Abstract
The Error Related Negativity (ERN) is a neural marker of performance monitoring that has been inconsistently linked to anxiety risk in children. One avenue for understanding inconsistencies is to investigate other neural dynamics linked to ERN. In this study, we investigated interactions between ERN and the theta frequency band, which is associated with attentional control and theorized to contribute ERN, in association with childhood anxiety risk.
Fifty-nine 3-year-old children provided usable EEG data during a modified go/no-go task. Associations between ERN and anxious behaviors in preschoolers were moderated by theta power during incorrect trials. Specifically, when theta power was low, greater ERN predicted more social withdrawal; when theta power was high, ERN and social withdrawal were unrelated. Our findings suggest that ERN and theta may jointly contribute to anxiety risk in early childhood.
Keywords: ERN, theta, anxiety risk, preschool
The error related negativity (ERN) is an event-related potential (ERP) that is visible as a negative deflection in the averaged electroencephalograph (EEG) recording 50-100 ms following the commission of an error (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). The ERN has been the subject of numerous empirical studies aimed at delineating its precise function and association(s) with behavior (e.g., Brooker, Buss, & Dennis, 2011; Brooker & Buss, 2014; DuPuis et al., 2015; Holroyd & Coles, 2002; Jonkman et al., 2007; Ladouceur et al., 2007; Meyer et al., 2012; Torpey, Hajcak, & Klein, 2009; Torpey et al., 2012). Although significant advances have been made, much remains to be understood about the ERN as part of a coordinated network of neural responses during development. In the current study, we examine ERN and theta power as interacting influences on early socioemotional development.
Although no longitudinal studies of the ERN exist in young children, a number of characteristics of the ERN appear to change across developmental periods. It is generally accepted that ERN is maximal at frontal electrode sites in adults (Davies, Segalowitz, & Gavin, 2004; Falkenstein et al., 1991), but may be distributed more broadly in children, including visible ERN in parietal locations (Brooker, Buss, & Dennis, 2011; Torpey, Hajcak, & Klein, 2009). This difference is presumably due to a maturational shift from a reliance on parietal brain regions for processes of self-monitoring to increasing dependence on the maturing frontal cortex (Durston et al., 2006). ERN amplitudes also appear to change over time, typically increasing with age (Du Puis et al., 2015, though see also Grammer, Carrasco, Gehring, & Morrison, 2014). The ERN in children peaks between −4 (Ladouceur, Dahl, & Carter, 2007) and −7.35 microvolts (Du Puis et al., 2015) while adult ERN peaks between −5 and −10 microvolts (Hajcak et al., 2005; Moser, Hajcak, & Simons, 2005). Developmental changes in ERN amplitudes are believed to reflect the maturation of neural structures, such as the anterior cingulate cortex (ACC), from which the ERN is believed to originate (Carter et al., 1998).
The ERN has been described as a neural marker of processes of error-monitoring (Falkenstein et al., 1991; Gehring et al., 1993), conflict detection (Carter, Braver, Barch, Botnivick, Noll, & Cohen, 1998), reinforcement learning (Holroyd & Coles, 2002), error-related distress (Bartholow, Pearson, Dickter, Fabiani, Gratton, & Sher, 2005; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003), and the motivational significance of errors (Hajcak, Moser, Yueng, and Simons, 2005). ERN amplitudes are augmented when accuracy, rather than speed, is emphasized during response-time tasks (Gehring et al., 1993), underscoring the link between ERN amplitudes and one’s self-monitoring of performance. Given a range of empirical support for more specific definitions, we have adopted a view of the ERN as a general neural mechanism of performance monitoring. As noted above, however, research largely comprises studies conducted with older children and adults. Developmental studies of the ERN remain rare, though the limited number of studies with children have produced findings similar to work conducted with adults. Greater ERN amplitudes are associated with more anxious behaviors in children ages 8-14 (Ladouceur, Dahl, & Carter, 2007) and greater negative affect (Hajcak & Foti, 2008) and increased sensitivity to the value of errors (Hajcak, Moser, Yeung, & Simons, 2005) in young adults. Greater ERN is also linked with poorer attentional orienting in 4-8 year-old children (Brooker, Buss, & Dennis, 2011), but better executive functioning and more positive academic outcomes in children between ages 5 and 8 (DuPuis, Ram, Willner, Karalunas, Segalowitz, & Gatzke-Kopp, 2015).
An important behavioral correlate of the ERN has been its link to anxiety symptoms in both children (McDermott, Perez-Edgar, Henderson, Chronis-Tuscano, Pine, & Fox, 2009; Meyer, Weinberg, Klein, & Hajcak, 2012; Santesso, Segalowitz, & Schmidt, 2006) and adults (Hajcak & Simons, 2002; Weinberg, Olvet, & Hajcak, 2010). Augmented ERNs are further visible in children with nonclinical levels of anxiety symptoms, but who may be at enhanced levels of risk during early (Brooker & Buss, 2014) or middle (Lahat, Lamm, Chronis-Tuscano, Pine, Henderson, & Fox, 2014) childhood. An explanation for this association is that the sensitivity threshold for detecting response errors may be lower for children who are at risk for anxiety problems relative to children who are not at risk (Torpey et al., 2012).
However, the evidence for associations between anxiety and ERN in children has not been entirely consistent. One study of children between ages 8 and 13 reported that ERN was not associated with anxiety in children below age 11 (10.95 years; Meyer, Weinberg, Klein, & Hajcak, 2012). In fact, results suggested a marginally positive link between anxiety symptoms and smaller ERN in younger children. This finding led to speculation that the ERN may only denote anxiety in later adolescence. Similarly, previous work with six-year-old children has revealed enhanced ERN in clinically anxious children but muted ERN in children at enhanced risk for anxiety problems based on maternal symptoms (Meyer et al., 2012). Thus, it is additionally possible that ERN is more robustly associated with the presence of clinical symptoms than with risk for the development of problems. Other experts have posited that broad age ranges, and specifically the inclusion of older children in previous samples may be largely responsible for the presence of ERN effects (Crone & Ridderinkhof, 2011; Grammer et al., 2014). Thus, there remains a need to examine associations between ERN and anxiety risk in samples of only young children who have not yet developed clinical symptoms of disorder.
Another possible explanation for inconsistent associations between ERN and anxiety risk is that ERN is one of many possible contributors to anxiety problems in children. That is, putative moderators may obscure direct associations between ERN and markers of risk (Aiken & West, 1991). Indeed, other neural dynamics have been linked to the presence of the ERN. For example, there is evidence, from work with adults, that activity in the theta frequency band of the EEG is associated with ERN during processes that necessitate performance monitoring. Theta power is visible in the roughly 5-8 Hz band in adults and young children (Kikuchi et al., 2011). Measures of overall theta power can be decomposed into theta-1 (4.2-5.9 Hz) and theta-2 (6.4-7.8 Hz) frequencies (Kikuchi et al., 2011; Orekhova, Stroganoz, Posikera & Elam, 2006), which appear to reflect distinct forms of processing. Power in the theta-1 range is largely visible at frontocentral scalp locations and is increased during tasks that necessitate enhanced goal-directed attention in both preschoolers (Orekhova, Stroganoz, Posikera, & Elam, 2006) and adults (Jensen & Tesche, 2002). Given its distribution and associations with motivated cognition, this band of theta activity may be most closely linked to ERN. In contrast, power in the theta-2 range is largely visible in parietal regions (Orekhova et al., 2006) and appears to reflect language-related cognitive processing (Kikuchi et al, 2011).
The medial septum diagonal band of Broca (MS-DBB) model of theta provides an explanation of how theta power may be linked to the ACC and ERN (Canolty et al., 2006). The MS-DBB is considered to be the central area from which theta originates (Buzsaki, 2002). ERN generated from the ACC appears to be partially dependent on theta activity such that the ERN results from a partial phase-resetting and amplitude enhancement of EEG activity following an event (Luu et al., 2004; Trujillo and Allen, 2007). Specifically, theta oscillations interrupt the typical phase period in the ACC, resulting in a visible ERN. This idea is consistent with the postulate that the ERN is a combined product of activity in the ACC, PFC, and limbic system (Davies, Segalowitz, & Gavin, 2004). Functionally, this sequence of events has been proposed to indicate a theta-based signaling of the need for enhanced cognitive control (Cavanagh, Cohen, & Allen, 2009; Cavanagh & Frank, 2014).
By this logic, developmental changes in the ACC, which occur throughout childhood and adolescence (Kelly et al., 2008), should parallel maturational changes in both ERN and theta. Arguably the most prominent developmental change in the ACC during childhood involves significant synaptic pruning. This change enables more efficient information transfer across the cortex; it is also believed to underlie shifts from more diffuse neural activation in children to more localized recruitment of neural resources in adolescents and adults (Casey et al., 1997; Kelly et al., 2008) that are linked to changes in ERN distribution as described above. Developmental changes in theta are less well understood, but have been at least partially indicated by past work. The oscillations of slow-wave frequency bands, such as theta, decrease with age (Ulhaas, Roux, Rodriguez, Rotarska-Jagiela, & Singer, 2009). As with the ERN, this change may also reflect synaptic pruning across development and an increasing reliance on self-regulatory processes supported by a maturing cortex (Knyazev, Slobodskaya, & Wilson, 2004).
A small number of studies have investigated the link between theta and performance monitoring. Work using magnetoencephalography found increases in theta activity in the frontal midline immediately following error commission (Cheyne, Ferrari, & Cheyne, 2012). No increases in theta activity were observed for correct trials, leading to speculation that frontal theta might be specific to processes of error detection. However, other work has reported theta in both correct and erroneous responses (Luu, Tucker, & Makeig, 2004). Notably, when ERN was greater, non-phase-locked theta power was significantly enhanced relative to correct responses. Theta is also beginning to emerge as a correlate of some of the same behaviors as the ERN; for example, Cavanagh and Shackman (2014) recently reported that heightened trait anxiety is associated with greater frontal midline theta power.
Thus far, research linking childhood ERN with assessments of theta is limited. However, it is reasonable to suggest that theta reflects a putative moderator that may help to explain inconsistent associations between ERN and anxiety risk during childhood, as systems that produce ERN and theta activity develop. Therefore, this was the aim of the current study. We focused our work on the early preschool period, a time of rapid neural development (Casey, Giedd, & Thomas, 2000) and the earliest age at which ERN has been demonstrated to date (Grammer et al., 2014). Importantly, we included only 3-year-old children to avoid the possibility that effects might be driven by a specific age group within a broader sample. We predicted that ERN would be visible in our early-childhood sample and that associations between ERN and anxiety risk would be modulated by theta power. Because inconsistencies in past work led us to predict the presence of moderation, which can obscure main effects (Aiken & West, 1991) we did not predict a direct association between ERN and anxiety risk.
Method
Participants and Design
Participants (N = 107) were recruited as part of a larger study of developing cognition and emotion in preschoolers. Families with 3-year-old children (M = 3.60, SD = 0.20) were invited to participate via mailings based on local birth records, flyers, and in-person recruitment at public events. Families who agreed to participate were mailed questionnaire packets two weeks prior to the laboratory visit. The questionnaires included surveys of parent and child information ranging from emotion and coping measures to the family’s physical health. All children came to the lab accompanied by one parent. After consent was obtained, participants were escorted to a separate room for EEG data collection during a computerized go/no-go task. Following this, children participated in a series of behavioral tasks; these tasks are unrelated to the hypotheses of the current study and so are not reported here. Parents were present, but uninvolved, for all data collection procedures except for a parent-child interaction task that occurred at the end of the laboratory visit. Families received $30-$40 for their time and each child was given a small gift as a token of appreciation. On average, families spent a total of 90 minutes in the laboratory.
Go/No-go Task
Children completed a modified go/no-go task (Torpey et al., 2009), in which either a space ship (no-go stimulus) or an asteroid (go stimulus) was presented in the center of a 23-inch computer screen using Presentation stimulus delivery software (Neurobehavioral Systems, Inc.). Space ships were presented such that they were always vertically aligned. The child was instructed to push the response button to “shoot the asteroids, but to be careful not to shoot other space ships”. The full task comprised 2 blocks of 40 trials for a maximum of 80 trials for each child. Each block presented trials in a pseudorandomized order so that blocks contained roughly 60% go trials. Response time and accuracy were recorded for each trial.
Each trial began with the presentation of a gray fixation cross for 200 ms followed by the stimulus presentation for 1200 ms. Following this, the fixation cross was again presented in the center of the monitor for 300-800 ms prior to the beginning of the subsequent trial. Similar to other work with children (Steiben et al., 2007), an error rate of roughly 50% was maintained (25 correct and 27 incorrect responses, on average). through an automated procedure that decreased stimulus presentation time by 50 ms following 2 consecutive correct responses and increased stimulus presentation time by 50 ms following 2 consecutive incorrect responses. Final presentation times varied from 50 ms to 5000 ms.
Prior to beginning the experimental blocks, children received instructions for the task using laminated pictures of go and no-go stimuli. When children could demonstrate, using the pictures, that they understood the directions, they completed two practice blocks of 10 trials each. Children were reminded to respond to the task as quickly and accurately as possible before beginning the task. All children received a sticker after each block (practice and experimental blocks) was completed.
Error-related Negativity
EEG data were acquired during the go/no-go task using BioSemi Active 2 recording system. Continuous EEG was recorded through a 64 channel cap using Ag/AgCl-tipped electrodes arranged according to the American Electroencephalographic Society 10-20 labeling system. Children were capped and conductive gel was applied to each electrode site. Flat electrodes were also placed at the outer canthi of the left and right eye to collect horizontal eye movements and at the supra and infra orbital sites of the left eye to collect vertical eye movement. Two flat electrodes were also placed on the mastoids for later re-referencing.
Data were sampled at a rate of 2048 Hz. During recording, data were referenced to the Common Mode Sense and Driven Right Leg electrodes. Offline, all electrodes were re-referenced to the average of the right and left mastoids, high-pass filtered at 0.1 Hz, and corrected for eye movement or blinks (Gratton & Coles, 1983). Correct and incorrect trials were segmented (-200 to 600 ms), and then baseline corrected for 200 milliseconds prior to the response. Artifacts were marked in segmented data when one of the following criteria were met: a voltage step of more than 75 μV between data points, a voltage difference of 150 μV per within 200ms, amplitudes below 0.5 μV within a 50ms period, and activity that exceeded +100μV or −100 μV. Remaining segments were visually inspected for residual artifacts. Clean segments were averaged and low-pass filtered at 30 Hz. Peak negative amplitudes, or the most negative amplitude occurring between 0 and 100 ms were marked for error (ERN) and correct (Correct-trial negativity [CRN]) trials at Fz, FCz, Cz, Pz. The average number of correct trials used in individual averages was 15 and average number of incorrect trials was 30. ERN and CRN averages were not created for participants with less than six trials of usable data (n = 30; Olvet & Hajcak, 2009; Pontifex et al., 2010).
Theta Power
Theta power values were decomposed from the cleaned ERN averages. A Fast-Fourier transform was applied to each cleaned ERN segment and all clean segments were averaged. Power was computed in six distinct one hertz bins (Hamming window: 50% overlap) between 3.5-8.5 Hz, which reflects the range of theta in children (Kikuchi et al., 2011).
Anxious Behaviors
Mothers and fathers provided ratings of children’s anxious behaviors using the MacArthur Health and Behavior Questionnaire (HBQ; Essex et al., 2002), a 173-item parent-report questionnaire that uses dimensional ratings to assess emotional and behavioral symptoms, physical health, and social adjustment in young children. Given the emphasis of the current study on anxious behaviors associated with early anxiety risk in children, we focused on parent ratings of social withdrawal and social inhibition. Although the HBQ offers direct assessment of anxious symptoms that may also be considered indicative of risk for later diagnosis, the total numbers of anxiety symptoms reported for children near this age are still relatively low (Egger & Angold, 2006), which would reduce our ability to test for associations with other variables.
The social inhibition scale is made up of three items that ask parents to rate (0 = never true of my child, 2 = often or very true of my child) the degree to which their child is shy around and appears afraid of individuals with whom they are unfamiliar. The social withdrawal subscale asks parents to rate, for 9 items (0 = never true of my child, 2 = often or very true of my child ) not only inhibited behaviors but also the degree to which their child is interactive with peers and engages in social play with other children.
Reliability for maternal and paternal ratings of social withdrawal (mom α=0.76, dad α=0.79) and social inhibition (mom α=0.79, dad α=0.72) were deemed acceptable. Maternal and paternal ratings were correlated for both social withdrawal (r=0.56, p<0.01) and social inhibition (r=0.56, p<0.01) ratings. Therefore, in order to reduce possible rater bias, maternal and paternal ratings for each scale were mean composited.
Covariates
Previous research has suggested that the presence of depressive symptoms may lead to reductions in ERN amplitudes (Weinberg, Klein, & Hajcak, 2012). Therefore, in order to prevent reductions in ERN related to an extraneous variable and in order to isolate associations between ERN and anxiety risk in particular, we included children’s depressive symptoms as a covariate in moderation analyses. Similar to ratings of anxious behaviors, depressive symptoms were derived from parental ratings on the Depression scale of the HBQ (Essex et al., 2002). The depression scale comprises 7 items that ask parents to rate (0 = never true of my child, 2 = often or very true of my child) the degree to which their child displays commons symptoms of depression (e.g., anhedonia, lethargy, persistent sadness or crying, etc.). Similar to other HBQ scales, reliability for parent ratings of depressive symptoms was acceptable (mom α=0.61, dad α=0.73) and parental ratings did not differ across mothers and fathers (r = 0.52, p<0.01). Thus, maternal and paternal ratings were mean composited in order to reduce possible rater bias.
Missing Data
Participants excluded from the current analyses included families who did not complete questionnaires or children who did not provide usable EEG data. Unusable data resulted from excess movement or outside interference such as noise or electronic malfunctions (n=5). Data also excluded were due to the child refusing to complete the task (n=6) or refusing to be capped (n=3). The final sample included data from 98 children (Mage=3.56 years, SD= 0.35; 60% female), 59 of whom provided usable data on ERN and theta and 95 of whom provided usable data on anxious behaviors. A missing value analysis suggested that all data were missing completely at random (Little’s MCAR χ2=22.44, p>0.10). Because data were MCAR, a complete-case analysis strategy was adopted.
Plan for Analysis
Following a preliminary investigation of the data, analyses proceeded in a series of three steps. First, we confirmed the presence of ERN in 3-year-old children using a 2 (trial type) by 4 (electrode site) repeated measures ANOVA. Second, to validate the range of theta used in the current report, we conducted a 4 (electrode site) by 6 (frequency bin) repeated measures ANOVA. Finally, we used a 2-step hierarchical regression to test theta as a moderator of the association between ERN and anxious behaviors in preschoolers.
Results
Preliminary Analyses
As has been observed in previous reports, response times for incorrect trials (M = 419.58 ms) were significantly faster than those for correct trials (M = 682.15 ms; (t(99) = 8.08, p < 0.05). Consistent with the iterative procedure of the go/no-go task, roughly half (49.98%) of responses were correct and incorrect (51.01%) on average.
ERN
The presence of an ERN during preschool was verified using a 2 (trial type) by 4 (electrode site) repeated measures ANOVA. Greenhouse Geisser corrections were used to correct for violations of sphericity. Results suggested a main effect of electrode site (F(1.92, 100.47)=5.06, p<0.01, ηp2=0.09). Follow-up analyses using paired samples t-tests revealed that amplitudes at Pz (M =−2.02, SD=0.77) were significantly smaller (ts>2.10, ps<0.05), or more positive, than amplitudes at Cz (M= −2.67, SD=.85), FCz (M=−3.02, SD=.93), and Fz (M= −4.48, SD=0.76). ERN amplitudes at Cz, FCz, and Fz were not significantly different from each other (ts<1.67, ps>0.05) There was also a main effect of trial type (F(1,52) = 7.22, p<0.01, ηp2= 0.12), such that amplitudes for Incorrect trials, (t(53)=−2.68, p<0.01) were more negative (M=−4.85, SD=0.98) than those for Correct trials (M=−1.25, SD=0.99).
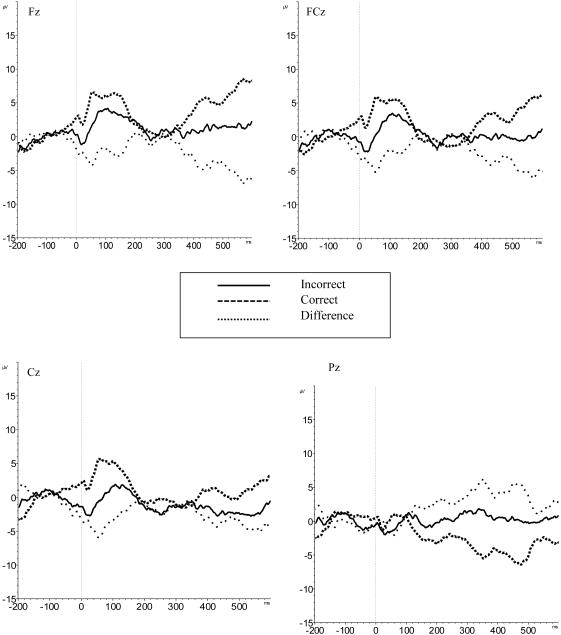
Importantly, a marginal interaction emerged between the electrode site and trial type, (F(1.98, 103) = 2.72, p=0.07, ηp2=0.05), reflecting an ERN at Fz (t(54) = −2.73, p< 0.05, d= 0.49) , FCz, (t(54) = −2.89, p < 0.05, d=0.51), and Cz, (t(54) = −2.77, p<0.05, d=0.50), but not at Pz (t(52) = −1.22, p > 0.05; Figure 1). Because the largest ERN effect (d =0.51) was observed at electrode FCz, subsequent analyses focused on amplitudes derived from the difference wave created by subtracting correct (CRN) from incorrect (ERN) values at this site (ΔERN = ERN – CRN). The use of a difference wave allows for the statistical elimination of neural activity that reflects processing that is common to both incorrect and correct trials (Luck, 2014).
Figure 1.
ERN waveforms
Theta
To validate the range used for theta, a 4 (electrode site) x 6 (bin) Repeated Measures ANOVA was used to determine frequencies at which theta was maximal. Greenhouse Geisser corrections were used to correct for violations of sphericity. Results revealed a significant effect of electrode site (F(2.28, 116.20) = 4.66, p <0.05, ηp2=.084) such that theta power was greater at Fz than at Pz (t(57)= 3.17, p<0.05) and FCz (t(57)=3.01, p<0.05). Theta power did not differ between Fz and Cz (t(57)=-1.79, p>0.05). Theta power was also significantly less at Pz than at FCz (t(57)= −4.86, p<.05), and Cz (t(57)= −5.72, p<.05); no significant differences in theta power were observed among the other electrodes.
A significant effect was also seen for frequency bin (F(2,255)= 21.77, p<.05, ηp2=.29). Paired samples t-tests revealed more power in bin one (2.5 -3.5 Hz) relative to bins two (3.5 - 4.5 Hz; t(57)= 4.66, p<.05, d=.59), three (4.5-5.5 Hz; t(57)=7.47, p<.05, d=.98), four (5.5 - 6.5 Hz; t(57)=10.02, p<.05, d=1.38), five (6.5 - 7.5 Hz; t(57)=11.69, p<.05, d=1.65), and six (7.5 - 8.5 Hz; t(57)=13.69, p<.05, d=1.79). More power was also observed in bin two than three, (t(57)= 3.09, p<.05, d=.33) four, (t(57)=5.32, p<.05, d=.72), five, (t(57)=6.17, p<.05, d=.93), and six (t(57)=7.25, p<.05, d=1.03). Bin three contained more theta power than bins four (t(57)=3.56, p<.05, d=.41), five (t(57)=4.62, p<.05, d=.63), and six (t(57)=5.65, p<.05, d=.71). More power was seen in bin four than bin five (t(57)= 2.12, p<.05, d=.20) and bin six (t(57)=2.19, p<.05, d=.26). Bins five and six were not significantly different from each other (t(57)= .493, p>.05) and the interaction between bin and electrode site was not significant (F(9.30,474.54)= .54, p>.05).
Based on these analyses and prior research (Kikuchi et al., 2011), we defined theta-1 as power in bins 1 through 3 (3.5 Hz-5.5 Hz) and theta-2 as power in bins 4 through 6 (6.5 Hz- 8.5 Hz). Divided in this way, theta-1 power was significantly greater (M=.01, SD= .33) than theta-2 power ((M= −.39, SD= .35; t(57)= 10.35, p <.05; d=1.17). Given greater power in the current sample and its previous associations with ERN, subsequent analyses focused on theta-1 only.
Interactions Between ERN and Theta Predicting Anxiety Risk
We used 2-step hierarchical regression to test theta-1 as a moderator of the association between ERN and preschoolers’ anxious behaviors. Main effects for depressive symptoms (covariate), ΔERN, and theta-1 power during incorrect trials were entered in model Step 1. Theta-1 power on incorrect trials was the focus of analyses given our hypotheses about associations between error-related activation and anxious behaviors. The interaction between ΔERN and theta-1 power was entered in Step 2. Predictor variables were centered prior to the creation of an interaction term. In order to allow an opportunity for internal replication for our results, scales of parent-reported anxious behaviors were tested as outcomes in separate models.
Parent-reported anxiety risk
The first model tested theta-1 power following incorrect responses as a moderator of the association between ΔERN and social withdrawal. Main effects for ΔERN (B=−0.01, SE(B)=0.02, p>0.10) and theta-1 (B=0.19, SE(B)=0.43, p>0.10) power were not significant. Results revealed a significant interaction (B=0.11, SE(B)=0.05, p<0.05) between ΔERN and theta-1 predicting social withdrawal during preschool. This interaction was probed by recentering theta-1 power at low (-1SD) and high (+1 SD) levels (Aiken & West, 1991). Probes showed that when theta-1 power was low, greater ΔERN predicted more social withdrawal symptoms (B= −0.06, SE= 0.03, p<0.05). In contrast, when theta-1 power was high, ΔERN and social withdrawal were unrelated (B= 0.02, SE= 0.02, p>0.10).
In the model predicting preschoolers’ social inhibition, main effects for ΔERN (B=−0.03, SE(B)=0.02, p>0.10) and theta-1 (B=0.46, SE(B)=0.45, p>0.10) power were not significant. However, a marginal interaction emerged between theta-1 and ΔERN predicting preschoolers’ social inhibition (B=0.10, SE(B)=0.05, p=0.06). Again, the interaction was probed by recentering theta-1 power at low (-1SD) and high (+1 SD) levels (Aiken & West, 1991). Probes revealed a negative association between ΔERN and social inhibition when theta-1 power was low (B=−0.07, SE=0.03, p<0.05). However, ΔERN was unrelated to social inhibition when theta-1 power was high (B=0.01, SE=0.03, p>0.10).
Post-hoc analyses
Given our pattern of findings, a logical follow-up question would ask whether the same pattern of findings emerge for theta-1 power during correct trials. Therefore, we reran regression analyses to test whether theta power during correct trials also appeared to moderate associations between ERN and anxious behaviors in preschoolers, potentially suggesting a lack of specificity of the current effects to error-related activation. No significant interactions between ERN and theta-1 power were observed during correct trials for social inhibition (B=0.07, SE(B)=0.05, p>0.10) or social withdrawal (B=0.08, SE(B)=0.05, p>0.10).
Discussion
The current findings provide a replication of previous work on the ERN in young children and an a novel extension that offers insight into the conditions under which ERN may be most strongly associated with anxiety risk in the form of anxious behaviors during preschool. First, our results suggest that 3-year-old children showed ERN at frontocentral sites, but not parietal sites. This finding is consistent with previous evidence that young children engage in response monitoring processes that are visible at the neural level (Brooker & Buss, 2014; Grammer et al., 2014; Torpey, Hajcak, & Klein, 2009). Importantly, this is the first study to present evidence of ERN in a sample of children who are all 3 years of age. This type of evidence, in which within-sample age differences are minimized, negates the possibility that ERN effects are driven by the inclusion of older children in the sample (Crone & Ridderinkhof, 2011). Similarly, our iterative go/no-go procedure, which held performance constant across participants, diminishes the likelihood that ERN effects reflect differences in task difficulty across development (Gehring, Liu, Orr, & Carp, 2015). Thus, although individual differences undoubtedly exist, we have increased confidence that, on average, ERN is present by age 3.
Our findings of ERN at Fz, FCz, and Cz electrodes are consistent with previous work suggesting that ERN in children has a broader scalp distribution than is typically observed for ERN in adults (Brooker & Buss, 2014 [ERN at Fz, Cz, Pz for high fear children]; Grammer et al., 2014 [ERN at Fz, FCz], Torpey et al., 2009 [ERN at Fz, Cz, Pz]). Previous research has shown that the neural processes believed to be reflected by the ERN develop across childhood and adolescence, becoming more localized over time before maximizing at frontocentral locations by the adolescent years (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006). Early distributions are believed to reflect the broader recruitment of neural networks in younger children. Our results also mirror previous amplitudes differences in ERN across maturation (Davies et al., 2004; DuPuis et al., 2015). That is, ERN amplitudes in this sample are somewhat smaller than are typically observed in work with adults. Overall, these findings reinforce developmental differences in self-monitoring between from childhood to adulthood that will be important for the design of future studies.
This study also offers additional insight into the presence of theta activity during the time period of the ERN. We found the greatest theta power following responses in frequencies between roughly 3 and 5 Hz, with more power at lower frequencies, consistent with previous work in children (Marshall, Fox, & the BEIP Core Group, 2004) and adults (Hall, Bernat, & Patrick, 2007). This frequency range overlaps with the expected rage for theta activity associated with goal-directed attentional control, as may be expected during a go/no-go task. Moreover, theta activity immediately following participant responses was maximal at the FCz electrode site, consistent with the localization of the ERN in broader studies. Although conclusions about precise neural generators are limited in EEG studies, this pattern of findings supports a suggestive association between ERN and theta power. Yet, although ERN showed the greatest effect size at FCz, it was not significantly different from ERN at Fz or Cz. Furthermore, theta activity did not differ between correct and incorrect trials. Thus, our results suggest that although theta mirrors neural activity associated with ERN, ERN and theta are likely complementary rather than redundant measures.
We did not find any direct associations between ERN and theta power. It is somewhat unclear whether this differs from previous research, as statistical associations (i.e., correlations) between ERN and theta have been suggested but are not consistently reported (e.g., Hall, Bernat & Patrick, 2007). ERN has been used as a proxy for theta power in frontal midline regions (Cavanagh & Shackman, 2014). However, ERN may also be only one peak in the oscillation of theta rhythms surrounding an incorrect response (Luu & Tucker, 2001), diminishing direct associations between ERN and theta measures. Furthermore, there is evidence that fluctuations in theta power may not be specific to error trials (Yordanova et al., 2004); other neural dynamics, such as delta, may be involved in discriminating error-specific activity. Thus, additional approaches in future investigations may be necessary to fully explicate the direct association between theta and ERN.
We did find evidence that theta power following incorrect responses moderated the relation between ERN and anxious behaviors during preschool. Specifically, when theta power was low, greater ERN predicted more anxious behaviors in preschoolers. In contrast, when theta power was high, ERN amplitudes and anxious behaviors were unrelated. There is evidence, from work with adults, that decreased frontal midline theta is associated with enhanced levels of anxiety (Suetsugi et al., 1998). Given that theta power typically increases in the presence of errors (Luu et al., 2004; Trujillo & Allen, 2007), it is possible that anxiety risk is associated with inefficient or ineffective signaling of a need for refined cognitive control strategies. That is, if enhanced theta power is necessary for initiating cognitive adjustments that lead to refined cognitive control (Cavanagh & Frank, 2014), enhanced ERN paired with diminished theta may reflect a signaling “mismatch” between ERN and theta power that prevents appropriate behavioral adjustments. This lack of adjustment is consistent with behavioral evidence that children who are at enhanced risk for anxiety problems do not appropriately modulate behavior to context (Buss, 2011) despite showing enhanced ERN (Brooker & Buss, 2014). Although this effect will need to be replicated in future work, our results offer additional insight into one possible mechanistic association between neural dynamics and preschool anxiety risk and one possible explanation for previous inconsistencies in reported associations between ERN and anxiety.
The current study offers novel information about the interactions between ERN and theta power in relation to anxious behaviors in preschoolers. However, this work is not without limitations. While our sample size was comparable to or larger than many studies of childhood ERN, our total N remains fairly small, diminishing our power for detecting small effects. Additionally, this work was done in a cross-sectional sample of nonclinical children. While a nonclinical approach is most desirable for studies of anxiety risk rather than anxiety disorders, it remains unclear whether putatively “at risk” children in the current sample will go on to develop problems with anxiety. Finally, we recognize that theta is likely not to be the only potential modulator of ERN amplitudes. Thus this work offers theta as one possible mechanism for resolving discrepancies in previous studies linking ERN to anxiety risk, but additional possibilities remain. Future research will be important for fully explicating this association.
In sum, we found that individual differences in theta power following errors may be one possible mechanism which modulates links between ERN and anxiety risk in preschoolers. Though additional work is needed, we believe that these results offer a starting point for understanding inconsistent findings between ERN and anxiety risk in young children and a refinement of the understanding of the neural pathways by which ERN is linked with early risk.
Highlights.
ERN is visible in children at age 3
Theta power moderates associations between ERN and early anxiety risk
Greater ERN is associated with greater anxiety risk when theta power is low.
ERN is unrelated to anxiety risk when theta power is high.
Acknowledgments
We would like to thank the families that participated in this study for their dedication and willingness to be a part of our work. We would also like to acknowledge research support provided by K01MH100240 (PI: Brooker) from the National Institute of Mental Health and P20GM104417 (PI: Harmsen) from the National Institute of General Medicine. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Confidence in the presence in these differences is increased by follow-up analyses showing that rerunning analyses substituting power the theta-2 range as our measure of theta produced nonsignficant results.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; Thousand Oaks, CA: 1991. [Google Scholar]
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology. 2005;42:33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Developmental Neuropsychology. 2014;39(1):1–8. doi: 10.1080/87565641.2013.826661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Dennis TA. Error-monitoring brain activity is associated with affective behaviors in young children. Developmental Cognitive Neuroscience. 2011;1:141–152. doi: 10.1016/j.dcn.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Developmental Neuropsychology. 2014;39(1):1–8. doi: 10.1080/87565641.2013.826661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology. 2011;47(3):804–819. doi: 10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313 doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi AB, Nystrom LE, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botnivik MM, Noll DN, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Shackamn AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology-Paris. 2014;109:1–3. 3–15. doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29(1):98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne DO, Ferrari P, Cheyne JA. Intended actions and unexpected outcomes: automatic and controlled processing in a rapid motor task. Frontiers in Human Neuroscience. 2012;(6):1–15. doi: 10.3389/fnhum.2012.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Ridderinkhof KR. The developing brain: From theory to neuroimaging and back. Developmental Cognitive Neuroscience. 2011;1(2):101–109. doi: 10.1016/j.dcn.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25- year-olds. Developmental Neuropsychology. 2004;25(3):3550–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- DuPuis D, Ram N, Willner CJ, Karalunas S, Segalowitz SJ, Gatzke-Kopp LM. Implications of ongoing neural development for the measurement of the error-related negativity in childhood. Developmental Science. 2015;18(3):452–468. doi: 10.1111/desc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: Presentation, nosology and epidemiology. Journal of Child Psychology and Psychiatry. 2006;47(3/4):313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Essex, et al. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArther health and behavior questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(5):588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II Error processing in choice reaction tasks. Electroencephalography and clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: Correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101(3):523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne) In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potentials. Oxford University Press; New York, NY: 2015. pp. 231–291. [Google Scholar]
- Grammer JK, Carrasco M, Gehring WJ, Morrison FJ. Age-related changes in error processing in young children: A school based investigation. Developmental Cognitive Neuroscience. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: Defensive motivation and the error-related negativity. Psychological Science. 2008;19(2):103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110(1):63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18(4):326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles GH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Short communication: Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, van Melis JJM, Kemner C, Markus CR. Methylphenidate improves deficient error evaluation in children with ADHD: An event related brain potential study. ScienceDirect. 2007;76:217–229. doi: 10.1016/j.biopsycho.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Martino AD, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DA, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2008;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Imashoiya H, Uno H, Fujita T. Error-related negativity in a visual go/no-go task: Children vs. adults. Developmental Neuropsychology. 2007;31(2):181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Shitamichi K, Yoshimura Y, Ueno S, Remijm GB, Hirosawa T, Munesue T, Tsubokawa T, Haruta Y, Oi M, Higashida H, Minabe Y. Lateralized theta wave connectivity and language performance in 2- to 5- year-old children. The Journal of Neuroscience. 2011;31(42):14984–14988. doi: 10.1523/JNEUROSCI.2785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskaya HR, Wilson GD. Shohov SP, editor. Personality and brain oscillations in the developmental perspective. Advances in Psychology Research. 2004;29:1–34. [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10(6):874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano Al., Pine DS, Henderson HA, Fox DA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(4):447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-related Potential Technique. 2nd MIT Press; Cambridge, MA: 2014. [Google Scholar]
- Luu P, Tucker DM. Regulating action: Alternating activation of midline frontal and motor cortical networks. Clinical Neurophysiology. 2001;112:1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA, the BEIP Core Group A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience. 2004;16(8):1327–1338. doi: 10.1162/0898929042304723. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42:261–268. doi: 10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophyiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stronganova TA, Posikera IN, Elam M. EEG theta rhythm in infants and preschool children. Clinical Neurophysiology. 2006;117:1047–1062. doi: 10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu C, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47(4):767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive disorder. Developmental Neuropsychology. 2006;29(3):431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation fro subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Suetsugi M, Mizuki Y, Ushijima I, Yamada M, Imaizumi J. Anxiolytic effects of low-dose clomipramine in highly anxious healthy volunteers assessed by frontal midline theta activity. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1998;22(1):97–112. doi: 10.1016/s0278-5846(97)00182-6. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsycholoyg. 2009;34(6):749–761. doi: 10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Developmental Psychobiology. 2012;54:139–150. doi: 10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJB. Theta EEG dynamics of the error-related negativity. Clinical Neurophysiology. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical netwokrs. Trends in Cognitive Science. 2009;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology. 2012;121(4):885–896. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;22:590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]