Abstract
Universal testing for Lynch syndrome is now a routine component of the diagnostic work-up of endometrial cancer patients. The purpose of this study was to identify prospectively the barriers to universal screening based on a tissue testing approach (microsatellite instability analysis, immunohistochemistry for DNA mismatch repair proteins, and MLH1 methylation analysis). Endometrial carcinoma patients (n=213) prospectively underwent microsatellite instability and immunohistochemistry testing for expression of DNA mismatch repair proteins. Patients with low (MSI-L) or high (MSI-H) levels of tumor microsatellite instability or immunohistochemical loss of MLH1 (and absent MLH1 methylation), MSH2, MSH6, or PMS2 were referred to a genetic counselor for consideration of germline testing. Six discordances (3.1% of tested cases) between immunohistochemistry and microsatellite instability were identified. Half of these exhibited heterogeneous immunohistochemical loss of MLH1/PMS2 and were microsatellite stable (MSS). Of the remaining cases, one was MSS with immunohistochemical loss of MSH6, one was MSS with immunohistochemical loss of MLH1/PMS2 and absent MLH1 promoter methylation, and one was MSI-H with intact expression of DNA MMR proteins. Four patients had MSI-L tumors with intact immunohistochemical protein expression; the clinical significance of MSI-L in endometrial cancer is unclear. Eight patients did not have germline mutations despite tissue testing suggesting Lynch syndrome. Including cases with insufficient tissue for testing and patients declining tissue or germline testing, we encountered significant barriers to universal screening in 13.6% of screened patients (29/213) that preclude designation of a tumor as sporadic or hereditary.
Keywords: Lynch Syndrome, endometrial cancer, microsatellite instability, DNA mismatch repair
INTRODUCTION
Endometrial cancer (EC) is the most common gynecologic cancer in the United States (1). Risk factors for endometrial cancer include increasing age, obesity, hypertension, diabetes, menstrual irregularities, nulliparity and unopposed estrogen (2). Another significant risk factor for EC is Lynch Syndrome, an inherited cancer syndrome due to a germline mutation in one of the four DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6 and PMS2), which accounts for 2–6% of all endometrial cancers. For women with Lynch Syndrome, the lifetime risk of EC is 64%, and the lifetime risk of colorectal cancer is 54% (3). Prior studies have shown that use of clinical screening (patient age and family history of cancer) alone misses a substantial subset of endometrial cancer patients that may harbor a germline Lynch Syndrome mutation (4, 5).
PCR-based microsatellite instability (MSI) testing, immunohistochemistry (IHC) evaluation of expression for the DNA MMR proteins, and PCR-based MLH1 methylation analysis have emerged as useful clinical laboratory tests to screen endometrial cancer patients for Lynch Syndrome. Tumors with high levels of microsatellite instability (MSI-H) or immunohistochemical loss of expression of DNA MMR proteins in the absence of MLH1 gene methylation are suggestive of Lynch Syndrome. Many recently published studies have advocated for universal screening of endometrial carcinomas with MSI and/or IHC (4, 6, 7). The American College of Obstetricians and Gynecologists and the Society of Gynecologic Oncology have recently issued a practice bulletin recommending that universal tissue testing as a rational approach for identifying women at risk for Lynch-associated endometrial cancer (8). With the adoption of universal testing, clinical and diagnostic conundrums may potentially emerge, including discordances between MSI and IHC results, tumor testing suggestive of Lynch Syndrome with negative germline testing, and germline testing that results in a variant of unknown significance (9, 10). The incidences of these problems in endometrial cancer testing are not well documented. In addition, it is known that a subset of Lynch-associated endometrial cancers may have low levels of MSI (MSI-L). The detection of MSI-L in colorectal cancer does not typically warrant a genetic counseling referral unless there is IHC loss of a MMR protein or the patient has an informative family history (11).
The purpose of this study was to prospectively identify the incidence of clinically and diagnostically significant challenges when universal tissue testing is applied to patients with endometrial carcinoma.
MATERIALS AND METHODS
Patient population and study design
Institutional Review Board approval was obtained for this prospective study, and a waiver of informed consent granted as universal testing is the standard clinical practice at our institution. Women ages 18 and older with newly diagnosed endometrial cancer undergoing surgery at The University of Texas MD Anderson Cancer Center (Houston, TX) from August 2012-August 2014 underwent tumor testing on their pathology specimens. Patient demographics were retrieved from the electronic medical record and included age at endometrial cancer diagnosis, body mass index (BMI), FIGO surgical stage, tumor histology, FIGO tumor grade (non-endometrioid tumors were considered grade 3), depth of myometrial invasion, and tumor location within the uterus (corpus vs. lower uterine segment). Data extraction was performed primarily by author ASB and validated by author KLR. Tumor testing consisted of immunohistochemistry for the expression of DNA MMR proteins MLH1, MSH2, MSH6, and PMS2, with MLH1 promoter methylation analysis in cases of IHC loss of MLH1, and PCR-based microsatellite instability (MSI) testing. Pre-analytic, analytic, and post-analytic barriers to definitively classifying an endometrial carcinoma as sporadic or Lynch syndrome related were recorded.
Molecular analyses
Immunohistochemistry (IHC) for the expression of the DNA MMR proteins was performed in a Clinical Laboratory Improvement Amendments (CLIA) approved laboratory using previously described methods (9). The absence of nuclear staining in tumor cells with retained stromal staining was classified as loss of expression for the corresponding DNA MMR protein. Endometrial carcinomas exhibiting loss of MSH2, MSH6, or PMS2 were considered suggestive of a Lynch Syndrome (LS) associated tumor. For cases in which there was IHC loss of MLH1 protein expression, the PCR-based MLH1 promoter methylation assay was utilized to distinguish between sporadic epigenetic silencing of MLH1, methylated, and suspected MLH1 loss due to LS, unmethylated as previously described (12). Briefly, DNA isolated from formalin-fixed, paraffin-embedded endometrial carcinoma tissue sections was treated with bisulfite to convert unmethylated cytosines to uracil using the Zymo EZ DNA Methylation-Gold Kit according to the manufacturer’s instructions (Zymo Research, Orange, CA). Methylation of MLH1 was assessed by methylation-specific PCR followed by capillary electrophoresis using FAM labeled reverse primer and unlabelled forward primers (Integrated DNA Technology). The following primer sequences were used: methylated forward, 5′-GAT AGC GAT TTT TAA CGC-3′, unmethylated forward, 5′-AGA GTG GAT AGT GAT TTT TAA TGT-3′ and labeled reverse primer, 5′-FAM-TCT ATA AAT TAC TAA ATC TCT TC-3′. The forward primers were designed to distinguish the methylated amplicon from the unmethylated by difference in size. The bisulfite treated DNA was then subjected to PCR using primers specific for methylated and unmethylated DNA. The methylated PCR product of 85 bp was separated from unmethylated PCR product of 91 bp by capillary electrophoresis using an ABI Prism 3130 Genetic Analyzer. MSI was assessed using a panel of 6 National Cancer Institute (Bethesda, MD) recommended microsatellites with the addition of TGFBR2. A tumor with allelic shift in 3 or more markers was designated as MSI-high (MSI-H), 1–2 markers as MSI-low (MSI-L), and no allelic shift as microsatellite stable (MSS). For cases with insufficient tissue for molecular analysis, a referral to genetic counselor was based on patient clinical characteristics and family history.
Genetic counseling referral and germline testing
Patients with endometrial carcinomas with IHC loss of MLH1, MSH2, MSH6, or PMS2 with lack of MLH1 methylation (for patients with IHC loss of MLH1) were referred to a genetic counselor for consideration of germline testing, no matter the MSI testing results. Patients with MSI-H cancers lacking MLH1 methylation were also referred to genetic counselors, no matter the IHC results. There are no established guidelines for patients with MSI-L endometrial cancers. If MSI-L was associated with IHC loss of a MMR protein and lack of MLH1 methylation, patients were referred to genetic counseling and germline testing. Patients with MSI-L with intact IHC protein expression were also referred to genetic counselors, who might recommend germline testing if the family history of cancer was informative. Germline testing of mismatch repair genes was performed by commercial clinical laboratories, usually Ambry Genetics or Myriad Genetics Briefly, next-generation sequencing was performed using the Illumina HiSeq2500 (Illumina Inc., San Diego, Calif). Sanger sequencing was performed for any regions with insufficient read depth coverage. Large rearrangements were identified using quantitative dosage analysis of the data obtained from next-generation sequencing. In addition, deletions and duplications were identified using a custom microarray comparative genomic hybridization (CGH) chip (Agilent Technologies, Santa Clara, California). Multiplex ligation-dependent probe amplification analysis for large rearrangements in PMS2 was performed to distinguish homologous pseudo genes and actual gene regions. Variants were classified using American College of Medical Genetics and Genomics recommendations (13, 14).
Statistical analysis
Summary statistics were calculated to describe the clinical and demographic characteristics of the study population using Stata v14.1 software (College Station, Texas). To determine the concordance between immunohistochemistry and MSI-H, we calculated the proportion that agree and disagree along with their 95% confidence intervals.
RESULTS
There were 213 surgeries performed for endometrial carcinoma during the study period. Clinical and pathologic characteristics of the study population are listed in Table 1. The median age at diagnosis was 61.3 years with a range of 23–86. Most women were diagnosed at age greater than 50, were obese with a BMI greater than 30, had cancers with endometrioid histology (74.2%) and had early stage disease. Thus, this patient cohort is a good representation of the endometrial cancer patient population in general.
Table 1.
Clinical and pathologic characteristics of women undergoing surgery for endometrial cancer at The University of Texas MD Anderson Cancer Center between August 2012-August 2014.
| Characteristic | N | % |
|---|---|---|
| Age at Diagnosis | ||
| N | 213 | |
| Mean | 61.3 ±10.6 | |
| Median (Min-Max) | 61.0 (23.0–86.0) | |
| BMI | ||
| Mean | 35.2 ± 10.6 | |
| Median (Min-Max) | 33.8 (15.5–74.4) | |
| BMI | ||
| Underweight (< 18.0) | 2 | 0.9 |
| Normal (18.0 – 24.9) | 31 | 14.6 |
| Overweight (25.0–29.9) | 48 | 22.5 |
| Obese (≥30.0) | 132 | 62.0 |
| FIGO Stage | ||
| I | 151 | 70.9 |
| II | 13 | 6.1 |
| III | 29 | 13.6 |
| IV1 | 20 | 9.4 |
| Histology | ||
| Endometrioid | 158 | 74.2 |
| Serous | 15 | 7.0 |
| Clear cell | 4 | 1.9 |
| Mixed | 31 | 14.6 |
| Carcinosarcoma | 3 | 1.4 |
| Other | 2 | 0.9 |
| Grade | ||
| 1 | 19 | 8.9 |
| 2 | 139 | 65.3 |
| 3 | 55 | 25.8 |
| Depth of Myometrial Invasion | ||
| 0 | 41 | 19.3 |
| <50 | 112 | 52.6 |
| >50 | 60 | 28.2 |
| Tumor Location2 | ||
| C | 205 | 97.2 |
| LUS | 3 | 1.4 |
| C & LUS | 3 | 1.4 |
One patient was documented in the electronic medical record as “advanced” and therefore recorded as Stage IV disease for statistical purposes
For two patients, the exact site of tumor within the uterus is not known. C, uterine corpus; LUS, lower uterine segment
Microsatellite instability analysis was successfully performed in 199 cases, and immunohistochemistry was carried out in 203 cases. Reasons for not performing the tests included failure of insurance to authorize testing, patient declining tumor screening, and insufficient tissue to perform the evaluation. Results of MSI and IHC testing are summarized in Table 2. Of the evaluable MSI testing cases, 71.9% were MSS, 25.1% MSI-H, and 3% MSI-L. Of the cases with IHC testing, 22.8% of patients had tumors with loss of MLH1/PMS2, 1.0% loss of MSH2/MSH6, 1.5% loss of MSH6, and 1.5% loss of PMS2. 72.6% of patients had tumors with intact staining for all MMR proteins.
Table 2.
Summary of microsatellite Instability (MSI) and Immunohistochemistry (IHC) results.
| MSI Status | Number | %* |
|---|---|---|
| MSI-Low | 6 | 3.0 |
| MSI-Stable | 143 | 71.9 |
| MSI-High | 50 | 25.1 |
|
| ||
| IHC Result | ||
|
| ||
| Intact MLH1, MSH2, MSH6, PMS2 | 146 | 72.6 |
| Loss of MLH1 & PMS2 | 46 | 22.8 |
| With MLH1 methylation | 43 | 93.5 |
| Without MLH1 methylation | 3 | 6.5 |
| Loss of MSH2 & MSH6 | 2 | 1.0 |
| Loss of MSH6 only | 3 | 1.5 |
| Loss of PMS2 only | 3 | 1.5 |
Percent of tested endometrial cancers (MSI – 199; Immunohistochemistry – 203)
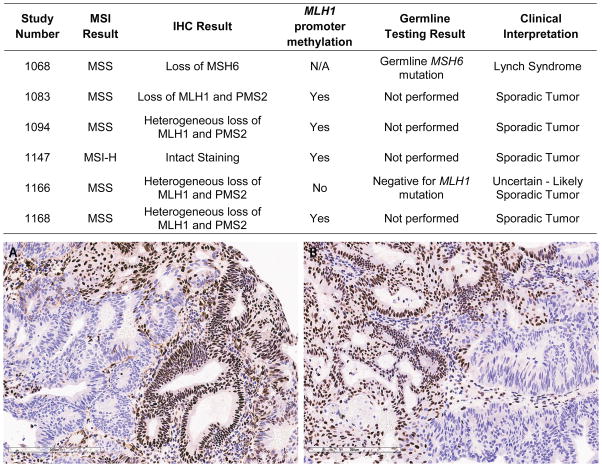
Detailed discordance and concordance data for MSI and IHC are summarized in Table 3 and Figure 1. Overall concordance and discordance between IHC and MSI was 96.9% and 3.1%, respectively. Cases exhibiting MSI-H results had a concordant loss of IHC expression in 98.0% of cases. For the one discordant case, IHC exhibited heterogeneous loss of MLH1/PMS2 and had methylation of the MLH1 promoter and was thus considered a sporadic tumor (Figure 1). The lowest level of agreement occurred in tumors with IHC loss of at least one MMR protein, with only 89.1% of these tumors being MSI-H. MSS cases were concordant with intact IHC protein expression in 96.5% of cases. Of the 5 discordant cases, 4 exhibited heterogeneous loss of MLH1/PMS2 with 3 having MLH1 promoter methylation and therefore considered sporadic tumors. The one patient with a tumor with unmethylated MLH1 promoter underwent genetic testing for a germline mutation in the MLH1 gene and this was found to be negative. The final discordant case exhibited IHC loss of MSH6, and genetic testing revealed a germline mutation in MSH6. For EC tumors with intact IHC expression for all proteins, there was concordance with a MSS result in 96.5% of cases. Of the 5 discordant cases, one tumor was MSI-H with a methylated MLH1 promoter and the remaining 4 were MSI-L. All MSI-L cases are discussed in more detail below. The table in Figure 1 summarizes the tumor testing and germline testing results, when applicable, for cases with discordant IHC and MSI results.
Table 3.
Concordance and discordance between microsatellite instability (MSI) and immunohistochemistry (IHC) results in endometrial adenocarcinomas.
| Number | % | (95% CI) | |
|---|---|---|---|
| MSI-High | |||
| IHC shows loss of expression of at least one MMR protein | 49 | 98.0 | (89.4 – 99.9) |
| IHC shows intact expression of all 4 proteins | 1 | 2.0 | (0.0 – 10.6) |
| MSI-Stable | |||
| IHC shows loss of expression of at least one MMR protein | 5 | 3.5 | (1.2 – 8.0) |
| IHC shows intact expression of all 4 proteins | 137 | 96.5 | (92.0 – 98.8) |
| IHC loss of at least one MMR protein | |||
| MSI-Low | 1 | 1.8 | (0.0 – 9.7) |
| MSI-Stable | 5 | 9.1 | (3.0 – 20.0) |
| MSI-High | 49 | 89.1 | (77.8 – 95.9) |
| IHC shows intact expression of all 4 proteins | |||
| MS-Low | 4 | 2.8 | (0.8 – 7.1) |
| MS-Stable | 137 | 96.5 | (92.0 – 98.8) |
| MS-High | 1 | 0.7 | (0.0 – 3.9) |
| Overall Agreement | |||
| Concordance1 | 186 | 96.9 | (93.3 – 98.8) |
| Discordance2 | 6 | 3.1 | (1.2 – 6.7) |
IHC shows loss of expression of at least one MMR protein and MSI-High or IHC shows intact expression of all 4 proteins and MS-Stable
IHC shows loss of expression of at least one MMR protein and MS-Stable or IHC shows intact expression of all 4 proteins and MSI-High
Figure 1.
The table summarizes endometrial cancer patients with discordances between MSI and IHC tissue testing results. The tumor in the photomicrograph demonstrates heterogeneous loss of MLH1 (A) and PMS2 (B) protein expression by immunohistochemistry. Retained protein expression is indicated by red-brown nuclear staining; loss of protein expression is apparent in tumor cells with blue nuclei. Loss of PMS2 protein expression is secondary to the primary defect in MLH1, as MLH1 and PMS2 typically exist as dimers in the nucleus.
Six patients had MSI-L tumors (Table 4). Five of these had intact IHC staining of all four DNA MMR proteins, and five were age less than 60 at the time of diagnosis, thus meeting the Society of Gynecologic Oncology (SGO) clinical screening criteria for referral to a genetic counselor. The insurance carrier declined genetic testing for one of these patients. For two patients, no MSH6 germline mutations were identified. One patient had a germline MSH6 variant of uncertain significance. The fifth patient with an MSI-L tumor and intact MMR IHC was not offered genetic testing, as she had a synchronous ovarian granulosa cell tumor, which can associated with endometrial hyperplasia and cancer. One patient with an MSI-L endometrial cancer had a tumor with IHC loss of MLH1 and PMS2 and MLH1 promoter methylation, which is consistent with a sporadic tumor.
Table 4.
Microsatellite instability low (MSI-L) endometrial adenocarcinomas with associated immunohistochemistry (IHC), family history and germline test results
| Study Number | MSI Result | IHC Result | Age at Diagnosis | FDR with LATs1 | Germline Testing Result | Clinical Interpretation |
|---|---|---|---|---|---|---|
| 1009 | MSI-L | Intact Staining | 59 | Daughter: precancerous colon polyp at age 48 | No MSH6 mutation detected | Presumed sporadic |
| 1021 | MSI-L | Intact Staining | 59 | No | No MSH6 mutation detected | Presumed sporadic |
| 1066 | MSI-L | Loss of MLH1/PMS2 MLH1 methylation present | 62 | No | Not performed | Presumed sporadic |
| 1108 | MSI-L | Intact MLH1, MSH2, MSH6 staining2 | 53 | No |
MSH6
VUS3 |
Uncertain |
| 1115 | MSI-L | Intact Staining | 52 | No | Insurance denied testing | Uncertain, likely sporadic |
| 1129 | MSI-L | Intact Staining | 54 | No | Not offered genetic testing4 | Presumed sporadic |
FDR, First Degree Relative; LAT, Lynch Syndrome Associated Tumors (colorectal, stomach, endometrial ovarian, biliary, sebaceous adenomas)
IHC for PMS2 unsuccessful; both tumor and internal positive control lacked staining
VUS, variant of unknown significance
Endometrial cancer was believed to be secondary to the concurrent ovarian granulosa cell tumor
Table 5 summarizes the germline testing results, when available, of all endometrial cancer cases with tissue testing suggesting possible Lynch Syndrome. Included is one endometrial cancer patient who had genetic testing prior to her hysterectomy because of the diagnosis of colorectal adenocarcinoma at a young age; a PMS2 germline mutation was detected, so no subsequent tissue testing was performed. Seven germline Lynch Syndrome mutations were identified in this cohort, representing 3.4% of patients undergoing some type of tissue testing. Note that tissue testing identified 12/213 (5.6%) patients as being suspected of having a potential Lynch germline mutation. For 5/12 of these patients, either germline testing did not identify a mutation or was declined by insurance.
Table 5.
Summary of patients with tissue testing results concerning for Lynch Syndrome with corresponding germline results, if available
| Study # | Age at Diagnosis | Family History | MSI | IHC Result | Germline Testing |
|---|---|---|---|---|---|
| 1023 | 68 | Brother CRC1 at 55 yrs | MSI-H | Loss of PMS2 | Insurance declined testing |
| 1050 | 65 | Sister CRC at 53; P2 aunt X 2 ovarian or endometrial cancer; P uncle CRC | MSI-H | Loss of MSH6 |
MSH6
c.3238_3239delCT, p.L1080VfsX12 |
| 1051 | 49 | P aunt CRC 50s; p nephew CRC 50s; sister Endometrial cancer at 49 | MSI- H | Loss of PMS2 | PMS2 deletion of exon 10 |
| 1060 | 75 | Sister with cancer, type unknown to patient | MSI-H | Loss of MSH6 | Negative for MSH2 or MSH6 |
| 1068 | 62 | None | MSS | Loss of MSH6 | MSH6 c.2805_2806delTG, p.D396LfsX2 |
| 1088 | 47 | P uncle and father with CRC; P aunt with ovarian cancer | MSI- H | Loss of MLH1/PMS2 No MLH1 methylation |
MLH1 deletion of exons 2–3 |
| 1126 | 69 | None | MSI-H | Loss PMS2 | Negative for MLH1 or PMS2 |
| 1131 | 59 | Mother gastric cancer at 71 | MSI-H | Loss of MLH1/PMS2 No MLH1 methylation |
Insurance declined testing |
| 1147 | 86 | None | MSI-H | Intact staining of all four proteins with MLH1 methylation | Patient declined genetic counseling |
| 1165 | 40 | PGM CRC60s, P Aunt CRC 40s, P Aunt CRC 60s, P Cousin CRC at 28 | MSI- H | Loss of MLH1/PMS2 No MLH1 methylation |
MLH1
C.298C>T, p.R100 |
| 1204 | 42 | Mother CRC 60s, MGM CRC 60s, M3 Uncle CRC 50s, Maternal uncle CRC 50s | MSI-H | Loss of MLH1/PMS2 No MLH1 methylation |
MLH1 deletion in exons 16– 19 |
| 1206 | 54 | None | MSI-H | Loss of MSH2/MSH6 | Negative for MSH2 |
| 1212 | 64 | None | MSI- H | Loss of MSH2/MSH6 | Patient declined testing |
| 1181 | 79 | Sister with CRC at 65; sister with gyn cancer in 40s | MSI- H | Loss of MLH1/PMS2 MLH1 methylation not performed - insurance declined | MLH1 VUS4 IVS12-10T>G |
| 11595 | 23 | Personal history of CRC at 17 | Not performed | Not performed | PMS2 |
CRC, colorectal cancer
P, paternal
M, maternal
VUS – Variant of unknown significance; this patient had a strong family history of Lynch-associated cancers and a personal history of colorectal cancer.
Young patient with history of colon cancer prior to diagnosis of endometrial cancer. Constitutional mismatch repair deficiency was suspected by the genetics counselors, so no tissue testing was ordered. Genetic testing was performed at the time of colon cancer diagnosis, and documentation confirms a PMS2 mutation but specific mutation is not available.
Various clinical and diagnostic challenges that can occur with the implementation of universal tissue testing for Lynch Syndrome were identified in this study. These challenges are significant, as they prevented us from definitively classifying these cancers as hereditary or sporadic. These include pre-testing factors (n=10, 4.7%; patient does not want testing; insurance denies reimbursement for testing; not enough tumor for testing), IHC/MSI discordances (n=6, 2.8%), MSI-L with intact MMR protein expression by IHC (n=5, 2.3%), and post-tissue testing issues (n=8, 3.8%; insurance denies reimbursement for germline testing; patient declines germline testing; VUS detected; no germline mutation identified). In sum, we encountered clinical or diagnostic challenges when utilizing a universal tissue testing approach to screen for Lynch Syndrome in 13.6% (29/213) of endometrial cancer patients.
DISCUSSION
National organizations support screening patients with endometrial adenocarcinoma for LS either through family history or tissue-based screening modalities. There have been several studies evaluating the effectiveness of universal tumor testing and its ability to identify endometrial cancers secondary to LS; however, there have not been any published studies evaluating the clinical and diagnostic challenges that result from a universal tumor testing approach. To maximize capturing clinical and diagnostic challenges that can emerge through universal tumor testing, data were prospectively collected on a sequential, unselected cohort of endometrial carcinoma patients who underwent hysterectomy. The demographic and pathologic characteristics in this study population are similar to published national data, thus findings from this study can presumably be applied to other EC patients (15). Results from this cohort also show tumor testing results of MSI-H in 25.1% of cases, with 93.5% of these with MLH1 promoter methylation, a finding consistent with other published literature evaluating both colorectal and endometrial carcinomas (12, 16–21). Using the universal tumor screening approach, clinical and diagnostic challenges in definitively designating an endometrial cancer patient as sporadic or Lynch Syndrome would be expected to occur in approximately 13.6% of patients.
Looking first at tumor testing strategies, the choice of best tumor-based screening method for Lynch Syndrome is not clear. For endometrial cancer, the National Comprehensive Cancer Network (NCCN) guidelines recommend IHC or MSI screening of all women less than age 50 or those with a significant past medical history of family history concerning for Lynch Syndrome (22). The American College of Obstetrics and Gynecology and the Society of Gynecologic Oncology practice guidelines recommend that all women should undergo comprehensive clinical screening or molecular tumor based testing, deferring the choice of specific approaches to individual practices (8). These national guidelines do not favor one form of tissue testing over the other. Goodfellow et al., in a recent large cooperative group study of over 1,000 endometrial cancer patients, recommend a combination of MSI, IHC, and reflexive MLH1 promoter methylation for all patients with endometrial cancer with endometrioid histology, regardless of age, suggesting that cases of Lynch Syndrome could be missed if one method was used in place of another (6).
Microsatellite instability testing by itself has an overall sensitivity for detecting germline LS mutations of 83% with a range of 25–93% (23). These numbers are largely derived from colorectal cancer family registries and colorectal cancer literature. The greatest sensitivity is for detecting MSI-H associated with MLH1 and MSH2 germline mutations and to a lesser degree MSH6 and PMS2. If an institution were to solely use MSI testing as a tissue screen for LS, as is the case for many of the colorectal carcinoma screening protocols, the number of missed clinically significant LS cases in our cohort is 1/199 (0.5%). Drawbacks to using only MSI are that it is more expensive (2016 Medicare reimbursement associated with CPT81391 =$394.44), and it is technically more complex than IHC. Additionally, it does not target the possible gene of interest and subsequent evaluation would include either adding IHC to identify the source of mismatch repair defect or germline testing of all four DNA MMR genes.
Immunohistochemistry for the four DNA mismatch repair proteins has an overall sensitivity for detecting germline LS mutations of 94% with a range of 92–100% (23). These numbers are also extrapolated from the colorectal cancer literature and some studies which include endometrial cancers. IHC with reflexive MLH1 promoter methylation for cases with MLH1 protein loss has been proposed to be more cost effective than IHC alone (12). 2016 Medicare reimbursement for DNA MMR is $233.81 (CPT 88360), and PCR-based MLH1 promoter methylation is $159.64. If an institution chose to only perform IHC with reflexive MLH1 promoter methylation in indicated cases, the number of missed LS cases based on our prospective population is 2/203 (0.98%). Benefits of this approach include that it is a reliable indicator for identifying at-risk individuals, targets gene of interest for subsequent germline testing, is less expensive than MSI, is simpler to perform, and the presence of an internal positive control with each IHC sample. Drawbacks to IHC are ambiguous results such as heterogeneous staining which occurred in 4/203 (2.0%) of our patient population as well as false negative results in which a non-functional protein is translated and stains positive on IHC. While the latter circumstance was not encountered in this study, it was reported by Goodfellow et al. and has been occasionally encountered during the clinical practice of one of the authors (RRB) (6). While the numbers of missed Lynch Syndrome cases are relatively small when considering IHC or MSI as single screening tests, one does need to consider that each missed patient may have multiple siblings and children who would also be at risk for having a deleterious germline mutation. This results in a larger number of missed opportunities for early intervention and cancer screenings.
There have been two major studies examining the concordance between MSI and IHC. A retrospective analysis by Bartley et al. found discordance between MSI and IHC results in 13 of 591 (2.2%) cases; nearly all of the examined tumors were colorectal, with only seven endometrial cancers in the entire cohort. Two of the thirteen identified discordances were endometrial adenocarcinomas (9). A prospective study by Leenen et al. found a 100% concordance between MSI and IHC in a series of 179 endometrial carcinomas (7). In a large cooperative group study of over 900 endometrial cancer patients, 2.0% of endometrial cancers were MSI-high with intact IHC expression of MMR proteins (6). In our prospective EC population, MSI and IHC were discordant in 3.9% of cases.
In addition to discordances between tumor testing methodologies, there are other sources of diagnostic difficulty. For example, MSI-L is not clearly associated with a risk for Lynch Syndrome, but some tumors that are MSI-L have a corresponding germline mutation in one of the DNR MMR genes. In the study by Goodfellow et al., their cohort of 1043 had a 2.8% incidence of MSI-L tumors. Patients with tumors that were MSI-L with intact IHC did not undergo germline sequencing, and there were no tumors with MSI-L and a loss of expression on IHC (6). In another study, de Leeuw et al. found that 3/37 (8.1%) of the MSH6 mutation carriers with endometrial carcinoma had an MSI-L tumor and concomitant IHC loss of MSH6 (26). In the prospective study in the Netherlands, there were no cases of MSI-L tumors but their MSI testing included 5 microsatellites rather than the 7 microsatellites in the Bethesda Panel (7). Our study detected MSI-L detected in 3% of patients; one of these patients had a germline MSH6 variant of unknown significance but no definite MSH6 deleterious mutations. Of the small number of MSI-L endometrial carcinomas reported in the published literature, the number with a germline mutation is low but not zero (27). Kuismanen et al. examined endometrial cancers from families with known MLH1 or MSH2 germline mutations and found that, compared to colon cancers arising in these families, there was a lower proportion of unstable microsatellites, with 23% being MSS (28). For institutions using only MSI testing as a screening method, patients with MSI-L tumors may benefit from reflexive IHC or germline testing for MSH6 mutation.
Heterogeneous protein expression on IHC presents another tumor testing interpretive challenge (Figure 1). Our cohort showed 2.0% (4/203) of tumors expressed a partial loss of protein expression for MLH1 and PMS2. These tumors either had associated MLH1 methylation or no germline mutation detected. Patient numbers are small for this finding, but they do suggest that a heterogeneous pattern on IHC is not associated with Lynch Syndrome.
Tumor testing results associated with germline MSH6 mutations are less consistent than other Lynch Syndrome germline mutations. Individuals with an MSH6 mutation often have a unique phenotype in that probands are often older at diagnosis and endometrial cancer is more commonly seen then colorectal cancer in the family history (24, 27). The study by Goodfellow et. al had 21 endometrial cancer cases with IHC loss of MSH6.(6) Of these, 15 were MSI-H, 1 was MSI-L, and 5 were MSS. Seven of nine confirmed MSH6 germline mutations were from patients who had tumors that were MSI-H cases with corresponding IHC loss of MSH6. Two other confirmed patients with MSH6 mutations were also MSI-H; one had intact IHC for DNA MMR proteins and one had inconclusive IHC results. MSI-L cases did not undergo genetic testing in their study. Our cohort had two germline MSH6 mutations and one variant of unknown significance. For these, one was MSI-H with concordant loss of MSH6, one was MSS with discordant loss of MSH6, and one was MSI-L with intact IHC expression of all four MMR proteins.
Other potential tissue testing problems have been reported, but they were not encountered in our study. The Mayo Clinic group recently identified 22/13,100 (0.2%) cases of MSH6 heterogeneous loss by IHC using a retrospective analysis (29). At least 23% of these were associated with germline mutations in MMR genes other than MSH6. Goodfellow et al. identified 2 cases of germline PMS2 mutations (one variant of undetermined significance and one deleterious) in which the PMS2 IHC showed intact positive protein expression, but there was IHC loss of MSH2 and MSH6 (6).
After receiving tumor testing results suggestive of Lynch Syndrome (MSI-H and/or IHC loss of DNA MMR proteins), a referral to a genetic counselor with subsequent germline testing is typically performed. The recommendations are clear when a germline LS mutation is identified. Challenges that ensue for the genetic counselor are when there is no identifiable germline mutation or a variant of unknown significance occurs. Prior studies have shown that approximately 60% of tumors that have testing consistent with a diagnosis of LS will have a germline mutation subsequently identified (30, 31). In our series, 13/213 (6.1%) of endometrial carcinomas exhibited tumor testing suggestive of Lynch Syndrome, and 6/13 (46.2%) of these had a germline mutation confirmed. For the patients without a germline mutation, guidelines for the patient’s subsequent colon cancer screening and recommendations for family members are less clear. One study showed that the risk for subsequent cancers in these cases was lower than patients with a germline mutation but higher than the general population (32). There have also been some studies delving in to etiology of this phenomenon. Two studies have recently identified somatic mutations of MMR genes in endometrial and colorectal cancers, but the exact incidence of such mutations is not certain (33, 34).
In this study, 13.6% of endometrial cancer cases encountered clinical or diagnostic challenges which limited our ability to definitively classify a patient as LS or sporadic. Note that the number of instances in each of the pre-analytic (patient or insurance declining screening), analytic (insufficient tissue, ambiguous results, discordance between IHC and MSI), and post-analytic (patient or insurance declines genetic counseling/testing, informative tumor testing with negative germline testing, or germline testing showing a variant of unknown significance) categories is relatively small. However, it is clear that problems can arise at numerous steps along the path to universal testing. It should also be noted here that only some of these problems can likely be resolved. For example, over time medical insurance companies will become more educated as to the relationship of endometrial cancer to Lynch syndrome. With more cumulative experience, we will likely be able to confidently classify MSI-low/intact MMR IHC cases as sporadic. Implementation of somatic sequencing of MMR genes will help to classify some of the cases with no germline mutation as sporadic. Other problems, however, such as IHC/MSI discordance and detection of a germline VUS will continue to limit our ability to accurately classify a subset of patients.
Acknowledgments
Funding Sources: NIH 2P50 CA098258-06 SPORE in Uterine Cancer (RRB), NIH T32 Training Grant (ASB), M.D. Anderson Cancer Center Support Grant NIH CA016672
Footnotes
Conflict of Interest Disclosures: None for all authors.
References
- 1.Group USCSW. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. 2010. [Google Scholar]
- 2.ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstetrics and gynecology. 2005;106(2):413–25. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 3.Backes FJ, Cohn DE. Lynch syndrome. Clinical Obstetrics and Gynecology. 2011;54(2):199–214. doi: 10.1097/GRF.0b013e3182185a41. [DOI] [PubMed] [Google Scholar]
- 4.Bruegl AS, Djordjevic B, Batte B, Daniels M, Fellman B, Urbauer D, et al. Evaluation of clinical criteria for the identification of Lynch syndrome among unselected patients with endometrial cancer. Cancer Prev Res (Phila) 2014;7(7):686–97. doi: 10.1158/1940-6207.CAPR-13-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan P, Mulligan AM, Aronson M, Ferguson SE, Bapat B, Semotiuk K, et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. Cancer. 2012;118(3):681–8. doi: 10.1002/cncr.26323. [DOI] [PubMed] [Google Scholar]
- 6.Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers From GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol. 2015;33(36):4301–8. doi: 10.1200/JCO.2015.63.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leenen CH, van Lier MG, van Doorn HC, van Leerdam ME, Kooi SG, de Waard J, et al. Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer </= 70 years. Gynecologic oncology. 2012;125(2):414–20. doi: 10.1016/j.ygyno.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Committee on Practice B-G, Society of Gynecologic O. ACOG Practice Bulletin No. 147: Lynch syndrome. Obstet Gynecol. 2014;124(5):1042–54. doi: 10.1097/01.AOG.0000456325.50739.72. [DOI] [PubMed] [Google Scholar]
- 9.Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer prevention research. 2012;5(2):320–7. doi: 10.1158/1940-6207.CAPR-11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batte BA, Bruegl AS, Daniels MS, Ring KL, Dempsey KM, Djordjevic B, et al. Consequences of universal MSI/IHC in screening ENDOMETRIAL cancer patients for Lynch syndrome. Gynecol Oncol. 2014;134(2):319–25. doi: 10.1016/j.ygyno.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilar E, Mork ME, Cuddy A, Borras E, Bannon SA, Taggart MW, et al. Role of microsatellite instability-low as a diagnostic biomarker of Lynch syndrome in colorectal cancer. Cancer Genet. 2014;207(10–12):495–502. doi: 10.1016/j.cancergen.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruegl AS, Djordjevic B, Urbauer DL, Westin SN, Soliman PT, Lu KH, et al. Utility of MLH1 methylation analysis in the clinical evaluation of Lynch Syndrome in women with endometrial cancer. Curr Pharm Des. 2014;20(11):1655–63. doi: 10.2174/13816128113199990538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggington JM, Bowles KR, Moyes K, Manley S, Esterling L, Sizemore S, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet. 2014;86(3):229–37. doi: 10.1111/cge.12315. [DOI] [PubMed] [Google Scholar]
- 14.Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, et al. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 2008;10(4):294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 15.Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR. Trends in endometrial cancer incidence rates in the United States, 1999–2006. Journal of women's health. 2011;20(8):1157–63. doi: 10.1089/jwh.2010.2529. [DOI] [PubMed] [Google Scholar]
- 16.Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17(18):2413–7. doi: 10.1038/sj.onc.1202178. [DOI] [PubMed] [Google Scholar]
- 17.Salvesen HB, MacDonald N, Ryan A, Iversen OE, Jacobs IJ, Akslen LA, et al. Methylation of hMLH1 in a population-based series of endometrial carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6(9):3607–13. [PubMed] [Google Scholar]
- 18.Simpkins SB, Bocker T, Swisher EM, Mutch DG, Gersell DJ, Kovatich AJ, et al. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Human molecular genetics. 1999;8(4):661–6. doi: 10.1093/hmg/8.4.661. [DOI] [PubMed] [Google Scholar]
- 19.McCourt CK, Mutch DG, Gibb RK, Rader JS, Goodfellow PJ, Trinkaus K, et al. Body mass index: relationship to clinical, pathologic and features of microsatellite instability in endometrial cancer. Gynecologic oncology. 2007;104(3):535–9. doi: 10.1016/j.ygyno.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Westin SN, Lacour RA, Urbauer DL, Luthra R, Bodurka DC, Lu KH, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(36):5965–71. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zauber NP, Denehy TR, Taylor RR, Ongcapin EH, Marotta SP, Sabbath-Solitare M, et al. Microsatellite instability and DNA methylation of endometrial tumors and clinical features in young women compared with older women. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20(9):1549–56. [PubMed] [Google Scholar]
- 22.Network NCC;Pages2016.
- 23.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. The Journal of molecular diagnostics : JMD. 2008;10(4):293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer research. 2006;66(15):7810–7. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 25.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Leeuw WJ, Dierssen J, Vasen HF, Wijnen JT, Kenter GG, Meijers-Heijboer H, et al. Prediction of a mismatch repair gene defect by microsatellite instability and immunohistochemical analysis in endometrial tumours from HNPCC patients. J Pathol. 2000;192(3):328–35. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH701>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, et al. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A. 2003;100(10):5908–13. doi: 10.1073/pnas.1030231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuismanen SA, Moisio AL, Schweizer P, Truninger K, Salovaara R, Arola J, et al. Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am J Pathol. 2002;160(6):1953–8. doi: 10.1016/S0002-9440(10)61144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham RP, Kerr SE, Butz ML, Thibodeau SN, Halling KC, Smyrk TC, et al. Heterogenous MSH6 loss is a result of microsatellite instability within MSH6 and occurs in sporadic and hereditary colorectal and endometrial carcinomas. Am J Surg Pathol. 2015;39(10):1370–6. doi: 10.1097/PAS.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 30.Moline J, Mahdi H, Yang B, Biscotti C, Roma AA, Heald B, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecol Oncol. 2013;130(1):121–6. doi: 10.1016/j.ygyno.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, Sanchez-Heras AB, et al. Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PLoS One. 2013;8(11):e79737. doi: 10.1371/journal.pone.0079737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, Zapater P, Castillejo A, Barbera VM, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144(5):926–32. e1. doi: 10.1053/j.gastro.2013.01.044. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 33.Geurts-Giele WR, Leenen CH, Dubbink HJ, Meijssen IC, Post E, Sleddens HF, et al. Somatic aberrations of mismatch repair genes as a cause of microsatellite-unstable cancers. J Pathol. 2014;234(4):548–59. doi: 10.1002/path.4419. [DOI] [PubMed] [Google Scholar]
- 34.Haraldsdottir S, Hampel H, Tomsic J, Frankel WL, Pearlman R, de la Chapelle A, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147(6):1308–16. e1. doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]