0.8) | 0.10 |
| CDRS-R total score | 8.3 (2.1)a | 3.8 (0.7)b | 3.4 (0.6)b | 210.12*** |
| CDRS-R composites | ||||
| Affective | 2.9 (0.9)a | 1.3 (0.3)b | 1.2 (0.3)b | 134.52*** |
| Cognitive | 2.5 (0.7)a | 1.2 (0.3)b | 1.1 (0.3)b | 124.75*** |
| Neurovegetative | 2.9 (0.9)a | 1.3 (0.3)b | 1.1 (0.2)b | 157.30*** |
p < .001;
p < .05.
Within rows, values with different superscripts differ significantly at p < .05.
Note: MDD = major depressive disorder; CDRS-R = Children’s Depression Ratings Scale-Revised.
Table 2.
Correlations Among Depressive Symptom Composites, Maltreatment History, and Demographic Variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Age | -- | |||||||
| 2. Tanner Stage | .67** | -- | ||||||
| 3. Sex | −.05 | −.16* | -- | |||||
| 4. Ethnicity | −.18* | −.13 | −.08 | -- | ||||
| 5. Maltreatment History | −.10 | −.004 | −.003 | .11 | -- | |||
| 6. Depressive Symptom Severity | −.14 | −.05 | .01 | .03 | .14 | -- | ||
| 7. Affective Symptom Composite | −.13 | −.06 | .02 | .01 | .14 | .95** | -- | |
| 8. Cognitive Symptom Composite | −.10 | .01 | −.01 | .01 | .15 | .93** | .83** | -- |
| 9. Neurovegetative Symptom Composite | −.15 | −.08 | .003 | .06 | .12 | .95** | .83** | .83** |
p < .05;
p< .001.
Note: Sex (1 = female; 2 = male); Ethnicity (0 = Non-Hispanic; 1 = Hispanic); Maltreatment History (0 = No; 1 = Yes).
3.1 Piecewise Multilevel Models of Pre- and Post-TSST Cortisol Levels
Preliminary analyses on the full sample without substantive predictors revealed that, on average, cortisol levels decreased over time during the resting period (b = −0.003, SE = 0.001, p < .001), anticipatory cortisol levels were increasing at the start of the TSST (b = 0.033, SE = 0.002, p < .001), and cortisol levels showed deceleration during the course of the post-TSST collection (b = −0.0004, SE = 0.00003, p < .001). For descriptive purposes and to replicate prior research examining the impact of MDD on cortisol responses, group status (MDD, high-risk, low-risk) was examined as a predictor of resting slopes, anticipatory reactivity, and recovery. Between-group differences were observed for anticipatory cortisol reactivity (b = −0.006, SE = 0.003, p = .024) and cortisol recovery (b = 0.0001, SE = 0.00004, p = .023), but not for cortisol levels prior to the TSST (b = −0.010, SE = 0.094, p = .919) or resting cortisol slopes (b = 0.001, SE = 0.001, p = .426). Simple slope analyses revealed that anticipatory cortisol reactivity was higher for low-risk (b = 0.039, SE = 0.003, p < .001) than for high-risk (b = 0.032, SE = 0.002, p < .001) or MDD youth (b = 0.026, SE = 0.004, p < .001). Simple slope analyses revealed more rapid deceleration in post-TSST cortisol levels (i.e., faster recovery) among low-risk youth (b = −0.001, SE = 0.00004, p < .001) than for high-risk (b = −0.0004, SE = 0.00003, p < .001) or MDD youth (b = −0.0003, SE = 0.0001, p < .001).
3.2 Depressive Symptom Severity as a Predictor of Cortisol Levels
Depressive symptom severity was positively associated with cortisol levels immediately prior to the TSST (b = .159, SE = 0.079, p = .045) and significantly moderated anticipatory cortisol reactivity (b = −0.010, SE = 0.002, p < .001) and quadratic change in post-TSST cortisol levels (b = 0.0001, SE = 0.00003, p < .001). Simple slope analysis revealed that anticipatory cortisol reactivity was lower among youth with higher (+1 SD) depressive symptom severity (b = 0.02, SE = 0.004, p < .001) compared to youth with lower (−1 SD) depressive symptom severity (b = 0.04, SE = 0.003, p < .001). Regions of significance indicated that that the depressive symptom severity X anticipatory cortisol reactivity interaction was significant for youth scoring less than 2.3 SD above the mean and greater than 6.6 SD above the mean. Simple slope analyses also revealed that there was more rapid deceleration in post-TSST cortisol levels (i.e., faster recovery) among youth with lower (−1 SD) depressive symptom severity (b = −0.0005, SE = 0.0001, p < .001) compared to youth with higher (+1 SD) depressive symptom severity (b = −0.0003, SE = 0.0001, p < .001). Regions of significance indicated that the depressive symptom severity X cortisol recovery interaction was significant for youth scoring less than 2.4 SD above the mean and greater than 9.9 SD above the mean. There was a non-significant trend for depressive symptom severity moderating resting cortisol levels (b = 0.001, SE = 0.001, p = .051). Results of this model indicated that there was significant variation in cortisol parameters that remained to be explained after accounting for depressive symptom severity; subsequent analyses examined whether depressive symptom composites could account, in part, for this variation.
3.3 Depressive Symptom Composites as Predictors of Cortisol Levels
This model simultaneously included all three composites as predictors of pre- and post-TSST cortisol levels and change to determine the effect of each composite over and above the effect of the other composites and maltreatment history (Table 3).2 Maltreatment history was associated with lower cortisol levels immediately prior to the TSST (b = −0.519, SE = 0.168, p = .003) but was not significantly associated with changes in resting cortisol levels (b = −0.002, SE = 0.001, p = .229), anticipatory cortisol reactivity (b = −0.003, SE = 0.005, p = .510), or quadratic change in post-TSST cortisol levels (b = −0.00001, SE = 0.0001, p = .853).
Table 3.
Multilevel Model Predicting Cortisol Responses to the TSST
| Predictors | Coefficient b (SE) |
|---|---|
| Intercept (baseline cortisol level), β00 | −3.214 (.094)** |
| Maltreatment, β01 | −0.519 (.168)* |
| Affective Composite, β02 | 0.126 (.163) |
| Cognitive Composite, β03 | 0.057 (.167) |
| Neurovegetative Composite, β04 | −0.007 (.155) |
| Resting (linear rate of change during acclimation to laboratory), β10 | −0.002 (.001)* |
| Maltreatment, β11 | −0.002 (.001) |
| Affective Composite, β12 | 0.001 (.001) |
| Cognitive Composite, β13 | −0.0001 (.001) |
| Neurovegetative Composite, β14 | 0.00004 (.001) |
| Anticipatory Reactivity (linear rate of change at start of TSST), β20 | 0.034 (.003)** |
| Maltreatment, β21 | −0.003 (.005) |
| Affective Composite, β22 | 0.020 (.005)** |
| Cognitive Composite, β23 | 0.007 (.005) |
| Neurovegetative Composite, β24 | −0.037 (.005)** |
| Recovery (trajectory curvature post-TSST), β30 | −0.0004 (.00004)** |
| Maltreatment, β31 | −0.00001 (.0001) |
| Affective Composite, β32 | −0.0002 (.0001)** |
| Cognitive Composite, β33 | −0.0001 (.0001) |
| Neurovegetative Composite, β34 | 0.0005 (.0001)** |
p<.001;
p<.01.
Note: TSST = Trier Social Stress Test.
Affective symptoms
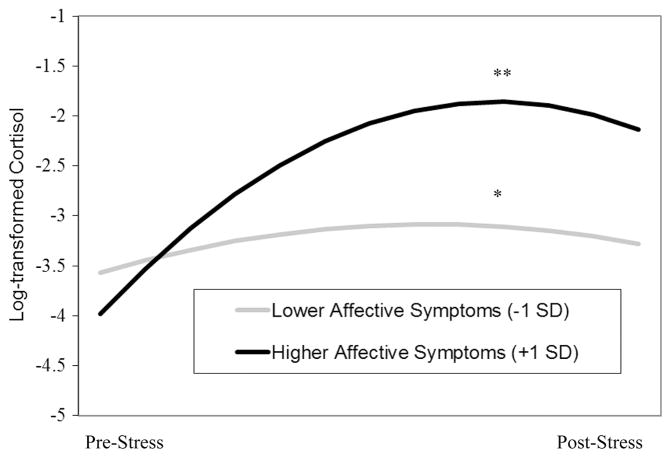
Affective symptom composite scores significantly moderated anticipatory cortisol reactivity (b = 0.020, SE = 0.005, p < .001) and quadratic change in post-TSST cortisol levels (b = −0.0002, SE = 0.0001, p < .001) (Figure 1). Simple slope analysis indicated higher anticipatory cortisol reactivity among youth with higher (+1 SD) affective symptom composite scores (b = 0.05, SE = 0.006, p < .001) compared to youth with lower (−1 SD) affective symptom composite scores (b = 0.01, SE = 0.006, p = .014). Regions of significance indicated that that the affective symptom composite X anticipatory cortisol reactivity interaction was significant for youth scoring less than 3.3 SD below the mean and greater than 1.1 SD below the mean. Simple slope analysis also revealed more rapid deceleration in post-TSST cortisol levels (i.e., faster recovery) among youth with higher (+1 SD) affective symptom composite scores (b = −0.0006, SE = 0.0001, p < .001) compared to youth with lower (−1 SD) affective symptom composite scores (b = −0.0002, SE = 0.0001, p = .003) (Figure 1). Regions of significance indicated that the affective symptom composite X cortisol recovery interaction was significant for youth scoring less than 4.9 SD below the mean and greater than 1.2 SD below the mean. Affective symptom composite scores were not significantly associated with cortisol levels immediately prior to the TSST (b = 0.126, SE = 0.163, p = .440) or with changes in resting cortisol levels (b = 0.001, SE = 0.001, p = .303).
Figure 1.
Interaction of affective symptoms and sampling time predicting quadratic changes in cortisol levels during the TSST (recovery). **p < .001; *p < .01.
Cognitive symptoms
Cognitive symptom composite scores were not significantly associated with changes in resting cortisol levels (b = −0.0001, SE = 0.001, p = .969), cortisol levels immediately prior to the TSST (b = 0.057, SE = 0.167, p = .735), anticipatory cortisol reactivity (b = 0.007, SE = 0.005, p = .169), or quadratic change in post-TSST cortisol levels (b = −0.0001, SE = 0.0001, p = .200).
Neurovegetative symptoms
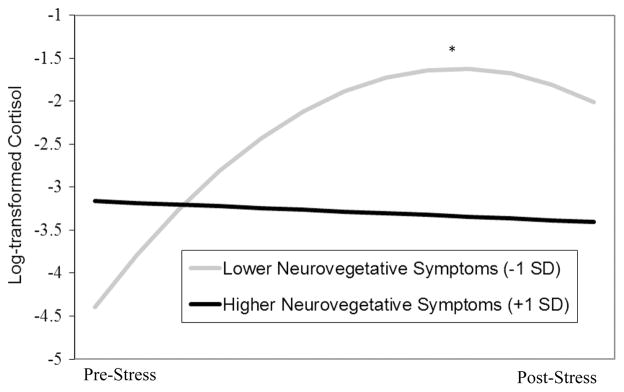
Neurovegetative symptom composite scores significantly moderated anticipatory cortisol reactivity (b = −0.037, SE = 0.005, p < .001) and quadratic change in post-TSST cortisol levels (b = 0.0005, SE = 0.0001, p < .001) (Figure 2). Simple slope analysis indicated that anticipatory cortisol levels were increasing at the beginning of the TSST among youth with lower (−1 SD) neurovegetative symptom composite scores (b = 0.07, SE = 0.005, p < .001) but were not changing significantly for youth with higher (+1 SD) neurovegetative symptom composite scores at the beginning of the TSST (b = −0.003, SE = 0.005, p = .544). Regions of significance indicated that that the neurovegetative symptom composite X anticipatory cortisol reactivity interaction was significant for youth scoring less than 0.7 SD above the mean and greater than 1.2 SD above the mean. Simple slope analysis also revealed that whereas youth with lower (−1 SD) neurovegetative symptom composite scores showed deceleration in post-TSST cortisol levels (b = −0.001, SE = 0.0001, p < .001), youth with higher (+1 SD) neurovegetative symptom composite scores did not exhibit any acceleration or deceleration in their post-TSST cortisol levels (b = 0.0000, SE = 0.0001, p = .428) (Figure 2). Regions of significance indicated that that the neurovegetative symptom composite X cortisol recovery interaction was significant for youth scoring less than 0.7 SD above the mean and greater than 1.2 SD above the mean. Neurovegetative symptom composite scores were not significantly associated with cortisol levels immediately prior to the TSST (b = −0.007, SE = 0.155, p = .966) or with change in resting cortisol levels (b = 0.00004, SE = 0.001, p = .974).
Figure 2.
Interaction of neurovegetative symptoms and sampling time predicting quadratic changes in cortisol levels during the TSST (recovery). *p < .001.
4. Discussion
Major depression is characterized by significant heterogeneity in symptom presentation (van Loo et al., 2012) and variability in the strength and direction of HPA axis alterations (Lopez-Duran et al., 2009). The MDD diagnosis may encompass multiple depressive subtypes with distinct phenomenology, pathophysiology, and treatment response (Halbreich, 2006). The present study is the first to investigate whether depressive symptom composites explain variability in adolescents’ cortisol responses to psychosocial stress. Although adolescents with more severe overall depressive symptoms (and those with current MDD) exhibited blunted anticipatory cortisol reactivity to the TSST and slower cortisol recovery, which is consistent with prior work in adolescents (Harkness et al., 2011), depressive symptom composite scores were associated with contrasting cortisol response patterns. Whereas affective symptoms of MDD were associated with greater anticipatory cortisol reactivity and more rapid cortisol recovery, neurovegetative symptoms were associated with blunted anticipatory cortisol reactivity and more protracted recovery. Depressive symptom composites predicted cortisol response patterns over and above maltreatment history, which prior work has established is an important determinant of HPA reactivity (MacMillan et al., 2009). Taken together, these findings suggest that discrepancies between studies investigating cortisol responses in depressed youth could be driven by sample differences in affective and neurovegetative symptoms.
Adolescents with higher affective symptom composite scores exhibited greater anticipatory cortisol reactivity and more rapid cortisol recovery than adolescents with lower affective symptom composite scores. These findings extend prior work that showed higher cortisol stress responses in anhedonic compared to hedonic depressed preschoolers (Luby et al., 2003, 2004) and complement studies that associated melancholic features of depression (including anhedonia) with both elevated diurnal cortisol secretion (Stetler and Miller, 2011) and greater cortisol nonsuppression to the dexamethasone suppression test (for a review see Rush and Weissenburger, 1994). Studies in youth have generally not found an association between depression status and altered response to corticotropin-releasing hormone challenge (Lopez-Duran et al., 2009), suggesting that suprahypothalamic factors – including cognitive appraisals (for a review see Denson et al., 2009) – may help to explain why youth with more affective symptoms show heightened acute cortisol responses to social stressors.
The present findings add to a growing literature on blunted cortisol responses in individuals with stress-related conditions (Petrowski et al., 2010) and suggest that both overall depression severity and neurovegetative symptoms, in particular, play an important role in determining cortisol responses in these diverse populations. One important avenue for future research is to identify which neurovegetative symptoms are most closely tied to blunted cortisol responses in depressed youth. Sleep disturbances have been differentially associated with cortisol stress reactivity in boys and girls (Pesonen et al., 2012). Recent evidence suggested that blunted cortisol responses to psychosocial stress were associated with physical complaints - including musculoskeletal pain - in young adults (Paananen et al., 2015). Fatigue has also been associated with lower cortisol responses to stress in non-depressed adults (Lennartsson et al., 2015). Understanding the mechanisms linking fatigue and blunted cortisol reactivity is critical given that depressed adolescents with prolonged fatigue exhibited greater disability and health service use than adolescents with either prolonged fatigue or depression alone (Lamers et al., 2013). The relative impact of insomnia versus hypersomnia and increased versus decreased appetite on cortisol responses could not be addressed because all symptoms were included in the neurovegetative composite.
The arrival index reflects how much an individual’s cortisol levels decrease from the moment s/he first presents to the laboratory until immediately prior to the TSST. In healthy individuals, higher arrival indices predicted greater cortisol reactivity to the TSST and higher self-reported anxiety levels following the TSST (Balodis et al., 2010). Elevated anticipatory cortisol levels prior to a social challenge predicted higher depressive symptoms in children exposed to peer victimization (Rudolph et al., 2011). In the present study, slower declines in resting cortisol levels prior to the TSST were associated with higher depressive symptom severity; however, none of the depressive symptom composites accounted for unique variance in resting cortisol slopes over and above the other composites.
Limitations of the present study provide directions for future research. First, the cross-sectional design does not permit inferences regarding whether cortisol responses predispose youth to, are an epiphenomenon of, or reflect a biological scar of, depressive symptom composites. Second, despite careful selection of participants to minimize psychiatric and medical comorbidities, methodological rigor in the implementation of the TSST protocol, and use of an interview-based measure of depressive symptoms, cortisol responses to psychosocial stress are influenced by a host of factors that could not all be accounted for in the present study (e.g., wake time and same-day exercise levels). Third, although pubertal stage did not significantly predict cortisol responses in the present study, this may have been due to the relatively small proportion of pre-pubertal youth that were enrolled. Fourth, although maltreatment history was not associated with change in cortisol levels during the TSST protocol (i.e., resting, anticipatory reactivity, recovery), future studies should examine whether maltreatment history interacts with depressive symptom composites to influence cortisol responses to the TSST; the current study was not sufficiently powered to test such 3-way interactions.
In summary, the present study highlights the contrasting associations between affective versus neurovegetative symptoms and cortisol responses to psychosocial stress in adolescents varying in their presentation of depressive symptoms. Importantly, these distinct and opposing cortisol response patterns were masked when focusing solely on the role of depressive symptom severity and MDD status, and occurred in spite of a strong, positive correlation between affective and neurovegetative symptom composites. Individual differences in affective and neurovegetative symptoms may prove useful in identifying depressed youth at risk for negative mental and physical health conditions associated with hyper- or hypo-cortisolism, respectively. In addition, youth who present with predominantly affective versus neurovegetative symptoms may benefit from interventions that seek to correct heightened versus blunted cortisol responses to social stressors. In light of recent evidence that changes in specific depressive symptoms predicted increased likelihood of developing MDD during adolescence (Kouros et al., 2016), future studies should examine whether changes in cortisol stress response indices over time are differentially associated with risk for specific depressive symptom composites or depressive subtypes.
Highlights.
Altered cortisol responses to stress are common in depressed youth.
However, the strength and direction of cortisol responses are highly variable.
Relations between depressive symptom profiles and cortisol responses were examined.
Affective symptoms predicted higher cortisol reactivity and more rapid recovery.
Neurovegetative symptoms predicted lower cortisol reactivity and slower recovery.
Acknowledgments
This research was funded in part by grants from the National Institute of Health (R01 DA014037, R01 DA015131, R01 DA017804, R01 DA017805, R01 MH062464, R01 MH068391, K01 MH101403), by the Sarah M. and Charles E. Seay Chair in Child Psychiatry at UT Southwestern Medical Center (Uma Rao), and by the Betsey R. Bush Endowed Professorship in Behavioral Health at the University of Tennessee (Uma Rao). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Findings for this model were unchanged when group status (MDD, high-risk, low-risk) was included as an additional Level 2 predictor of resting cortisol levels, anticipatory cortisol reactivity, and cortisol recovery.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Antonijevic IA. Depressive disorders: Is it time to endorse different pathophysiologies? Psychoneuroendocrinology. 2006;31:1–15. doi: 10.1016/j.psyneuen.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Wynne-Edwards KE, Olmstead MC. The other side of the curve: Examining the relationship between pre-stressor physiological responses and stress reactivity. Psychoneuroendocrinology. 2010;35:1363–1373. doi: 10.1016/j.psyneuen.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy: A 30-year retrospective. Am Psychol. 1991;46:368–375. doi: 10.1037//0003-066x.46.4.368. [DOI] [PubMed] [Google Scholar]
- Booij SJ, Bouma EMC, de Jonge P, Ormel J, Oldehinkel AJ. Chronicity of depressive problems and the cortisol response to psychosocial stress in adolescents: The TRAILS study. Psychoneuroendocrinology. 2013;38:659–666. doi: 10.1016/j.psyneuen.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer DH. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Lawrence Erlbaum Associates; New Jersey: 2003. [Google Scholar]
- Colich NL, Kircanski K, Foland-Ross LC, Gotlib IH. HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology. 2015;55:94–101. doi: 10.1016/j.psyneuen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Spratt EG, Hooper SR. Neurodevelopmental biology associated with childhood sexual abuse. J Child Sex Abus. 2011;20:548–587. doi: 10.1080/10538712.2011.607753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- Dieleman GC, van der Ende J, Verhulst FC, Huizink AC. Perceived and physiological arousal during a stress task: Can they differentiate between anxiety and depression? Psychoneuroendocrinology. 2010;35:1223–1234. doi: 10.1016/j.psyneuen.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Espejo EP, Hammen CL, Connolly NP, Brennan PA, Najman MJ, Bor W. Stress sensitization and adolescent depressive severity as a function of childhood adversity: A link to anxiety disorders. J Abnorm Child Psychol. 2007;35:287–299. doi: 10.1007/s10802-006-9090-3. [DOI] [PubMed] [Google Scholar]
- Ford MB, Collins NL. Self-esteem moderates neuroendocrine and psychological responses to interpersonal rejection. J Pers Soc Psychol. 2010;98:405–419. doi: 10.1037/a0017345. [DOI] [PubMed] [Google Scholar]
- Ge X, Kim IJ, Brody GH, Conger RD, Simons RL, Gibbons FX, Cutrona CE. It’s about timing and change: Pubertal transition effects on symptoms of major depression among African American youths. Dev Psychol. 2003;39:430–439. doi: 10.1037/0012-1649.39.3.430. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: Measurement issues and prospective effects. J Clin Child Adolesc Psychol. 2004;33:412–425. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Major depression is not a diagnosis, it is a departure point to a different diagnosis: Clinical and hormonal considerations. Psychoneuroendocrinology. 2006;31:16–22. doi: 10.1016/j.psyneuen.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biol Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Henry R, Hammen C. The Childhood Adversity Interview. University of California at Los Angeles; Los Angeles, CA: 1998. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-aged Children-Present and Lifetime versions (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kouros CD, Morris MC, Garber J. Within-person changes in individual symptoms of depression predict subsequent depressive episodes in adolescents: A prospective study. J Abnorm Child Psychol. 2016;44:483–494. doi: 10.1007/s10802-015-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Ratings scales to assess depression in school-aged children. Acta Peadopsychiatr Int J Child Adolesc Psychiatry. 1981;46:305–315. [PubMed] [Google Scholar]
- Lamers F, Hickie I, Merikangas KR. Prevalence and correlates of prolonged fatigue in a U.S. sample of adolescents. Am J Psychiatry. 2013;170:502–510. doi: 10.1176/appi.ajp.2012.12040454. [DOI] [PubMed] [Google Scholar]
- Lennartsson AK, Sjörs A, Währborg P, Ljung T, Jonsdottir IH. Burnout and hypocortisolism: A matter of severity? A study on ACTH and cortisol responses to acute psychosocial stress. Front Psychiatry. 2015;6:8. doi: 10.3389/fpsyt.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Schneiderman N. Piecewise latent growth curve modeling of systolic blood pressure reactivity and recovery from the cold pressor test. Psychophysiology. 2001;38:951–960. doi: 10.1111/1469-8986.3860951. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: Evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment. Results of the Youth Mood Project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in boys. Arch Dis Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie J. Psychometric properties of the Children’s Depression Rating Scale-Revised in Adolescents. J Child Adolesc Psychopharmacol. 2010;20:513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Plessow F, Kirschbaum C, Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: Evaluation of salivary cortisol pulse detection in panel designs. Psychosom Med. 2013;75:832–840. doi: 10.1097/PSY.0000000000000002. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: Social-evaluative threat and prior depressive episodes as moderators. J Affect Disord. 2012;143:223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Frodl T, Dinan TG. A review of atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology. 2012;37:1589–1599. doi: 10.1016/j.psyneuen.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Paananen M, O’Sullivan P, Straker L, Beales D, Coenen P, Karpinnen J, Pennell C, Smith A. A low cortisol response to stress is associated with musculoskeletal pain combined with increased pain sensitivity in young adults: A longitudinal cohort study. Arthritis Res Ther. 2015;17:355. doi: 10.1186/s13075-015-0875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Kajantie E, Heinonen K, Pyhälä R, Lahti J, Jones A, Matthews KA, Eriksson JG, Strandberg T, Räikkönen K. Sex-specific associations between sleep problems and hypothalamic-pituitary-adrenocortical axis activity in children. Psychoneuroendocrinology. 2012;37:238–248. doi: 10.1016/j.psyneuen.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Petrowski K, Herold U, Joraschky P, Wittchen HU, Kirschbaum C. A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with panic disorder with concurrent normal cortisol awakening responses. Psychoneuroendocrinology. 2010;35:414–421. doi: 10.1016/j.psyneuen.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Poznanski E, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) Manual. Western Psychological Services; Los Angeles, CA: 1996. [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Giles DE, Rao R, Kaufman J, Nelson B. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biol Psychiatry. 1996;40:474–484. doi: 10.1016/0006-3223(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows. Scientific Software International, Inc; Lincolnwood, IL: 2004. [Google Scholar]
- Roberts JE, Monroe SM. Vulnerable self-esteem and social processes in depression: Toward an interpersonal model of self-esteem regulation. In: Joiner T, Coyne J, editors. The interactional nature of depression: Advances in interpersonal approaches. American Psychological Association; Washington, DC: 1999. pp. 149–187. [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology. 2011;214:209–219. doi: 10.1007/s00213-010-1879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Weissenburger JE. Melancholic symptom features and DSM-IV. Am J Psychiatry. 1994;151:489–498. doi: 10.1176/ajp.151.4.489. [DOI] [PubMed] [Google Scholar]
- Sheets ES, Craighead WE. Comparing chronic interpersonal and noninterpersonal stress domains as predictors of depression recurrence in emerging adults. Behav Res Ther. 2014;63:36–42. doi: 10.1016/j.brat.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies LA, Margolin G, Susman EJ, Gordis EB. Adolescents’ cortisol reactivity and subjective distress in response to family conflict: The moderating role of internalizing symptoms. J Adolesc Health. 2011;49:386–392. doi: 10.1016/j.jadohealth.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Dev Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Belden AC, Spitznagel E, Dietrich R, Luby JL. Blunted stress cortisol reactivity and failure to acclimate to familiar stress in derepssed and sub-syndromal children. Psychiat Res. 2013;210:575–583. doi: 10.1016/j.psychres.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan B. Predictors of anticipatory cortisol reactivity to subsequent stressors. Physiol Behav. 2015;149:239–246. doi: 10.1016/j.physbeh.2015.06.011. [DOI] [PubMed] [Google Scholar]
- van Loo HM, de Jonge P, Romeijn JW, Kessler RC, Schoevers RA. Data-driven subtypes of major depressive disorder: A systematic review. BMC Med. 2012;10:156. doi: 10.1186/1741-7015-10-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigfield A, Eccles JS, MacIver D, Reuman DA, Midgley C. Transitions during early adolescence: Changes in children’s domain-specific self-perceptions and general self-esteem across the transition to junior high school. Dev Psychol. 1991;27:552–565. [Google Scholar]
- Willett JB, Singer JD, Martin NC. The design and analysis of longitudinal studies of development and psychopathology in context: Statistical models and methodological recommendations. Dev Psychopathol. 1998;10:395–426. doi: 10.1017/s0954579498001667. [DOI] [PubMed] [Google Scholar]