Abstract
Osteocalcin (OC) is a vitamin K-dependent protein synthesized during bone formation. Mice injected with the undercarboxylated form of OC (ucOC) had more skeletal muscle mass and less fat mass than sham-treated controls, suggesting a unique metabolic role for ucOC. UcOC decreases in response to vitamin K supplementation. Our objective was to determine the effect of reducing ucOC on change in lean tissue and fat mass in older community-dwelling adults (n=401, mean±SD 69±6 years) using data from a randomized controlled trial of vitamin K supplementation Over 3 years, serum ucOC was reduced by 58% in women and by 61% in men randomized to vitamin K, while in the control group ucOC decreased by 1% in women and 4% in men (supplementation*time p<0.001 in men and women). However, there were no differences in the change in appendicular lean mass (calculated as arm lean mass + leg lean mass)or total body fat mass between women randomized to vitamin K and control over 3 years (supplementation*time p-values all ≥0.18)or between men randomized to vitamin K and control (supplementation*time p-values all ≥0.54). Consistent with these findings, ucOC was not associated cross-sectionally with appendicular lean mass or fat mass in men or women after adjustment for total OC at baseline (all p ≥0.12). These findings indicate the undercarboxylated form of OC is not implicated in age-related changes in skeletal muscle or adipose tissue mass in older community-dwelling adults.
Osteocalcin (OC) is a non-collagenous protein produced exclusively by osteoblasts during bone formation. OC undergoes a post-translational modification so that its glutamic acid (uc) residues are carboxylated to gamma-carboxy-glutamic acid (gla) residues. This carboxylation requires vitamin K as an enzymatic co-factor (1;2). Carboxylated osteocalcin (cOC) is able to bind calcium. A unique role for undercarboxylated OC (ucOC) in regulating energy metabolism has been proposed based on observations that ucOC injection improved insulin resistance and reduced fat mass in osteocalcin knockout(3) and wild-type (4;5)mice. Attempts to translate these findings to humans have yielded equivocal results (6–13). More recently, an additional role for ucOC in enhancing skeletal muscle mass and function has been suggested because mice lacking a neuromuscular gap junction protein (connexin43) that were injected with human ucOC had increased muscle area and grip strength compared to the same knockout mice injected with saline (14).
UcOC is reduced by vitamin K supplementation (1;2). If increasing ucOC favorably affects muscle and/or fat mass in humans, as mouse models propose(3–5;14), one would expect reducing ucOC with vitamin K supplementation would promote lean mass loss and fat gain. To test this, we compared the change in lean and fat mass (measured using dual x-ray absorptiometry) between older community-dwelling men and women randomized to receive vitamin K supplementation and those randomized to the control group over 3 years(15) As. indicated in the primary analysis of this trial, vitamin K supplementation reduced serum ucOC by 58% and 61% in women and men, respectively (15). Overall, appendicular lean mass (calculated as arm lean mass + leg lean mass) decreased by 8% in women and 6% in men, while total body fat mass increased by 2% in women and 1% in men.
METHODS
Data were obtained from a 3-year randomized controlled parallel-arm trial designed to test the effect of vitamin K supplementation on age-related bone loss and coronary artery calcification, conducted from 2002–2007. The study design and participation have been described previously in detail (15;16). Briefly, 164 community-dwelling men and 237 women aged 60–80 years were randomized to receive 500 μg/day phylloquinone (vitamin K1) or control for three years (supplemental Figure 1). Randomization was sex-stratified. All participants received 600 mg elemental calcium and 400 IU vitamin D3. Participants and investigators with participant contact were blinded to the intervention. Exclusion criteria included a kidney stone in the past 5 years; hyperthyroidism; bilateral hip surgery; therapy with a bisphosphonate, calcitonin, estrogen, tamoxifen, testosterone, or warfarin in the previous 6 months; known coronary disease; prior open heart surgery; atrial fibrillation; pacemaker; femoral neck BMD more than 1.8 standard deviation below the mean for subjects of the same age and sex; laboratory evidence of kidney or liver disease; and inability to provide informed consent (15). All participants signed a written informed consent, and this study was approved by the Institutional Review Board at Tufts University. This study was registered with ClinicalTrials.gov (NCT00183001).
Body composition
Lean body mass, arm and leg lean mass, fat mass and % body fat were measured using dual x-ray absorptiometry (DXA; GE Lunar Prodigy, Madison WI)at baseline, and at the 6, 12, 24, and 36-month follow-up visits. Appendicular lean mass was calculated as arm lean mass + leg lean mass. Weight was measured using a digital scale.
Physical activity was estimated using the Physical Activity Scale for the Elderly (17).
Biochemical measures
All blood samples were drawn after a ≥10-h fast, and dedicated aliquots of plasma and serum were stored at −80 C until the time of analysis. Serum total OC and ucOC were measured by RIA, using the method of Gundberg et al (18). The antibody recognizes both carboxylated and undercarboxylated osteocalcin. cOC was separated from ucOC by adsorption on hydroxyapatite. Total OC was determined in the serum before adsorption and ucOC was measured in the adsorbed serum. The total CV for the three control sera with an average total OC result of 6.4, 14.7, and 23.8 μg/liter were 8.8, 8.9, and 7.6%, respectively(15). The circulating total OC and ucOC measured using this hydroxyapatite binding assay were verified using mass spectrometry in 30 vitamin K supplemented and 29 control participants (2).
Statistical analyses
UcOC measures were transformed using the natural log to reduce skewness. Baseline body composition and osteocalcin measures were compared between the two randomized groups using students T-test. Mixed models with an autoregressive correlation structure were used to evaluate whether the change in lean mass, appendicular lean mass, fat mass, and % body fat over three years differed between those randomized to vitamin K and those randomized to control. Initial models were unadjusted, and subsequent models included baseline measures of appendicular lean and fat mass as covariates to account for between group baseline differences. The change in body weight and BMI were analyzed similarly. In sensitivity analyses, non-adherent participants (defined as those who did not take at least 85% of supplements, based on direct pill count) were excluded (n=65 women and 24 men). We analyzed the cross-sectional association of serum ucOC with appendicular lean mass and total fat mass at baseline using general linear regression. Because the ucOC depends on the total amount of OC we adjusted by including total OC as a covariate and considered the adjusted model our main cross-sectional model. We also calculated the correlation between the 3-year change in ucOC and change in total OC in the vitamin K treated and control groups using Pearson coefficients. Men and women were analyzed separately because body composition differs by sex. All analyses were carried out using SAS v 9.3. Statistical significance was set at an alpha of 0.05.
RESULTS
Pertinent baseline characteristics of men and women have previously been described (15;16), and are shown in Table 1. The women randomized to receive vitamin K supplementation, on average, weighed 4.3 kg more at baseline than the women randomized to receive the placebo (p=0.017; Table 1). Women in the vitamin K supplement group also had significantly more body fat than women in the control group at baseline (p=0.013), but the two groups did not differ with respect to appendicular and total body lean mass. Self-reported physical activity was similar between the two groups of women and men (15). Serum OC measures were also balanced between the two groups(15). Men randomized to receive vitamin K had 1.0 kg more appendicular lean mass, on average, compared to men randomized to the control group (p=0.055). Otherwise, the two groups of men were balanced with respect to body weight, body fat, and OC (Table 1).
Table 1.
Participant characteristics at baseline (mean ± SD)
| Women | Men | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Vitamin K1 supplement a (n=134) | Control a (n=133) | p-value | Vitamin K1 supplement a (n=95) | Control a (n=90) | p-value | |
| Age (years) c | 68±6 | 68±5 | 0.929 | 69±5 | 69±6 | 0.877 |
| Weight (kg)c | 74.3±14.8 | 70.0±14.2 | 0.017 | 85.3±14.6 | 84.2±14.6 | 0.590 |
| BMI (kg/m2) | 28.6±5.7 | 27.2±5.1 | 0.041 | 28.2±4.7 | 27.8±4.0 | 0.534 |
| Physical activity score b,c | 123±54 | 127±62 | 0.578 | 130±66 | 140±62 | 0.691 |
| Appendicular lean mass (kg) | 17.3±2.6 | 16.8±2.3 | 0.145 | 25.9±3.3 | 24.9±3.4 | 0.055 |
| Total body lean mass (kg) | 40.0±53.2 | 39.1±46.7 | 0.120 | 57.4±6.4 | 55.8±6.8 | 0.095 |
| Total body fat (kg) | 31.4±10.3 | 28.2±10.3 | 0.013 | 24.8±10.0 | 25.1±8.9 | 0.824 |
| % body fat | 41.5±6.9 | 39.3±7.5 | 0.015 | 29.2±6.3 | 28.2±7.3 | 0.347 |
| Serum total OC (ng/ml) c | 9.0±3.2 | 8.8±3.2 | 0.522 | 7.7±2.7 | 7.7±2.9 | 0.920 |
| Serum ucOC (ng/ml) c,d | 4.1±2.7 | 4.0±2.5 | 0.803 | 3.2±1.9 | 2.9±1.7 | 0.285 |
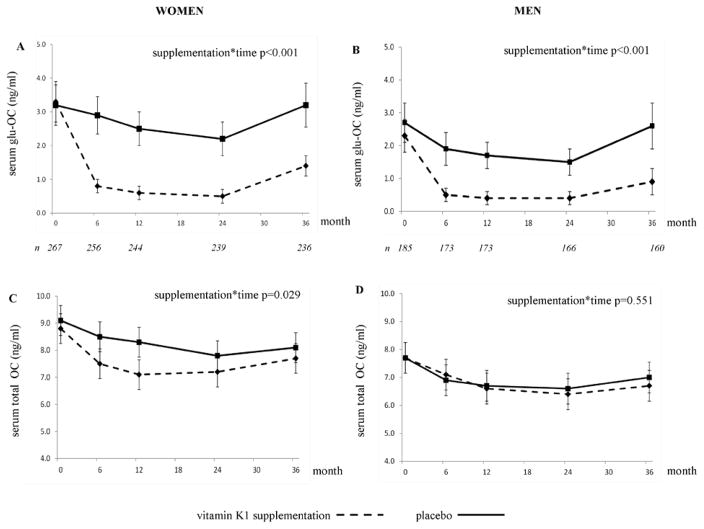
During the first 6-months of vitamin K supplementation, the serum ucOC decreased by 78% in women and by 76% in men. After 3-years, serum ucOC was reduced by 58% and 61% in women and men, respectively, randomized to receive vitamin K(Figures 1A and 1B). In the control group, the serum ucOC changes were as follows in women: 9% decrease at 6 months; 1% decrease at 3-years; and men: 30% decrease at 6 months, 4% decrease at 3-years. During the first year, women randomized to vitamin K had a greater reduction in total OC than women in the control group, but the total OC was similar in the two groups at 3-years (Figure 1C). In men, the total OC changed similarly in the vitamin K and control groups (Figure 1D) (15).
Figure 1.
Change in serum ucOC (ng/ml) (A, B)and total OC (ng/l) (C, D). UcOC data are geometric means with 95% confidence interval; total OC data are arithmetic means with 95% confidence interval of the mean.
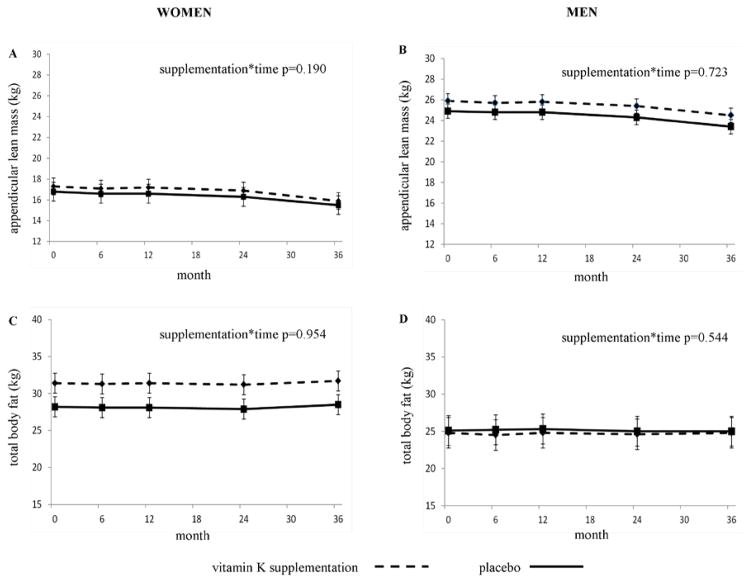
In women, the 3-year change in appendicular lean mass was not different between those randomized to vitamin K (−1.4 kg) and those randomized to control (−1.3 kg) (supplementation*time p=0.190 (unadjusted); p=0.180 (adjusted for baseline appendicular lean mass)) (Figure 2A). Furthermore, the 3-year change in total fat mass was not different between those randomized to vitamin K (+0.2 kg) and those randomized to control(+0.4 kg) (supplementation*time p=0.954 (unadjusted); p=0.931 (adjusted for baseline fat mass)) (Figure 2C). Similarly in men, the 3-year change in appendicular lean mass was not different between those randomized to vitamin K (−1.4 kg) and randomized to control(−1.5 kg) (supplementation*time p=0.723 (unadjusted); p=0.713 (adjusted for baseline appendicular lean mass)) (Figure 2B). Consistent with these findings, the 3-year change in total fat mass was not different between men randomized to vitamin K (+0.05 kg) and men randomized to control (−0.02 kg) (supplementation*time p=0.544 (unadjusted); p=0.618 (adjusted for baseline fat mass)) (Figure 2D). Results were similar when baseline appendicular lean mass and baseline fat mass were adjusted for (Figure 2). The changes in percent body fat, total lean mass, body weight and BMI did not differ between the vitamin K-supplemented and control groups in men or women (all supplementation*time p≥0.209). When analyses were restricted to women and men who took at least 85% of their vitamin K supplements (based on direct pill count), the results were similar(men: supplementation*time p-values ranged 0.15 to 0.94; women: supplementation*time p-values ranged 0.358 to 0.969).
Figure 2.
Change in appendicular lean mass (A, B) and total body fat mass (C, D) in women and men randomized to vitamin K supplementation and control over 3 years. Data are arithmetic means with 95% confidence interval of the mean.
At baseline, serum ucOC was not associated with appendicular lean mass or total fat mass in women (unstandard beta coefficients: appendicular lean mass = −0.05; total body fat mass = 0.76; p =0.112 and 0.666 respectively) or men (unstandard beta coefficients: appendicular lean mass = −0.21; total body fat mass = −0.10; p = 0.329 and 0.862 respectively)after adjustment for total OC. Similarly, when ucOC was expressed as a % of the total OC that was undercarboxylated (referred to as ucOC% or ucOC%), it was not associated with lean or fat mass in women or men (all p ≥0.209). In the unadjusted models serum ucOC was marginally inversely associated with appendicular lean mass and total fat mass in women (unstandard beta coefficients: appendicular lean mass = −0.12; total body fat mass = −0.48; p = 0.047 and 0.054 respectively), but not men (unstandard beta coefficients: appendicular lean mass = −0.14; total body fat mass = −0.46; p = 0.316 and 0.233 respectively). The 3-year change in ucOC was positively correlated with the 3-year change in total OC in the vitamin K treated and control groups of women and men (Pearson coefficients: women randomized to vitamin K r=0.66; women randomized to control r=0.76; men randomized to vitamin K r=0.62; men randomized to control r=0.55; all p<0.001).
DISCUSSION
Emerging evidence suggests lowering ucOC may reduce skeletal muscle mass (14;19)and increase fat mass (3–5;20) so we compared the 3-year change in lean and fat mass between community-dwelling older women and men randomized to receive vitamin K supplementation and those randomized to placebo because vitamin K supplementation reduces ucOC (15). In our study, vitamin K supplementation reduced serum ucOC by 58–61% in women and men over 3 years (15), but there were no corresponding changes in lean mass or fat mass between the vitamin K supplementation and control groups in either women or men. These findings do not support the premise that reducing the ucOC in the circulation leads to changes in lean or fat mass in older adults. The primary dietary sources of vitamin K in Western diets are green leafy vegetables. Therefore, high dietary vitamin K intake is characteristic of generally healthy diets (21). For higher serum ucOC to be associated with higher skeletal muscle mass and lower fat mass, diets high in green leafy vegetables would be adversely associated with these outcomes. To the best of our knowledge, there are no studies that have linked high vegetable intakes to higher body fat and/or less lean mass in humans.
In a previous vitamin K randomized trial conducted in post-menopausal women, there was no change in body weight or BMI in the women whose circulating ucOC decreased by 72% through intake of high dose vitamin K(menaquinone-4, 45 mg/d) for 3 years, whereas statistically significant increases in body weight and BMI were noted in the women who had no change in ucOC in response to no vitamin K supplementation (22;23). In contrast, in post-menopausal women treated with parathyroid hormone for osteoporosis, the total OC and ucOC concentrations both increased over 3-months(by 177% and 242% respectively) and a higher 3-month increase in ucOC was associated with a larger decrease in body weight and fat mass over 12-months(10). It was not reported whether the change in total OC was similarly associated with weight change, so it is not known if this association was unique to ucOC or reflects an overall effect of bone turnover. Following a 20-week weight-loss intervention in which post-menopausal women lost an average of 11 kg body weight (and 4% body fat), there was no change in the ucOC concentration or ucOC%, suggesting reducing OC carboxylation is not necessary for weight loss or fat loss in post-menopausal women (9). Overall, the results of our analysis are consistent with the finding that lowering serum ucOC by vitamin K supplementation does not lead to weight or fat gain in post-menopausal women(22), and we have extended these observations to older men.
We also did not find ucOC to be associated with or promote changes in appendicular lean mass or fat mass when the total OC was accounted for either as a covariate in the model or by expressing serum ucOC as a % of the total OC. This adjustment is critical because the circulating total OC reflects bone turnover and is independent of vitamin K for its synthesis (24). The amount of ucOC in circulation depends first on the amount of OC synthesized and released into circulation (total OC) and then on the post-translational availability of vitamin K to carboxylate the protein. All participants in our study received supplemental calcium and vitamin D so the observed decrease in total OC in all participants was expected(15). However in women, the decrease in total OC over the first year was significantly greater in the vitamin K group compared to the control group, which is an observation that is consistent with other vitamin K supplementation interventions in which participants received calcium and vitamin D (25;26). At the 3-year follow-up, the total OC was similar in both groups of women, albeit lower than baseline, which is also consistent with other interventions over a 3-year duration (26). More importantly, the change in total OC was significantly positively correlated with the change in ucOC in both groups(vitamin K supplement and control)of women and men. These findings highlight the importance of taking into account total OC and the contribution of vitamin K to the carboxylation of OC before drawing conclusions about the importance of ucOC to body composition or other metabolic outcomes.
The findings from mouse studies that indicated ucOC acts as a hormone influencing body composition and energy metabolism (3–5;14;27;28)have translated poorly to humans. This may be attributable, in part, to species differences in OC gene expression and carboxylation. As reviewed previously, mice have 3 genes encoding OC whereas humans have one(12;24). In mice, circulating OC is usually fully carboxylated (24). In humans, OC can be carboxylated at 1, 2, or all glutamic acid 3 residues (2). However, it is rarely completely carboxylated and rarely completely uncarboxylated (2;24). For example, in a sub-sample of participants in this trial whose serum OC forms were measured using state-of-the-art mass spectrometry, 500μg/day phylloquinone (which is approximately five times more than the Adequate Intake (AI) set by the United States Institute of Medicine (29)) did not result in complete carboxylation of OC. Rather, supplementation resulted in a greater fractional abundance of cOC and less ucOC (2). Conversely, reducing dietary vitamin K intakes to 10 μg/day for one month (corresponding to ~10% of the AI(29)) did not result in complete uncarboxylation of OC (2). The physiological relevance of injecting mice with completely uncarboxylated synthetic human osteocalcin (3–5;14)to evaluate an effect on body composition and energy metabolismis therefore questionable. That serum ucOC is readily modified by diet additionally challenges the proposed hormonal function of ucOC (27;28)because circulating hormone levels are typically tightly controlled.
This trial was not designed specifically to test the effect of reducing ucOC on lean mass or fat mass, which we acknowledge is a limitation. This is balanced by the robustness of the double-blind, placebo-controlled randomized vitamin K supplementation study design because vitamin K supplementation reduced the serum ucOC in half over 3 years (15), allowing us to test the effect of reducing ucOC on change in lean and fat mass in older men and women. At baseline, women were not balanced with respect to body weight and body fat between the supplementation and control groups, with women randomized to receive vitamin K weighing more and having more body fat than women in the control group. Men randomized to receive vitamin K had 1.0 kg more appendicular lean mass than men randomized to control, a difference that was borderline statistically significant. If our analyses supported our overall hypothesis, in women the between group difference in body fat would have increased over time and in men the between group difference in appendicular lean mass would have decreased over time. Instead these between group differences remained constant over 3 years. Inclusion of these baseline measures as covariates in our analyses did not change our results. Participants in our study were generally healthy based on being free of osteoporosis and known cardiovascular disease at baseline and they did not lose femoral neck or lumbar spine bone mineral density over 3-years (15). However, the overall rates of appendicular lean mass loss we observed are higher than reported in other studies of community-dwelling older adults (30;31)so it is unlikely that the participants’ health status would have blunted an ability to detect an association between serum ucOC and change in lean mass. The vitamin K supplements used in our study contained phylloquinone, which is found in green leafy vegetables and vegetable oils and is the primary dietary form of vitamin K in Western diets. Menaquinones (known collectively as vitamin K2) are found in smaller amounts in animal-based and fermented foods (32). In postmenopausal women who received supplements containing either 1000 μg/day phylloquinoneor 45 mg/day menaquinone-4, serum %ucOC was reduced by the same amount in both groups over one year (25). It is unlikely our results would be different if participants received a different dose and/or form of vitamin K. All the men and women in our study received 600 mg of elemental calcium and 400 IU of vitamin D3 (15), which likely contributed to the lack of bone loss (15). Vitamin D has been proposed to improve muscle function, although definitive data to support this role are not yet available (33;34). Whether or not vitamin D status influences the association between ucOC and muscle is not known. In a previous cross-sectional analysis of 90 post-menopausal women at risk for hip fracture, higher ucOC% was associated with better quadriceps and hip abduction strength (35). Muscle mass and strength are highly correlated and loss of muscle mass predicts loss of strength (30;36), so it stands to reason that ucOC would be similarly associated with muscle mass and strength. Strength measures were not available in our study, so we were unable to evaluate this formally. At this time a biological mechanism through which ucOC would affect strength without affecting muscle mass is unclear.
In conclusion, we have shown that reducing the serum ucOC through vitamin K supplementation over a 3 year period does not promote lean tissue loss or fat gain in older community-dwelling men and women. The ucOC was also not significantly associated with lean or fat mass at baseline in men or women when total OC was accounted for. Overall these findings suggest OC carboxylation is not implicated in age-related changes in skeletal muscle mass or fat mass in humans.
Acknowledgments
This work was supported by the National Institutes of Health [R01AG14759, R01HL69272, K01AR063167] and also received support from the US Department of Agriculture, Agricultural Research Service, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the USDA. Author’s roles: Study design and implementation: SLB, BDH, CMG; Data analysis and interpretation: MKS, BDH, SLB, CMG; Drafting manuscript: MKS; Revising manuscript: BDH, SLB, CMG; Approving final manuscript: all. MKS, BDH, SLB take responsibility for integrity of data analysis.
Reference List
- 1.Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003 Aug;133(8):2565–9. doi: 10.1093/jn/133.8.2565. [DOI] [PubMed] [Google Scholar]
- 2.Rehder DS, Gundberg CM, Booth SL, Borges CR. Gamma-carboxylation and fragmentation of osteocalcin in human serum defined by mass spectrometry. Mol Cell Proteomics. 2015 Jun;14(6):1546–55. doi: 10.1074/mcp.M114.047621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007 Aug 10;130(3):456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008 Apr 1;105(13):5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012 Feb;50(2):568–75. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iki M, Tamaki J, Fujita Y, Kouda K, Yura A, Kadowaki E, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int. 2012 Feb;23(2):761–70. doi: 10.1007/s00198-011-1600-7. [DOI] [PubMed] [Google Scholar]
- 7.Srichomkwun P, Houngngam N, Pasatrat S, Tharavanij T, Wattanachanya L, Khovidhunkit W. Undercarboxylated osteocalcin is associated with insulin resistance, but not adiponectin, during pregnancy. Endocrine. 2015 Dec 26; doi: 10.1007/s12020-015-0829-x. [DOI] [PubMed] [Google Scholar]
- 8.Zwakenberg SR, Gundberg CM, Spijkerman AM, van der AD, van der Schouw YT, Beulens JW. Osteocalcin Is Not Associated with the Risk of Type 2 Diabetes: Findings from the EPIC-NL Study. PLoS One. 2015;10(9):e0138693. doi: 10.1371/journal.pone.0138693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centi AJ, Booth SL, Gundberg CM, Saltzman E, Nicklas B, Shea MK. Osteocalcin carboxylation is not associated with body weight or percent fat changes during weight loss in post-menopausal women. Endocrine. 2015 Dec;50(3):627–32. doi: 10.1007/s12020-015-0618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer AL, Sellmeyer DE, Schwartz AV, Rosen CJ, Vittinghoff E, Palermo L, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1-84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study) J Clin Endocrinol Metab. 2011 Dec;96(12):E1982–E1989. doi: 10.1210/jc.2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth SL, Centi AJ, Gundberg C. Bone as an endocrine organ relevant to diabetes. Curr Diab Rep. 2014 Dec;14(12):556. doi: 10.1007/s11892-014-0556-3. [DOI] [PubMed] [Google Scholar]
- 12.Gundberg CM, Lian JB, Booth SL. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv Nutr. 2012 Mar;3(2):149–57. doi: 10.3945/an.112.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu DM, Guo XZ, Tong HJ, Tao B, Sun LH, Zhao HY, et al. Association between osteocalcin and glucose metabolism: a meta-analysis. Osteoporos Int. 2015 Jun 19; doi: 10.1007/s00198-015-3197-8. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Grimston S, Civitelli R, Thomopoulos S. Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice. J Bone Miner Res. 2015 Apr;30(4):596–605. doi: 10.1002/jbmr.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008 Apr;93(4):1217–23. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea MK, O’Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009 Jun;89(6):1799–807. doi: 10.3945/ajcn.2008.27338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 18.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998 Sep;83(9):3258–66. doi: 10.1210/jcem.83.9.5126. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Hanson E, Betik AC, Brennan-Speranza TC, Hayes A, Levinger I. Hindlimb Immobilization, but not Castration, Induces Reduction of Undercarboxylated Osteocalcin Associated with Muscle Atrophy in Rats. J Bone Miner Res. 2016 Jun 13; doi: 10.1002/jbmr.2884. [DOI] [PubMed] [Google Scholar]
- 20.Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner WS, et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest. 2014 Apr;124(4):1–13. doi: 10.1172/JCI72323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braam L, McKeown N, Jacques P, Lichtenstein A, Vermeer C, Wilson P, et al. Dietary phylloquinone intake as a potential marker for a heart-healthy dietary pattern in the Framingham Offspring cohort. J Am Diet Assoc. 2004 Sep;104(9):1410–4. doi: 10.1016/j.jada.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Knapen MH, Schurgers LJ, Shearer MJ, Newman P, Theuwissen E, Vermeer C. Association of vitamin K status with adiponectin and body composition in healthy subjects: uncarboxylated osteocalcin is not associated with fat mass and body weight. Br J Nutr. 2012 Sep 28;108(6):1017–24. doi: 10.1017/S000711451100626X. [DOI] [PubMed] [Google Scholar]
- 23.Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007 Jul;18(7):963–72. doi: 10.1007/s00198-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol. 2013 Jan;9(1):43–55. doi: 10.1038/nrendo.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binkley N, Harke J, Krueger D, Engelke J, Vallarta-Ast N, Gemar D, et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res. 2009 Jun;24(6):983–91. doi: 10.1359/JBMR.081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med. 2008 Oct 14;5(10):e196. doi: 10.1371/journal.pmed.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011 Jun;54(6):1291–7. doi: 10.1007/s00125-011-2155-z. [DOI] [PubMed] [Google Scholar]
- 28.Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014 Nov 1;561:137–46. doi: 10.1016/j.abb.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 30.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006 Oct;61(10):1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 31.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001 May;56(5):B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 32.Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 2013 Jul;4(4):463–73. doi: 10.3945/an.113.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011 Mar;22(3):859–71. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 34.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014 Nov;99(11):4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 35.Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, et al. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone. 2014 Jul;64:8–12. doi: 10.1016/j.bone.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003 Mar;51(3):323–30. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]